Abstract
The identification that primary aldosteronism is a common cause of resistant hypertension is a significant advance in our ability to care for patients with hypertension. Primary aldosteronism is common, and when unrecognized is associated with increased incidence of adverse cardiovascular outcomes. Identification of primary aldosteronism is based upon use of the plasma aldosterone level, plasma renin activity and the aldosterone:renin ratio (ARR). Differentiation between unilateral and bilateral autonomous adrenal aldosterone production then guides further therapy, with use of mineralocorticoid receptor blockers for those with bilateral autonomous adrenal aldosterone production and laparoscopic adrenalectomy for those with unilateral autonomous aldosterone production. In this review, we discuss in detail the pathogenesis of primary aldosteronism-induced hypertension and potassium disorders, the evaluation of the patient with suspected primary aldosteronism and the management of primary aldosteronism, both through medications and through surgery.
Introduction
The goal of medicine is to prevent or minimize the chance of disease. Hypertension is one of modern medicine’s great successes. High blood pressure substantially increases all cause morbidity and mortality, including myocardial infarction, congestive heart failure, cerebrovascular accident (CVA), renal failure, claudication, limb loss, and death. Multiple randomized, double-blind, placebo-controlled clinical trials demonstrate that controlling hypertension decreases the risk of these complications, and the benefit is proportional to the individual’s risk. Clearly, controlling blood pressure substantially benefits the patient’s future health.
Evidence developed over the past decades has led to the recognition that autonomous adrenal aldosterone production, termed primary aldosteronism, is surprisingly common in hypertensive patients. As many as 5–13% of patients with hypertension have primary aldosteronism1–3, and the likelihood increases with the severity of hypertension4. Indeed, prospective studies show that 14–23% of patients with resistant hypertension, typically defined as inadequately controlled blood pressure despite the use of at least three antihypertensive medications, including a diuretic, at pharmacologically appropriate doses and dosing intervals, have primary aldosteronism4–8. Simultaneously, autonomous aldosterone production leads to altered potassium homeostasis, which may contribute to the hypertension.
Primary aldosteronism is by no means the only cause of resistant hypertension. Other causes include renovascular hypertension, other adrenal disorders, including both pheochromocytoma and Cushing syndrome, and specific genetic defects leading to altered renal sodium and potassium transport. The latter, in particular, also lead to potassium homeostasis disorders. We briefly review the screening for these conditions in the evaluation of the patient with resistant hypertension; excellent recent reviews of the evaluation and management of renovascular hypertension are available to the interested reader9–11, and discussion of genetic disorders leading to altered renal sodium and potassium transport and thereby causing resistant hypertension are available in this issue of Seminars in Nephrology.
Mechanism through which aldosterone alters blood pressure
Aldosterone regulates blood pressure by several mechanisms (Figure 1). These include effects on the kidneys, vasculature, central nervous system (CNS) and through endocrine hormones. No single effect is sufficient to explain the hypertension that occurs in primary hyperaldosteronism; taken together they explain why primary hyperaldosteronism causes hypertension that can be so difficult to control. Indeed, a recent study exemplifies the central role of aldosterone in blood pressure regulation. Mice were generated which lacked aldosterone synthase, a key enzyme in aldosterone production. These mice exhibited an approximately 40-fold increase in plasma renin activity and a 3.5-fold increase in angiotensin II levels, yet their mean arterial pressure was decreased substantially, from 110 ± 2 mmHg to 96 ± 2 mmHg12. Thus, the lack of aldosterone synthesis results in hypotension, despite a secondary significant stimulation of the renin-angiotensin system.-.
Figure 1.
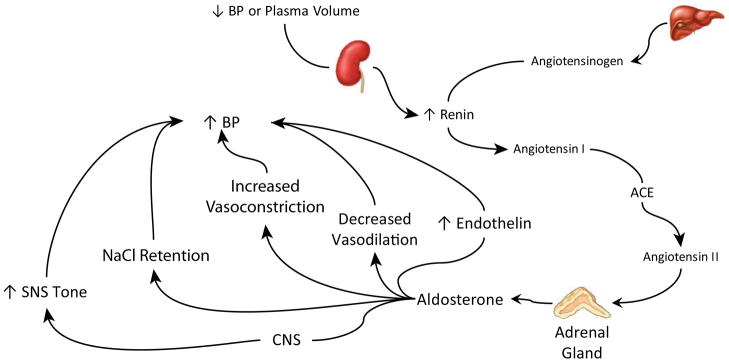
Mechanisms through which aldosterone increases blood pressure. Aldosterone production is regulated in large through the renin-angiotensin system, acting through the AT1 receptor to stimulate adrenal aldosterone production. Aldosterone has multiple effects that regulate blood pressure. These include actions in the CNS, likely mediated through increased sympathetic nervous system tone, direct stimulation of renal NaCl retention, increased arterial vasoconstriction, decreased arterial vasodilatory responses and stimulation of endothelin.
Aldosterone has mediates multiple renal effects that regulate blood pressure. First, aldosterone stimulates renal sodium chloride retention by increasing expression of the thiazide-sensitive sodium-chloride cotransporter in the distal convoluted tubule (DCT), the amiloride-sensitive epithelial sodium channel (ENaC), in the collecting duct and the chloride-reabsorbing protein, pendrin, in the cortical collecting duct13–15. Moreover, the coordinate effect of aldosterone on both ENaC and pendrin has synergistic effects on blood pressure and plasma volume regulation16. It is important to recognize, however, that aldosterone-induced plasma volume expansion cannot be the only mechanism through which aldosterone increases blood pressure. In particular, case reports of individuals with end-stage renal disease and no residual renal function demonstrate that aldosterone, completely independent of renal-mediated NaCl retention, can cause severe hypertension through activation of the mineralocorticoid receptor17.
Hypokalemia occurs either as a result of total body K+ depletion or through an alteration in the distribution of potassium between intracellular and extracellular compartments 18. In the cortical collecting duct, aldosterone consistently stimulates sodium reabsorption and potassium secretion19,20 However, despite this increase in renal cortical potassium secretion, total renal potassium excretion typically does not change 21–23. Within the renal cortex aldosterone increases expression of the potassium channels which secrete potassium24,25 but also stimulates K absorptive pumps (H, K-ATPases) in the renal cortex and medulla 26. A major component of the hypokalemia observed with aldosterone excess may actually reflect aldosterone’s stimulation of the ubiquitous Na+-K+-ATPase, resulting in transcellular shifts of potassium into cells, decreasing extracellular potassium22,23,27. As discussed elsewhere, potassium depletion predisposes to hypertension through a variety of mechanisms28–30.
Some of aldosterone’s effect on blood pressure are related to its effects on vascular tone. Aldosterone increases both basal vascular tone and vascular reactivity to circulating vasoconstrictors, including norepinephrine, epinephrine, angiotensin II and vasopressin31,32. Simultaneously, aldosterone decreases flow-mediated vasodilatation, perhaps due to decreased nitric oxide production resulting from decreased endothelial nitric oxide synthase expression33,34. Finally, aldosterone causes perivascular fibrosis, and increases vascular expression of endothelin35.
Part of the effects of aldosterone on blood pressure regulation appear to be mediated through effects in the central nervous system (CNS). Complete systems for both aldosterone synthesis and mineralocorticoid receptor expression are present in the CNS36–38. CNS aldosterone levels parallel salt-sensitive hypertension in rats39, and aldosterone administered directly in the CNS, via intra-cerebroventricular catheters, increases blood pressure, even using doses which when administered peripherally do not alter blood pressure40. Salt-sensitive hypertension is, at least in part, due to aldosterone produced directly in the CNS39 and low-salt diets increase expression in the CNS of the rate limiting enzyme for aldosterone production, CYP11B236. Indeed, intracerebroventricular administration of mineralocorticoid receptor antagonists blocks the development and can reverse salt-sensitive hypertension41. Some data suggest that peripheral aldosterone increases blood pressure through activation of a CNS MR receptor42. The mechanisms for activation of a CNS aldosterone-MR axis increases blood pressure are not fully understood, but may involve stimulation of sympathetic hyperactivity43,44.
Aldosterone mediates its physiologic and pathophysiologic effects predominantly by activating the mineralocorticoid receptor (MR)45. The MR is located in the inactive state in the cytoplasm; aldosterone binding to MR promotes a conformational change and translocation to the nucleus where it regulates gene transcription. Aldosterone also has nongenomic effects, which develop independent of MR, and may involve activation of GPR3046,47.
Cortisol is a naturally synthesized glucocorticoid with an affinity for the MR similar to that of aldosterone, but is present in plasma at ~100-fold greater levels than aldosterone. The enzyme, 11-beta-hydroxysteroid dehydrogenase type 2 (11–β-HSD2) is expressed in the aldosterone-sensitive distal nephron and collecting duct, metabolizes cortisol to cortisone, which binds to MR poorly, thereby preventing glucocorticoid-dependent activation of the MR48. Either the genetic deficiency or ingestion of inhibitors of 11–β-HSD can result in excessive activation of the MR, and the development of severe hypertension48. Aldosterone also has non-genomic effects, but their role in mineralocorticoid-dependent blood pressure regulation remains unclear49,50.
Effects of unrecognized primary aldosteronism
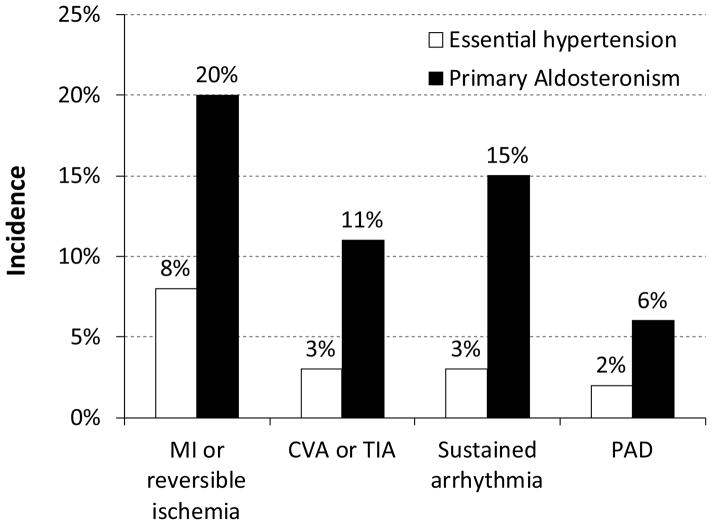
Identification of primary aldosteronism is important because this simplifies management of what can otherwise be very difficult to control hypertension and because unrecognized primary aldosteronism is associated with increased risk of serious adverse cardiovascular complications. As shown in Figure 2, when patients with untreated primary aldosteronism are compared to patients with essential hypertension, the risk of previous myocardial infarction or acute coronary syndrome is increased ~2.5-fold, cerebrovascular event or TIA is increased ~3–4-fold, sustained cardiac arrhythmia is increased ~5-fold and the peripheral arterial disease is increased ~3-fold51. Renal disease is also increased in patients with primary aldosteronism, with greater amounts of urinary albumin excretion and greater rates of microalbuminuria52. However, after primary aldosteronism is diagnosed and treatment is begun, whether with medications or with adrenalectomy, the risk of future cardiovascular events is no different than in patients with essential hypertension51. Thus, identification and treatment of primary aldosteronism both improves blood pressure control and appears to reduce the excess cardiovascular risk associated with primary aldosteronism.
Figure 2.
Risk of cardiovascular events in patients with unrecognized primary aldosteronism. Incidence of previous cardiovascular events was determined at time of diagnosis of primary aldosteronism in 54 consecutive patients, and compared with 323 patients matched for age, sex, body mass index and estimated duration of hypertension51. Incidence of previous myocardial infarction or reversible ischemia, cerebrovascular accident (CVA) or transient ischemic attack (TIA), sustained cardiac arrhythmia and peripheral arterial disease (PAD) was assessed, and in each case was significantly greater (P<0.05) than in patients with essential hypertension.
Normal regulation of adrenal aldosterone production
A number of factors regulate adrenal aldosterone production. Several of these factors are directly related to aldosterone’s role in blood pressure regulation, and include acute and chronic NaCl depletion, intravascular volume depletion and angiotensin II53,54. Atrial natriuretic peptide, which is increased in volume expanded conditions, inhibits aldosterone production55. Chronic hyperkalemia stimulates aldosterone production and chronic hypokalemia inhibits production, consistent with the role of aldosterone in regulating total body potassium stores and renal clearance of potassium. Whether this is due to a specific effect of K+ on the adrenal cortex or reflects stimulation of K+ sensors elsewhere that regulate adrenal cortical aldosterone synthesis remains an incompletely addressed issue. There is a significant circadian variation in plasma aldosterone levels, which is independent of renin and angiotensin II, and corresponds with circadian variations in both blood pressure and plasma potassium56,57. Corticotrophin (ACTH) stimulates aldosterone production58, but the physiologic role of this regulation is currently not clear. Atrial natriuretic peptide inhibits both basal and hormone-stimulated aldosterone secretion, and this may contribute to the expected natriuresis in volume-overloaded conditions59,60. A large number of other stimuli and inhibitors of adrenal aldosterone production have been identified, but their role in normal physiologic conditions is thought to be relatively minor53,54.
Identification of primary aldosteronism
Screening for and confirming the diagnosis of primary aldosteronism has undergone fundamental alteration in recent years. Until relatively recently, screening was limited to evaluating patients who had a combination of resistant hypertension and hypokalemia, and involved only evaluation of the plasma aldosterone. Confirmation then required demonstration of a failure to suppress aldosterone production in the presence of either a NaCl load or exogenous mineralocorticoid administration. However, this paradigm has been largely replaced by one in which there is recognition that autonomous adrenal aldosterone production, even in the absence of an elevated absolute level of plasma aldosterone, can lead to resistant hypertension.
Autonomous adrenal aldosterone production indicates adrenal aldosterone production is excessive for the identifiable regulators of adrenal aldosterone production related to blood pressure regulation. Chronic changes in serum potassium, particularly when induced by changes in dietary potassium intake, increase aldosterone secretion, and this involves both direct effects on adrenal aldosterone secretion and indirect effects mediated through increased renin release61. The second major regulator of aldosterone secretion is AngII, which acting through the angiotensin II, type 1 (AT1) receptor, stimulates aldosterone secretion54. Thus, significant aldosterone release in the non-volume depleted individual who does not have hyperkalemia and in absence of renin-mediated AngII production indicates autonomous aldosterone production, and is generally referred to as primary aldosteronism. However, altered sensitivity of the adrenal gland to one or more of these stimuli may underlie, at least in part, the development of excessive aldosterone secretion in many individuals. In particular, recent studies show that genetic alterations in a specific K+ channel, KCNJ5, leads to increased adrenal aldosterone release in response to even normal levels of extracellular K+, and thereby to development of primary aldosteronism 62–64. Other studies show that, at least in genetically modified mice, that gain-of-function mutation in the angiotensin II type 1A receptor cause autonomous aldosterone production and hypertension, i.e., primary aldosteronism65. The frequency with which this occurs in humans, and whether gain-of-function mutations in the down-stream signaling pathway for aldosterone contribute to the genesis of primary aldosteronism, are important, but as yet unanswered, questions.
Consequently, screening for primary aldosteronism involves simultaneous assessment of plasma aldosterone, renin and potassium. Renin is the upstream enzyme responsible for conversion to angiotensinogen to angiotensin I, which is then converted by angiotensin converting enzyme to AngII, and there thereby is a strong correlation between renin and AngII. Renin is used in clinical settings because measurement of AngII levels is not clinically available due to technical difficulties in its measurement. Plasma renin activity measurements are generally preferred to immunoreactive renin measurements because of greater accuracy when low levels are present. Because increases in potassium are associated with stable, if not increased plasma renin activity, the presence of aldosterone levels that do not parallel plasma renin activity indicates autonomous adrenal aldosterone production, i.e., primary aldosteronism. Thus, plasma aldosterone levels in the normal individual should parallel the plasma renin activity, with a modifying effect by plasma potassium.
Interpretation of the plasma aldosterone and plasma renin activity is typically performed by calculation of the “aldosterone-to-renin ratio,” or “ARR.” Using the typical units for plasma aldosterone (ng/dl) and plasma renin activity, ng Ang I/ml/hr, the normal ARR is ~10, with normal typically stated to extend to as high as 25. Thus, an ARR greater than 25 is often taken as screening evidence for primary aldosteronism.
Use of the ARR without consideration of actual values of aldosterone and plasma renin activity can be problematic. A plasma aldosterone of 5 ng/dl, essentially a measurement at the lower limit of normal, in combination with a plasma renin activity of 0.1 ng/ml/hr, the lower limit of reporting for most laboratories, results in an ARR of 50, yet the patient actually is exhibiting near complete suppression of both aldosterone secretion and plasma renin activity. In this case, autonomous adrenal aldosterone production is unlikely. Consequently, ARR should not be used in isolation, but should be used in conjunction with assessment of the actual plasma aldosterone level. The optimal level of plasma aldosterone to use is unclear, however. Some reports recommend using plasma aldosterone levels of >12–15 ng/dl and >15 ng/dl66,67, whereas others do not recommend a formal cut-off68. In part this is because a significant number, 36–48%, of individuals with primary aldosteronism have plasma aldosterone levels between 9–16 ng/dl4,69 and ~20% of individuals with unilateral autonomous adrenal aldosterone production have levels <15 ng/dl69.
Effect of concomitant medications on ARR testing for primary aldosteronism
One of the important issues when assessing a patient for primary aldosteronism is the possibility that anti-hypertensive medications can alter the effectiveness of screening tests. If these medications have substantial effects, and anti-hypertensive regimens need to be changed prior to screening, then substantial delays may ensue, and some clinicians or patients may decide to forgo testing because of the complexity of changing the antihypertensive regimen. In one study ~2% (1/55) of patients using amlodipine and 23% (4/17) using irbesartan had false-negative ARR measurements; no false negative tests were seen with beta-adrenergic receptor blockers, alpha-adrenergic receptor blockers or angiotensin converting enzyme inhibitors70. Other studies, however, have not found that angiotensin receptor blockers or calcium channel blockers lead to false-negative ARR testing.71. The likelihood of false-positive tests appears to be greatest with the use of beta-adrenergic receptor blockers, which can decrease plasma renin activity, typically by ~50%, without altering plasma aldosterone levels71, but neither the frequency with which this leads to a false-positive elevation in the ARR nor the time-course of the reversal of this effect after discontinuing this medication class is clear.
How the clinician interpret these findings is not clear. Certainly, in an ideal setting, one might discontinue all antihypertensive medications prior to ARR testing. However, this certainly is not routinely feasible, nor is there good data on how long medications need to be discontinued prior to ARR testing. Even adjustment of antihypertensive medication regimen to avoid medications that alter testing results can difficult, and can result in serious complications, sometimes requiring hospitalization, in 10% or more of patients72. A reasonable clinical approach is to perform ARR testing without altering medications, but to be cognizant that medications can influence testing interpretation, particularly if a borderline elevation, i.e., 25–50, is identified.
Confirmation of primary aldosteronism
Several approaches are used to confirm the diagnosis of primary aldosteronism. Those most commonly performed include showing lack of suppression of plasma aldosterone levels with either NaCl loading, either oral or intravenous, or with exogenous mineralocorticoid administration73. At present, none of these is considered a “gold standard” confirmatory test. Moreover, these maneuvers can worsen blood pressure control and are contraindicated in patients with severe uncontrolled hypertension. A captopril challenge test has also been used in the past, but is less frequently used because of increasing reports of false negative or equivocal tests68,74.
The clinical importance in routine clinical practice of confirming the diagnosis of primary aldosteronism requires consideration of both the risks and benefits of confirmatory testing. As noted above, none of the confirmatory tests have 100% sensitivity or specificity. Many of the tests require maneuvers that can raise blood pressure, which is problematic in the management of the patient who already has poorly controlled hypertension. The captopril challenge test is optimally performed in patients not receiving inhibitors of the renin angiotensin system, requiring discontinuation of many routinely used anti-hypertensive medications, such as beta-blockers, ACE-I, ARB and direct renin inhibitors, which can lead to worsening of their hypertension control. Moreover, patients with resistant hypertension generally respond well to mineralocorticoid receptor (MR) antagonists with significant improvement in their blood pressure75,76. Response is generally slow, with a maximum response to each dose change requiring as much as a month to develop. In a recent clinical trial, ~75% of patients treated with escalating doses of spironolactone responded, as defined by a diastolic BP less than 90 mm Hg or a decrease in diastolic BP of at least 10 mmHg; maximal response was seen at 12–16 weeks of treatment 77. It is also important to recognize that pre-treatment plasma renin activity and aldosterone do not predict the magnitude of blood pressure improvement78,79. Because of these factors, some centers do not routinely perform confirmatory testing prior to initiating treatment with MR blockers for patients with an ARR and primary aldosteronism.
Differentiation of unilateral from bilateral autonomous adrenal aldosterone production
Autonomous adrenal aldosterone production can result from a variety of histologic and pathophysiologic processes. The two most common are bilateral adrenal hyperplasia (BAH) and aldosterone-producing adenoma (APA), which typically account for 90% or more of all identified causes. Rare causes include unilateral adrenal hyperplasia and bilateral aldosterone-producing adenoma. Because treatment depends on whether the disease is unilateral or bilateral, accurate differentiation is critical.
In the past, differentiating unilateral from bilateral aldosterone production was based on the observation that hyperplastic adrenal tissue, as seen in bilateral adrenal hyperplasia, exhibited increased sensitivity to AngII stimulation of aldosterone production, while APA was relatively insensitive. These tests involved saline suppression tests, with measurement of plasma aldosterone prior to and after infusion of 1.25 L of normal saline over two hours, and postural stimulation tests, with measurement of plasma aldosterone after being recumbent overnight and then following four hours of upright posture. However, the technical difficulties in performing these tests and the recognition that they had significant error rates for discriminating between unilateral and bilateral autonomous adrenal aldosterone production has led to most centers no longer using them.
Abdominal imaging, using CT imaging, is typically the first step in the evaluation of whether autonomous aldosterone production is unilateral or bilateral. Using high-resolution imaging with thin slices between serial images is important in order to avoid the possibility of failing to identify a small adenoma. MR imaging can be performed, but typically does not have the resolution or ability to perform slices as thin as can be obtained by CT imaging. Accordingly, CT imaging is the preferred initial imaging tool. Imaging is effective only in evaluating for the possibility of an adenoma, and does not differentiate successfully between hyperplastic and normal adrenal cortical tissue.
It is important for the clinician to recognize, however, that imaging techniques are not able to differentiate an APA from a non-functional adenoma and that they may fail to identify both unilateral adrenal hyperplasia and an APA below the resolution of the imaging modality. For example, recent studies suggest that 20–30% of adenomas identified by CT imaging in patients with primary aldosteronism are non-functional, and that 20–30% of patients without an adenoma identifiable by CT imaging will have evidence by adrenal vein aldosterone sampling of unilateral aldosterone production80–82. The likelihood of an adenoma being non-functional in patients with primary aldosteronism appears to be age-related, and at autopsy, an adrenal mass is found in approximately 3% of those 50 years of age or greater83. Why the frequency of nonfunctional adrenal adenomas appears to be greater in those with primary aldosteronism than in the general population is not clear. Thus, in patients in whom an APA is likely, confirmatory testing with adrenal vein sampling (AVS) for aldosterone should be performed. This is a technically difficult procedure, however, and failure rates, most commonly due to failure to cannulate the right adrenal vein, can be significant; failure rates can be greater than 90% in centers that do not perform this procedure frequently84. Implementation of standardized procedures increase the success rate, but failure of successful cannulation remains common, occurring in 5–30% of patients undergoing the procedure84–86.
Interpretation of AVS results requires biochemical confirmation of successful cannulation of both adrenal veins. Because of the anatomic position of the right adrenal vein arising from the inferior vena cava, while the left adrenal vein arises from the left renal vein, failure to cannulate the right adrenal vein is common, and may be difficult to identify from fluoroscopic imaging during the AVS procedure. Accordingly, biochemical confirmation, demonstrating high levels of cortisol in the adrenal vein sample significantly greater than in the inferior vena cava (IVC), is critical. This is calculated using the formula: SIL/R = [Cortisol]L/R ÷ [Cortisol]IVC, where L/R indicates left or right adrenal vein, respectively. A minimal selectivity index of 1.1, i.e., adrenal vein cortisol more than 110% of IVC cortisol concentration, is taken to indicate successful adrenal vein sampling, and the greater the selectivity index, the greater the confidence of successful adrenal vein sampling.
Corticotrophin hormone (ACTH) or a synthetic analogue is frequently administered during the AVS procedure. This serves to stimulate adrenal cortisol production, increasing the difference between adrenal vein and inferior vena cava cortisol levels, increasing the selectivity index and thereby assisting in determining whether the adrenal vein was successfully cannulated85,87–89. However, ACTH also stimulates aldosterone production, sometimes by as much as 20-fold. Some evidence suggests that the effect of ACTH on aldosterone production may be greater in normal adrenal tissues than in APA, leading to an increased risk of misclassifying whether a patient with an APA actually has BAH, and sometimes attributing the APA to the incorrect side89.
These many issues complicate the interpretation of AVS results. First, the procedure is technically difficult, and should be performed in specialized centers by a specific interventional radiologist using a standardized procedure. Second, radiologic interpretation of adrenal vein cannulation is not sufficient to identify biochemical evidence of adrenal vein sampling. Third, stimulation of cortisol production with ACTH or an analogue increases the ease of confirming successful adrenal vein sampling, but because of effects on aldosterone production may lead to misleading interpretations as to whether aldosterone secretion is unilateral or bilateral and, if unilateral, which side is the predominant source. Thus, interpretation requires careful evaluation of the biochemical studies resulting from AVS in conjunction with imaging modalities.
Other tests have been used to differentiate bilateral and unilateral autonomous aldosterone production. Physiologic testing based on the differential sensitivity of hyperplastic and adenomatous adrenal cortical tissue to AngII, such as saline suppression testing and postural stimulation testing, have significant error rates, as much as 20%, and are not routinely used as a basis for determining whether surgical intervention is appropriate. Assessment of urinary steroid metabolites, such as aldosterone-18-glucoronide is both problematic and has low sensitivity90,91. Adrenal scintigraphy, using 131I-norcholesterol (NP-59), may have value in selected patients, but may yield incorrect information regarding whether disease is unilateral or bilateral in as many as 24% of cases92. Whether NP-59 combined SPECT/CT imaging results in improved accuracy will require further testing93.
Therapy of primary aldosteronism
Once the diagnosis of primary aldosteronism has been made and a determination has been made as to whether autonomous adrenal aldosterone production is unilateral or bilateral, therapy is rather straightforward. With unilateral aldosterone production, surgical removal results cure of the hypertension in 30–50% and significant improvements in blood pressure control in almost all of the remainder. The preferred surgical procedure is laparoscopic adrenalectomy, which should be performed by a surgeon experienced with this procedure. If bilateral autonomous aldosterone production is identified, bilateral adrenalectomy is contraindicated because of the consequent adrenal insufficiency of other adrenal hormones, such as cortisol, catecholamines and adrenal-derived sex steroid hormones. Unilateral adrenalectomy can be performed, but typically results in the expected 50% reduction in plasma aldosterone levels. In most cases, this reduction in plasma aldosterone is not sufficient to induce a significant improvement in blood pressure control.
Patients with bilateral autonomous aldosterone production should be treated with mineralocorticoid receptor (MR) blockers. They should be started at modest doses, and titrated slowly. In contrast to patients with mild essential hypertension, in whom MR blockers have minimal effect on blood pressure, in patients with primary aldosteronism blood pressure improvements of 0.5 – 0.7 mmHg per mg spironolactone are not unusual in the author’s experience. Most patients find that the responses are delayed, and dose adjustments should be done once a month until an optimal dose is identified.
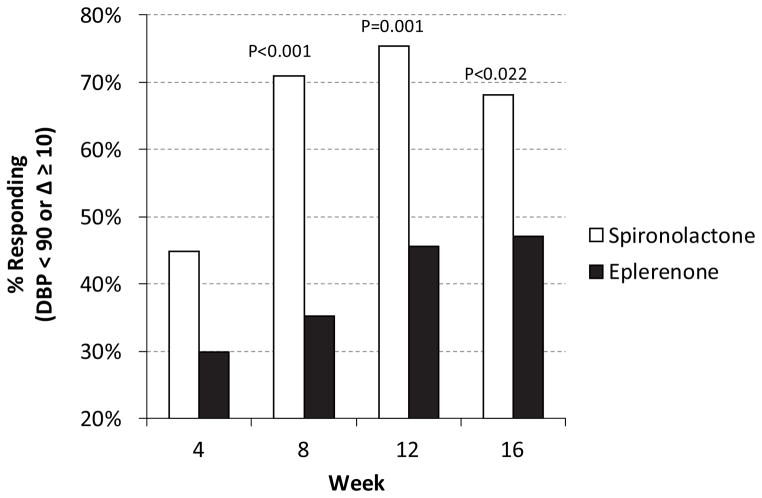
Two MR blockers are in widespread use in the United States, spironolactone and eplerenone. Spironolactone has off-target effects on sex-steroid hormone receptors, which can lead to a dose-dependent risk of breast enlargement (gynecomastia) in men and breast tenderness (gynecodynia) and menstrual irregularities in women; eplerenone is more selective for the MR and the risk of these adverse effects is less, but not completely eliminated. These differences in side-effects led many to prefer the use of eplerenone to spironolactone for several years. However, a recent clinical trial was reported in which patients with primary aldosteronism were randomized to use of either spironolactone or eplerenone77. Spironolactone was significantly more effective than eplerenone at improving blood pressure, with almost twice as great a reduction in blood pressure and a substantially greater proportion of patients experiencing a significant improvement in blood pressure (Figure 3). The difference in response is likely due to pharmacologic differences, as metabolites of spironolactone are biologically active, and have relatively long half-lives, whereas eplerenone has a relatively short half-life of ~4 hours, and its metabolites are inactive94,95. Overall side-effects were similar, although spironolactone therapy caused more male gynecomastia and female gynecodynia than did eplerenone. Accordingly, spironolactone may be the preferred agent for medical treatment of primary aldosteronism.
Figure 3.
Results of randomized controlled trial of spironolactone versus eplerenone in the treatment of primary aldosteronism. Patients with primary aldosteronism were randomized to either spironolactone or eplerenone therapy. Dose was increased at pre-specified time intervals until hypertension control was obtained. At each time point, the likelihood of significantly improved blood pressure control, pre-defined as either a DBP < 90 mmHg or a decrease of at least 10 mmHg was significantly greater in individuals treated with spironolactone77. Not shown is that mean improvement in blood pressure at each time point was ~2–2.5-fold greater in patients randomized to spironolactone than in those randomized to eplerenone.
There are additional side-effects of MR blocker therapy with which the clinician should be familiar. Muscle cramps are not uncommon, are observed with both spironolactone and eplerenone and may be quite distressful to the patient. In general, changing from one MR blocker to the other will provide symptomatic relief. A second important side-effect with which to be familiar are neuropsychiatric side-effects. These are often non-specific, and can include symptoms of feeling out of touch with reality, difficulty thinking, feeling lost and disoriented, unawareness of what is going on, general weakness, difficulty planning and organizing, and tiredness and feeling weary77. While seen more commonly with eplerenone than with spironolactone, they are seen with both77. Other studies have suggested that MR blockers can alter selective attention and performance in various memory tasks96. In the author’s experience, many of these effects are temporary and resolve within a few weeks. Finally, hyperkalemia is common when MR blockers are used for conditions other than primary aldosteronism, such as mortality reduction in patients with congestive heart failure97,98, but is relatively uncommon in patients with primary aldosteronism. However, it can occur late in therapy, often following years of MR blocker administration, and may require either a decrease in the MR dose or addition of diuretics.
Recommended evaluation of the patient with possible primary aldosteronism
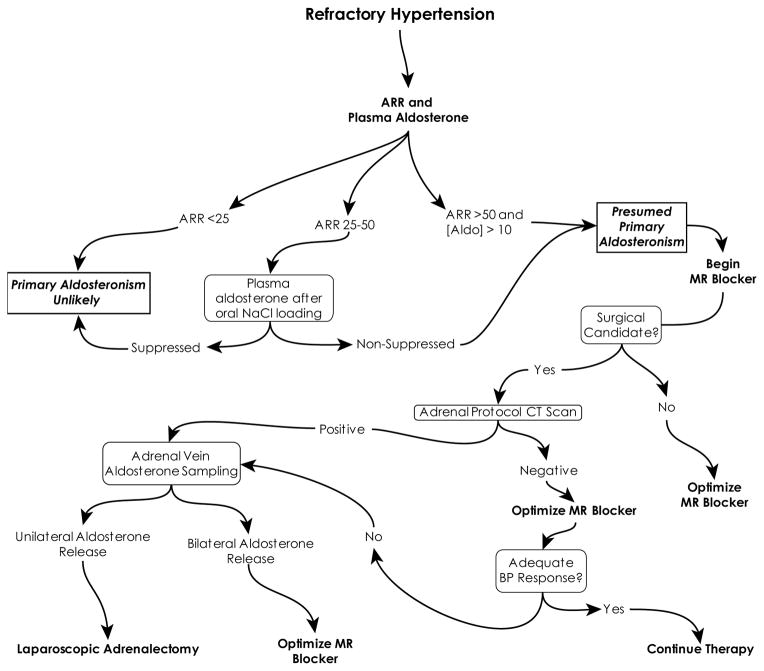
Because untreated primary aldosteronism is associated with adverse outcomes and treated primary aldosteronism responds very well to medical therapy, and may potentially be curative if unilateral aldosterone production is identified, aggressive case identification and treatment may be beneficial. Current evidence suggests that all patients with resistant hypertension, defined as inadequately controlled blood pressure despite documented compliance with a three medication regiment dosed appropriately and including a diuretic, and patients with either spontaneous or easily provoked hypokalemia, should be screened for primary aldosteronism with measurement of plasma aldosterone and plasma renin activity and calculation of the ARR. The author then uses the following protocol for the evaluation of these patients (Figure 4). If the ARR is markedly elevated, >50, in conjunction with a non-suppressed plasma aldosterone, greater than 10 ng/dl, then a presumptive diagnosis of primary aldosteronism can be made and treatment with the MR blocker spironolactone instituted. Confirmatory testing can be considered, but the benefit is not clear, as patients who meet these criteria are likely to have an excellent blood pressure response to MR blockers. Patients who are good surgical candidates and willing to consider adrenalectomy, if indicated, then undergo high-resolution CT imaging with thin sectioning though the adrenal glands to screen for an adrenal adenoma. Patients found to have an adenoma then undergo adrenal vein sampling to determine whether the adenoma is functional or non-functional. In addition, patients without an adenoma but with a poor response to MR blocker therapy can be considered for AVS. Because AVS is technically difficult, the procedure should be performed in specialized centers. Patients with unilateral aldosterone secretion can undergo laparoscopic adrenalectomy, with excellent likelihood of either cure or significant improvement in their blood pressure.
Figure 4.
Evaluation and management of suspected primary aldosteronism. See text for details.
Conclusion
Primary aldosteronism is a frequent cause of resistant hypertension, which should be considered in essentially all patients with resistant hypertension. Screening involves measurement of plasma aldosterone and plasma renin activity and calculation of the ARR, but interpretation of the results involves consideration of each of these components, not just the ARR. CT imaging followed by adrenal vein sampling for aldosterone enables differentiation between unilateral and bilateral autonomous adrenal aldosterone production. Patients with unilateral autonomous adrenal aldosterone production who are good surgical candidates can undergo laparoscopic adrenalectomy, with excellent likelihood of either cure or substantial improvement in their hypertension. Those with bilateral autonomous aldosterone production can be treated with MR blockers and can expect a substantial improvement in their blood pressure. Spironolactone may be a preferred agent because of evidence of greater efficacy than observed with eplerenone.
Acknowledgments
The preparation of this report was supported by funds from the NIH (DK-045788) and the Merit Review Grant program of the Department of Veterans Affairs (1I01BX000818). The author thanks the many talented nephrology fellows at the University of Florida for their insightful questions regarding the management of patients with primary aldosteronism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lim PO, Rodgers P, Cardale K, Watson AD, Macdonald TM. Potentially high prevalence of primary aldosteronism in a primary-care population. Lancet. 1999;353:40. doi: 10.1016/S0140-6736(05)74868-6. [DOI] [PubMed] [Google Scholar]
- 2.Stowasser M, Taylor PJ, Pimenta E, Ahmed AH, Gordon RD. Laboratory investigation of primary aldosteronism. Clin Biochem Rev. 2010;31:39–56. [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A Prospective Study of the Prevalence of Primary Aldosteronism in 1,125 Hypertensive Patients. Journal of the American College of Cardiology. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 4.Mosso L, Carvajal C, Gonzalez A, Barraza A, Avila F, Montero J, et al. Primary Aldosteronism and Hypertensive Disease. Hypert. 2003;42:161–165. doi: 10.1161/01.HYP.0000079505.25750.11. [DOI] [PubMed] [Google Scholar]
- 5.Gallay BJ, Ahmad S, Xu L, Toivola B, Davidson RC. Screening for primary aldosteronism without discontinuing hypertensive medications: plasma aldosterone-renin ratio. Am J Kidney Dis. 2001;37:699–705. doi: 10.1016/s0272-6386(01)80117-7. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism Among Black and White Subjects With Resistant Hypertension. Hypert. 2002;40:892–896. doi: 10.1161/01.hyp.0000040261.30455.b6. [DOI] [PubMed] [Google Scholar]
- 7.Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22:2217–2226. doi: 10.1097/00004872-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Umpierrez GE, Cantey P, Smiley D, Palacio A, Temponi D, Luster K, et al. Primary Aldosteronism in diabetic subjects with resistant hypertension. Diabetes Care. 2007;30:1699–1703. doi: 10.2337/dc07-0031. [DOI] [PubMed] [Google Scholar]
- 9.Meier P. Atherosclerotic renal artery stenosis: update on management strategies. Curr Opin Cardiol. 2011;26:463–471. doi: 10.1097/HCO.0b013e32834a6fe8. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner I, Lerman LO. Renovascular hypertension: screening and modern management. Eur Heart J. 2011;32:1590–1598. doi: 10.1093/eurheartj/ehq510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Textor SC. Issues in renovascular disease and ischemic nephropathy: beyond ASTRAL. Curr Opin Nephrol Hypertens. 2011;20:139–145. doi: 10.1097/MNH.0b013e328342bb35. [DOI] [PubMed] [Google Scholar]
- 12.Makhanova N, Lee G, Takahashi N, Sequeira Lopez ML, Gomez RA, Kim HS, et al. Kidney function in mice lacking aldosterone. Am J Physiol Renal Physiol. 2006;290:F61–F69. doi: 10.1152/ajprenal.00257.2005. [DOI] [PubMed] [Google Scholar]
- 13.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, et al. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypert. 2003;42:356–362. doi: 10.1161/01.HYP.0000088321.67254.B7. [DOI] [PubMed] [Google Scholar]
- 14.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blazer-Yost BL, Liu X, Helman SI. Hormonal regulation of ENaCs: insulin and aldosterone. Am J Physiol. 1998;274:C1373–C1379. doi: 10.1152/ajpcell.1998.274.5.C1373. [DOI] [PubMed] [Google Scholar]
- 16.Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, et al. Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1202671109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazory A, Weiner ID. Primary hyperaldosteronism in a patient with end stage renal disease. Nephrol Dial Transplant. 2007;22:917–919. doi: 10.1093/ndt/gfl702. [DOI] [PubMed] [Google Scholar]
- 18.Young DB, Jackson TE. Effects of aldosterone on potassium distribution. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1982;243:R526–R530. doi: 10.1152/ajpregu.1982.243.5.R526. [DOI] [PubMed] [Google Scholar]
- 19.Palmer LG, Frindt G. Aldosterone and potassium secretion by the cortical collecting duct. Kidney Int. 2000;57:1324–1328. doi: 10.1046/j.1523-1755.2000.00970.x. [DOI] [PubMed] [Google Scholar]
- 20.O’Neil RG. Aldosterone regulation of sodium and potassium transport in the cortical collecting duct. Semin Nephrol. 1990;10:365–374. [PubMed] [Google Scholar]
- 21.Pan YJ, Young DB. Experimental aldosterone hypertension in the dog. Hypert. 1982;4:279–287. doi: 10.1161/01.hyp.4.2.279. [DOI] [PubMed] [Google Scholar]
- 22.Rabinowitz L. Aldosterone and potassium homeostasis. Kidney Int. 1996;49:1738–1742. doi: 10.1038/ki.1996.258. [DOI] [PubMed] [Google Scholar]
- 23.Young DB, Jackson TE. Effects of aldosterone on potassium distribution. Am J Physiol. 1982;243:R526–R530. doi: 10.1152/ajpregu.1982.243.5.R526. [DOI] [PubMed] [Google Scholar]
- 24.Wald H, Garty H, Palmer LG, Popovtzer MM. Differential regulation of ROMK expression in kidney cortex and medulla by aldosterone and potassium. Am J Physiol. 1998;275:F239–F245. doi: 10.1152/ajprenal.1998.275.2.F239. [DOI] [PubMed] [Google Scholar]
- 25.Beesley AH, Hornby D, White SJ. Regulation of distal nephron K+ channels (ROMK) mRNA expression by aldosterone in rat kidney. J Physiol. 1998;509 (Pt 3):629–634. doi: 10.1111/j.1469-7793.1998.629bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenlee MM, Lynch IJ, Gumz ML, Cain BD, Wingo CS. Mineralocorticoids stimulate the activity and expression of renal H+, K+-ATPases. J Am Soc Nephrol. 2011;22:49–58. doi: 10.1681/ASN.2010030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bia MJ, DeFronzo RA. Extrarenal potassium homeostasis. Am J Physiol. 1981;240:F257–F268. doi: 10.1152/ajprenal.1981.240.4.F257. [DOI] [PubMed] [Google Scholar]
- 28.Ray PE, Suga S, Liu XH, Huang X, Johnson RJ. Chronic potassium depletion induces renal injury, salt sensitivity, and hypertension in young rats. Kidney Int. 2001;59:1850–1858. doi: 10.1046/j.1523-1755.2001.0590051850.x. [DOI] [PubMed] [Google Scholar]
- 29.Suga SI, Phillips MI, Ray PE, Raleigh JA, Vio CP, Kim YG, et al. Hypokalemia induces renal injury and alterations in vasoactive mediators that favor salt sensitivity. Am J Physiol Renal Physiol. 2001;281:F620–F629. doi: 10.1152/ajprenal.2001.281.4.F620. [DOI] [PubMed] [Google Scholar]
- 30.Barri YM, Wingo CS. The effects of potassium depletion and supplementation on blood pressure: a clinical review. Am J Med Sci. 1997;314:37–40. doi: 10.1097/00000441-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Finch L, Haeusler G. Vascular resistance and reactivity in hypertensive rats. Blood Vessels. 1974;11:145–158. doi: 10.1159/000158008. [DOI] [PubMed] [Google Scholar]
- 32.Berecek KH, Stocker M, Gross F. Changes in renal vascular reactivity at various stages of deoxycorticosterone hypertension in rats. Circ Res. 1980;46:619–624. doi: 10.1161/01.res.46.5.619. [DOI] [PubMed] [Google Scholar]
- 33.Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109:2857–2861. doi: 10.1161/01.CIR.0000129307.26791.8E. [DOI] [PubMed] [Google Scholar]
- 34.Taddei S, Virdis A, Mattei P, Salvetti A. Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypert. 1993;21:929–933. doi: 10.1161/01.hyp.21.6.929. [DOI] [PubMed] [Google Scholar]
- 35.Gumz ML, Popp MP, Wingo CS, Cain BD. Early Transcriptional Effects of Aldosterone in a Mouse Inner Medullary Collecting Duct Cell Line. Am J Physiol Renal Physiol. 2003;285:F664–F673. doi: 10.1152/ajprenal.00353.2002. [DOI] [PubMed] [Google Scholar]
- 36.Ye P, Kenyon CJ, MacKenzie SM, Seckl JR, Fraser R, Connell JMC, et al. Regulation of Aldosterone Synthase Gene Expression in the Rat Adrenal Gland and Central Nervous System by Sodium and Angiotensin II. Endocrinology. 2003;144:3321–3328. doi: 10.1210/en.2003-0109. [DOI] [PubMed] [Google Scholar]
- 37.Gomez-Sanchez EP, Gomez-Sanchez CE. Is aldosterone synthesized in the CNS regulated and functional? Trends in Endocrinology & Metabolism. 2003;14:444–446. doi: 10.1016/j.tem.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Herman JP. Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell Mol Neurobiol. 1993;13:349–372. doi: 10.1007/BF00711577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang BS, White RA, Jeng AY, Leenen FHH. Role of central nervous system aldosterone synthase and mineralocorticoid receptors in salt-induced hypertension in Dahl salt-sensitive rats. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2009;296:R994–R1000. doi: 10.1152/ajpregu.90903.2008. [DOI] [PubMed] [Google Scholar]
- 40.Kageyama Y, Bravo EL. Hypertensive mechanisms associated with centrally administered aldosterone in dogs. Hypert. 1988;11:750–753. doi: 10.1161/01.hyp.11.6.750. [DOI] [PubMed] [Google Scholar]
- 41.Gomez-Sanchez EP, Fort C, Thwaites D. Central mineralocorticoid receptor antagonism blocks hypertension in Dahl S/JR rats. American Journal of Physiology - Endocrinology And Metabolism. 1992;262:E96–E99. doi: 10.1152/ajpendo.1992.262.1.E96. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Sanchez EP, Fort CM, Gomez-Sanchez CE. Intracerebroventricular infusion of RU28318 blocks aldosterone-salt hypertension. American Journal of Physiology - Endocrinology And Metabolism. 1990;258:E482–E484. doi: 10.1152/ajpendo.1990.258.3.E482. [DOI] [PubMed] [Google Scholar]
- 43.Kontak AC, Wang Z, Arbique D, Adams-Huet B, Auchus RJ, Nesbitt SD, et al. Reversible sympathetic overactivity in hypertensive patients with primary aldosteronism. J Clin Endocrinol Metab. 2010;95:4756–4761. doi: 10.1210/jc.2010-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang BS, Wang H, Leenen FH. Chronic central infusion of aldosterone leads to sympathetic hyperreactivity and hypertension in Dahl S but not Dahl R rats. Am J Physiol Heart Circ Physiol. 2005;288:H517–H524. doi: 10.1152/ajpheart.00651.2004. [DOI] [PubMed] [Google Scholar]
- 45.Fuller PJ, Young MJ. Mechanisms of Mineralocorticoid Action. Hypert. 2005;46:1227–1235. doi: 10.1161/01.HYP.0000193502.77417.17. [DOI] [PubMed] [Google Scholar]
- 46.Wendler A, Albrecht C, Wehling M. Nongenomic actions of aldosterone and progesterone revisited. Steroids. 2012;77:1002–1006. doi: 10.1016/j.steroids.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Gros R, Ding Q, Sklar LA, Prossnitz EE, Arterburn JB, Chorazyczewski J, et al. GPR30 Expression Is Required for the Mineralocorticoid Receptor-Independent Rapid Vascular Effects of Aldosterone. Hypert. 2011;57:442–451. doi: 10.1161/HYPERTENSIONAHA.110.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogerson FM, Fuller PJ. Mineralocorticoid action. Steroids. 2000;65:61–73. doi: 10.1016/s0039-128x(99)00087-2. [DOI] [PubMed] [Google Scholar]
- 49.Funder JW. Non-genomic actions of aldosterone: role in hypertension. Curr Opin Nephrol Hypertens. 2001;10:227–230. doi: 10.1097/00041552-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Funder JW. The Nongenomic Actions of Aldosterone. Endocr Rev. 2005;26:313–321. doi: 10.1210/er.2005-0004. [DOI] [PubMed] [Google Scholar]
- 51.Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, et al. Cardiovascular Outcomes in Patients With Primary Aldosteronism After Treatment. Arch Intern Med. 2008;168:80–85. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 52.Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, et al. Renal Damage in Primary Aldosteronism. Hypert. 2006;48:232–238. doi: 10.1161/01.HYP.0000230444.01215.6a. [DOI] [PubMed] [Google Scholar]
- 53.Quinn SJ, Williams GH. Regulation of Aldosterone Secretion. Annu Rev Physiol. 1988;50:409–426. doi: 10.1146/annurev.ph.50.030188.002205. [DOI] [PubMed] [Google Scholar]
- 54.SPAT A, HUNYADY L. Control of Aldosterone Secretion: A Model for Convergence in Cellular Signaling Pathways. Physiol Rev. 2004;84:489–539. doi: 10.1152/physrev.00030.2003. [DOI] [PubMed] [Google Scholar]
- 55.Laragh JH. Atrial Natriuretic Hormone, the Renin-Aldosterone Axis, and Blood Pressure-Electrolyte Homeostasis. N Engl J Med. 1985;313:1330–1340. doi: 10.1056/NEJM198511213132106. [DOI] [PubMed] [Google Scholar]
- 56.Armbruster H, Vetter W, Beckerhoff R, Nussberger J+, Vetter H, Siegenthaler W. DIURNAL VARIATIONS OF PLASMA ALDOSTERONE IN SUPINE MAN: RELATIONSHIP TO PLASMA RENIN ACTIVITY AND PLASMA CORTISOL. Acta Endocrinologica. 1975;80:95–103. doi: 10.1530/acta.0.0800095. [DOI] [PubMed] [Google Scholar]
- 57.LIGHTMAN SL, JAMES VHT, LINSELL C, MULLEN PE, PEART WS, Sever PS. Studies of diurnal changes in plasma renin activity, and plasma noradrenaline, aldosterone and cortisol concentrations in man. Clinical Endocrinology. 1981;14:213–223. doi: 10.1111/j.1365-2265.1981.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 58.Kaplan NM. The biosynthesis of adrenal steroids: effects of angiotensin II, adrenocorticotropin, and potassium. J Clin Invest. 1965;44:2029–2039. doi: 10.1172/JCI105310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnston CI, Hodsman PG, Kohzuki M, Casley DJ, Fabris B, Phillips PA. Interaction between atrial natriuretic peptide and the renin angiotensin aldosterone system: endogenous antagonists. The American Journal of Medicine. 1989;87:24S–28S. doi: 10.1016/0002-9343(89)90087-9. [DOI] [PubMed] [Google Scholar]
- 60.Ganguly A. Atrial natriuretic peptide-induced inhibition of aldosterone secretion: a quest for mediator(s) American Journal of Physiology - Endocrinology And Metabolism. 1992;263:E181–E194. doi: 10.1152/ajpendo.1992.263.2.E181. [DOI] [PubMed] [Google Scholar]
- 61.Bauer JH, Gauntner WC. Effect of potassium chloride on plasma renin activity and plasma aldosterone during sodium restriction in normal man. Kidney Int. 1979;15:286–293. doi: 10.1038/ki.1979.37. [DOI] [PubMed] [Google Scholar]
- 62.Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, et al. K+ Channel Mutations in Adrenal Aldosterone-Producing Adenomas and Hereditary Hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Sanchez CE, Gomez-Sanchez EP. Mutations of the Potassium Channel KCNJ5 Causing Aldosterone-Producing Adenomas. Hypert. 2011 doi: 10.1161/HYPERTENSIONAHA.111.186205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mulatero P, Tauber P, Zennaro MC, Monticone S, Lang K, Beuschlein F, et al. KCNJ5 Mutations in European Families With Nonglucocorticoid Remediable Familial Hyperaldosteronism. Hypert. 2011 doi: 10.1161/HYPERTENSIONAHA.111.183996. [DOI] [PubMed] [Google Scholar]
- 65.Billet S, Bardin S, Verp S, Baudrie V+, Michaud A, Conchon S, et al. Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J Clin Invest. 2007;117:1914–1925. doi: 10.1172/JCI28764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, et al. Guidelines for the diagnosis and treatment of primary aldosteronism -The Japan Endocrine Society 2009. Endocrine Journal. 2011;58:711–721. doi: 10.1507/endocrj.ej11-0133. [DOI] [PubMed] [Google Scholar]
- 67.Seiler L, Rump LC, Schulte-Monting J, Slawik M, Borm K, Pavenstadt H, et al. Diagnosis of primary aldosteronism: value of different screening parameters and influence of antihypertensive medication. Eur J Endocrinol. 2004;150:329–337. doi: 10.1530/eje.0.1500329. [DOI] [PubMed] [Google Scholar]
- 68.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 69.Stowasser M, Gordon RD. Primary aldosteronism--careful investigation is essential and rewarding. Mol Cell Endocrinol. 2004;217:33–39. doi: 10.1016/j.mce.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Mulatero P, Rabbia F, Milan A, Paglieri C, Morello F, Chiandussi L, et al. Drug Effects on Aldosterone/Plasma Renin Activity Ratio in Primary Aldosteronism. Hypert. 2002;40:897–902. doi: 10.1161/01.hyp.0000038478.59760.41. [DOI] [PubMed] [Google Scholar]
- 71.Seifarth C, Trenkel S, Schobel H, Hahn EG, Hensen J. Influence of antihypertensive medication on aldosterone and renin concentration in the differential diagnosis of essential hypertension and primary aldosteronism. Clin Endocrinol (Oxf) 2002;57:457–465. doi: 10.1046/j.1365-2265.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- 72.Fischer E, Beuschlein F, Bidlingmaier M, Reincke M. Commentary on the Endocrine Society Practice Guidelines: Consequences of adjustment of antihypertensive medication in screening of primary aldosteronism. Reviews in Endocrine & Metabolic Disorders. 2011;12:43–48. doi: 10.1007/s11154-011-9163-7. [DOI] [PubMed] [Google Scholar]
- 73.Weiner ID, Linus S, Wingo CS. Endocrine causes of hypertension: aldosterone. In: Johnson RJ, Fluege J, Feehally J, editors. Comprehensive Clinical Nephrology. USA: Saunders; 2010. pp. 469–476. [Google Scholar]
- 74.Mulatero P, Bertello C, Garrone C, Rossato D, Mengozzi G, Verhovez A, et al. Captopril Test Can Give Misleading Results in Patients With Suspect Primary Aldosteronism. Hypert. 2007;50:e26–e27. doi: 10.1161/HYPERTENSIONAHA.107.093468. [DOI] [PubMed] [Google Scholar]
- 75.Chapman N, Dobson J, Wilson S, Dahlöf Br, Sever PS, Wedel H, et al. Effect of Spironolactone on Blood Pressure in Subjects With Resistant Hypertension. Hypert. 2007;49:839–845. doi: 10.1161/01.HYP.0000259805.18468.8c. [DOI] [PubMed] [Google Scholar]
- 76.de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of Spironolactone Therapy in Patients With True Resistant Hypertension. Hypert. 2010;55:147–152. doi: 10.1161/HYPERTENSIONAHA.109.140988. [DOI] [PubMed] [Google Scholar]
- 77.Parthasarathy HK, Menard J, White WB, Young WF, Jr, Williams GH, Williams B, et al. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011;29:980–990. doi: 10.1097/HJH.0b013e3283455ca5. [DOI] [PubMed] [Google Scholar]
- 78.Mahmud A, Mahgoub M, Hall M, Feely J. Does Aldosterone-to-Renin Ratio Predict the Antihypertensive Effect of the Aldosterone Antagonist Spironolactone? American Journal Of Hypertension. 2005;18:1631–1635. doi: 10.1016/j.amjhyper.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. American Journal Of Hypertension. 2003;16:925–930. doi: 10.1016/s0895-7061(03)01032-x. [DOI] [PubMed] [Google Scholar]
- 80.Magill SB, Raff H, Shaker JL, Brickner RC, Knechtges TE, Kehoe ME, et al. Comparison of adrenal vein sampling and computed tomography in the differentiation of primary aldosteronism. J Clin Endocrinol Metab. 2001;86:1066–1071. doi: 10.1210/jcem.86.3.7282. [DOI] [PubMed] [Google Scholar]
- 81.Mulatero P, Bertello C, Rossato D, Mengozzi G, Milan A, Garrone C, et al. Roles of Clinical Criteria, Computed Tomography Scan, and Adrenal Vein Sampling in Differential Diagnosis of Primary Aldosteronism Subtypes. Journal of Clinical Endocrinology & Metabolism. 2008;93:1366–1371. doi: 10.1210/jc.2007-2055. [DOI] [PubMed] [Google Scholar]
- 82.Kempers MJE, Lenders JWM, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus ARMM, et al. Systematic Review: Diagnostic Procedures to Differentiate Unilateral From Bilateral Adrenal Abnormality in Primary Aldosteronism. Ann Intern Med. 2009;151:329–337. doi: 10.7326/0003-4819-151-5-200909010-00007. [DOI] [PubMed] [Google Scholar]
- 83.NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”) NIH Consens State Sci Statements. 2002;19:1–25. [PubMed] [Google Scholar]
- 84.Vonend O, Ockenfels N, Gao X, Allolio B, Lang K, Mai K, et al. Adrenal Venous Sampling: Evaluation of the German Conn’s Registry. Hypert. 2011;57:990–995. doi: 10.1161/HYPERTENSIONAHA.110.168484. [DOI] [PubMed] [Google Scholar]
- 85.Rossi GP, Pitter G, Bernante P, Motta R, Feltrin G, Miotto D. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone (ACTH) stimulation. J Hypertens. 2008;26:989–997. doi: 10.1097/HJH.0b013e3282f9e66a. [DOI] [PubMed] [Google Scholar]
- 86.Auchus RJ, Michaelis C, Wians FH, Jr, Dolmatch BL, Josephs SC, Trimmer CK, et al. Rapid cortisol assays improve the success rate of adrenal vein sampling for primary aldosteronism. Ann Surg. 2009;249:318–321. doi: 10.1097/SLA.0b013e3181961d77. [DOI] [PubMed] [Google Scholar]
- 87.Monticone S, Satoh F, Giacchetti G, Viola A, Morimoto R, Kudo M, et al. Effect of Adrenocorticotropic Hormone Stimulation During Adrenal Vein Sampling in Primary Aldosteronism. Hypert. 2012;59:840–846. doi: 10.1161/HYPERTENSIONAHA.111.189548. [DOI] [PubMed] [Google Scholar]
- 88.Rossi GP, Sacchetto A, Chiesura-Corona M, De Toni R, Gallina M, Feltrin GP, et al. Identification of the Etiology of Primary Aldosteronism with Adrenal Vein Sampling in Patients with Equivocal Computed Tomography and Magnetic Resonance Findings: Results in 104 Consecutive Cases. Journal of Clinical Endocrinology & Metabolism. 2001;86:1083–1090. doi: 10.1210/jcem.86.3.7287. [DOI] [PubMed] [Google Scholar]
- 89.Seccia TM, Miotto D, De Toni R, Pitter G, Mantero F, Pessina AC, et al. Adrenocorticotropic Hormone Stimulation During Adrenal Vein Sampling for Identifying Surgically Curable Subtypes of Primary Aldosteronism. Hypert. 2009;53:761–766. doi: 10.1161/HYPERTENSIONAHA.108.128553. [DOI] [PubMed] [Google Scholar]
- 90.Schirpenbach C, Seiler L, Maser-Gluth C, R++diger F, Nickel C, Beuschlein F, et al. Confirmatory testing in normokalaemic primary aldosteronism: the value of the saline infusion test and urinary aldosterone metabolites. Eur J Endocrinol. 2006;154:865–873. doi: 10.1530/eje.1.02164. [DOI] [PubMed] [Google Scholar]
- 91.Maser-Gluth C, Reincke M, Allolio B, Schulze E. Metabolism of glucocorticoids and mineralocorticoids in patients with adrenal incidentalomas. European Journal of Clinical Investigation. 2000;30:83–86. doi: 10.1046/j.1365-2362.2000.0300s3083.x. [DOI] [PubMed] [Google Scholar]
- 92.Volpe C, Enberg U, Sjogren A, Wahrenberg H, Jacobsson H, Torring O, et al. The role of adrenal scintigraphy in the preoperative management of primary aldosteronism. Scand J Surg. 2008;97:248–253. doi: 10.1177/145749690809700308. [DOI] [PubMed] [Google Scholar]
- 93.Yen RF, Wu VC, Liu KL, Cheng MF, Wu YW, Chueh SC, et al. 131I-6β–Iodomethyl-19-Norcholesterol SPECT/CT for Primary Aldosteronism Patients with Inconclusive Adrenal Venous Sampling and CT Results. Journal of Nuclear Medicine. 2009;50:1631–1637. doi: 10.2967/jnumed.109.064873. [DOI] [PubMed] [Google Scholar]
- 94.Sica DA. Pharmacokinetics and Pharmacodynamics of Mineralocorticoid Blocking Agents and their Effects on Potassium Homeostasis. Heart Failure Reviews. 2005;10:23–29. doi: 10.1007/s10741-005-2345-1. [DOI] [PubMed] [Google Scholar]
- 95.Struthers A, Krum H, Williams GH. A Comparison of the Aldosterone-blocking Agents Eplerenone and Spironolactone. Clin Cardiol. 2008;31:153–158. doi: 10.1002/clc.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cornelisse S, Joels M, Smeets T. A randomized trial on mineralocorticoid receptor blockade in men: effects on stress responses, selective attention, and memory. Neuropsychopharmacology. 2011;36:2720–2728. doi: 10.1038/npp.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tamirisa KP, Aaronson KD, Koelling TM. Spironolactone-induced renal insufficiency and hyperkalemia in patients with heart failure. Am Heart J. 2004;148:971–978. doi: 10.1016/j.ahj.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]