Summary
Few families of signaling factors have been implicated in the control of development. Here, we identify the neuropeptides nociceptin and somatostatin, a neurotransmitter and neuroendocrine hormone, as a class of developmental signals in both chick and zebrafish. We show that signals from the anterior mesendoderm are required for the formation of anterior placode progenitors, with one of the signals being somatostatin. Somatostatin controls ectodermal expression of nociceptin, and both peptides regulate Pax6 in lens and olfactory progenitors. Consequently, loss of somatostatin and nociceptin signaling leads to severe reduction of lens formation. Our findings not only uncover these neuropeptides as developmental signals but also identify a long-sought-after mechanism that initiates Pax6 in placode progenitors and may explain the ancient evolutionary origin of neuropeptides, predating a complex nervous system.
Highlights
-
•
Anterior mesendoderm promotes sensory progenitor specification
-
•
Neuropeptides promote lens and olfactory epithelium fate
-
•
Neuropeptides induce Pax6, the master regulator of eye formation
Placode progenitors generate major components of sense organs. Lleras-Forero et al. show that neuropeptides control early placode development: mesendoderm-derived somatostatin promotes ectodermal nociceptin expression. Together, both peptides regulate Pax6 in lens and olfactory progenitors. Consequently, loss of somatostatin and nociceptin signaling leads to severe reduction of lens formation.
Introduction
In the vertebrate head, multipotent progenitor cells give rise to crucial components of sense organs and sensory ganglia (Schlosser, 2010, Streit, 2007). They reside in the ectoderm next to the anterior neural plate, where they are induced by FGFs combined with Wnt and BMP attenuation (Ahrens and Schlosser, 2005, Brugmann et al., 2004, Litsiou et al., 2005). Although they generate cells as diverse as lens fibers and olfactory sensory neurons, they are initially competent to form any placode and share a common developmental program: at neurula stages, all sensory progenitors are specified as lens and diversify later under the influence of inductive signals from surrounding tissues (Bailey et al., 2006, Baker et al., 1999, Bhattacharyya and Bronner-Fraser, 2008, Gallagher et al., 1996, Groves and Bronner-Fraser, 2000, Henry and Grainger, 1987, Jacobson, 1963, Martin and Groves, 2006, Schlosser, 2010, Streit, 2007).
One of the key factors initiating the lens program is the paired box transcription factor Pax6 (Cvekl and Duncan, 2007, Lang, 2004). Consistent with its expression in lens and olfactory precursors (anterior placode progenitors [aPPs]), Pax6 mutations in humans and mice lead to severe eye and olfactory abnormalities (Grindley et al., 1995, van Heyningen and Williamson, 2002). Indeed, Pax6 is one of the most striking examples of a master regulator: its misexpression not only induces ectopic eyes in flies and vertebrates, but its function is so conserved that vertebrate Pax6 does so in Drosophila (Chow et al., 1999, Halder et al., 1995). It is therefore surprising that the tissues and signals that initiate Pax6 expression—and thus aPP specification—are unknown. Here, we demonstrate that the anterior mesendoderm is required for aPP formation and regulates Pax6. It does so by secreting the neuropeptide somatostatin (SST), which in turn activates nociceptin (Noc) in the overlying ectoderm. Together, these neuropeptides control aPP fate as an early step in lens and olfactory epithelium development.
Results
Identification of Somatostatin and Nociceptin as Potential Developmental Signals
Consistent with its function, Pax6 is initially confined to future lens and olfactory cells (aPPs). However, chick posterior placode progenitors (pPPs; inner ear and epibranchial ganglia) upregulate Pax6 within only 5 hr when cultured in isolation and ultimately turn into lenses (Bailey et al., 2006), providing an experimental paradigm to screen for new Pax6 regulators. Transcriptome comparison of four different cell populations (HH6 aPPs and pPPs before and after 5 hr culture) reveals 136 Pax6 coregulated transcripts. Among these, only four encode signaling molecules, including the propeptide for the opioid-related Noc (Figure S1 available online); the receptor for another neuropeptide, SST, is enriched in aPPs. This raises the intriguing possibility that in addition to their well-known functions in the adult nervous system and neuroendocrine modulation (Gahete et al., 2010), they may also play a role during development.
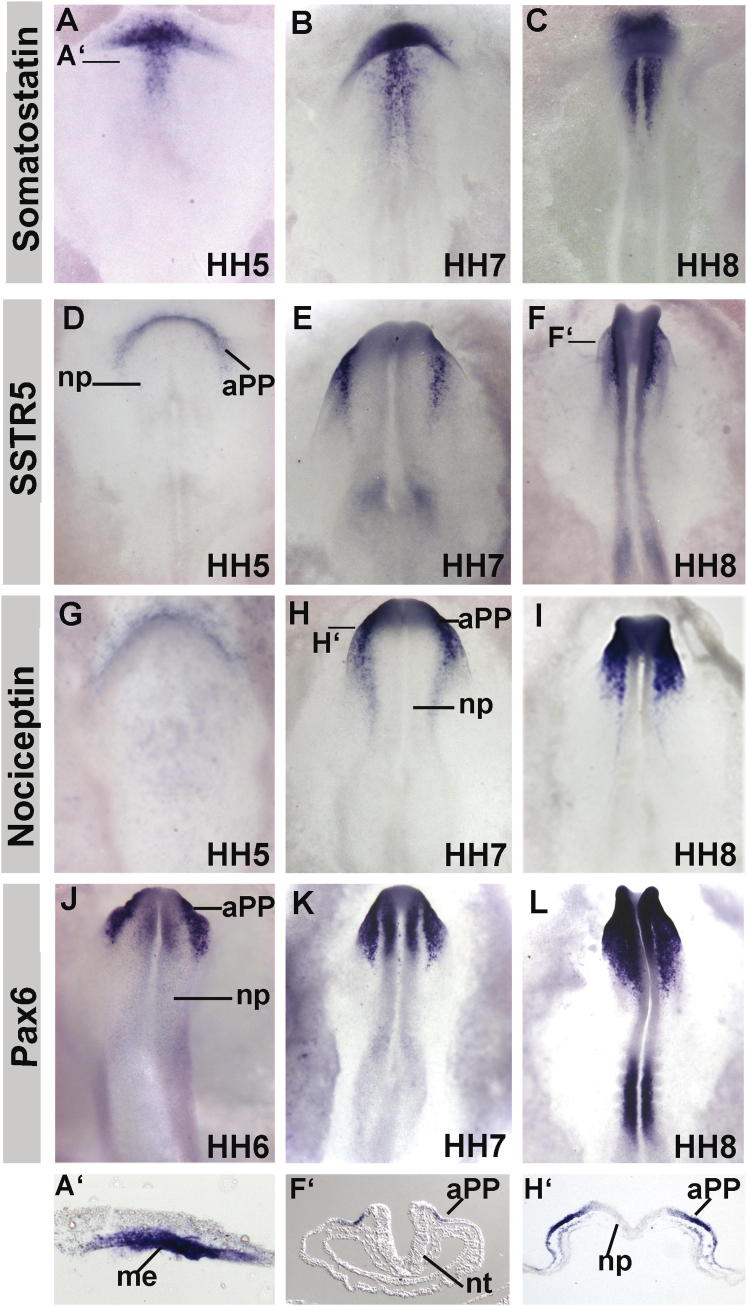
We therefore surveyed the expression of Noc and SST and their receptors from primitive streak to early somite stages. The SST prepropeptide and the processed peptide are expressed in the anterior mesendoderm underlying Pax6+ aPPs (Figures 1A–1C, 1A′, S1Ca–S1Cf, S2A, and S2B). In contrast, its receptor SSTR5 and Noc (Figures 1D–1I, 1F′, and 1H′) are restricted to aPPs in the overlying ectoderm, where they colocalize with Pax6 (Figures 1J–1L); both are downregulated rapidly in aPPs after the HH8. Thus, Noc and SSTR5 represent aPP-specific transcripts. Like Noc mRNA, the processed peptide is present in the ectoderm (Figures S1Cg–S1Cl). Noc signaling is mediated by its cognate G protein-coupled receptor opiate receptor-like 1 (OPRL1) and, due to a change in the N-terminal amino acid in most nonmammalian vertebrates, by δ, κ, and μ opioid receptors (Danielson et al., 2001) (Figures S1Aa and S1Ab). At neural plate stages, all opioid and other SST (STTR1, STTR3, STTR4) receptors are broadly expressed in the ectoderm including placode progenitors (Figures S2Ac–S2Aj and S3Aa–S3Al). Thus, the expression of both neuropeptides and their receptors is consistent with a role in initiating Pax6 expression and in specifying progenitors for the olfactory epithelium and the crystalline lens.
Figure 1.
Expression of SST, SSTR5, Noc, and Pax6
In situ hybridization for SST (A–C ventral view; A′ transverse section), SSTR5 (D–F dorsal view; F′ transverse section), Noc (G–I dorsal view; I′ transverse section), and Pax6 (J–L dorsal view). Stages are indicated in the right corner of each panel. Black lines in (A)–(L) indicate the level of the sections. aPP, anterior placode progenitors; me, mesendoderm; np, neural plate; nt: neural tube. See also Figure S1.
Somatostatin Signaling from the Anterior Mesendoderm Promotes Placode Progenitor Fate
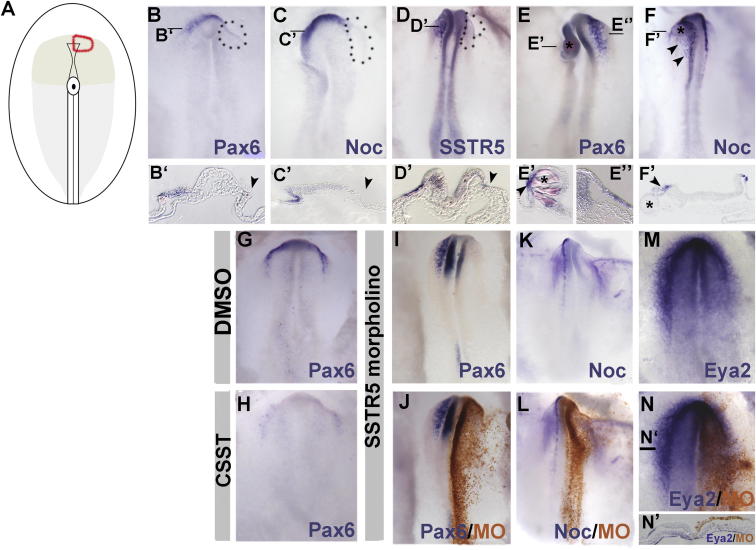
SST is expressed in the anterior mesendoderm, a tissue implicated in forebrain patterning (Dale et al., 1997, Foley et al., 1997, Wilson and Houart, 2004, Withington et al., 2001). To test whether mesendoderm-derived signals are required for aPP identity, we ablated this tissue unilaterally in HH4+/HH5− embryos (Figures 2A, S2Ba, and S2Bb). We find that Pax6 (1 out of 14, 7% Pax6+; Figures 2B and 2B′) and the general PP marker Eya2 (0 out of 4 Eya2+; Figure S2Be) are absent 5–6 hr thereafter, whereas sham-operated embryos are normal (Figure S2Bf). Likewise, Noc transcripts are reduced at 5–6 hr and completely lost 16 hr after ablation (0 out of 7 Noc+; Figures 2C and 2C′), as is SSTR5 (0 out of 9 SSTR+; Figures 2D and 2D′). In contrast, the posterior ectoderm marker Gbx2 (n = 4) and nonneural ectoderm marker Dlx5 (n = 4) are unaffected (Figures S2C and S2D). Can SST rescue the expression of aPP markers? After mesendoderm removal, local exposure to SST-, but not DMSO-, coated beads (Figures S2Bg–S2Bh′) rescues Pax6 (3 out of 3 Pax6+; Figures 2E–2E″) and Noc expression (5 out of 5 Noc+; Figures 2F and 2F′) after 16 hr culture. Thus, the anterior mesendoderm provides key signals to promote aPP identity, SST being one of these signals.
Figure 2.
SST Is Required for aPP Character
(A) Unilateral ablation of axial and paraxial mesendoderm (red line); light gray indicates mid- and hindgut endoderm, and gray shows foregut endoderm.
(B–F) Pax6 (B and B′), Noc (C and C′), and SSTR5 (D and D′) after mesendoderm ablation (dotted lines); Pax6 (E and E′) and Noc (F and F′) after mesoderm ablation and SST bead graft. SST-coated beads (∗) restore Pax6 (E–E″) and Noc (F and F′) after ablation (arrowheads). Lines in (B)–(F) indicate the level of sections shown in (A′)–(E′).
(G and H) Pax6 after DMSO (G) or CSST (H) treatment.
(I–N) Pax6 (I and J), Noc (K and L), and Eya2 (M, N, and N′) in SSTR5 morphants. (J), (L), and (N) show the same embryos as in (I), (K), and (M), respectively, after MO detection (brown).
See also Figure S2.
To assess whether SST signaling is required for aPP character, we used two different approaches. First, HH4 embryos were cultured with the SST antagonist cyclosomatostatin (CSST) or vehicle control (DMSO). At HH6/HH7, Pax6 expression is absent in CSST (4 out of 18, 22% Pax6+; Figure 2H) but present in DMSO-treated controls (6 out of 6 Pax6+; Figure 2G). Second, we asked whether the receptor SSTR5 mediates SST function. Control or SSTR5 translation-blocking morpholinos (MOs) were electroporated into future aPPs at HH4. Like mesendoderm ablation, SSTR5 knockdown leads to a loss of Pax6 (1 out of 13, 8% Pax6+; Figures 2I and 2J), Noc (5 out of 19, 26% Noc+; Figures 2K and 2L), and Eya2 (0 out of 6 Eya2+; Figures 2M, 2N, and 2N′) at HH6-8, whereas control MOs have no effect (Figures S2Bi–S2Bn). Together, these results show that SST signaling from the anterior mesendoderm is crucial for the specification of lens and olfactory progenitors by controlling the onset of Pax6 and other PP-specific transcripts.
Signals from the Posterior Head Mesoderm Repress aPP Markers
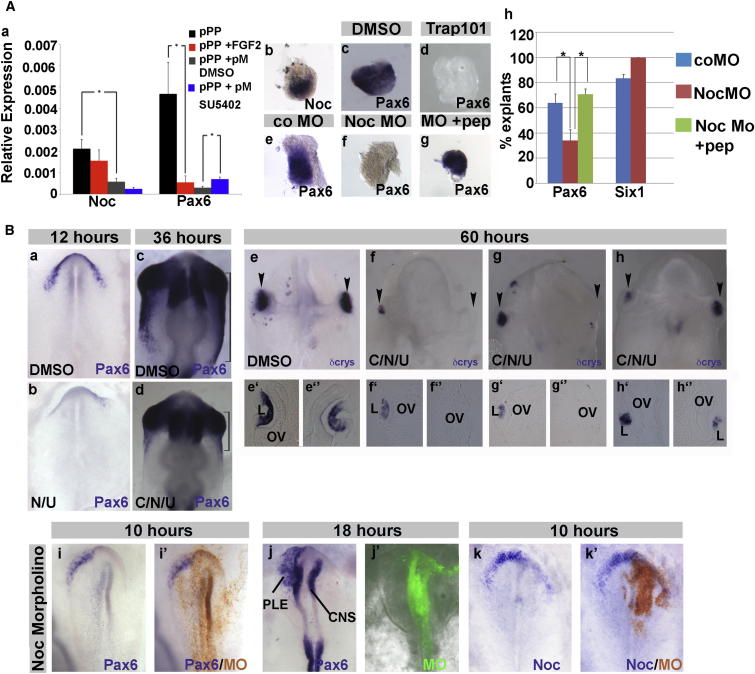
Although Pax6 and Noc are upregulated in explanted pPPs (Figures S1 and 3A), they are restricted to the aPP region in the embryo (Figure 1), suggesting that anterior fates are actively repressed in vivo. A possible source for such repressive signals is the mesoderm underlying pPPs. To test this, we analyzed Pax6 and Noc expression in pPP explants cultured with and without posterior mesoderm (pM). Indeed, we find that both transcripts are repressed by mesoderm-derived signals (Figure 3Aa). pPPs normally give rise to otic and epibranchial placodes with FGFs being potent inducers of a common otic-epibranchial progenitor domain (Freter et al., 2008, Groves and Bronner-Fraser, 2000, Martin and Groves, 2006, Yang et al., 2013). To test whether FGF signaling is sufficient for aPP inhibition, we cultured pPP explants with FGF2; this induces the otic-epibranchial marker Pax2 (data not shown) (Freter et al., 2008, Groves and Bronner-Fraser, 2000, Martin and Groves, 2006, Yang et al., 2013). Although Noc expression does not change significantly when compared to controls, Pax6 is significantly repressed (Figure 3Aa). To test whether mesoderm-derived FGF signaling is required for Pax6 repression, we treated pPP/pM explants with the FGF antagonist SU5402; this rescues Pax6 expression partially but does not restore the expression levels observed in the absence of mesoderm (Figure 3Aa). Together, these findings show that posterior head mesoderm plays a role in patterning the placodal domain by simultaneously promoting posterior and repressing anterior character. Although FGF signaling is involved in this process, other unidentified pathways must cooperate.
Figure 3.
Noc Is Required for aPP Character
(A) Regulation of aPP markers in vitro. (a) Quantification of Noc and Pax6 expression by NanoString nCounter in pPP explants cultured alone (black), with FGF2 (red), with posterior head mesoderm (pM; gray), or with posterior head mesoderm and SU5402 (blue). Bars represent means of normalized values ± SE. The asterisk (∗) indicates significant differences: p < 0.05. (b) Cultured pPP explants initiate Noc. (c and d) Pax6 after DMSO (c) or TRAP101 (d) treatment. (e–g) Pax6 in explants cultured with control MOs (e), Noc splice-blocking MOs (f), or Noc MOs and peptide (g). (h) Graph showing Noc knockdown effect on Pax6 and Six1. Bars represent mean values ± SE. The asterisk (∗) indicates significant differences: p < 0.05. See also Figure S3.
(B) Noc is required for aPP fate in vivo. (a and b) HH4+/HH5− embryos cultured for 12 hr in DMSO (a) or opioid receptor inhibitors (b; N/U: naloxone, UFP101). (c and d) Pax6 in HH4+/HH5− embryos treated with DMSO (c) or CSST (C) for 36 hr, naloxone (N) and UFP101 (U); compare brackets in (c) and (d). (e–h) δ-crystallin expression in embryos cultured for 60 hr from HH4+/HH5− with DMSO (e) or CSST (C), naloxone, and UFP101 (f–h). (f)–(h) show the range of phenotypes, and (e′)–(h″) show sections through the left and right lens (L) regions of the same embryos shown in (e)–(h). OV, optic vesicle. (i–k′) After electroporation of Noc ATG MOs at HH4−, embryos were cultured for 10 hr (i and i′, and k and k′) or 18 hr (j and j′). Brown (i′ and k′) and green (j′) indicate electroporated cells.
Nociceptin Promotes aPPs
Noc was identified as a Pax6 coregulated gene (Figure S1); both genes are coexpressed in aPPs (Figure 1), and like Pax6, Noc is rapidly upregulated in explanted pPPs (n = 19 out of 22; 86% Noc+; Figure 3Ab). To test whether Pax6 upregulation depends on Noc signaling, we used an OPRL1 antagonist. Pax6 transcripts are present in DMSO-treated control pPP explants (17 out of 23, 73% Pax6+; Figure 3Ac) but absent when Noc signaling is inhibited (11 out of 31, 35% Pax6+; Figure 3Ad). Likewise, Noc splice MOs, but not control MOs, prevent Pax6 initiation (Figures 3Ae, 3Af, and 3Ah; controls, 39 out of 64, 61% Pax6+; experimental, 27 out of 73, 34% Pax6+). This effect is rescued by the addition of Noc peptide (13 out of 17, 76% Pax6+; Figures 3Ag, 3Ah, and S3Ca), demonstrating the specificity of the MOs. In contrast, the generic PP marker Six1 does not change after Noc knockdown (Figure 3Ah, 23 out of 23 Six1+; Figure S3Ca), indicating that Noc specifically promotes anterior character.
To confirm that Noc is required for aPP specification in vivo, we used two strategies. First, HH4 chick embryos were treated with OPRL1 and opiate receptor antagonists; this leads to loss of Pax6 at HH6 (Figure 3Bb; 0 out of 8 Pax6+), unlike DMSO-treated controls (Figure 3Ba; 8 out of 11, 72% Pax6+). Second, we electroporated translation- or splice-blocking Noc MOs, alone or in combination, into stage HH4 chick embryos, targeting future lens and olfactory cells. These produce identical phenotypes: at early somite stages, the expression of general placode progenitor markers (Figures S3Be–S3Bh′; Six1+, n = 8; Eya2+, n = 7), the neural crest marker Pax7 (Figures S3Ba, S3Bb, and S3Bb’; n = 5), and Otx2 (Figures S3Bc, S3Bd, and S3Bd′; n = 7), an anterior ectoderm marker expressed prior to Pax6 and Noc, are all unaffected. However, like in vitro, in Noc morphants, Pax6 is severely reduced in lens and olfactory progenitors at head process and early somite stages (Figures 3Bi–3Bj′, 4 out of 14, 29% Pax6+; Figure S3Cb) as is Noc itself (0 out of 4 Noc+; Figures 3Bj and 3Bj′), whereas control MOs have little effect (Figures S2I–S2L; 7 out of 10, 70% Pax6+; 7 out of 11, 64% Noc+). In contrast, the CNS domain of Pax6 is unaffected as is the expression of Six3 (n = 9; data not shown) and Ganf (n = 2; data not shown) in the forebrain. Thus, Noc does not influence neural plate formation but is required to regulate anterior placode fates by controlling its own expression and that of the master regulator Pax6.
So far, our results show that mesendoderm-derived SST initiates Noc expression in the overlying ectoderm and that both signals are required for aPP-specific gene expression. Do SST and Noc act in a linear pathway? If so, nociceptin should rescue the SST phenotype. We therefore ablated anterior mesendoderm (the source of SST) or knocked down SSTR5 followed by a graft of Noc-coated beads. Activation of Noc signaling does not rescue Pax6 expression in the absence of SST (Figure S3D; n = 13). Thus, the two neuropeptides act in parallel, and both are required for Pax6 expression and, consequently, for aPP specification.
Lens Defects in the Absence of SST and Noc Signaling
SST and Noc are transiently expressed in the anterior mesendoderm and anterior preplacodal ectoderm, respectively, and participate in aPP specification by regulating Pax6 (see above). Does the loss of aPP character affect placode formation at later stages? To assess this, we treated embryos with inhibitors of Noc and SST signaling starting at HH4/HH5−. This reduces Pax6 in the surface ectoderm, but not in the brain, at HH10/HH11 (Figures 3Bc and 3Bd; 7 out of 14, 50% Pax6 reduction; controls: 13 out of 14, 93% normal). At embryonic day 3 (E3), phenotypes vary slightly, with only 12.4% (n = 24) showing normal expression of the lens differentiation marker δ-crystallin compared to 70% of DMSO-treated controls (n = 20). Lens placodes or vesicles are absent uni- (12.4%) or bilaterally (16.6%) or are substantially smaller (58.3%; Figures 3Bf–3Bh and 3Bf′–3Bh″) than in controls (Figures 3Be and 3Be′; n = 20; 30% with unilateral small placode). Although patches of δ-crystallin show typical placode morphology, they remain small and never invaginate to form a vesicle. In severe cases, optic vesicle morphology is affected because lens-derived signals are required for its normal development (Coulombre and Coulombre, 1964, Yamamoto and Jeffery, 2000, Chow and Lang, 2001). Thus, SST and Noc signaling are critical for aPP specification and therefore for normal lens formation.
Somatostatin and Nociceptin Functions Are Conserved in Zebrafish
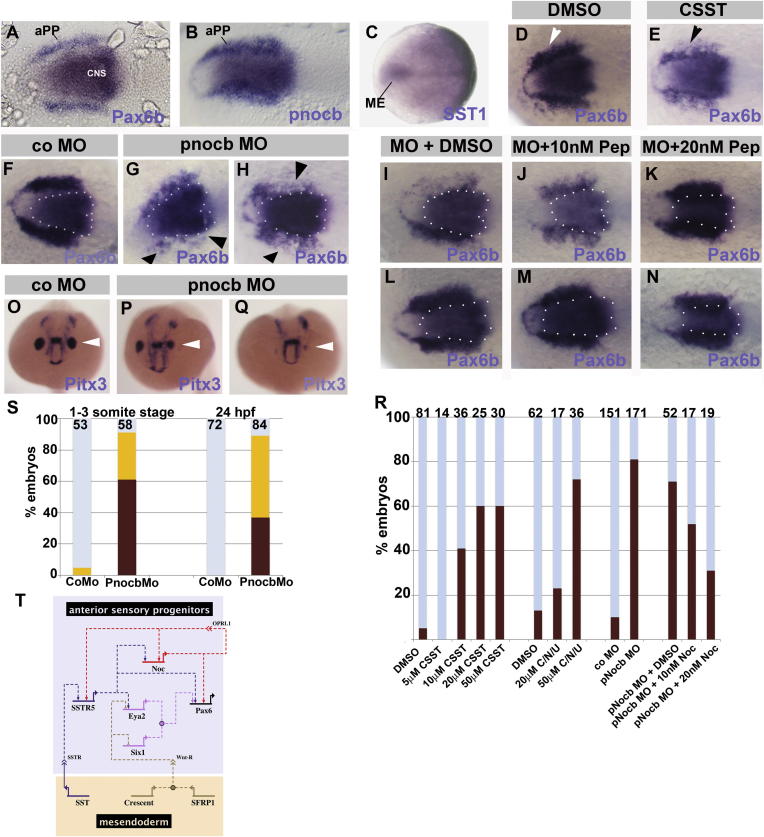
To assess whether neuropeptide function in anterior placode precursor formation is conserved across species, we turned to zebrafish and cloned prepronociceptin b (pnocb). As in chick, pnocb is coexpressed with Pax6b in aPPs (Figures 4A and 4B), whereas SST1 (Devos et al., 2002) is expressed in the mesendoderm (Figure 4C). Like in chick, inhibition of SST signaling using the antagonist CSST leads to disruption of Pax6b at neural plate stages in a dose-dependent manner (Figures 4D, 4E, and 4O). Noc knockdown using two different MOs alone or in combination results in uni- or bilateral reduction of Pax6b (Figures 4G, 4H, and 4O; 32 out of 171, 19% normal Pax6b) and Pitx3 (Figure 4P; 5 out of 58, 9% Pitx3+) in aPPs at neural plate stages. Pax6b expression is rescued by growing morphants in the presence of Noc peptide (Figures 4I–4K and 4O). In pnocb morphants, the general PP markers Eya1 and Six1 are unaffected (data not shown), suggesting that, like in chick, aPPs retain placode progenitor identity but lose their anterior character. In contrast to Pax6b reduction in aPPs, its expression in the neural plate is unaffected, as are the neural markers Rx3, Emx1, Six3, and Irxb until at least the ten-somite stage (data not shown; n > 25 for each marker). These results show that nociceptin signaling primarily affects placode, but not neural plate, development.
Figure 4.
SST and Noc Control aPPs in Zebrafish
(A–C) Expression of Pax6b (A), pnocb (B), and SST1 (C) in zebrafish at early somite stages: dorsal views, anterior to the left.
(D and E) Pax6b reduction by CSST (E; arrowhead), but not by DMSO (D; white arrowhead).
(F–H) Embryos were injected with control (F) or pnocb MOs (G and H); the latter show Pax6b reduction (arrowheads in G and H). Dotted lines indicate CNS expression of Pax6b.
(I–N) pnocb ATG (I–K) or control (L–N) MO-injected embryos were incubated in DMSO (I and L) or Noc peptide (J, K, M, and N). Dotted lines indicate CNS Pax6b expression.
(O–Q) Embryos were injected with control (O) or pnocb MOs (P and Q). At 24 hpf (frontal views), Pitx3 expression reveals asymmetric, small (P; arrowhead) or almost absent lenses (Q; arrowhead).
(R) Graph shows Pax6b reduction after SST inhibition, SST and Noc inhibition, in Noc morphants and Noc morphants + Noc peptide. Numbers for each treatment are at the top. Brown bars indicate embryos with phenotype, blue bars show normal embryos.
(S) Quantification of pnocb morphants with Pitx3 reduction (yellow) or loss (brown) at early somite stages or 24 hpf.
(T) Model for neuropeptide function.
Consistent with the loss of early Pax6b, Noc morphants show variable eye phenotypes after 24 hr: their lenses are smaller, asymmetric, or absent (n = 84; Figures 4L–4N and 4P), and as a consequence, the optic vesicles are reduced in size. Simultaneous inhibition of SST and Noc phenocopies the loss of each pathway individually (n = 54; data not shown). Thus, like in chick, Noc is coexpressed with Pax6b in placode progenitors at the border of the anterior neural plate, whereas SST1 is expressed in the underlying mesendoderm. Both contribute to the specification of aPPs, and their loss leads to abnormal eye development in chick and zebrafish.
Discussion
Our findings reveal a function for the anterior mesendoderm in controlling aPP fates as an early step for lens and olfactory development. We identify two neuropeptides, SST and Noc, mediating this process in amniotes and anamniotes (Figure 4T). Mesendoderm-derived SST promotes aPP identity in the overlying ectoderm by regulating Eya2 and Noc. In turn, Noc controls its own expression and, together with SST, the onset of Pax6, a key regulator of eye and olfactory fates.
Mice lacking Noc, SST, and their receptors have been generated (Köster et al., 1999, Low et al., 2001, Nishi et al., 1997, Zeyda et al., 2001, Zeyda and Hochgeschwender, 2008). Adult animals do not display obvious sense organ phenotypes, for which several other explanations are possible. First, the mutants have not been examined for defects in lens and olfactory progenitors—the effects of the mutants may be transient or subtle. With respect to SST, a robust phenotype would only be expected when all four SSTRs expressed in sensory progenitors are ablated. Furthermore, a second peptide, cortistatin, is often coexpressed with SST, signals through all SSTRs, and may thus compensate for the absence of SST (Gahete et al., 2008, Zeyda and Hochgeschwender, 2008). Finally, the mammalian Noc prepropeptide contains a second peptide, nocistatin, a Noc antagonist, which is absent in nonmammalian vertebrates (Danielson et al., 2001, Okuda-Ashitaka et al., 1998). This may account for subtle or lack of phenotype in mice. Here, using different tools including tissue ablation, morpholinos, and drugs to interfere with SST and Noc function, we reveal their role in aPP specification. Whether neuropeptides have a similar function in rodents and other mammals remains to be discovered; however, our results are consistent in both amniotes and anamniotes.
Once placode progenitors are specified, they have an autonomous tendency to form a lens when cultured in isolation, regardless of their later fate (Bailey et al., 2006). They do so by initiating Pax6 followed by the transcription factors controlling lens-specific δ-crystallin. However, in the embryo, pPPs never express Pax6 and never contribute to the lens, suggesting that, in these cells, aPP character must be actively repressed. Here, we show that this is indeed the case: signals from the posterior head mesoderm inhibit aPP-specific genes partly through FGF (this study). When grafted anteriorly, the same mesoderm induces posterior character in the adjacent ectoderm (Kil et al., 2005). In contrast, anterior mesendoderm promotes anterior placode identity, and we show that SST signaling participates in this process by initiating Noc and Pax6 expression. Thus, signals from the underlying mesoderm play a crucial role in patterning the placode progenitor field along the rostro-caudal axis.
In agreement with their role in aPP specification, SST, Noc, and their respective receptors are expressed prior to Pax6, and their loss leads to severe reduction of the lens. However, they are likely to act in concert with other signals to impart anterior identity: Wnt and BMP attenuation is important for placode progenitor induction (Ahrens and Schlosser, 2005, Brugmann et al., 2004, Litsiou et al., 2005), whereas retinoic acid, BMP7, and FGF-like signals have been implicated in forebrain patterning (Dale et al., 1997, Halilagic et al., 2003, Sanchez-Arrones et al., 2012). Our data show that SST and Noc specifically control placodal fate, without affecting neural plate patterning. Together, these observations suggest that spatial and temporal integration of different pathways is crucial in aPP specification and in establishing the differences between neural and nonneural components of the cranial sensory nervous system.
Neuropeptides and neurotransmitters are ancient signaling molecules already present in primitive deuterostomes and other invertebrates (Buznikov et al., 2005, Buznikov et al., 2010, Coates et al., 2000, Conzelmann et al., 2011, Elphick, 2010, Elphick and Thorndyke, 2005, Hauser et al., 2006, Hauser et al., 2008). They predate the origin of the CNS. In these systems, they control proliferation and growth (Lauder, 1993); neurotransmitters like serotonin control oocyte maturation and cleavage as well as neuronal differentiation and function (Buznikov et al., 2005, Buznikov et al., 2010). In vertebrates, nonneuronal functions of serotonin include craniofacial development and left-right asymmetry (Levin et al., 2006, Reisoli et al., 2010). The opioid system appeared about 450 million years ago with the emergence of gnathostomes (Larhammar et al., 2009), whereas other neuropeptides were already present earlier (Coates et al., 2000, Elphick, 2010, Elphick and Thorndyke, 2005, Hauser et al., 2006, Hauser et al., 2008). Our findings define unexpected functions for SST and Noc signaling in vertebrate embryos outside the CNS, where they influence cell fate and tissue morphogenesis, suggesting that these may be the ancestral roles of these small molecules, whose functions in the nervous system were co-opted much later during evolution.
Experimental Procedures
Chick Embryo Experiments
All chick experiments involve embryos younger than E10 and do not require a UK Home Office license. Embryos were cultured in New (Stern and Ireland, 1981) or Cornish (Nagai et al., 2011) pastry culture. Mesendoderm ablations were performed at HH5−. Beads coated with SST, nociceptin, or DMSO (control) were grafted into the ablated area.
CSST (cyclo(7-aminoheptanoyl-Phe-D-Trp-Lys-Thr[Bzl]); Sigma-Aldrich) and naloxone (Sigma-Aldrich) were prepared in DMSO (1 mM), UFP101 (Sigma-Aldrich) in H2O (1 mM). For inhibition studies, embryos were preincubated for 1 hr in SST and/or Noc antagonists (1 μM each) or DMSO (0.1%; control) and cultured in their presence for 5–60 hr. For knockdown, experiments were performed using MOs (Gene Tools), which were electroporated as described by Voiculescu et al. (2008).
HH5+-6 pPP explants were cultured as described by Bailey et al. (2006). Collagen gels and culture media were supplemented as required with nociceptin antagonists, nociceptin, MOs, FGF2, or SU5402. For coculture with posterior head mesoderm, pPP and mesoderm were dissected separately and recombined before culture.
Embryos and explants were processed for in situ hybridization (Streit et al., 1998) using DIG-labeled antisense probes. For histological sections, embryos were embedded in paraffin and sectioned transversally at 15 μm.
NanoString nCounter
For each experimental condition, eight to ten pPP explants were lysed in lysis buffer (Ambion). Total RNA was hybridized with capture and reporter probes according to the nCounter Gene Expression Assay Manual. Following washing, target/probe complexes were immobilized for data collection in the nCounter Digital Analyzer. Each experiment was repeated three times on independent occasions. Mean value ± SD for Pax6 and Noc was extracted from the normalized data.
Cloning of Zebrafish pnocb and Functional Experiments
All zebrafish studies were performed with approval from the UK Home Office under a HO project license to C.H. Zebrafish pnocb was cloned by RT-PCR from 48 hpf embryo cDNA. pnoc or control MOs were injected at the one- to four-cell stage at a concentration of 1.8 ng/embryo. Embryos were grown at 28°C until the desired stage. For inhibition and rescue experiments, dechorionated embryos were incubated in appropriate compounds from 4 to 5 hpf until they had reached the desired stage.
Acknowledgments
The authors thank Ewa Kolano-Merlin and Vicky Snowden for excellent technical support, Timothy Grocott and Tatjana Sauka-Spengler for help with NanoString nCounter, and Claudio Stern, Ben Steventon, and Timothy Grocott for critical reading of the manuscript. This work was supported by a Wellcome Trust project grant to A.S., a KCL ORS award to L.L.-F., and MRC and Wellcome Trust project grants to C.H.
Published: July 29, 2013
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and three figures and can be found with this article online at http://dx.doi.org/10.1016/j.devcel.2013.07.001.
Accession Numbers
The Gene Expression Omnibus accession number for the microarray data reported in this paper is GSE48116.
Supplemental Information
References
- Ahrens K., Schlosser G. Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev. Biol. 2005;288:40–59. doi: 10.1016/j.ydbio.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Bailey A.P., Bhattacharyya S., Bronner-Fraser M., Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev. Cell. 2006;11:505–517. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Baker C.V., Stark M.R., Marcelle C., Bronner-Fraser M. Competence, specification and induction of Pax-3 in the trigeminal placode. Development. 1999;126:147–156. doi: 10.1242/dev.126.1.147. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Bronner-Fraser M. Competence, specification and commitment to an olfactory placode fate. Development. 2008;135:4165–4177. doi: 10.1242/dev.026633. [DOI] [PubMed] [Google Scholar]
- Brugmann S.A., Pandur P.D., Kenyon K.L., Pignoni F., Moody S.A. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development. 2004;131:5871–5881. doi: 10.1242/dev.01516. [DOI] [PubMed] [Google Scholar]
- Buznikov G.A., Peterson R.E., Nikitina L.A., Bezuglov V.V., Lauder J.M. The pre-nervous serotonergic system of developing sea urchin embryos and larvae: pharmacologic and immunocytochemical evidence. Neurochem. Res. 2005;30:825–837. doi: 10.1007/s11064-005-6876-6. [DOI] [PubMed] [Google Scholar]
- Buznikov G.A., Nikitina L.A., Bezuglov V.V., Francisco M.E., Boysen G., Obispo-Peak I.N., Peterson R.E., Weiss E.R., Schuel H., Temple B.R. A putative ‘pre-nervous’ endocannabinoid system in early echinoderm development. Dev. Neurosci. 2010;32:1–18. doi: 10.1159/000235758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R.L., Lang R.A. Early eye development in vertebrates. Annu. Rev. Cell Dev. Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Chow R.L., Altmann C.R., Lang R.A., Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126:4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- Coates D., Siviter R., Isaac R.E. Exploring the Caenorhabditis elegans and Drosophila melanogaster genomes to understand neuropeptide and peptidase function. Biochem. Soc. Trans. 2000;28:464–469. [PubMed] [Google Scholar]
- Conzelmann M., Offenburger S.L., Asadulina A., Keller T., Münch T.A., Jékely G. Neuropeptides regulate swimming depth of Platynereis larvae. Proc. Natl. Acad. Sci. USA. 2011;108:E1174–E1183. doi: 10.1073/pnas.1109085108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombre A.J., Coulombre J.L. Lens development. I. Role of the lens in eye growth. J. Exp. Zool. 1964;156:39–47. doi: 10.1002/jez.1401560104. [DOI] [PubMed] [Google Scholar]
- Cvekl A., Duncan M.K. Genetic and epigenetic mechanisms of gene regulation during lens development. Prog. Retin. Eye Res. 2007;26:555–597. doi: 10.1016/j.preteyeres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J.K., Vesque C., Lints T.J., Sampath T.K., Furley A., Dodd J., Placzek M. Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell. 1997;90:257–269. doi: 10.1016/s0092-8674(00)80334-7. [DOI] [PubMed] [Google Scholar]
- Danielson P.B., Hoversten M.T., Fitzpatrick M., Schreck C., Akil H., Dores R.M. Sturgeon orphanin, a molecular “fossil” that bridges the gap between the opioids and orphanin FQ/nociceptin. J. Biol. Chem. 2001;276:22114–22119. doi: 10.1074/jbc.M011741200. [DOI] [PubMed] [Google Scholar]
- Devos N., Deflorian G., Biemar F., Bortolussi M., Martial J.A., Peers B., Argenton F. Differential expression of two somatostatin genes during zebrafish embryonic development. Mech. Dev. 2002;115:133–137. doi: 10.1016/s0925-4773(02)00082-5. [DOI] [PubMed] [Google Scholar]
- Elphick M.R. NG peptides: a novel family of neurophysin-associated neuropeptides. Gene. 2010;458:20–26. doi: 10.1016/j.gene.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Elphick M.R., Thorndyke M.C. Molecular characterisation of SALMFamide neuropeptides in sea urchins. J. Exp. Biol. 2005;208:4273–4282. doi: 10.1242/jeb.01910. [DOI] [PubMed] [Google Scholar]
- Foley A.C., Storey K.G., Stern C.D. The prechordal region lacks neural inducing ability, but can confer anterior character to more posterior neuroepithelium. Development. 1997;124:2983–2996. doi: 10.1242/dev.124.15.2983. [DOI] [PubMed] [Google Scholar]
- Freter S., Muta Y., Mak S.S., Rinkwitz S., Ladher R.K. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–3424. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- Gahete M.D., Durán-Prado M., Luque R.M., Martínez-Fuentes A.J., Vázquez-Martínez R., Malagón M.M., Castaño J.P. Are somatostatin and cortistatin two siblings in regulating endocrine secretions? In vitro work ahead. Mol. Cell. Endocrinol. 2008;286:128–134. doi: 10.1016/j.mce.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Gahete M.D., Cordoba-Chacón J., Duran-Prado M., Malagón M.M., Martinez-Fuentes A.J., Gracia-Navarro F., Luque R.M., Castaño J.P. Somatostatin and its receptors from fish to mammals. Ann. N Y Acad. Sci. 2010;1200:43–52. doi: 10.1111/j.1749-6632.2010.05511.x. [DOI] [PubMed] [Google Scholar]
- Gallagher B.C., Henry J.J., Grainger R.M. Inductive processes leading to inner ear formation during Xenopus development. Dev. Biol. 1996;175:95–107. doi: 10.1006/dbio.1996.0098. [DOI] [PubMed] [Google Scholar]
- Grindley J.C., Davidson D.R., Hill R.E. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- Groves A.K., Bronner-Fraser M. Competence, specification and commitment in otic placode induction. Development. 2000;127:3489–3499. doi: 10.1242/dev.127.16.3489. [DOI] [PubMed] [Google Scholar]
- Halder G., Callaerts P., Gehring W.J. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Halilagic A., Zile M.H., Studer M. A novel role for retinoids in patterning the avian forebrain during presomite stages. Development. 2003;130:2039–2050. doi: 10.1242/dev.00423. [DOI] [PubMed] [Google Scholar]
- Hauser F., Williamson M., Cazzamali G., Grimmelikhuijzen C.J. Identifying neuropeptide and protein hormone receptors in Drosophila melanogaster by exploiting genomic data. Brief. Funct. Genomics Proteomics. 2006;4:321–330. doi: 10.1093/bfgp/eli003. [DOI] [PubMed] [Google Scholar]
- Hauser F., Cazzamali G., Williamson M., Park Y., Li B., Tanaka Y., Predel R., Neupert S., Schachtner J., Verleyen P., Grimmelikhuijzen C.J. A genome-wide inventory of neurohormone GPCRs in the red flour beetle Tribolium castaneum. Front. Neuroendocrinol. 2008;29:142–165. doi: 10.1016/j.yfrne.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Henry J.J., Grainger R.M. Inductive interactions in the spatial and temporal restriction of lens-forming potential in embryonic ectoderm of Xenopus laevis. Dev. Biol. 1987;124:200–214. doi: 10.1016/0012-1606(87)90472-6. [DOI] [PubMed] [Google Scholar]
- Jacobson A.G. The determination and positioning of the nose, lens, and ear. III. Effects of reversing the antero-posterior axis of epidermis, neural plate and neural fold. J. Exp. Zool. 1963;154:293–303. doi: 10.1002/jez.1401540305. [DOI] [PubMed] [Google Scholar]
- Kil S.H., Streit A., Brown S.T., Agrawal N., Collazo A., Zile M.H., Groves A.K. Distinct roles for hindbrain and paraxial mesoderm in the induction and patterning of the inner ear revealed by a study of vitamin-A-deficient quail. Dev. Biol. 2005;285:252–271. doi: 10.1016/j.ydbio.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Köster A., Montkowski A., Schulz S., Stübe E.M., Knaudt K., Jenck F., Moreau J.L., Nothacker H.P., Civelli O., Reinscheid R.K. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc. Natl. Acad. Sci. USA. 1999;96:10444–10449. doi: 10.1073/pnas.96.18.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R.A. Pathways regulating lens induction in the mouse. Int. J. Dev. Biol. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Dreborg S., Larsson T.A., Sundström G. Early duplications of opioid receptor and Peptide genes in vertebrate evolution. Ann. N Y Acad. Sci. 2009;1163:451–453. doi: 10.1111/j.1749-6632.2008.03672.x. [DOI] [PubMed] [Google Scholar]
- Lauder J.M. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Levin M., Buznikov G.A., Lauder J.M. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev. Neurosci. 2006;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- Litsiou A., Hanson S., Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Low M.J., Otero-Corchon V., Parlow A.F., Ramirez J.L., Kumar U., Patel Y.C., Rubinstein M. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J. Clin. Invest. 2001;107:1571–1580. doi: 10.1172/JCI11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K., Groves A.K. Competence of cranial ectoderm to respond to Fgf signaling suggests a two-step model of otic placode induction. Development. 2006;133:877–887. doi: 10.1242/dev.02267. [DOI] [PubMed] [Google Scholar]
- Nagai H., Lin M.C., Sheng G. A modified cornish pasty method for ex ovo culture of the chick embryo. Genesis. 2011;49:46–52. doi: 10.1002/dvg.20690. [DOI] [PubMed] [Google Scholar]
- Nishi M., Houtani T., Noda Y., Mamiya T., Sato K., Doi T., Kuno J., Takeshima H., Nukada T., Nabeshima T. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J. 1997;16:1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda-Ashitaka E., Minami T., Tachibana S., Yoshihara Y., Nishiuchi Y., Kimura T., Ito S. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature. 1998;392:286–289. doi: 10.1038/32660. [DOI] [PubMed] [Google Scholar]
- Reisoli E., De Lucchini S., Nardi I., Ori M. Serotonin 2B receptor signaling is required for craniofacial morphogenesis and jaw joint formation in Xenopus. Development. 2010;137:2927–2937. doi: 10.1242/dev.041079. [DOI] [PubMed] [Google Scholar]
- Sanchez-Arrones L., Stern C.D., Bovolenta P., Puelles L. Sharpening of the anterior neural border in the chick by rostral endoderm signalling. Development. 2012;139:1034–1044. doi: 10.1242/dev.067934. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Making senses development of vertebrate cranial placodes. Int. Rev. Cell Mol. Biol. 2010;283:129–234. doi: 10.1016/S1937-6448(10)83004-7. [DOI] [PubMed] [Google Scholar]
- Stern C.D., Ireland G.W. An integrated experimental study of endoderm formation in avian embryos. Anat. Embryol. (Berl.) 1981;163:245–263. doi: 10.1007/BF00315703. [DOI] [PubMed] [Google Scholar]
- Streit A. The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int. J. Dev. Biol. 2007;51:447–461. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- Streit A., Lee K.J., Woo I., Roberts C., Jessell T.M., Stern C.D. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–519. doi: 10.1242/dev.125.3.507. [DOI] [PubMed] [Google Scholar]
- van Heyningen V., Williamson K.A. PAX6 in sensory development. Hum. Mol. Genet. 2002;11:1161–1167. doi: 10.1093/hmg/11.10.1161. [DOI] [PubMed] [Google Scholar]
- Voiculescu O., Papanayotou C., Stern C.D. Spatially and temporally controlled electroporation of early chick embryos. Nat. Protoc. 2008;3:419–426. doi: 10.1038/nprot.2008.10. [DOI] [PubMed] [Google Scholar]
- Wilson S.W., Houart C. Early steps in the development of the forebrain. Dev. Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withington S., Beddington R., Cooke J. Foregut endoderm is required at head process stages for anteriormost neural patterning in chick. Development. 2001;128:309–320. doi: 10.1242/dev.128.3.309. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Jeffery W.R. Central role for the lens in cave fish eye degeneration. Science. 2000;289:631–633. doi: 10.1126/science.289.5479.631. [DOI] [PubMed] [Google Scholar]
- Yang L., O’Neill P., Martin K., Maass J.C., Vassilev V., Ladher R., Groves A.K. Analysis of FGF-dependent and FGF-independent pathways in otic placode induction. PLoS One. 2013;8:e55011. doi: 10.1371/journal.pone.0055011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyda T., Hochgeschwender U. Null mutant mouse models of somatostatin and cortistatin, and their receptors. Mol. Cell. Endocrinol. 2008;286:18–25. doi: 10.1016/j.mce.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Zeyda T., Diehl N., Paylor R., Brennan M.B., Hochgeschwender U. Impairment in motor learning of somatostatin null mutant mice. Brain Res. 2001;906:107–114. doi: 10.1016/s0006-8993(01)02563-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.