Abstract
Objective
The importance of the cholinergic system for cognitive function has been well-documented in animal and human studies. The objective of this study was to elucidate the cognitive and functional connectivity changes associated with enhanced acetylcholine (ACh) levels. We hypothesized older adults with mild memory deficits would show behavioral and functional network enhancements with an acetylcholinesterase inhibitor treatment (donepezil) when compared to a placebo control group.
Methods
We conducted a 3-month, double-blind, placebo-controlled study on the effects of donepezil in twenty-seven older adults with mild memory deficits. Participants completed a delayed recognition memory task. FMRI scans were collected at baseline prior to treatment and at 3-month follow-up while on a 10 mg daily dose of donepezil or placebo.
Results
Donepezil treatment significantly enhanced the response time for face and scene memory probes when compared to the placebo group. A group-by-visit interaction was identified for the functional network connectivity of the left fusiform face area (FFA) with the hippocampus and inferior frontal junction, such that the treatment group showed increased connectivity over time when compared to the placebo group. Additionally, the enhanced functional network connectivity of the FFA and hippocampus significantly predicted memory response time at 3-month follow-up in the treatment group.
Interpretation
These findings suggest that increased cholinergic transmission improves goal-directed neural processing and cognitive ability and may serve to facilitate communication across functionally-connected attention and memory networks. Longitudinal fMRI is a useful method for elucidating the neural changes associated with pharmacological modulation and is a potential tool for monitoring intervention efficacy in clinical trials.
Introduction
The neurotransmitter acetylcholine (ACh) plays a critical role in cognition, supporting perception, attention, and learning and memory. Animal studies have revealed the role of ACh in attention and memory through local manipulation of cholinergic function. For instance, sustained attention tasks in rodents have been associated with upregulation of ACh and enhanced activity in cortical sensory and prefrontal cognitive control regions1,2. In contrast, lesions to cholinergic input to the prefrontal cortex and other neocortical regions in animals have demonstrated severe impairments in stimulus detection, working memory and spatial localization tasks3–5.
Human neuroimaging studies have also shown behavioral and neural effects of cholinergic upregulation, with reports of both enhancements and decrements6,7. A recent meta-analysis by Bentley and colleagues (2011) reviewed several pharmacological-neuroimaging studies, highlighting key differences in experimental design8. They reported that relative changes in neural activity were often related to task goals, such that attended stimuli resulted in increased activity while passively viewed or irrelevant stimuli commonly resulted in no change or decreased activity. In addition, the differences in reported findings varied based on drug type, brain region, study population, and drug duration, such that acute exposure of ACh upregulation had a greater impact on neural activity than prolonged exposure9. Furthermore, while healthy subjects with normal cholinergic function showed detrimental effects, hypocholinergic populations, such as individuals with Alzheimer’s disease (AD), showed beneficial effects with increased ACh levels. In AD patients, prefrontal, occipital, and hippocampal regions have shown increases in regional BOLD activity during a face-encoding task after 6 days of galantamine administration10. Additionally, increased fusiform gyrus activity has also been documented during face encoding after acute exposure to rivastigmine in AD patients11.
Previous studies on patients with mild cognitive impairment (MCI), a preclinical stage of AD, have also demonstrated cognitive and neural improvements after undergoing cholinergic treatments, including improved task accuracy and response time, enhanced neuropsychological test performance, and increased task-related brain activity11–13. MCI patients showed increased regional activity in prefrontal cortex during an n-back working memory task after 6 weeks of donepezil treatment when compared to a healthy control group13. Neuroimaging studies of ACh upregulation in MCI patients have mostly examined changes in regional whole-brain activity, leaving the possibility of changes in functional network connectivity unexplored. A recent study in rodents found that pharmacological manipulation using medetomidine, an alpha-two adrenergic agonist, modified large-scale brain networks in a dose-dependent manner, while not influencing regional neural activity14. Thus, functional network connectivity may be a more sensitive approach for detecting neuropharmacological changes due to the effect of cholinergic upregulation on spatially-distinct but functionally-related networks8,15,16.
The present study aimed to elucidate cholinergic interactions associated with attention and memory by examining changes in functional network connectivity in a donepezil treatment group relative to a placebo control group. All participants were older adults with mild memory impairment and represent a vulnerable population at-risk for further cognitive decline. The inclusion of a placebo control group offered additional insight into the naturally occurring changes that take place over time without an intervention, thus serving as an important control comparison. We hypothesized that increased ACh concentration through acetylcholinesterase inhibition (donepezil) would enhance behavioral performance and fMRI task-related functional network connectivity in a treatment group when compared to the placebo control group. Using a delayed recognition memory task, we interrogated functional connectivity in prefrontal cortex, parietal lobe and the hippocampus, areas responsive to ACh upregulation during attentionally-demanding stimulus encoding8,15–18.
Methods
Recruitment
Twenty-seven older adults with mild memory deficits between 50–80 years old were recruited for this study from the University of California, San Francisco Memory and Aging Center or through community screening. All participants gave written informed consent to participate, which was approved by the University of California, San Francisco Committee for Human Research. Participants were screened and diagnosed after a comprehensive neurological and neuropsychological evaluation. Screening for depression was done using the 30-item Geriatric Depression Scale (GDS) (self-report). Diagnosis of mild memory impairment was determined by consensus between the neurologist and neuropsychology specialist. Participants met the core clinical criteria for “Mild Cognitive Impairment due to Alzheimer’s disease” published by Albert and colleagues (2011) as a part of the international workgroup convened by the Alzheimer’s Association and the National Institute on Aging. These criteria include 1) concern regarding a change in cognition, 2) impairment in one or more cognitive domains, 3) preservation of independence in functional abilities, and 4) not demented19. Participants endorsed subjective memory decline over the past year and demonstrated objective memory impairment (≥ 1 SD below age- and education- matched normative values) on verbal or visual memory testing. Participants were excluded if they met criteria for dementia (DSM-IV), a history of a neurological disorder, current psychiatric illness, head trauma with loss of consciousness greater than 10 minutes, severe sensory deficits, or substance abuse. All participants were also screened for medical contraindications and were ineligible if they used donepezil, NMDA antagonists, antidepressants, anti-anxiety, narcotics, anticonvulsants, or antipsychotics.
Neuropsychological testing
Participants were administered a comprehensive screening battery of neuropsychological tests assessing memory, executive function, and visuospatial ability. Tests included the California Verbal Learning Test II (20-minute delayed recall)20, modified WMS-III Logical memory (15-minute delay)21, Benson figure (copy and 10-minute delayed recall)22, modified trail-making test A and B (time to complete)23, design fluency (number of unique designs in 60s)24, modified Stroop interference (number correct in 60s)23, letter fluency (D words in 60s)23, forward and backward digit span (longest length) and digit symbol (number correct in 60s)25.
Experimental paradigm
The experiment consisted of three conditions: participants were instructed to (1) Remember Faces and Ignore Scenes, (2) Remember Scenes and Ignore Faces, and (3) Passively View the Faces and Scenes (as a control condition). In all conditions, participants viewed four sequentially presented novel, grayscale images (2 faces and 2 scenes presented in a randomized order). Each image was presented for 800 msec, with a 200-msec blank-screen interstimulus interval. Presentation of stimuli was followed by a 9-sec delay period and a 1-second face or scene probe stimulus. Participants pressed a button to indicate whether the stimulus matched one of the previously presented stimuli. In the Passive View task, an arrow was presented, and participants pressed a button to indicate the direction of the arrow (Figure 1). Of note, in the passive view condition the same perceptual information was presented in the absence of an overt task, creating two important controls26–34. There was a physiological/neural control, which was the BOLD response during the passive face/scene viewing (‘encoding period’), used as a baseline for encoding-selective activity in the other tasks. There was also a response time control, which was the time to respond to the direction of an arrow presented during the ‘retrieval period’. This was used as a behavioral control of response time for the other tasks. As a simple discrimination task, it served as a baseline to indicate if treatment-induced changes in RT during the delayed-recognition tasks were selective or simply due to a generalized increase in response times. There were a total of 9 sessions (3 per condition) for each scan, lasting 4.5 minutes. Each session contained 10 trials of one task condition. Conditions were presented in a counterbalanced order across the study. Each participant practiced the task prior to the fMRI scanning session. Grayscale images of faces and natural scenes were 225 pixels wide and 300 pixels tall (14×18 cm), subtended 3 degrees of visual angle from fixation, and were presented foveally. All cue images were novel throughout the fMRI experiment and across visits.
Figure 1. Experimental Paradigm.

Participants were instructed to remember faces or scenes over a delay period, followed by a matched forced choice yes/no response. Reaction time and accuracy were recorded as performance measures. A passive view condition was included to control for bottom-up effects associated with treatment.
Scanning and drug dose schedule
Eligible participants were randomly assigned in a double-blind manner to either a placebo or treatment group. The randomization list was computer-generated, and a research assistant who was not affiliated with the study gave the assignments. All participants were given a de-identified subject number, and the association between subject number and group designation was stored in a sealed envelope. Participants completed a baseline fMRI scan prior to treatment. Following the scan, participants in the placebo group received a bottle of sugar pills, and participants in the treatment group received a bottle of 5 mg donepezil pills. This dose was to be taken daily for 1 month for drug stabilization and titration, consistent with current standard of care for donepezil treatment. A second fMRI scan was completed at this time (1 month post-baseline). Participants were then titrated to 10 mg of donepezil or placebo to be taken daily for 2 months, and a third fMRI scan was completed (3 months post-baseline). The study lasted for a total of 3 months, and the goal of the study was to examine baseline and 3 month follow-up differences at 10 mg of donepezil.
Behavioral performance analysis
Participants performed a forced choice yes/no recognition response for each trial, to indicate whether the probe matched one of the previously viewed stimuli (or the direction of an arrow during the passive view condition). These responses resulted in two behavioral measures: response time (RT) for the memory probe and accuracy (ACC) for stimulus recognition. To control for bottom-up effects due to treatment (e.g., elevated arousal), we used the passive view condition as an internal control within each subject. Thus, for the RT behavioral measure, the computed values for each participant were “faces RT – passive view RT” or “scenes RT – passive view RT.” ACC was the raw accuracy score computed as percent correct. To explore the effect of treatment over time, we conducted a 2-way Repeated Measures ANOVA with two groups and two timepoints, resulting in a 2×2 factorial design to examine the interaction of group-by-visit. The 2×2 ANOVA was completed to directly assess the differences between baseline and 3 month follow-up. Possible titration effects of 5 mg of donepezil or placebo after 1 month of treatment were also assessed for interactions for baseline vs. 1 month follow-up and 1 month vs. 3 month follow-up, as secondary analyses.
fMRI data acquisition
All fMRI data was collected on a Siemens 3T MAGNETOM Trio. Echo planar imaging data was acquired (FA=770, TE = 28 ms, TR = 2 s) with 29 interleaved axial slices and 1.8×1.8×3 mm voxels (FOV = 23 cm; 128 × 128 matrix). All pre-preprocessing of the data was conducted in SPM5. Raw blood oxygen level dependent (BOLD) data were corrected for slice-timing acquisition and motion-artifacts. A 5 mm isotropic Gaussian smoothing kernel was applied prior to modeling the data. High-resolution T1-MPRAGE images were also acquired (1 mm3 voxels).
Separate regressors were modeled for each stage of the trial (encode, delay, and probe) and convolved with a canonical Gaussian hemodynamic response function. Each session was modeled separately and included regressors for the encode, delay, and probe stages, which were modeled separately for correct and incorrect trials. In addition there were 6 motion regressors for the X, Y, Z directions and pitch, roll, and yaw rotations to control for possible subject movement. There were a total of 108 regressors in the GLM model for the univariate analysis. The instruction period at the start of each block was removed from the analysis. Correct trials were subjected to further analysis.
fMRI localizer scan
To allow us to independently localize stimulus-selective regions in each participant’s visual association cortex, participants performed a brief functional localizer task before beginning the main experiment. Participants were presented with alternating 16-s blocks of face stimuli, scene stimuli, and rest periods (7 blocks of each type) and were instructed to attend to the stimuli and to indicate with a button press whenever they noticed an immediate (1-back) repeat of a stimulus. Face-selective ROIs were defined within the fusiform face area (FFA) of the fusiform gyrus35, and scene-selective ROIs were defined within the parahippocampal place area (PPA) of the parahippocampal/anterior lingual gyrus36 for each participant. Each participant’s native space ROI was defined as the cluster of 35 contiguous voxels within each anatomical region with the highest cluster t-value on a face-scene contrast for FFA and scene-face contrast for PPA31, 37–40. The FFA and PPA ROI clusters were based on 35 contiguous voxels identified at p<0.01 to reach a whole-brain cluster extent correction of p<.05.
fMRI functional connectivity analysis
Functional connectivity maps of the regions of interest were generated by extracting beta values for every stage of every trial and correlating these values using the beta series correlation method41, 42. The beta- series correlation method was a functional connectivity analysis approach that utilized trial-by-trial variability to measure covariance in activity between spatially disparate regions, and offered a powerful tool for assessing network interactions during sub-stages of a trial26, 37–39, 41–45. Single-subject z-transformed maps were normalized to the MNI space (2 mm3 voxels) and Gaussian smoothed (5 mm FWHM) for the group analysis. Functional network connectivity during the encode stage was examined within the prefrontal cortex, parietal lobe and hippocampus. To account for possible non-specific perfusion effects of donepezil, an additional analysis using primary motor cortex (M1) as a control region was conducted. The peak MNI coordinates (−37 −21 58) from a large meta-analysis on 126 motor studies46 was used to form a 4 mm sphere (36 voxels) around the peak. Monte Carlo simulations of 1000 permutations were conducted to control for multiple tests and a minimum cluster size of 24 voxels was accepted for a cluster-corrected significance of p<0.05 for each ROI (WFU Pickatlas toolbox)47. To explore the neural effects of treatment over time specific to the cognitive task, a 3-way ANOVA with two groups (placebo, treatment), 2 timepoints (baseline, follow-up), and 2 conditions (face/scene, passive view) was performed, resulting in a 2×2×2 factorial design with main effects for group, visit, and condition in order to examine the interaction of group-by-visit-by-condition.
Results
Demographics and Neuropsychological Screening Tests
Our groups did not differ on any demographic or neuropsychological screening test. As expected, all participants performed significantly lower on tests of memory, with respect to age- and education-matched normative values as indicated by asterisks in Table 1. There were no other significant differences. One participant was excluded from the study and analysis due to poor drug tolerability at 10 mg of donepezil. All other participants reported study compliance.
Table 1.
Demographics and Neuropsychological Testing
| Subject Demographics | Placebo | Treatment | Group difference | |
|---|---|---|---|---|
| N (M/F) | 13 (7/6) | 13 (8/5) | 0.69 | |
| Age | 69.2 (8.2) | 63.8 (7.4) | 0.10 | |
| Handedness (Left/Right) | 2/11 | 1/12 | 0.54 | |
| Education | 16.3 (2.0) | 16.9 (2.5) | 0.50 | |
| Geriatric Depression Scale | 4.5 (3.9) | 7.3 (6.1) | 0.16 | |
|
| ||||
| Neuropsychological Tests | ||||
|
| ||||
| Global Memory | MMSE (max. 30) | 28.4 (1.9) | 28.3 (0.9) | 0.89 |
| CVLT Long Delay free recall (max. 16) | 5.4 (3.5)* | 6.9 (3.2)* | 0.26 | |
| Delayed Benson figure recall (max. 17) | 7.9 (3.7)* | 9.4 (4.1)* | 0.36 | |
| Logical Memory Immediate (max. 25) | 9.4 (3.8)* | 10.5 (3.2)* | 0.47 | |
| Logical Memory Delayed (max. 25) | 6.6 (3.6)* | 8.9 (3.8)* | 0.18 | |
| Digit Span Backward | 5.3 (1.3) | 4.5 (1.1) | 0.12 | |
| Attention/Processing Speed Executive Function | Digit Span Forward | 6.7 (1.1) | 6.7 (0.8) | 0.99 |
| WAIS-III Digit Symbol (90 sec.) | 42.5 (8.7) | 45.6 (11.9) | 0.65 | |
| Number Sequencing (max. 150 sec.) | 36.4 (12.1) | 30.8 (10.3) | 0.27 | |
| Mod. Trailmaking Test B (max. 300 sec.) | 83.4 (36.5) | 84.8 (21.3) | 0.92 | |
| Stroop Interference (# correct in 60 sec.) | 41.3 (10.2) | 40.7 (11) | 0.92 | |
| Verbal fluency (D words in 60 sec.) | 14.9 (4.5) | 13.5 (5.1) | 0.49 | |
| Visuospatial | Copy of Benson figure (max. 17) | 15.3 (1.2) | 15.0 (1.3) | 0.52 |
There were no significant group differences on demographics or neuropsychological tests (as shown by p-values).
indicates significant within-group differences from age- and education- matched normative values at p<0.05. As expected based on diagnostic criteria, the memory scores for both the placebo and treatment group were significantly lower than normative values. Values are presented as the mean and standard deviation in parentheses. The p-values of group differences were computed from a 2-sample t-test or chi-squared test.
Behavioral Performance
For response time on the face memory task, there was a significant group-by-visit interaction for baseline vs. 3 month follow-up [F(1,24)=6.86, p<0.04, Cohen’s d (d)=1.07]. As a secondary analysis to explore for possible titration effects at 1 month follow-up, additional 2×2 ANOVA were computed for each pairwise comparison. There was no significant interaction for baseline vs. 1 month follow-up [F(1,24)=2.55, p=0.12, d=0.52] or 1 month vs. 3 month follow-up [F(1,24)=1.28, p=0.27, d=0.39]. For response time on the scene memory task, there was a significant group-by-visit interaction for baseline vs. 3 month follow-up [F(1,24)=4.76, p<0.04, d=0.89] (Figure 2). When probing the pairwise comparisons with 1 month follow-up, again there was no significant interaction for baseline vs. 1 month follow-up [F(1,24)=0.18, p=0.68, d=0.15] or 1 month vs. 3 month follow-up [F(1,24)=2.99, p=0.10, d=0.62].
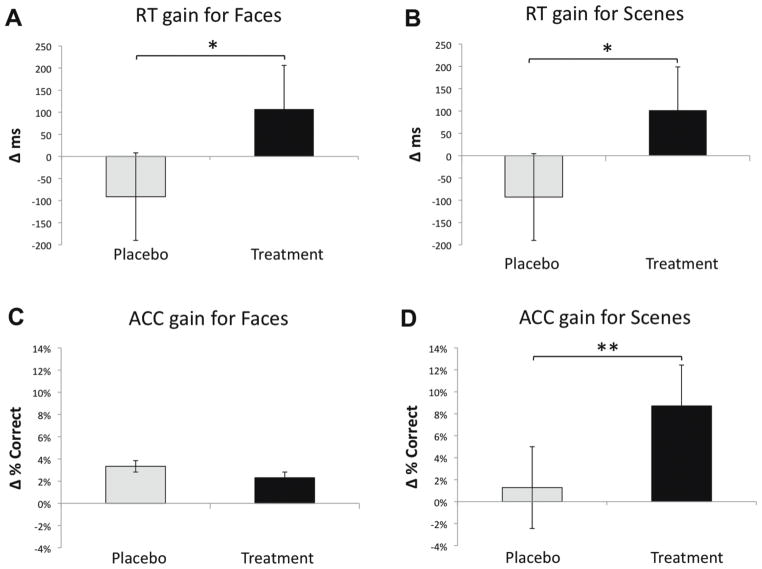
Figure 2. Response Time and Accuracy Change Scores.
Behavioral data showing the difference between baseline and follow-up for response time and accuracy in the two groups. 2-way repeated measures ANOVA assessing group-by-visit interactions for reaction time (A, B) and accuracy (C, D) for faces and scenes. Gain scores represent the behavioral improvement between baseline and follow-up. Single asterisks (*) represent a significant interaction (p<0.05), and double asterisks (**) represent a non-significant trend (p<0.10). The error bars represent standard error of the mean.
RT change, calculated as baseline – 3-month follow-up, revealed an RT gain for the treatment group of 107 ms for faces (baseline: 796 ms, follow-up: 689 ms) and 102 ms for scenes (baseline: 643 ms, follow-up: 541 ms), while there was an RT loss for the placebo group of −91 ms for faces (baseline: 625 ms, follow-up: 714 ms) and −93 ms for scenes (baseline: 586 ms, follow-up: 679 ms). The change in RT for the treatment group was significantly different from the placebo group for faces [t(24)=2.62, p<0.02, d=1.07] and scenes [t(24)=2.18, p<0.04, d=0.89]. Interestingly, these results revealed that the placebo group actually became slower three months later, presumably reflecting that this was a vulnerable population for further cognitive decline. Of note, RT on a simple discrimination task, used as a control task, was not different between the baseline and 3 month follow-up in the treatment [t(12)= −0.78, p=0.45, d=0.45] or placebo [t(12)=0.65, p=0.53, d=0.38] groups. Additionally, there were no significant RT differences between groups at baseline for any condition.
There were no significant 2×2 interactions for group by visit for ACC of face stimuli. There was a trend for a group-by-visit interaction for ACC of scene stimuli in the analysis of baseline vs. 3 month follow-up [F(1,24)=2.96, p=0.10, d=0.63] and baseline vs. 1 month follow-up [F(1,24)=3.40, p=0.08, d=0.76.]. The ACC gain (calculated as follow-up – baseline), suggested the treatment group showed a trend to perform better after treatment. Similar to RT, there were no significant ACC differences between groups at baseline for any condition.
We next assessed within-group changes in performance over time in post-hoc pair-wise comparisons. For the treatment group, there was a significant improvement in RT for faces (t(12)=1.97, p=0.04, d=1.14) and scenes (t(12)=1.73, p=0.05, d=1.00), in addition to a significant improvement in ACC for scenes (t(12)= −3.16, p=0.004, d=−1.82). There was no difference in ACC for faces. For the placebo group, there was a significant RT slowing for faces (t(12)= −1.73, p=0.05, d=1.00). There was no significant change in RT for scenes or ACC for faces or scenes in the placebo group.
As a secondary RT analysis, we investigated the difference in RT across all participants at baseline for correctly remembered vs. incorrectly remembered trials. The RT for correctly remembered trials were faster than incorrectly remembered trials (t(25)= −8.23, p<0.0001, d=3.29). This supports that RT reflects memory performance for the delayed recognition task48, 49.
Overall, the treatment group showed significant enhancement in performance on two behavioral measures (RT for faces and RT for scenes), and the placebo group showed a significant decrement on one behavioral measure (RT for faces). It is important to note that the RT gain in the treatment group was obtained with values corrected by an independent measure of RT in the discrimination control task; thus the findings represent changes in cognitive-specific memory performance. Furthermore, there was no change in control task RT from baseline to follow-up in the treatment (or placebo) group, suggesting that treatment did not result in general motoric enhancements or arousal.
Fusiform Face Area (FFA) and Parahippocampal Place Area (PPA) BOLD signal
To investigate the neural effects associated with the significant behavioral changes, we assessed group-by-visit interactions for the FFA and PPA for baseline vs. 3 month follow-up (herein, denoted as “Baseline” and “Follow-up”, respectively). To account for physiological changes associated with bottom-up effects of cholinergic enhancement, we used BOLD measures during the stimulus-viewing period of the passive view condition as a control for the encoding-period of the face and scene memory conditions (e.g., activity for face memory – activity for passive view). There were no main effects of group or visit on univariate activity in the FFA or PPA. We found a significant group-by-visit interaction for the left FFA [F(1,24)=5.47, p<0.03, d=0.85] (Figure 3). The right FFA did not show a significant change associated with treatment [F(1,24)=1.30, p=0.27, d=0.39]. In addition, the left and right PPA did not show a significant change associated with treatment [F(1,24)=0.53, p=0.48, d=0.29 and F(1,24)=0.67, p=0.42, d=0.32, respectively]. To guide the network connectivity analysis based on these findings, we subjected the left FFA ROI to functional connectivity analysis.
Figure 3. Increased Activity in the Left FFA ROI for Face Encoding.
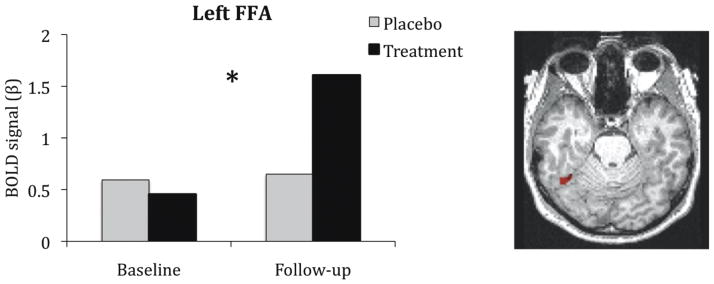
The BOLD signal in the left FFA region of interest, defined by an independent localizer task. The left FFA showed a significant group-by-visit interaction, as indicated by the asterisk (*), in which the BOLD signal increased for the treatment group and remained constant in the placebo control group, relative to baseline. This ROI displayed on a representative native T1-image was used as a seed for functional connectivity analysis to explore network-based changes associated with ACh upregulation.
Functional Network Connectivity
Using the FFA as a seed region, we performed a region of interest analysis in the prefrontal cortex, parietal lobe, and hippocampus using a three-way ANOVA of group (treatment, placebo) × visit (baseline, follow-up) × condition (face memory (FM), passive view (PV)) to explore neural changes during the encode stage after treatment. Functional connectivity in these regions were analyzed if they survived correction for multiple comparisons and showed no group differences at the baseline visit. No regions in the parietal lobe survived permutation testing to correct for multiple comparisons and showed no group differences at baseline.
For the prefrontal cortex analysis, the right inferior frontal junction (IFJ) showed a significant 3-way interaction (F(1,24)=7.38, p=0.01, d=0.68). To explore this 3-way interaction further, the FM and PV conditions were analyzed separately. There was a significant group-by-visit interaction for FM, such that functional connectivity increased in the treatment group after 3 months when compared to the placebo group [F(1,24)=9.56, p<0.005, d=1.25] (Figure 4a). The group-by-visit interaction for PV was not significant F(1,24)=0.33, p=0.57, d=0.24), nor were the main effects of group or visit significant, suggesting that the enhanced connectivity with the IFJ was specific to face memory processing.
Figure 4. Enhanced Network Connectivity in the Hippocampus and IFJ with FFA.
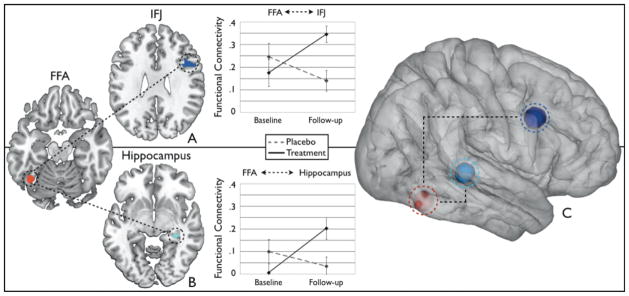
The FFA network connectivity to the hippocampus and inferior frontal junction reveal significant enhancements after treatment (solid lines) when compared to the placebo group (dashed lines) (p<.05). These regions have been implicated in memory and cognitive control networks with known cholinergic projections from the medial septal nuclei and nucleus basalis of the basal forebrain, respectively Increases in the functional connectivity of (A) FFA and IFJ and (B) FFA and hippocampus from baseline to follow-up in the treatment group. (C) Schematic of the network connectivity of memory and attention systems with the FFA. The ROIs are illustrated using 3-D spheres around the peak voxel rendered onto a 3-D brain.
For the hippocampal analysis, the right hippocampus showed a significant 3-way interaction (F(1,24)=5.17, p=.03, d=0.55). To explore this further, the FM and PV conditions were analyzed separately. There was a significant group-by-visit interaction for FM, such that functional connectivity increased after treatment when compared to the placebo group [F(1,24)=6.67, p<0.02, d=1.01] (Figure 4b). The group-by-visit interaction for PV was not significant F(1,24)=0.59, p=.45, d=0.31), nor were the main effects of group or visit significant, also suggesting that the enhanced connectivity in the hippocampus was specific to face memory processing.
An M1 control region did not show a significant group-by-visit interaction (F(1,24)=0.03, p=0.86, d=0.06) or main effect of group (F(1,24)=0.11, p=0.74, d=0.13) or time (F(1,24)=1.13, p=0.26, d=0.47), further suggesting that the effects reported for FFA connectivity with the IFJ and hippocampus are task-specific and not a product of global enhancement. Figure 4c illustrates the 3-D projections of the IFJ, hippocampus, and FFA regions rendered on a 3-D brain.
Although we found a significant interaction in the ANOVA for the behavioral and functional connectivity data analyzed separately, we also assessed a direct relationship between the two measures to strengthen our interpretation that the changes in functional connectivity were related to task performance. Across-participant correlation analyses revealed that there was a significant relationship between the RT for faces and the functional connectivity of the FFA and hippocampus in the treatment group at the follow-up visit (r=−0.68, p=0.01, d=−1.85), which was not found in the placebo group (r=0.16, p=0.62, d=0.32) (Figure 5). These data suggest that the relationship between faster face RT and increased functional connectivity to the hippocampus are associated with cholinergic treatment.
Figure 5. Enhanced Functional Connectivity of FFA and Hippocampus Predicts Performance.
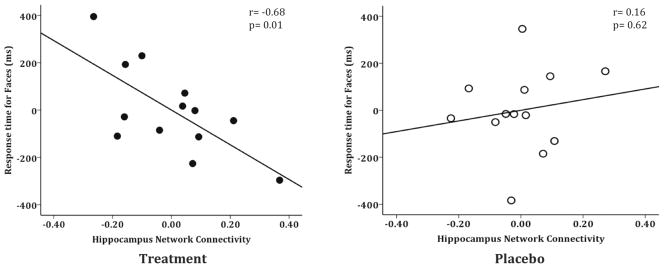
The treatment group demonstrated a significant neurobehavioral relationship between the functional connectivity of the FFA and hippocampus and response time for faces at the follow-up visit (r=−0.68, p=0.01) (solid dots). The placebo control group did not show a significant relationship (r=0.16, p=0.62) (open dots). Displayed partial regression plots are controlled for face response time at baseline. These data suggest that enhanced communication between the functionally-connected regions of the FFA and hippocampus predicts better performance.
Discussion
The goal of the present study was to elucidate the neurobiological changes associated with cholinergic modulation in older adults with mild memory impairment after a 3-month treatment period. Our findings revealed cognitive and functional network enhancements after donepezil treatment, which were not shown in the placebo control group. The treatment group had improved response time and enhanced functional network connectivity of the hippocampus and inferior frontal junction with the FFA. This result was specific to attentionally-demanding face memory processing, as there was no observed effect when passively viewing the same stimuli without an overt memory task. The treatment group demonstrated a significant group-by visit interaction and neurobehavioral correlation at the 3-month visit, while the placebo group did not show a significant correlation. In fact, the placebo group demonstrated signs of cognitive decline on the memory task. This is the first study to show that ACh upregulation remediates functional network connectivity to the hippocampus relative to baseline function and that the reinstated function predicts improved delayed recognition performance after donepezil treatment.
Previous studies on the effect of cholinergic modulation in MCI patients have primarily shown increased regional brain activity in several areas, including visual association cortex, frontal cortex, and the hippocampus10,13,50. Some studies identified a neural change after treatment in lieu of a cognitive effect11, reported a cognitive or neural benefit without a predictive relationship10, or produced significant within-group treatment effects without a placebo control13,51. The present study extends the existing body of literature on ACh upregulation in older adults with mild memory deficits by demonstrating functional network enhancements and associated cognitive benefits in a donepezil treatment group compared to a placebo control group over a 3-month period. Importantly, the placebo control group, which was comprised of older adults with mild memory deficits similar to the treatment group at baseline, actually demonstrated a slowing of response time on the delayed recognition task after 3 months. Thus, the treatment not only prevented decline in performance that was exhibited if there was no treatment, but also resulted in significantly faster responses on the task. This revealed the robust influence of ACh upregulation that could not have been demonstrated without a placebo control group. The study design also allowed for investigation of goal-directed effects of ACh upregulation. Our findings showed both increased regional activity (i.e., FFA) and enhanced functional connectivity (FFA-to-HPC, IFJ) during the task-relevant condition of face memory and not during the passive view condition. This is consistent with previous studies that documented the specificity of neural changes associated with task-relevant goals, such that attended stimuli resulted in increased activity while passively viewed stimuli resulted in no change8.
The role of the hippocampus in face encoding has been shown in single cell recordings and electroencephalography studies of non-human primates52,53 and human functional neuroimaging studies54,55. The activity of the hippocampus during encoding differs in patients with memory deficits, such that individuals with early memory dysfunction have a hyperactive neural signal (i.e., MCI) while those with more severe memory impairment have a hypoactive neural signal (i.e., AD)56,57. The role of the hyperactive signal is still under investigation, as some believe it is compensatory for degradation in task performance, while others view it as an indicator of neuronal dysfunction. A recent study demonstrated that reducing hippocampal activity to a control level using antiepileptic levetiracepam treatment improves task performance58. Our data support that re-establishing functional connectivity between the hippocampus and stimulus-selective visual cortex relates to improved cognition. A study by Goveas and colleagues also provide interesting evidence for increased intrinsic (resting state) hippocampal connectivity with the prefrontal cortex in AD patients after 12 weeks of donepezil treatment, which correlated with improved cognition on the AD Assessment Scale-cognitive subscale (ADAS-cog)59. This study was open-label and did not have a placebo control group, so the possibility of a placebo or learning effect is unknown. Another study by this group also reported similar increases in intrinsic connectivity between the medial prefrontal cortex and middle and posterior cingulate gyri in AD patients, which again correlated with ADAS-cog60. Ricciardi and colleagues conducted a recent study examining the effects of physostigmine in healthy young adults during a task-on selective attention task61. They report decreased connectivity between task-specific ventral visual regions and prefrontal and parietal regions. These findings are consistent with previous studies, which concluded that cholinergic upregulation in healthy populations results in decreased BOLD signal due to enhanced neural efficiency8. These studies support the importance of understanding what neural mechanisms maybe involved in ACh upregulation and highlights potential differences in at-risk populations with memory impairment.
The role of the cholinergic system in cognitive control tasks supported by regions of the middle frontal gyrus has been shown in animal studies on distraction and sustained attention1,62. In humans, the right IFJ has been implicated in similar cognitive control tasks of attentionally-mediated perceptual and memory processing63. Strikingly, a study by Zanto and colleagues44 demonstrated the causal role of IFJ in goal-directed perceptual processing, such that perturbation of the IFJ activity resulted in impaired task performance.
The inferior frontal junction receives projections from the nucleus-basalis while the hippocampus receives cholinergic innervations from the medial septal region of the basal forebrain. This anatomical division of basal forebrain projections to separate cortical regions may support distinct cognitive networks for memory and attention8. Our findings support this model as we attribute the FFA-hippocampus network to face memory encoding and FFA-IFJ connectivity to cognitive control mechanisms of face encoding. Moreover, the recruitment of cognitive control regions for enhanced memory encoding has been suggested to combat performance decline or maintain task rules in the presence of distraction64.
Changes in cholinergic function may have an impact on the pathogenesis of age-associated or pathological memory impairment. Tonic increases in cholinergic signaling are implicated in supporting top-down control of attention, and attentional effort (for a review see Hasselmo and Sarter, 2011)65. In its role as a neuromodulator, ACh increases gain functions by causing post synaptic changes in neuronal excitability, and coordinates the firing of groups of neurons66–69. The basal forebrain undergoes degeneration with age70, and degeneration of cholinergic neurons of the basal forebrain is one of the earliest pathological events in AD71–76. Enhanced basal forebrain degeneration can be detected at the earliest signs of AD-related impairment70. Age-related cognitive impairment may be caused by loss of cholinergic modulation that normally supports downstream neuronal excitability and enhances synchronized activity across populations of neurons.
Possible hemodynamic confounds are a consistent concern in pharmaco-fMRI, as it is still unclear how perfusion influences functional neuroimaging measurements (i.e., BOLD signal)8. However, while donepezil can increase cerebral blood flow77, we believe it is unlikely that the effects we currently describe are a result of these confounds. Our critical predictions regarding ACh rely on condition × drug and region × drug interactions, not main effects78. The influence of donepezil on functional connectivity is also unlikely to arise from hemodynamic confounds because these measures rely on correlated activity and not overall activity changes. Furthermore, the lack of a treatment effect in our M1 control region supports that our conclusions are not likely due to global perfusion effects associated with ACh upregulation.
Additional longitudinal studies would be useful in examining the persistence of treatment benefits. Exploring the changes in brain network communication associated with treatment could be a sensitive approach to disease monitoring, treatment efficacy, or proof of concept preclinical trials. Longitudinal task fMRI is a potential tool for tracking cognitive and functional network enhancements associated with any therapeutic intervention.
Acknowledgments
This work was supported by Pfizer/Eisai (A.G.) and K01AG034175 (J.P.). We thank Drs. Joaquin Anguera, Ezequiel Morsella, Peter Wais, and David Ziegler for providing feedback on earlier versions of this manuscript.
References
- 1.Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. Journal of Neuroscience. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St Peters M, Demeter E, Lustig C, et al. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. Journal of Neuroscience. 2011;31:9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins TW, Everitt BJ, Marston HM, et al. Comparative effects of ibotenic acid- and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. Behavioural Brain Research. 1989;35:221–240. doi: 10.1016/s0166-4328(89)80143-3. [DOI] [PubMed] [Google Scholar]
- 4.Chudasama Y, Dalley JW, Nathwani F, et al. Cholinergic modulation of visual attention and working memory: dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learning and Memory. 2004;11:78–86. doi: 10.1101/lm.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croxson PL, Kyriazis DA, Baxter MG. Cholinergic modulation of a specific memory function of prefrontal cortex. NatNeurosci. 2011;14:1510–1512. doi: 10.1038/nn.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furey ML, Pietrini P, Haxby JV, et al. Cholinergic stimulation alters performance and task-specific regional cerebral blood flow during working memory. Proc Natl Acad Sci USA. 1997;94:6512–6516. doi: 10.1073/pnas.94.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuah LY, Chee MW. Cholinergic augmentation modulates visual task performance in sleep-deprived young adults. Journal of Neuroscience. 2008;28:11369–11377. doi: 10.1523/JNEUROSCI.4045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentley P, Driver J, Dolan RJ. Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Progress in Neurobiology. 2011;94:360–388. doi: 10.1016/j.pneurobio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goekoop R, Scheltens P, Barkhof F, Rombouts SA. Cholinergic challenge in Alzheimer patients and mild cognitive impairment differentially affects hippocampal activation--a pharmacological fMRI study. Brain. 2006;129:141–157. doi: 10.1093/brain/awh671. [DOI] [PubMed] [Google Scholar]
- 10.Goekoop R, Rombouts SA, Jonker C, et al. Challenging the cholinergic system in mild cognitive impairment: a pharmacological fMRI study. Neuroimage. 2004;23:1450–1459. doi: 10.1016/j.neuroimage.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Rombouts SA, Barkhof F, Van Meel CS, Scheltens P. Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;73:665–671. doi: 10.1136/jnnp.73.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: insights from functional MRI studies. Neuropsychologia. 2008;46:1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saykin AJ, Wishart HA, Rabin LA, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127:1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- 14.Nasrallah FA, Tan J, Chuang KH. Pharmacological modulation of functional connectivity: alpha2-adrenergic receptor agonist alters synchrony but not neural activation. Neuroimage; 60:436–446. doi: 10.1016/j.neuroimage.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Bentley P, Driver J, Dolan RJ. Modulation of fusiform cortex activity by cholinesterase inhibition predicts effects on subsequent memory. Brain. 2009;132:2356–2371. doi: 10.1093/brain/awp176. [DOI] [PubMed] [Google Scholar]
- 16.Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 17.Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Dickerson BC. Functional magnetic resonance imaging of cholinergic modulation in mild cognitive impairment. Curr Opin Psychiatry. 2006;19:299–306. doi: 10.1097/01.yco.0000218602.25346.c6. [DOI] [PubMed] [Google Scholar]
- 19.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement; 7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 21.Wechsler DA. Wechsler Memory Scale - Revised. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- 22.Possin KL, Laluz VR, Alcantar OZ, et al. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer’s disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011;49:43–48. doi: 10.1016/j.neuropsychologia.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Delis D, Kaplan EB, Kramer J. The Delis-Kaplan Executive Function System. The Psychological Corporation; 2001. [Google Scholar]
- 25.Wechsler DA. Test Manual. New York: Psychological Corporation; 1981. Wechsler Adult Intelligence Scale - Revised. [Google Scholar]
- 26.Gazzaley A, Rissman J, Cooney J, et al. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17 (Suppl 1):i125–135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gazzaley A, Cooney JW, McEvoy K, et al. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- 28.Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- 29.Gazzaley A, Clapp W, Kelley J, et al. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci USA. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clapp WC, Gazzaley A. Distinct mechanisms for the impact of distraction and interruption on working memory in aging. Neurobiol Aging. 2010;33:134–148. doi: 10.1016/j.neurobiolaging.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clapp WC, Rubens MT, Gazzaley A. Mechanisms of working memory disruption by external interference. Cereb Cortex. 2009;20:859–872. doi: 10.1093/cercor/bhp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanto TP, Hennigan K, Ostberg M, et al. Predictive knowledge of stimulus relevance does not influence top-down suppression of irrelevant information in older adults. Cortex. 2010;46:564–574. doi: 10.1016/j.cortex.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wendelken C, Baym CL, Gazzaley A, Bunge SA. Neural indices of improved attentional modulation over middle childhood. Dev Cogn Neurosci; 1:175–186. doi: 10.1016/j.dcn.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutman AM, Clapp WC, Chadick JZ, Gazzaley A. Early top-down control of visual processing predicts working memory performance. J Cogn Neurosci; 22:1224–1234. doi: 10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- 37.Bollinger J, Rubens MT, Zanto TP, Gazzaley A. Expectation-driven changes in cortical functional connectivity influence working memory and long-term memory performance. J Neurosci. 2010;30:14399–14410. doi: 10.1523/JNEUROSCI.1547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bollinger J, Rubens MT, Masangkay E, et al. An expectation-based memory deficit in aging. Neuropsychologia. 2011;49:1466–1475. doi: 10.1016/j.neuropsychologia.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chadick JZ, Gazzaley A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nat Neurosci. 2011;14:830–832. doi: 10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clapp WC, Rubens MT, Sabharwal J, Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proc Natl Acad Sci USA. 2009;108:7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gazzaley A, Rissman J, D’Esposito M. Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci. 2004;4:580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- 42.Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 43.Kalkstein J, Checksfield K, Bollinger J, Gazzaley A. Diminished top-down control underlies a visual imagery deficit in normal aging. J Neurosci; 31:15768–15774. doi: 10.1523/JNEUROSCI.3209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wais PE, Rubens MT, Boccanfuso J, Gazzaley A. Neural mechanisms underlying the impact of visual distraction on retrieval of long-term memory. J Neurosci. 2010;30:8541–8550. doi: 10.1523/JNEUROSCI.1478-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31:1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 48.Macleod C, Nelson T. Response Latency and Response Accuracy as Measures of Memory. Acta Psychol (Amst) 1984;57:215–235. [Google Scholar]
- 49.Heathcote A, Freeman E, Etherington J, et al. A dissociation between similarity effects in episodic face recognition. Psychon Bull Rev. 2009;16:824–831. doi: 10.3758/PBR.16.5.824. [DOI] [PubMed] [Google Scholar]
- 50.Petrella JR, Krishnan S, Slavin MJ, et al. Mild cognitive impairment: evaluation with 4-T functional MR imaging. Radiology. 2006;240:177–186. doi: 10.1148/radiol.2401050739. [DOI] [PubMed] [Google Scholar]
- 51.Goekoop R, Barkhof F, Duschek EJ, et al. Raloxifene treatment enhances brain activation during recognition of familiar items: a pharmacological fMRI study in healthy elderly males. Neuropsychopharmacology. 2006;31:1508–1518. doi: 10.1038/sj.npp.1300956. [DOI] [PubMed] [Google Scholar]
- 52.Bruce C, Desimone R, Gross CG. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. Journal of Neurophysiology. 1981;46:369–384. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- 53.Jutras MJ, Fries P, Buffalo EA. Gamma-band synchronization in the macaque hippocampus and memory formation. Journal of Neuroscience. 2009;29:12521–12531. doi: 10.1523/JNEUROSCI.0640-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greicius MD, Krasnow B, Boyett-Anderson JM, et al. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- 55.Haxby JV, Ungerleider LG, Horwitz B, et al. Face encoding and recognition in the human brain. Proc Natl Acad Sci USA. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- 58.Bakker A, Krauss GL, Albert MS, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goveas JS, Xie C, Ward BD, et al. Recovery of hippocampal network connectivity correlates with cognitive improvement in mild Alzheimer’s disease patients treated with donepezil assessed by resting-state fMRI. J Magn Reson Imaging; 34:764–773. doi: 10.1002/jmri.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li W, Antuono PG, Xie C, et al. Changes in regional cerebral blood flow and functional connectivity in the cholinergic pathway associated with cognitive performance in subjects with mild Alzheimer’s disease after 12-week donepezil treatment. Neuroimage; 60:1083–1091. doi: 10.1016/j.neuroimage.2011.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ricciardi E, Handjaras G, Bernardi G, et al. Cholinergic enhancement reduces functional connectivity and BOLD variability in visual extrastriate cortex during selective attention. Neuropharmacology; 64:305–313. doi: 10.1016/j.neuropharm.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology. 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- 63.Demeter E, Hernandez-Garcia L, Sarter M, Lustig C. Challenges to attention: a continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. Neuroimage. 2011;54:1518–1529. doi: 10.1016/j.neuroimage.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarter M, Paolone G. Deficits in attentional control: cholinergic mechanisms and circuitry-based treatment approaches. Behav Neurosci. 2011;125:825–835. doi: 10.1037/a0026227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology; 36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 67.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 68.Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- 69.Kawai H, Lazar R, Metherate R. Nicotinic control of axon excitability regulates thalamocortical transmission. Nat Neurosci. 2007;10:1168–1175. doi: 10.1038/nn1956. [DOI] [PubMed] [Google Scholar]
- 70.Grothe M, Heinsen H, Teipel S. Longitudinal measures of cholinergic forebrain atrophy in the transition from healthy aging to Alzheimer’s disease. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pearson RC, Sofroniew MV, Cuello AC, et al. Persistence of cholinergic neurons in the basal nucleus in a brain with senile dementia of the Alzheimer’s type demonstrated by immunohistochemical staining for choline acetyltransferase. Brain Res. 1983;289:375–379. doi: 10.1016/0006-8993(83)90046-x. [DOI] [PubMed] [Google Scholar]
- 72.Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 73.Whitehouse PJ, Price DL, Struble RG, et al. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 74.Whitehouse PJ, Struble RG, Clark AW, Price DL. Alzheimer disease: plaques, tangles, and the basal forebrain. Ann Neurol. 1982;12:494. doi: 10.1002/ana.410120517. [DOI] [PubMed] [Google Scholar]
- 75.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 76.Bowen DM, Smith CB, White P, Davison AN. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99:459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- 77.Tateno M, Kobayashi S, Utsumi K, et al. Quantitative analysis of the effects of donepezil on regional cerebral blood flow in Alzheimer’s disease by using an automated program, 3DSRT. Neuroradiology. 2008;50:723–727. doi: 10.1007/s00234-008-0401-y. [DOI] [PubMed] [Google Scholar]
- 78.Buckner RL, Snyder AZ, Sanders AL, et al. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;12 (Suppl 2):24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]