Abstract
Multiple system atrophy (MSA) is a fatal, rapidly progressive neurodegenerative disease with limited symptomatic treatment options. Discrimination of MSA from other degenerative disorders crucially depends on the presence of early and severe cardiovascular autonomic failure (CAF). We have previously shown that neuropathologic lesions in the central autonomic nuclei similar to the human disease are present in transgenic MSA mice generated by targeted oligodendroglial overexpression of α-syn using the PLP promoter. We here explore whether such lesions result in abnormalities of heart rate variability (HRV) and circadian rhythmicity which are typically impaired in MSA patients.
HRV analysis was performed in five month old transgenic PLP-α-syn (tg) MSA mice and age-matched wild type controls. Decreased HRV and alterations in the circadian rhythmicity were detected in the tg MSA group. The number of choline-acetyltransferase-immunoreactive neurons in the nucleus ambiguus was significantly decreased in the tg group, whereas the levels of arginine-vasopressin neurons in the suprachiasmatic and paraventricular nucleus were not affected. Our finding of impaired HRV and circadian rhythmicity in tg MSA mice associated with degeneration of the nucleus ambiguus suggests that a cardinal non-motor feature of human MSA can be reproduced in the mouse model strengthening its role as a valuable testbed for studying selective vulnerability and assessing translational therapies.
Abbreviations: PLP, proteolipid protein promoter; MSA, multiple system atrophy; MSA-P, parkinsonian variant of MSA; MSA-C, cerebellar variant of MSA; α-syn, alpha-synuclein; SND, striatonigral degeneration; OPCA, olivopontocerebellar atrophy; GCI, (oligodendro-)glial cytoplasmic inclusions; SAOA, sporadic adult onset ataxia; ECG, electrocardiogram; UMSARS, unified MSA rating scale; wt, wild type; tg, transgenic; RMSSD, root mean square of successive RR interval differences; LF, low frequency; HF, high frequency; MR, magnetic resonance; SPECT, single-photon emission computed tomography; PET, positron emission tomography; ChAT, choline-acetyltransferase; AVP, arginine-vasopressin; TH, tyrosine hydroxylase
Keywords: Multiple system atrophy, Transgenic animal model, Alpha-synuclein, Heart rate variability, Autonomic failure
Highlights
► In the current study we assessed the cardiovascular phenotype of tg MSA mice. ► The α-syn-overexpressing MSA mice had decreased HRV. ► We detected depletion of cholinergic neurons in the nucleus ambiguus in MSA mice. ► The reduced HRV might be associated with neurodegeneration in the nucleus ambiguus.
Introduction
Multiple system atrophy (MSA) is a rapidly progressive neurodegenerative disease with unclear etiology. The disease can be classified into 2 motor subtypes: a parkinsonian variant (MSA-P) that accounts for 80% of cases in the western world and is caused by striatonigral degeneration (SND) and the cerebellar subtype (MSA-C) that results from olivopontocerebellar atrophy (OPCA) and is the predominant MSA subtype in Asia (Wenning and Stefanova, 2009). Besides the motor symptoms progressive autonomic failure is an important feature of MSA that is obligatory for the diagnosis (Gilman et al., 2008; Jecmenica-Lukic et al., 2012). The spectrum of autonomic failure in MSA includes disturbances of the cardiovascular system (e.g. orthostatic hypotension, and diminished heart rate variability), impaired respiratory function, urinary and erectile dysfunction, sudomotor disturbances (anhidrosis) or circadian rhythm disturbances mostly resulting from degeneration of the neuronal networks in the brainstem and preganglionic efferent autonomic systems (Benarroch, 2002, 2003; Ozawa, 2007).
Heart rate variability (HRV) is a physiological phenomenon that describes the variation of the distance (time) between consecutive R waves (R-R Interval) in an ECG signal and reflects the balance between the sympathetic and parasympathetic systems (Malliani et al., 1991). HRV reduction has been associated with various disorders (Agelink et al., 2002; Bleil et al., 2008; Lavoie et al., 2004; Malpas and Maling, 1990; Martens et al., 2008). Several studies have been performed in MSA patients to investigate cardiac autonomic dysfunction describing HRV as a potential biomarker for distinguishing MSA from PD, progressive autonomic failure (PAF) and sporadic adult onset ataxias (SAOAs) (Abele et al., 2004; Diedrich et al., 2002; Furushima et al., 2012; Kitae et al., 2001; Kiyono et al., 2012; Toshihide et al., 2006). A recent study by Nishizawa and co-workers evaluated time-domain and frequency-spectrum measures of HRV in 14 MSA-C and 3 MSA-P patients and detected that all HRV parameters (RMSSD — root mean square of successive RR interval differences, HF — high frequency and LF — low frequency) except LF/HF ratio were decreased in MSA patients compared to healthy controls (Furushima et al., 2012). Importantly, inverse correlations between HRV and disease progression were shown in MSA patients (Furushima et al., 2012; Kitae et al., 2001). Decreased HRV and disturbed circadian rhythm were reported in MSA and PD patients compared to early-onset parkinsonism patients and healthy controls, with MSA showing more pronounced changes than PD (Kiyono et al., 2012; Toshihide et al., 2006).
The neuropathological correlate of CAF in MSA has been linked to a distributed degeneration of multiple autonomic areas including preganglionic sympathetic neurons in the intermediolateral cell column, cholinergic neurons in the nucleus ambiguus and dorsal motor nucleus of the vagus, as well as arginine-vasopressin neurons in the suprachiasmatic and paraventricular nucleus and TH neurons in the ventrolateral medulla and periventricular area (Benarroch, 2002, 2003; Benarroch et al., 2002, 2003, 2006a, 2006b; Ozawa, 2007; Scheer et al., 2003; Singewald and Philippu, 1996; Tsai et al., 1992). It is presently considered that MSA is a primary oligodendrogliopathy characterized by the presence of α-synuclein (α-syn)-positive (oligodendro-)glial cytoplasmic inclusions (GCIs) which play a major role in MSA pathogenesis (Gilman et al., 2008; Wenning et al., 2008). However, the exact role of α-syn with regard to autonomic regulation and cardiovascular dysfunction in MSA is still unclear.
Transgenic (tg) MSA mice with specific oligodendroglial expression of human α-syn (hα-syn) under the control of the proteolipid protein (PLP) promoter (Kahle et al., 2002) have proven useful to study MSA-related pathogenic mechanisms (Stefanova et al., 2005, 2007, 2011, 2012). The tg MSA mouse model features GCIs as well as mild, progressive SND and OPCA, microgliosis and motor impairment (Kahle et al., 2002; Stefanova et al., 2005; Stemberger et al., 2010). Analysis of regions associated with autonomic functions revealed neuronal loss in the Onuf's nucleus and progressive degeneration of cholinergic neurons in the nucleus ambiguus, laterodorsal tegmental nucleus and pedunculopontine tegmental nucleus (Stemberger et al., 2010). These primary neuropathological findings gave reason to speculate about possible autonomic failure in this tg MSA model and served as the rationale for this study. Therefore, in the current study we assessed the cardiovascular phenotype of adult MSA mice by analyzing HRV and circadian rhythm as well as central autonomic neuropathology related to α-syn overexpression.
Materials and methods
Animal groups and implantation of radio-telemetry transmitters
Five month old, male, transgenic mice expressing hα-syn under the control of the oligodendroglial proteolipid protein (PLP) promoter in C57BL/6-background [previously described (Kahle et al., 2002; Stefanova et al., 2005)] and age-matched C57BL/6 mice as wild type (wt) control group were individually housed 24 h before the onset of the surgery and for the duration of the whole behavioral testing. All mice were anesthetized with isoflurane (1.5–2%) and implanted with TA10ETAF10 transmitters (Data Sciences International, USA) as previously described (Gaburro et al., 2011; Stiedl and Spiess, 1997; Stiedl et al., 1999). Post-surgically, all animals were treated with buprenorphione, a semi-synthetic opioid (0.2 mg/kg i.p.) for three days. Animals were further allowed to recover for 10 days before conduction of the behavioral tests.
Locomotion and ECG data acquisition and analysis
Recordings of locomotor activity, heart rate, body temperature and ECG were acquired by the use of TA10ETAF10 radio-telemetry transmitters and the appropriate recording system. Animals were kept in their home cage throughout the 48-h observation period. Dataquest A.R.T. 4.1 software (Data Sciences International, St. Paul, USA) was used for data collection. For ECG data editing, the ECG signal was exported to Chart software (Chart 5.0, AD Instruments, Germany) as a text file as previously described (Gaburro et al., 2011). Data for locomotor activity, body temperature and heart rate were averaged in 1-h intervals. Ten 30-s intervals were used per animal to calculate the HRV (expressed in RMSSD) and to obtain values for frequency-spectrum analysis (high frequency — HF, low frequency — LF and the ration between LF and HF — LF/HF). The time-points for basal HRV were chosen between 09:00 and 13:00 for the light-phase and between 21:00 and 03:00 for the dark phase when basal HR levels should be highest (Li et al., 1999).
Histological analysis
After the behavioral recordings were completed, animals were perfused under deep thiopental anesthesia with 5 ml of phosphate buffered saline followed by 40 ml of ice-cold 4% paraformaldehyde (PFA) (pH 7.4) (Sigma). Brains were removed and post-fixed in 4% PFA over night at 4 °C. Afterwards, they were put in 30% sucrose until they sank, before they were frozen with 2-methylbutane and kept at − 80 °C until processing. The brains were sliced in three series of coronal, adjacent 40-μm sections on a freezing microtome (Leica, Nussloch, Germany) and stored free-floating in a cryoprotective solution at − 20 °C. The following antibodies were used in this study: polyclonal goat-anti-ChAT (1:3000, Chemicon, Temecula, CA), polyclonal rabbit-anti-AVP (1:500, Novus biological, Littleton, CO), secondary antibodies were biotinylated horse anti-goat IgG and biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame or, CA). All quantification analysis was done by a blinded observer using the optical disector method (Nikon Eclipse E800 microscope, Nikon camera DXM1200 Stereo Investigator Software, Micro Bright Field Europe, Magdeburg, Germany).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.3 and Statistica 8.0 (StatSoft, USA) Software. Analysis of variance (ANOVA) was performed with factor line wt and tg groups and repeated measures for time (day versus night) as the second factor for activity, body temperature and heart rate analysis. Two-way ANOVA was performed for analysis of the HRV using RMSSD and frequency-spectrum analysis (HF, LF, LF/HF). Data plotted in graphs are means ± S.E.M. Post-hoc Bonferroni t-test was applied where it is appropriate. * = p < 0.05; ** = p < 0.01; *** = p < 0.001.
Results
Circadian changes in locomotor activity, heart rate and core body temperature
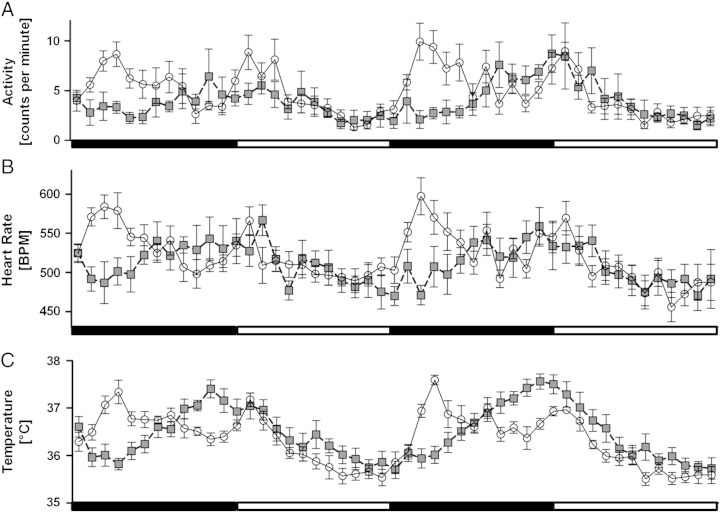
During the 48 h recording period, different patterns of spontaneous locomotor activity, heart rate and temperature were detected in five month old tg MSA mice as compared to age-matched wt controls. Repeated measures ANOVA revealed significantly lower values in the tg group during night regarding activity (F48, 768 = 2.3278, p < 0.0001; Fig. 1A), heart rate (F48, 768 = 3.2192, p < 0.0001; Fig. 1B) and core body temperature (F48, 768 = 5.5873, p < 0.0001; Fig. 1C).
Fig. 1.
Assessment of circadian rhythm parameters: five month old wt (white) and tg MSA (gray) animals show differences in day–night-rhythm in their home cage regarding A) locomotor activity [expressed as counts per minute (counts/min)], B) HR [expressed as beats per minute (BPM)] and C) temperature [°C]. Especially regarding the activity levels, tg MSA animals do not show the characteristic day–night-fluctuations e.g. their circadian rhythm is blunted and clearly differs from wt animals. Black bars indicate active night periods, and white bars indicate day phases. Data plotted are means ± S.E.M., n(tg) = 9, n(wt) = 6 animals.
Impaired HRV in tg MSA group
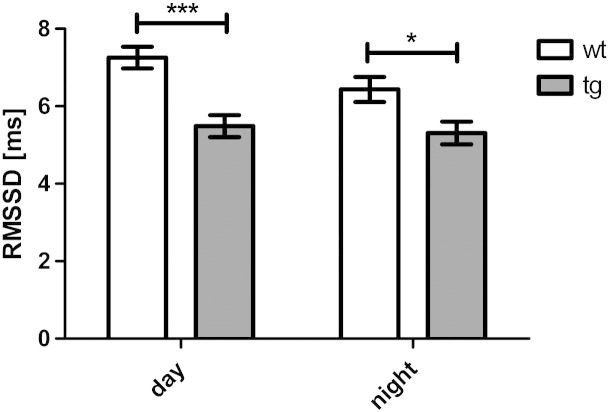
HRV measured in the time domain analysis (RMSSD) was significantly reduced in tg MSA animals as compared to control animals both during light and dark phases (F1, 635 = 26.956, p < 0.0001; Fig. 2). Accordingly, the respective frequency domain values HF and LF were reduced in tg MSA animals as compared to controls during day and night phases (HF: F1, 635 = 140.00, p < 0. 001 (Fig. 3A); LF: F1, 635 = 92.29, day: p < 0.001, night: p < 0.05 (Fig. 3B)). Lower HF and LF values were observed during light as compared to dark phase in the tg MSA group only (HF: F1, 635 = 18.71, tg: p < 0.001 (Fig. 3A); LF: F1, 635 = 19.33, tg: p < 0.001 (Fig. 3B)). The LF/HF ratio was significantly higher in tg MSA animals compared to the wt group during day phases (F1, 635 = 15.38, p < 0.001; Fig. 3C). In the wt group there was further significant increase of the LF/HF ratio in the dark versus light phase (F1, 635 = 4.178, p < 0.01).
Fig. 2.
Time-domain analysis of HRV (plotted as RMSSD [ms] — root mean square of successive RR interval differences) of five month old, male, wt and tg MSA animals. HRV is significantly decreased in transgenic animals at day and night. Data are means ± S.E.M. n(tg) = 9, n(wt) = 7 animals.
Fig. 3.
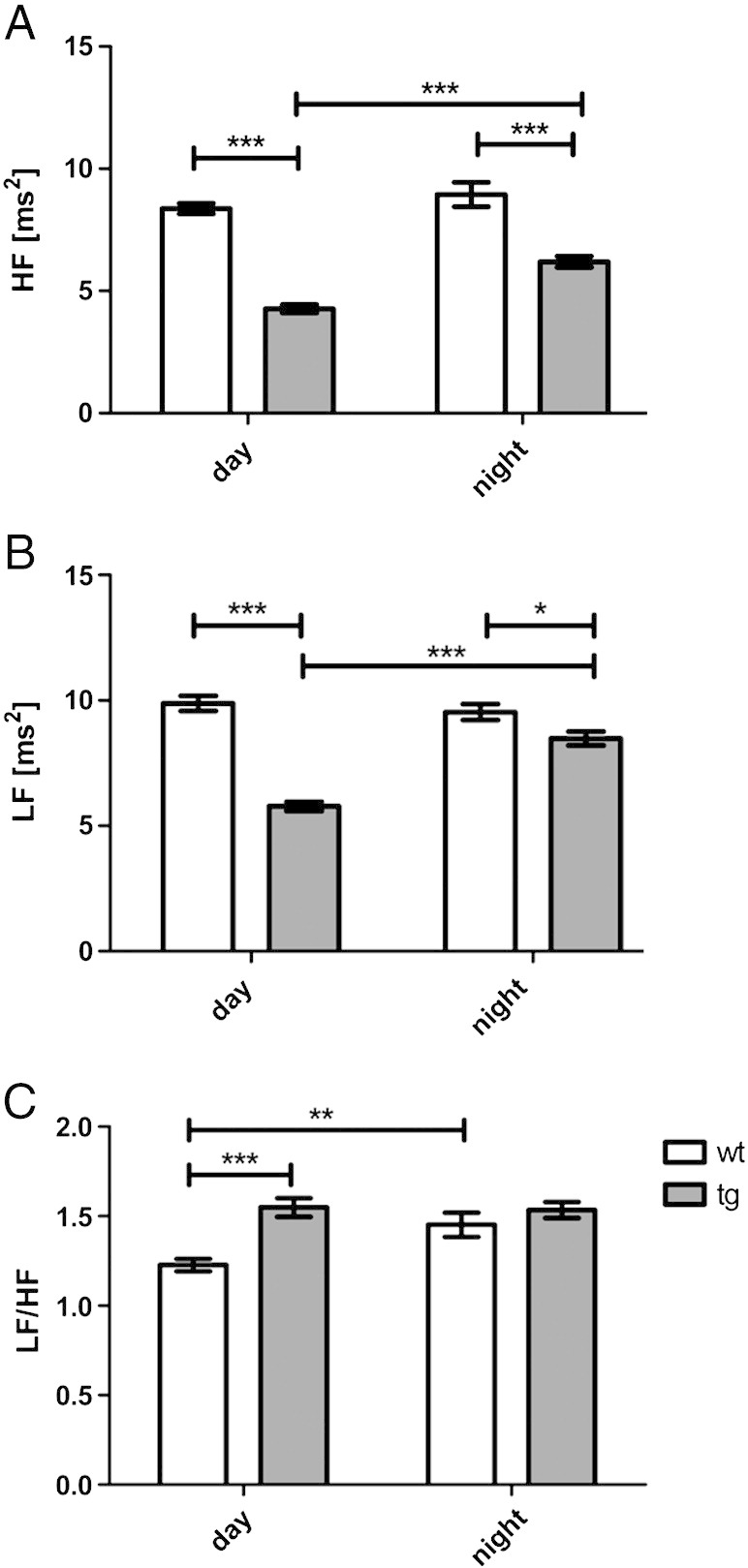
Frequency-spectrum analysis of five month old, male, wt and tg MSA animals. A) High frequency (HF) [ms2] representing vagal function, B) low frequency (LF) [ms2] illustrating sympathovagal balance and C) LF/HF ratio representing sympathovagal balance. Data are means ± S.E.M. n(tg) = 9, n(wt) = 7 animals.
Immunohistochemical analysis of neurodegeneration
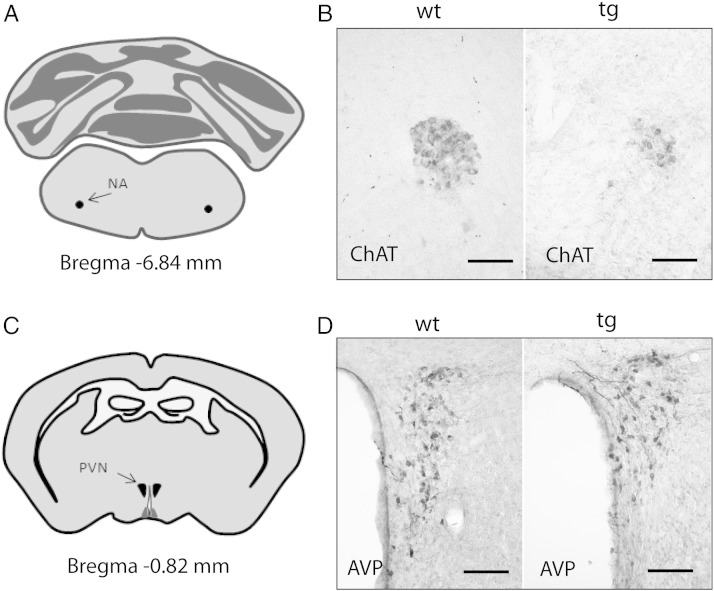
Quantification of ChAT-immunoreactive neurons in the nucleus ambiguus revealed a significant reduction in tg MSA animals (Figs. 4 and 5). However, the number of AVP-immunoreactive neurons in two further regions associated with cardiovascular regulation – the suprachiasmatic nucleus and the paraventricular nucleus – was not altered in tg MSA animals (Figs. 4 and 6).
Fig. 4.
Illustration of immunohistochemical labelings. A) Localization of the nucleus ambiguus (NA) and C) the paraventricular nucleus (PVN) and suprachiasmatic nucleus (SCN) is illustrated at selected Bregma levels. B) Choline-acetyltransferase (ChAT)-immunohistochemistry indicated loss of cholinergic neurons in the NA of tg animals. D) Representative microphotographs showing arginine-vasopressin (AVP) immunohistochemical stainings in the PVN of wild type (wt) and transgenic (tg) animals. Bars, 90 μm.
Fig. 5.
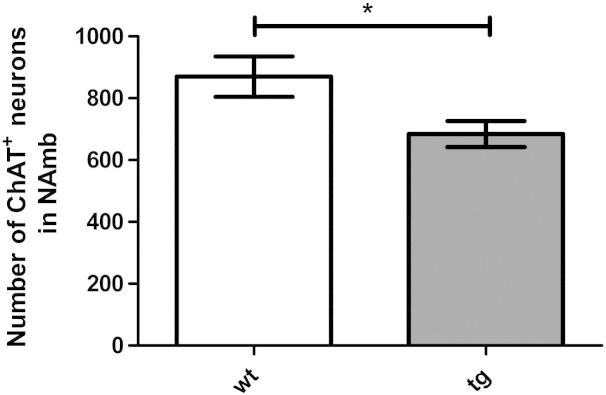
Number of choline-acetyltransferase (ChAT)-immunoreactive neurons is significantly decreased in the nucleus ambiguus of five month old transgenic animals. Data are means ± S.E.M. n(tg) = 7, n(wt) = 6 animals.
Fig. 6.
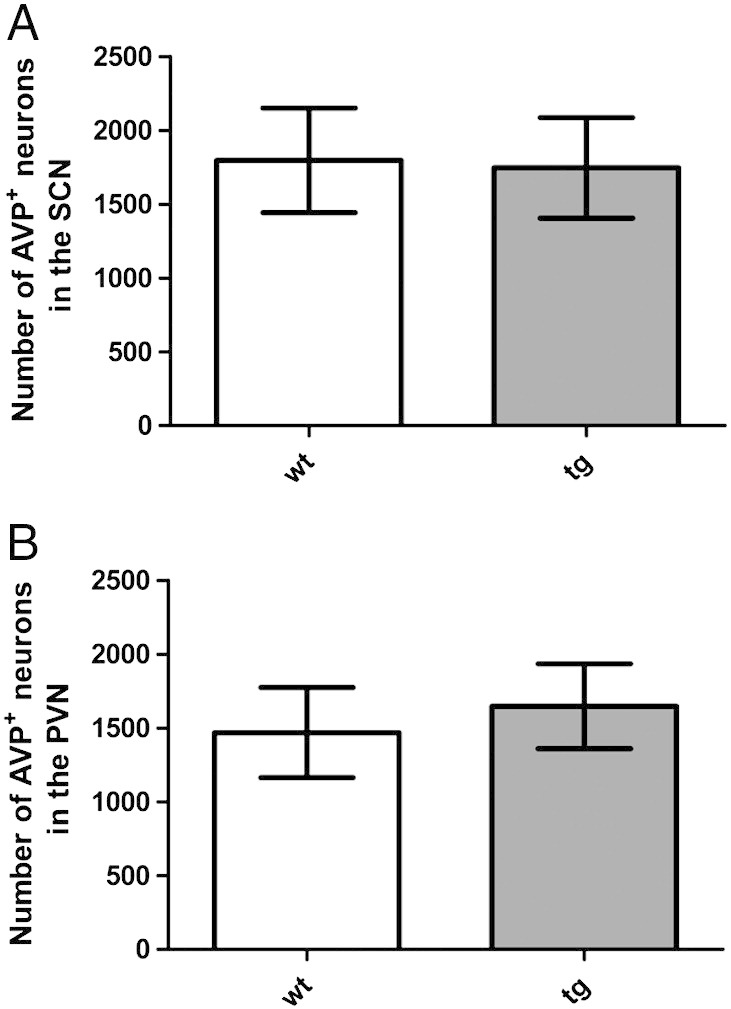
Number of arginine-vasopressin (AVP)-immunoreactive neurons is not affected in the suprachiasmatic nucleus (SCN) and paraventricular nucleus (PVN) of five month old transgenic animals. Data are means ± S.E.M. n(tg) = 9, n(wt) = 7 animals.
Discussion
This study aimed to analyze cardiac autonomic function in the well established PLP-tg mouse model of MSA (Kahle et al., 2002; Stefanova et al., 2005; Stemberger et al., 2010). Detailed characterization of animal models is of great importance to determine how closely they replicate the human disease pathology and define their relevance for investigating pathological disease mechanisms and testing potential therapeutical approaches. Detection of possible cardiovascular impairment as present in human MSA patients (Furushima et al., 2012; Kitae et al., 2001; Pagani et al., 1997; Wenning et al., 2008) and reflection of the previously reported degeneration of the central autonomic areas in the PLP-tg MSA mouse model were the main focus of the present study. We here corroborate our previous observation of selective degeneration involving the nucleus ambiguus. For the first time, we now demonstrate impaired heart rate variability and circadian rhythmicity as a functional read-out reflecting CAF and central autonomic neuropathology in the tg MSA mouse model.
Circadian changes in locomotor activity, heart rate and core body temperature
Home-cage circadian rhythmicity measured by assessing heart rate, core body temperature and locomotor activity in wt mice analyzed in this study were consistent with previously reported data (Gaburro et al., 2011; Sanchez-Alavez et al., 2011). Circadian rhythm measurements of tg animals differed from these patterns with reduced nocturnal activity, heart rate and temperature. Impaired circadian fluctuations as observed here are consistent with the human disease (Moreno-Lopez et al., 2011; Toshihide et al., 2006).
Impaired HRV in tg group
We observed reduced HRV in the tg MSA mice consistent with CAF reflecting central autonomic pathology observed in the nucleus ambiguus similar to human MSA (Abele et al., 2004; Diedrich et al., 2002; Furushima et al., 2012; Iodice et al., 2012; Kitae et al., 2001; Kiyono et al., 2012; Mathias, 2006; Plaschke et al., 1998; Toshihide et al., 2006). The reduced HRV was indicated by both time- and frequency-domain levels in the tg animal model again similar to the human disease (Furushima et al., 2012). The decrease of the HF components of HRV spectral analysis in tg MSA mice reflects what has been observed in MSA patients (Diedrich et al., 2002; Furushima et al., 2012).
LF has been reported to be regulated by neural activities of sympathetic components (Berger et al., 1989). As for human MSA patients (Diedrich et al., 2002; Furushima et al., 2012), a significant reduction of LF was detected in the tg MSA mice throughout the day and night. Furthermore, the ratio LF/HF reported to be higher in human MSA cases (Furushima et al., 2012) was also found to be significantly increased in tg MSA animals during the day as compared to controls indicating a dysregulation of sympathovagal balance (Diedrich et al., 2002; Furushima et al., 2012). A high LF/HF ratio principally suggests an increased sympathetic or decreased parasympathetic activity (Malliani et al., 1991; Montano et al., 1994; Pagani et al., 1997). Based on findings in MSA patients (Furushima et al., 2012; Kiyono et al., 2012), we conclude that in the current study changes in HRV patterns (HF, LF and ratio) may be also attributed to an impaired parasympathetic activity which matches with the neuropathological findings of cholinergic neurodegeneration in the nucleus ambiguus in this animal model and goes in line with reports from human MSA patients (Kiyono et al., 2012).
Immunohistochemical analysis of neurodegeneration
MSA brains show neurodegeneration in areas associated with CAF e.g. degeneration of the intermediolateral cell column, cholinergic depletion in the nucleus ambiguus and dorsal motor nucleus of the vagus, degeneration of arginine-vasopressin neurons in the suprachiasmatic and paraventricular nucleus, and loss of TH neurons in the ventrolateral medulla and periventricular area (Benarroch, 2002, 2003; Benarroch et al., 2002, 2003, 2006a, 2006b; Ozawa, 2007; Tsai et al., 1992). To further investigate whether cardiovascular autonomic dysfunction (decreased HRV) reflects central autonomic pathology in the tg MSA animal model, three of the brain regions affected by human MSA were chosen as the main regions of interest for this study: 1) the nucleus ambiguus associated with cardiovagal function (Benarroch et al., 2003, 2006a, 2006b; Ozawa, 2007), 2) the suprachiasmatic nucleus relevant for circadian rhythm and response to stress (Benarroch et al., 2006a, 2006b; Ozawa, 2007) and 3) the paraventricular nucleus involved in blood pressure maintenance, respiration and responses to stress (Benarroch, 2002, 2003; Benarroch et al., 2006a, 2006b; Ozawa, 2007). The functions of these areas are comparable between humans and rodents and have been used for histological analysis in HRV or circadian rhythm studies of other conditions such as PD or Huntington's disease (Dai et al., 1998; Koutcherov et al., 2000; Kudo et al., 2011a, 2011b; Rentero et al., 2002; Scheer et al., 2003; Sheward et al., 2010; Tsai et al., 1992; Yan et al., 2009). The reduction of ChAT-immunoreactive neurons in the nucleus ambiguus of the five month old tg MSA animals in this study, corroborates previous findings in the same model showing a slight trend towards reduction at 2 months and a marked decrease at 12 months of age. This loss of cholinergic neurons in the nucleus ambiguus has also been reported in human MSA patients and is associated with cardiovascular disturbances. The loss of AVP neurons described in the human disease (Benarroch et al., 2002, 2006a, 2006b) is thought to mediate CAF but could not be detected in the tg MSA model of this study.
Conclusion
This study was the first to show decreased HRV and impaired circadian rhythmicity in a tg animal model for MSA. The finding of reduced HRV in the hα-syn-overexpressing mouse model for MSA underscores the translational value of this animal model. How overexpression of hα-syn in oligodendrocytes leads to neurodegeneration in areas of autonomic regulation and thereby induces autonomic failure, is still unclear, however, our data indicate that the presence of α-syn aggregates is an essential key event in MSA pathology linked to central cardiovascular autonomic failure.
Acknowledgments
This work was funded by the Austrian Science Fund FWF DK SPIN (W1206), FWF SFB (F4404).
References
- Abele M. Spectral analysis of heart rate variability in multiple system atrophy and unexplained sporadic ataxia. J. Neurol. 2004;251:894–895. doi: 10.1007/s00415-004-0460-x. [DOI] [PubMed] [Google Scholar]
- Agelink M.W. Relationship between major depression and heart rate variability. Clinical consequences and implications for antidepressive treatment. Psychiatry Res. 2002;113:139–149. doi: 10.1016/s0165-1781(02)00225-1. [DOI] [PubMed] [Google Scholar]
- Benarroch E.E. New findings on the neuropathology of multiple system atrophy. Auton. Neurosci. 2002;96:59–62. doi: 10.1016/s1566-0702(01)00374-5. [DOI] [PubMed] [Google Scholar]
- Benarroch E.E. Brainstem in multiple system atrophy: clinicopathological correlations. Cell. Mol. Neurobiol. 2003;23:519–526. doi: 10.1023/A:1025067912199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. Depletion of mesopontine cholinergic and sparing of raphe neurons in multiple system atrophy. Neurology. 2002;59:944–946. doi: 10.1212/wnl.59.6.944. [DOI] [PubMed] [Google Scholar]
- Benarroch E.E. Preservation of branchimotor neurons of the nucleus ambiguus in multiple system atrophy. Neurology. 2003;60:115–117. doi: 10.1212/01.wnl.0000042087.07133.87. [DOI] [PubMed] [Google Scholar]
- Benarroch E.E. Differential involvement of hypothalamic vasopressin neurons in multiple system atrophy. Brain. 2006;129:2688–2696. doi: 10.1093/brain/awl109. [DOI] [PubMed] [Google Scholar]
- Benarroch E.E. Involvement of vagal autonomic nuclei in multiple system atrophy and Lewy body disease. Neurology. 2006;66:378–383. doi: 10.1212/01.wnl.0000196638.98781.bb. [DOI] [PubMed] [Google Scholar]
- Berger R.D. Transfer function analysis of autonomic regulation. I. Canine atrial rate response. Am. J. Physiol. 1989;256:H142–H152. doi: 10.1152/ajpheart.1989.256.1.H142. [DOI] [PubMed] [Google Scholar]
- Bleil M.E. Trait negative affect: toward an integrated model of understanding psychological risk for impairment in cardiac autonomic function. Psychosom. Med. 2008;70:328–337. doi: 10.1097/PSY.0b013e31816baefa. [DOI] [PubMed] [Google Scholar]
- Dai J. Postmortem tracing reveals the organization of hypothalamic projections of the suprachiasmatic nucleus in the human brain. J. Comp. Neurol. 1998;400:87–102. doi: 10.1002/(sici)1096-9861(19981012)400:1<87::aid-cne6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Diedrich A. Modulation of QT interval during autonomic nervous system blockade in humans. Circulation. 2002;106:2238–2243. doi: 10.1161/01.cir.0000035241.76918.6c. [DOI] [PubMed] [Google Scholar]
- Furushima H. Significance and usefulness of heart rate variability in patients with multiple system atrophy. Mov. Disord. 2012;27:570–574. doi: 10.1002/mds.24929. [DOI] [PubMed] [Google Scholar]
- Gaburro S. A mouse model of high trait anxiety shows reduced heart rate variability that can be reversed by anxiolytic drug treatment. Int. J. Neuropsychopharmacol. 2011;14:1341–1355. doi: 10.1017/S1461145711000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iodice V. Autopsy confirmed multiple system atrophy cases: Mayo experience and role of autonomic function tests. J. Neurol. Neurosurg. Psychiatry. 2012;83:453–459. doi: 10.1136/jnnp-2011-301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jecmenica-Lukic M. Premotor signs and symptoms of multiple system atrophy. Lancet Neurol. 2012;11:361–368. doi: 10.1016/S1474-4422(12)70022-4. [DOI] [PubMed] [Google Scholar]
- Kahle P.J. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitae S. Assessment of cardiovascular autonomic dysfunction in multiple system atrophy. Clin. Auton. Res. 2001;11:39–44. doi: 10.1007/BF02317801. [DOI] [PubMed] [Google Scholar]
- Kiyono K. Non-gaussianity of low frequency heart rate variability and sympathetic activation: lack of increases in multiple system atrophy and Parkinson disease. Front. Physiol. 2012;3:34. doi: 10.3389/fphys.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutcherov Y. Organization of the human paraventricular hypothalamic nucleus. J. Comp. Neurol. 2000;423:299–318. [PubMed] [Google Scholar]
- Kudo T. Circadian dysfunction in a mouse model of Parkinson's disease. Exp. Neurol. 2011;232:66–75. doi: 10.1016/j.expneurol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Kudo T. Dysfunctions in circadian behavior and physiology in mouse models of Huntington's disease. Exp. Neurol. 2011;228:80–90. doi: 10.1016/j.expneurol.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie K.L. Heart rate variability in coronary artery disease patients with and without panic disorder. Psychiatry Res. 2004;128:289–299. doi: 10.1016/j.psychres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Li P. Circadian blood pressure and heart rate rhythms in mice. Am. J. Physiol. 1999;276:R500–R504. doi: 10.1152/ajpregu.1999.276.2.R500. [DOI] [PubMed] [Google Scholar]
- Malliani A. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- Malpas S.C., Maling T.J. Heart-rate variability and cardiac autonomic function in diabetes. Diabetes. 1990;39:1177–1181. doi: 10.2337/diab.39.10.1177. [DOI] [PubMed] [Google Scholar]
- Martens E.J. Depression and anxiety as predictors of heart rate variability after myocardial infarction. Psychol. Med. 2008;38:375–383. doi: 10.1017/S0033291707002097. [DOI] [PubMed] [Google Scholar]
- Mathias C.J. Multiple system atrophy and autonomic failure. J. Neural Transm. Suppl. 2006:343–347. doi: 10.1007/978-3-211-45295-0_52. [DOI] [PubMed] [Google Scholar]
- Montano N. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation. 1994;90:1826–1831. doi: 10.1161/01.cir.90.4.1826. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez C. Excessive daytime sleepiness in multiple system atrophy (SLEEMSA study) Arch. Neurol. 2011;68:223–230. doi: 10.1001/archneurol.2010.359. [DOI] [PubMed] [Google Scholar]
- Ozawa T. Morphological substrate of autonomic failure and neurohormonal dysfunction in multiple system atrophy: impact on determining phenotype spectrum. Acta Neuropathol. 2007;114:201–211. doi: 10.1007/s00401-007-0254-1. [DOI] [PubMed] [Google Scholar]
- Pagani M. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- Plaschke M. Twenty-four-hour blood pressure profile and blood pressure responses to head-up tilt tests in Parkinson's disease and multiple system atrophy. J. Hypertens. 1998;16:1433–1441. doi: 10.1097/00004872-199816100-00006. [DOI] [PubMed] [Google Scholar]
- Rentero N. Activity patterns of cardiac vagal motoneurons in rat nucleus ambiguus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R1327–R1334. doi: 10.1152/ajpregu.00271.2002. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age (Dordr.) 2011;33:89–99. doi: 10.1007/s11357-010-9164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer F.A. Cardiovascular control by the suprachiasmatic nucleus: neural and neuroendocrine mechanisms in human and rat. Biol. Chem. 2003;384:697–709. doi: 10.1515/BC.2003.078. [DOI] [PubMed] [Google Scholar]
- Sheward W.J. Circadian control of mouse heart rate and blood pressure by the suprachiasmatic nuclei: behavioral effects are more significant than direct outputs. PLoS One. 2010;5:e9783. doi: 10.1371/journal.pone.0009783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N., Philippu A. Involvement of biogenic amines and amino acids in the central regulation of cardiovascular homeostasis. Trends Pharmacol. Sci. 1996;17:356–363. [PubMed] [Google Scholar]
- Stefanova N. Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am. J. Pathol. 2005;166:869–876. doi: 10.1016/s0002-9440(10)62307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov. Disord. 2007;22:2196–2203. doi: 10.1002/mds.21671. [DOI] [PubMed] [Google Scholar]
- Stefanova N. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am. J. Pathol. 2011;179:954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N. Systemic proteasome inhibition triggers neurodegeneration in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. Acta Neuropathol. 2012;124:51–65. doi: 10.1007/s00401-012-0977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemberger S. Targeted overexpression of human alpha-synuclein in oligodendroglia induces lesions linked to MSA-like progressive autonomic failure. Exp. Neurol. 2010;224:459–464. doi: 10.1016/j.expneurol.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl O., Spiess J. Effect of tone-dependent fear conditioning on heart rate and behavior of C57BL/6 N mice. Behav. Neurosci. 1997;111:703–711. doi: 10.1037//0735-7044.111.4.703. [DOI] [PubMed] [Google Scholar]
- Stiedl O. Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behav. Brain Res. 1999;104:1–12. doi: 10.1016/s0166-4328(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Toshihide H. Circadian rhythm of cardiovascular autonomic nervous function in Parkinson's disease, early-onset parkinsonism and multiple system atrophy. Int. Med. J. 2006;13:131–133. [Google Scholar]
- Tsai A. Loss of catecholaminergic neuron in the hypothalamus of multiple system atrophy. Rinsho Shinkeigaku. 1992;32:125–130. [PubMed] [Google Scholar]
- Wenning G.K., Stefanova N. Recent developments in multiple system atrophy. J. Neurol. 2009;256:1791–1808. doi: 10.1007/s00415-009-5173-8. [DOI] [PubMed] [Google Scholar]
- Wenning G.K. Multiple system atrophy: a primary oligodendrogliopathy. Ann. Neurol. 2008;64:239–246. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- Yan B. Diabetes induces neural degeneration in nucleus ambiguus (NA) and attenuates heart rate control in OVE26 mice. Exp. Neurol. 2009;220:34–43. doi: 10.1016/j.expneurol.2009.07.006. [DOI] [PubMed] [Google Scholar]