Abstract
Cytochrome P450 (CYP)2C19 catalyzes the bioactivation of the antiplatelet prodrug clopidogrel, and CYP2C19 loss-of-function alleles impair formation of active metabolites, resulting in reduced platelet inhibition. In addition, CYP2C19 loss-of-function alleles confer increased risks for serious adverse cardiovascular (CV) events among clopidogrel-treated patients with acute coronary syndromes (ACSs) undergoing percutaneous coronary intervention (PCI). Guideline updates include emphasis on appropriate indication for CYP2C19 genotype–directed antiplatelet therapy, refined recommendations for specific CYP2C19 alleles, and additional evidence from an expanded literature review (updates at http://www.pharmgkb.org).
This document is an update to the 2011 Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline on the clinical use of CYP2C19 genotype test results for patients requiring antiplatelet therapy.1 As of April 2013, a prospective randomized controlled trial on CYP2C19 genotype–directed antiplatelet therapy with clinical outcomes has yet to be reported. Consequently, these recommendations are based on evaluation of the currently available evidence. Specifically, the updated therapeutic recommendations are more focused on patients with acute coronary syndromes (ACSs) undergoing percutaneous coronary intervention (PCI) than the original guideline, with additional updates involving refined recommendations for variant and novel CYP2C19 alleles beyond *2 and further evidence from an expanded literature review. As in the 2011 guideline, recommendations for the use of other laboratory tests, such as platelet function monitoring, and cost-effectiveness assessments are beyond the scope of this document. This document does not focus on demographic or other clinical variables such as adherence to therapy, age, diabetes mellitus, obesity, smoking, and concomitant use of other drugs that may influence clopidogrel efficacy and clinical decision making. The CPIC of the National Institutes of Health's Pharmacogenomics Research Network and PharmGKB develops peer-reviewed gene/drug guidelines that are published and updated on www.pharmgkb.org every 2 years or as needed based on significant developments in the field.2
Focused Literature Review
A systematic literature review was conducted on CYP2C19 genotype and clopidogrel response (see Supplementary Materials and Methods online). Guidelines for antiplatelet therapy were developed based on interpretation of the available literature by the authors and experts in the field.
Drug: Clopidogrel
Background
Clopidogrel is a thienopyridine prodrug that requires hepatic biotransformation to form an active metabolite that selectively and irreversibly inhibits the purinergic P2RY12 receptor and thus platelet aggregation for the platelet's life span (~10 days). Only 15% of the prodrug is available for transformation to the active agent; the remaining 85% is hydrolyzed by esterases to inactive forms. Conversion of clopidogrel to its active metabolite requires two sequential oxidative steps involving several CYP enzymes (e.g., CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A4/5; Supplementary Figure S1 online). One small association study suggested that paraoxonase 1 (PON1) may also be involved in clopidogrel activation;3 however, this finding and the reported association between PON1 variant alleles and clopidogrel response variability was not replicated in multiple recent publications (for review, see ref. 4).
Gene: CYP2C19
Background
A gene summary on CYP2C19 has recently been published5 and is available online at PharmGKB: http://www.pharmgkb.org/gene/PA124#tabview=tab3&subtab=31. The hepatic CYP2C19 enzyme contributes to the metabolism of a large number of clinically relevant drugs such as antidepressants, benzodiazepines, mephenytoin, some proton pump inhibitors, and clopidogrel. Like many other CYP450 superfamily members, the CYP2C19 gene is highly polymorphic, with >25 known variant alleles (http://www.cypalleles.ki.se/cyp2c19.htm) (Supplementary Tables S1 and S2 online).5 The wild-type CYP2C19*1 allele is associated with functional CYP2C19-mediated metabolism. The most common CYP2C19 loss-of-function allele is *2 (c.681G>A; rs4244285), with allele frequencies of ~15% in Caucasians and Africans, and 29–35% in Asians (Supplementary Tables S3 and S4 online). Other CYP2C19 variant alleles with reduced or absent enzymatic activity have been identified (e.g., *3–*8); however, their frequencies are typically below 1%, with the exception of CYP2C19*3 (c.636G>A; rs4986893) in Asians (with an allele frequency of 2–9%; Supplementary Tables S3 and S4 online).
With respect to clopidogrel pharmacodynamics, CYP2C19 loss-of-function alleles are inherited as autosomal codominant traits because heterozygotes (e.g., *1/*2 and *1/*3) have platelet responsiveness to clopidogrel that lies between homozygous wild-type (i.e., *1/*1) and loss-of-function allele homozygotes or compound heterozygotes (e.g.,. *2/*2 and *2/*3).6,7,8,9,10,11 Therefore, on the basis of identified CYP2C19 genotypes, individuals typically are categorized as extensive metabolizers (EMs; *1/*1), intermediate metabolizers (IMs; e.g., *1/*2 and *1/*3), or poor metabolizers (PMs; e.g., *2/*2 and *2/*3) Table 1. The frequencies of CYP2C19 PMs are ~2–5% among Caucasians and Africans and ~15% in Asians (Supplementary Materials and Methods online).
Table 1. Assignment of likely CYP2C19 phenotypes based on genotypes.
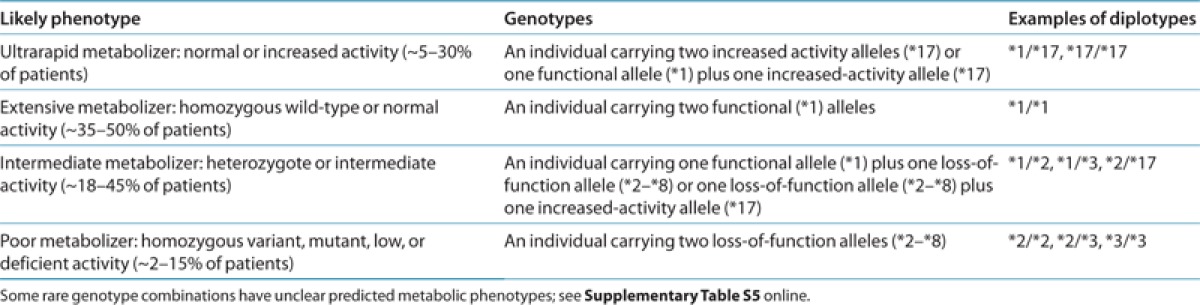
By contrast, the common CYP2C19*17 allele (c.-806C>T; rs12248560) results in increased activity as a consequence of enhanced transcription, with average multiethnic allele frequencies of ~3–21% (Supplementary Tables S3 and S4 online). Therefore, individuals who carry this allele may be categorized as ultrarapid metabolizers (e.g., *17/*17) Table 1. Some studies indicate that this allele results in enhanced platelet inhibition and clopidogrel response10,12,13,14 and perhaps an increased risk of bleeding complications.15,16 However, other studies have not identified an effect of CYP2C19*17.11,17,18,19,20 Discordant results in the literature may be due in part to the linkage disequilibrium that exists between *17 and *2. Specifically, the gain-of-function “T” allele of *17 always occurs on a haplotype that also harbors the wild-type “G” allele of *2, so the observed effect of the gain-of-function *17 allele may actually be due, in part, to the absence of the loss-of-function “A” allele of *2. As such, adequate evidence for an independent effect of this allele on clinical outcomes is lacking. Moreover, given that *17 is unable to completely compensate for the *2 loss-of-function allele,13 *2/*17 compound heterozygotes should be classified as IMs (see Supplementary Table S5 online).
Linking genetic variability to variability in drug-related phenotypes
Clopidogrel is commonly prescribed for ACSs and/or following PCI. However, responses to clopidogrel vary widely, with inhibition of adenosine diphosphate (ADP)-induced platelet aggregation being normally distributed across a broad range.11 Many studies have shown that CYP2C19*2 heterozygotes and homozygotes have reduced active clopidogrel metabolites and higher on-treatment platelet aggregation as compared with *1 homozygotes.6,7,8,10,11,21 Moreover, substantial evidence exists linking CYP2C19 genotype with clinical outcomes among clopidogrel-treated ACS patients, particularly those undergoing PCI,9,10,11,18,22,23,24 probably as a result of decreased formation of the active clopidogrel metabolite. Studies linking CYP2C19 genotype with variability in clopidogrel response are summarized in Supplementary Tables S6 and S7 online, and it is this body of evidence, rather than randomized clinical trials, that provides the basis for genotype-informed therapeutic recommendations when considering treatment with clopidogrel (Table 2). Of note, the most definitive studies showing a relationship between CYP2C19 genotype and clopidogrel response have predominantly been conducted in ACS patients, almost all of whom underwent PCI. Therefore, these recommendations do not apply to other situations in which clinicians may consider using clopidogrel, such as medical management of ACS, stroke, and peripheral artery disease.25,26
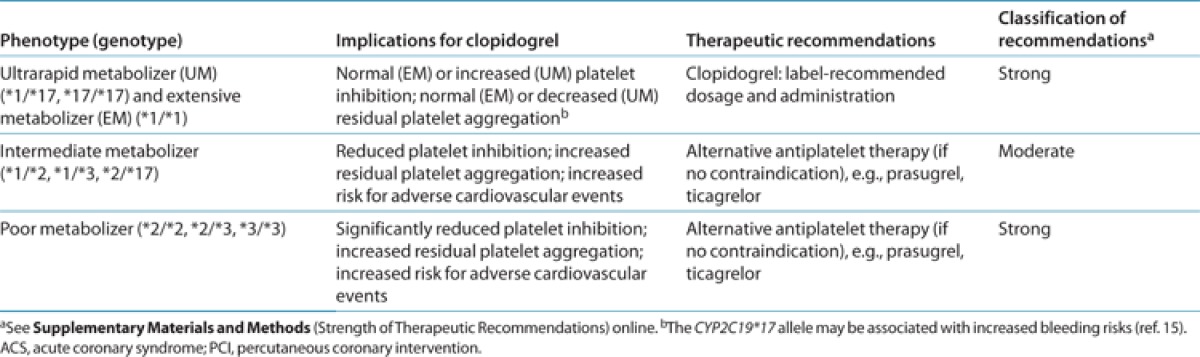
Table 2. Antiplatelet therapy recommendations based on CYP2C19 status when considering clopidogrel for ACS/PCI patients.
Large meta-analyses (Supplementary Table S7 online) have shown that clopidogrel-treated ACS patients undergoing PCI who are CYP2C19*2 heterozygotes or homozygotes have an increased risk for major adverse CV events as compared with *1 homozygotes (hazard ratio (HR) = 1.55, 95% confidence interval (CI) = 1.11–2.17 for heterozygotes; HR = 1.76, 95% CI = 1.24–2.50 for homozygotes) and increased risks of stent thrombosis (HR = 2.67, 95% CI = 1.69–4.22 for heterozygotes; HR = 3.97, 95% CI = 1.75–9.02 for homozygotes).27 Additional meta-analyses have replicated the association between CYP2C19 genotype and stent thrombosis, with reported odds ratios ranging from 1.75 to 3.82 among *2 heterozygotes and homozygotes (Supplementary Table S7 online).
As described above, a lack of effect of CYP2C19 loss-of-function alleles on adverse CV outcomes has been reported among clopidogrel-treated patients with lower clinical risks (e.g., in clinical trials with fewer coronary patients undergoing PCI with stenting and in patients receiving clopidogrel for atrial fibrillation and stroke).25 Consistent with these findings, meta-analyses that include studies with low frequencies of PCI, patients without coronary disease, follow-up periods beyond the duration of clopidogrel therapy, or non-CV outcomes have not supported a major role for CYP2C19 in clopidogrel response variability in these patient populations26 (Supplementary Table S7 online). Consequently, widespread adoption of CYP2C19-guided antiplatelet therapy for all patients is not recommended. Rather, this guideline is an example of indication-specific clinical pharmacogenetics whereby CYP2C19 genotype–directed antiplatelet therapy is limited predominantly to ACS patients managed with PCI (ACS/PCI).28 Although there are limited data regarding the potential role of CYP2C19 for elective PCI cases treated with clopidogrel, these guidelines may also be considered for these patients. However, the lack of indication for other US Food and Drug Administration–approved antiplatelet agents (e.g., prasugrel and ticagrelor) for treatment of elective PCI must be balanced with the boxed warning on the clopidogrel label recommending consideration of alternative antiplatelet therapy in CYP2C19 PMs with ACS or PCI.
The growing body of literature implicating CYP2C19*2 (and probably other loss-of-function alleles) in adverse clopidogrel responses prompted the Food and Drug Administration to implement a boxed warning on the clopidogrel label describing the relationship between CYP2C19 pharmacogenetics and drug response among ACS/PCI patients, particularly noting the diminished effectiveness in PMs. Because the Food and Drug Administration warning does not require genetic testing to initiate clopidogrel therapy, if a patient's genotype is unknown, the decision to perform CYP2C19 testing is up to the individual clinician.
Genetic test interpretation
Clinical genotyping tests are available that interrogate variant CYP2C19 alleles and predict an individual's CYP2C19 metabolizer phenotype (Supplementary Materials and Methods online). Each named star (*) allele is defined by the genotype at one or more specific single-nucleotide polymorphisms (Supplementary Table S1 online) and is associated with a level of enzyme activity (Supplementary Table S2 online). Table 1 and Supplementary Table S5 online summarize the assignment of the likely CYP2C19 phenotype based on common star allele diplotypes, and these assignments are used to link genotypes with personalized antiplatelet therapy.
Available genetic test options
A number of Clinical Laboratory Improvement Amendments–certified laboratories offer targeted genetic testing for CYP2C19*2, *3, and other variant alleles (see Supplementary Materials and Methods online). A voluntary listing of testing providers and related genetic test information is publically available through the Genetic Testing Registry of the National Institutes of Health: http://www.ncbi.nlm.nih.gov/gtr/. At the time of this writing, only the CYP2C19*2 and *3 alleles have been adequately studied with respect to clinical outcomes on clopidogrel, although other known loss-of-function alleles with lower frequencies (e.g., *4–*8) also likely influence clopidogrel response (see Supplementary Materials and Methods online).
CYP2C19 sequencing and novel variants
In addition to targeted genotyping, clinical laboratories may test CYP2C19 by direct sequencing, and clinical whole-exome and whole-genome sequencing programs are increasingly being deployed across academic medical centers and commercial laboratories. Sequencing-based tests capture the common CYP2C19 variants (e.g., *2 and *3) but will also identify both rare (e.g., *4–*8) and novel CYP2C19 variant alleles that have untested clinical significance with respect to clopidogrel response (Supplementary Materials and Methods online). Available evidence suggests that CYP2C19 and other drug metabolism genes are highly polymorphic. Although it is challenging to infer any association with clopidogrel response in the context of a novel CYP2C19 sequence variant, reported variants that are “pathogenic” or “likely pathogenic” (based on the American College of Medical Genetics and Genomics consensus nomenclature) may act in a manner biologically consistent with other common loss-of-function alleles (e.g., *2–*8) because these are sequence alterations that typically result in an upstream polypeptide frameshift, premature or mutated stop codon, or canonical splice-site mutation. Novel CYP2C19 variants classified as having “unknown significance” (e.g., missense alterations, in-frame insertions/deletions, and nonconserved nucleotide substitutions) or “likely benign” should not be assumed to mimic the biological consequences of known CYP2C19 loss-of-function alleles and their established roles in clopidogrel response variability.
Incidental findings
There are no diseases or conditions that have been convincingly linked to variation in the CYP2C19 gene independent of drug metabolism and response.
Other considerations
CYP2C19 loss-of-function alleles do not account for all the variability in clopidogrel response. Some studies have implicated variants in other genes associated with clopidogrel response (e.g., ABCB1, CES1, CYP2B6, CYP2C9, P2RY12, and PON1; see Supplementary Materials and Methods online for review); however, these have not been consistently replicated.
Therapeutic Recommendations
CPIC guidelines are designed to help clinicians understand how available genetic test results can be used to optimize drug therapy rather than to recommend in whom pharmacogenetic testing should be conducted. With the growing ease and availability of genetic testing and other sequencing programs, an increasing number of patients in the near future may already know their CYP2C19 genotype status at the time of treatment, and this document provides guidance on clinical management for those in whom genotype is available or for whom the clinician chooses to order a CYP2C19 genotyping test. With respect to other professional statements, the 2012 American College of Cardiology Foundation/American Heart Association ACS guidelines noted that genetic testing for CYP2C19 loss-of-function alleles may be considered on a case-by-case basis, especially for patients who experience recurrent ACS events despite ongoing therapy with clopidogrel.29 In addition, the committee recommended that genotyping might be considered if results of testing may alter management, which they suggest until better clinical evidence exists to provide a more scientifically derived recommendation.29
Optimal individualized antiplatelet treatment should maximize benefit by reducing risk of recurrent CV events while minimizing adverse effects such as bleeding. Prasugrel is an approved antiplatelet agent that was superior to clopidogrel in a large-scale randomized trial of ACS patients with planned PCI, with an HR for CV death, myocardial infarction, or stroke for prasugrel vs. clopidogrel of 0.81 (95% CI = 0.73–0.90, P < 0.001), as well as a 42% reduction in stent thrombosis.30 However, it may not represent a substitute for clopidogrel in all patients due to an increased risk of non-coronary artery bypass grafting TIMI major bleeding (HR = 1.32, 95% CI = 1.03–1.68; P = 0.03), including fatal bleeding (prasugrel = 0.4% vs. clopidogrel = 0.1%; P = 0.002);30 its contraindication in some patients (e.g., history of transient ischemic attack, stroke, or intracranial bleeding); and the lower expense of generic clopidogrel following the recent expiration of its patent. Of note, the benefit of prasugrel over clopidogrel was found to be greater in patients with a CYP2C19 loss-of-function allele,31 with no significant difference estimated in composite outcome risk between the two antiplatelet agents among CYP2C19 extensive metabolizers (i.e., *1/*1 patients).32
In addition to prasugrel, ticagrelor is a recently approved antiplatelet agent that also was superior to clopidogrel in a large-scale randomized trial of ACS patients with an HR for CV death, myocardial infarction, or stroke for ticagrelor vs. clopidogrel of 0.84 (95% CI = 0.77–0.92; P < 0.001),33 including a 26% reduction in stent thrombosis and 18% reduction in all-cause mortality. In the genetic substudy, as compared with clopidogrel, ticagrelor reduced the primary end point by 23% among patients carrying any CYP2C19 loss-of-function allele (8.6 vs. 11.2%; HR = 0.77, 95% CI = 0.60–0.99; P = 0.0380) and 14% among patients without any CYP2C19 loss-of-function allele (8.8 vs. 10.0%; HR = 0.86, 95% CI = 0.74–1.01), although this reduction did not reach statistical significance (P = 0.0608). However, formal interaction testing that evaluated if the effect of ticagrelor vs. clopidogrel varied by genotype was also not significant. Of note, the benefit of ticagrelor as compared with clopidogrel was subsequently shown to appear to be most pronounced among the subset of patients with CYP2C19 loss-of-function alleles who were undergoing PCI (carriers: 7.7 vs. 10.6%, HR = 0.71; noncarriers: 7.4 vs. 8.2%, HR = 0.90).34 In addition, it is not known to what extent twice-daily dosing may affect the efficacy of ticagrelor relative to clopidogrel in a real-world setting.
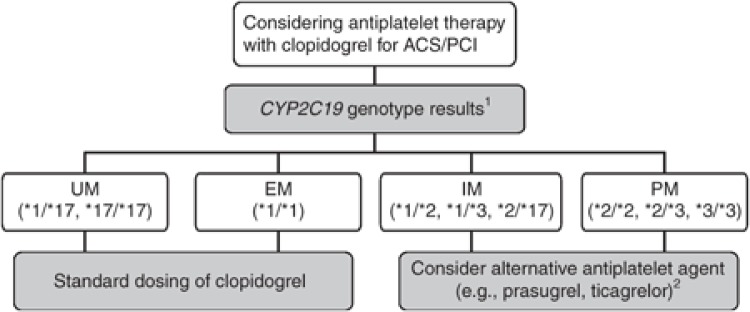
Despite the improvements in overall efficacy reported for prasugrel and ticagrelor as compared with clopidogrel, it is anticipated that clopidogrel will continue to be a widely prescribed medication for ACS/PCI patients. Genotype-directed therapy could identify patients who benefit most from alternative antiplatelet therapy. For clinicians considering treatment with clopidogrel, Table 2 and Figure 1 summarize the therapeutic recommendations for antiplatelet therapy based on CYP2C19 status. Standard dosing of clopidogrel, as recommended in the product insert, is warranted among ACS/PCI patients with a predicted CYP2C19 extensive metabolizer or ultrarapid metabolizer phenotype (i.e., *1/*1, *1/*17, and *17/*17).32 If genotyping from a Clinical Laboratory Improvement Amendments–certified laboratory identifies a patient as a CYP2C19 PM (i.e., *2/*2), current literature supports the use of an alternative antiplatelet agent (e.g., prasugrel or ticagrelor) when not contraindicated clinically.10,30,31,35,36,37
Figure 1.
Algorithm for suggested clinical actions based on CYP2C19 genotype when considering treatment with clopidogrel for ACS patients undergoing PCI (ACS/PCI). 1Other rare CYP2C19 genotypes exist beyond those illustrated (see Supplementary Materials and Methods online for other genotypes and frequencies). 2Note that prasugrel and ticagrelor are recommended only when not contraindicated clinically. ACS, acute coronary syndrome; EM, extensive metabolizer; IM, intermediate metabolizer; PCI, percutaneous coronary intervention; PM, poor metabolizer; UM, ultrarapid metabolizer.
The most challenging patient population to address is the CYP2C19 IM phenotype (e.g., *1/*2, *1/*3, and *2/*17). IMs have higher on-treatment residual platelet activity on average as compared with extensive metabolizers,6,7,8,10,11 and ACS/PCI CYP2C19*2 heterozygotes treated with clopidogrel have increased risks for serious adverse CV outcomes, including stent thrombosis27 (see Supplementary Materials and Methods online). Consequently, these data support switching to an alternative antiplatelet agent for IMs when not contraindicated. However, given the wide interindividual variability in residual platelet activity observed among clopidogrel-treated IMs, clinical judgment also taking into account other factors that may place an IM at increased risk of a CV event (or adverse bleeding event) must be considered to most effectively individualize therapy.
In addition, although these guidelines have been focused on CYP2C19*2 and *3, many clinical genotyping platforms include other variant alleles (e.g., *4–*8 and *17) that can alter a patient's predicted metabolizer phenotype interpretation (Supplementary Table S5 online). As mentioned above, the *4–*8 alleles have strong in vitro evidence for complete loss of function of the CYP2C19 enzyme (see Supplementary Materials and Methods online for references). Consequently, when these alleles are identified among ACS/PCI patients treated with clopidogrel, they should be considered as influencing clopidogrel metabolism and clinical outcomes consistent with the *2 loss-of-function allele.
Clopidogrel Dose Escalation
Recent studies that have evaluated a significantly increased clopidogrel loading and/or maintenance dosing strategy in both healthy subjects and ACS patients have reported improved platelet inhibition among CYP2C19*2 heterozygotes (IMs) based on ex vivo platelet aggregation but only nominal improvement among homozygotes (PMs) (see Supplementary Table S8 online). Large clinical trials that evaluated higher-dose clopidogrel in ACS/PCI patients with high on-treatment platelet reactivity have concluded that adjusting clopidogrel dose on the basis of platelet function monitoring alone does not reduce the incidence of death from CV causes, nonfatal myocardial infarction, or stent thrombosis.38,39 However, typically these trials only doubled the clopidogrel maintenance dose, which may not be adequate on the basis of recent studies that suggest that even higher doses may be required to achieve adequate platelet inhibition among CYP2C19*2 heterozygotes (see Supplementary Table S8 online). Given these data and the lack of clinical outcome studies for clopidogrel dose adjustment on the basis of CYP2C19 status alone, it is currently premature to support an increased dosing strategy based on CYP2C19 genotype. Future versions of these guidelines (updated on www.pharmgkb.org) will continue to incorporate results from ongoing clinical trials designed to address these and other emerging issues, including the potential role for platelet function monitoring.
Other Considerations
Diabetes mellitus, age, and body mass index are associated with high on-treatment residual platelet aggregation, and use of certain proton pump inhibitors may also affect clopidogrel response.
Potential benefits and risks for the patient
The potential benefits of CYP2C19 testing are that when considering treatment with clopidogrel in ACS/PCI patients, genotypes that confer a higher risk of a CV event on clopidogrel can be identified, and an alternative antiplatelet strategy can be instituted. Although there is mounting evidence associating deficient CYP2C19 with increased risks of adverse CV outcomes in clopidogrel-treated ACS/PCI patients, the absence of randomized clinical trial evidence that CYP2C19 genotyping improves outcomes must be acknowledged. Furthermore, although CYP2C19 genotyping is straightforward and reliable when performed in qualified laboratories, as with any laboratory test, an additional possible risk to the patient is an error in genotyping. Because genotypes are lifelong test results, any such error could have adverse health implications for the life of the patient.
Caveats: appropriate use and/or potential misuse of genetic tests
If pursuing CYP2C19 genotyping, one of the challenges is the need for rapid turnaround time of results. It would be advantageous to have the results before initiating antiplatelet therapy because the largest number of potentially preventable recurrent events occur early in treatment. For example, among other clinical and genetic factors, CYP2C19*2 has recently been associated with definite early stent thrombosis in a case–control study.22 Therefore, if CYP2C19 genotype is not already known from prior testing, early testing and expedited reporting would be advantageous. To address this issue, point-of-care genetic testing systems have been developed (see Supplementary Materials and Methods online), and some academic medical centers have deployed preemptive genotyping programs for selected patient populations.
Of note, as described above, these recommendations apply predominantly to ACS patients undergoing PCI. Current data do not support the use of CYP2C19 genotype data to guide treatment in other scenarios.25,26,40 In addition, at the time of this writing, there are no data available on the possible role of CYP2C19 in clopidogrel response in pediatric patient populations; however, there is no reason to suspect that CYP2C19 variant alleles would affect clopidogrel metabolism differently in children as compared with adults.
Acknowledgments
The authors acknowledge the critical input of the Clinical Pharmacogenetics Implementation Consortium (CPIC) of the Pharmacogenomics Research Network and PharmGKB, funded by the National Institutes of Health (NIH). This work was supported in part by NIH grants KL2TR000069 (S.A.S.), R24GM61374 (K.S. and T.E.K.), U01HL65962 (C.M.S.), U19HL065962-10 (D.M.R.), U01GM074492 (J.A.J.), U01HL105198 (A.R.S.), and U01GM92666 (CPIC). A.R.S. also acknowledges support from the Baltimore Veterans Administration Medical Center.
CPIC guidelines reflect expert consensus based on clinical evidence and peer-reviewed literature available at the time they are written and are intended only to assist clinicians in decision making and to identify questions for further research. New evidence may have emerged since the time a guideline was submitted for publication. Guidelines are limited in scope and are not applicable to interventions or diseases not specifically identified. Guidelines do not account for all individual variations among patients and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It remains the responsibility of the health-care provider to determine the best course of treatment for a patient. Adherence to any guideline is voluntary, with the ultimate determination regarding its application to be made solely by the clinician and the patient. CPIC assumes no responsibility for any injury to persons or damage to persons or property arising out of or related to any use of CPIC's guidelines, or for any errors or omissions.
S.A.S. receives support from NIH for antiplatelet pharmacogenomics research, is a consultant to USDS, and is an assistant director of a clinical laboratory that performs CYP2C19 testing. J.-S.H. has received research grant support from Biotronik and Medco Research Institute and consulting fees from Biotronik and Medco Health Solutions. J.L.M. receives research grant support through Brigham and Women's Hospital from Bayer Healthcare, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Johnson & Johnson, Sanofi-Aventis, Accumetrics, and Nanosphere, and is a consultant to Boehringer Ingelheim, Janssen, and American Genomics. D.M.R. receives support from the NIH for pharmacogenomics research and is a consultant to Merck, Novartis, Dai-Ichi, Sanofi, and Astellas. M.S.S. receives research grant support through Brigham and Women's Hospital from AstraZeneca, AstraZeneca/Bristol-Myers Squibb Alliance, Bristol-Myers Squibb/Sanofi-Aventis Joint Venture, Daiichi-Sankyo, Eisai, Merck, Sanofi-Aventis, Abbott Laboratories, Accumetrics, Nanosphere, and Roche Diagnostics, and is a consultant to AstraZeneca/Bristol-Myers Squibb Alliance, Merck, and Sanofi-Aventis. J.A.J. receives support from NIH for cardiovascular pharmacogenomics research, including clopidogrel. A.R.S. receives support from the NIH for antiplatelet pharmacogenomics research and is a consultant to USDS. The other authors declared no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
Supplementary Material
References
- Scott S.A., Clinical Pharmacogenetics Implementation Consortium et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin. Pharmacol. Ther. 2011;90:328–332. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relling M.V., Klein T.E. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin. Pharmacol. Ther. 2011;89:464–467. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman H.J., et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat. Med. 2011;17:110–116. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- Reny J.L., Combescure C., Daali Y., Fontana P., PON1 Meta-Analysis Group Influence of the paraoxonase-1 Q192R genetic variant on clopidogrel responsiveness and recurrent cardiovascular events: a systematic review and meta-analysis. J. Thromb. Haemost. 2012;10:1242–1251. doi: 10.1111/j.1538-7836.2012.04756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S.A., et al. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet. Genomics. 2012;22:159–165. doi: 10.1097/FPC.0b013e32834d4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulot J.S., et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- Brandt J.T., et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 2007;5:2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- Giusti B., et al. Cytochrome P450 2C19 loss-of-function polymorphism, but not CYP3A4 IVS10 + 12G/A and P2Y12 T744C polymorphisms, is associated with response variability to dual antiplatelet treatment in high-risk vascular patients. Pharmacogenet. Genomics. 2007;17:1057–1064. doi: 10.1097/FPC.0b013e3282f1b2be. [DOI] [PubMed] [Google Scholar]
- Collet J.P., et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- Mega J.L., et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- Shuldiner A.R., et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frére C., Cuisset T., Gaborit B., Alessi M.C., Hulot J.S. The CYP2C19*17 allele is associated with better platelet response to clopidogrel in patients admitted for non-ST acute coronary syndrome. J. Thromb. Haemost. 2009;7:1409–1411. doi: 10.1111/j.1538-7836.2009.03500.x. [DOI] [PubMed] [Google Scholar]
- Sibbing D., et al. Isolated and interactive impact of common CYP2C19 genetic variants on the antiplatelet effect of chronic clopidogrel therapy. J. Thromb. Haemost. 2010;8:1685–1693. doi: 10.1111/j.1538-7836.2010.03921.x. [DOI] [PubMed] [Google Scholar]
- Tiroch K.A., et al. Protective effect of the CYP2C19 *17 polymorphism with increased activation of clopidogrel on cardiovascular events. Am. Heart J. 2010;160:506–512. doi: 10.1016/j.ahj.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Sibbing D., et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121:512–518. doi: 10.1161/CIRCULATIONAHA.109.885194. [DOI] [PubMed] [Google Scholar]
- Li Y., Tang H.L., Hu Y.F., Xie H.G. The gain-of-function variant allele CYP2C19*17: a double-edged sword between thrombosis and bleeding in clopidogrel-treated patients. J. Thromb. Haemost. 2012;10:199–206. doi: 10.1111/j.1538-7836.2011.04570.x. [DOI] [PubMed] [Google Scholar]
- Geisler T., et al. CYP2C19 and nongenetic factors predict poor responsiveness to clopidogrel loading dose after coronary stent implantation. Pharmacogenomics. 2008;9:1251–1259. doi: 10.2217/14622416.9.9.1251. [DOI] [PubMed] [Google Scholar]
- Simon T., French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators et al. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- Sorich M.J., Polasek T.M., Wiese M.D. Systematic review and meta-analysis of the association between cytochrome P450 2C19 genotype and bleeding. Thromb. Haemost. 2012;108:199–200. doi: 10.1160/TH12-02-0095. [DOI] [PubMed] [Google Scholar]
- Lewis J., et al. The CYP2C19*17 variant is not independently associated with clopidogrel response. J. Thromb. Haemost 2013. e-pub ahead of print 29 June 2013. [DOI] [PMC free article] [PubMed]
- Hulot J.S., et al. CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics, and clinical efficacy in post-myocardial infarction patients. Circ. Cardiovasc. Interv. 2011;4:422–428. doi: 10.1161/CIRCINTERVENTIONS.111.963025. [DOI] [PubMed] [Google Scholar]
- Giusti B., et al. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am. J. Cardiol. 2009;103:806–811. doi: 10.1016/j.amjcard.2008.11.048. [DOI] [PubMed] [Google Scholar]
- Sibbing D., et al. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur. Heart J. 2009;30:916–922. doi: 10.1093/eurheartj/ehp041. [DOI] [PubMed] [Google Scholar]
- Cayla G., et al. Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. JAMA. 2011;306:1765–1774. doi: 10.1001/jama.2011.1529. [DOI] [PubMed] [Google Scholar]
- Paré G., et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N. Engl. J. Med. 2010;363:1704–1714. doi: 10.1056/NEJMoa1008410. [DOI] [PubMed] [Google Scholar]
- Holmes M.V., Perel P., Shah T., Hingorani A.D., Casas J.P. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306:2704–2714. doi: 10.1001/jama.2011.1880. [DOI] [PubMed] [Google Scholar]
- Mega J.L., et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.A., Roden D.M., Lesko L.J., Ashley E., Klein T.E., Shuldiner A.R. Clopidogrel: a case for indication-specific pharmacogenetics. Clin. Pharmacol. Ther. 2012;91:774–776. doi: 10.1038/clpt.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jneid H., et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2012;60:645–681. doi: 10.1016/j.jacc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Wiviott S.D., TRITON-TIMI 38 Investigators et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- Mega J.L., et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–2560. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- Sorich M.J., Vitry A., Ward M.B., Horowitz J.D., McKinnon R.A. Prasugrel vs. clopidogrel for cytochrome P450 2C19-genotyped subgroups: integration of the TRITON-TIMI 38 trial data. J. Thromb. Haemost. 2010;8:1678–1684. doi: 10.1111/j.1538-7836.2010.03923.x. [DOI] [PubMed] [Google Scholar]
- Wallentin L., PLATO Investigators et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- Hulot J.S., Collet J.P., Montalescot G. Genetic substudy of the PLATO trial. Lancet. 2011;377:637, author reply 637–637, author reply 638. doi: 10.1016/S0140-6736(11)60227-4. [DOI] [PubMed] [Google Scholar]
- Wallentin L., et al. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur. Heart J. 2008;29:21–30. doi: 10.1093/eurheartj/ehm545. [DOI] [PubMed] [Google Scholar]
- Pena A., et al. Can we override clopidogrel resistance. Circulation. 2009;119:2854–2857. doi: 10.1161/CIRCULATIONAHA.108.857722. [DOI] [PubMed] [Google Scholar]
- Montalescot G., et al. Prasugrel compared with high-dose clopidogrel in acute coronary syndrome. The randomised, double-blind ACAPULCO study. Thromb. Haemost. 2010;103:213–223. doi: 10.1160/TH09-07-0482. [DOI] [PubMed] [Google Scholar]
- Price M.J., GRAVITAS Investigators et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- Collet J.P., ARCTIC Investigators et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N. Engl. J. Med. 2012;367:2100–2109. doi: 10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]
- Wallentin L, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.