Abstract
Adequate asthma management depends on an accurate identification of asthma triggers. A review of the literature on trigger perception in asthma shows that individuals vary in their perception of asthma triggers and that the correlation between self-reported asthma triggers and allergy tests is only modest. In this paper, we provide an overview of psychological mechanisms involved in the process of asthma triggers identification. We identify sources of errors in trigger identification and targets for behavioral interventions that aim to improve the accuracy of asthma trigger identification and thereby enhance asthma control.
Introduction
Asthma and allergies are a major source of health problems. In Western and Westernized countries, prevalence of physician diagnosed asthma is about 9–12% [1–3], while 28% report at least 1 type of diagnosed allergic disorder (e.g, asthma, food allergy, rhinitis, dermatitis [2]. Allergic asthma and allergies have in common that symptoms occur in response to an allergic trigger (e.g. house dust mite, pollen). In asthma, non-allergic triggers such as air pollution, cigarette smoke, perfume, stress, negative emotions or physical activity may also trigger asthma symptoms [4]. Management of asthma and allergies consists of pharmacological management, combined with avoidance of triggers that cause symptom exacerbations [4, 5]. However, trigger avoidance interventions have shown mixed results, and systematic evaluations of interventions that focus on a specific environmental control measure for a specific trigger have shown only limited overall effects on symptoms and disease severity [6, 7].
Although it may be hard to generalize findings from the wide variety of trigger avoidance interventions that have been evaluated [8], we argue that one reason for the mixed success of trigger avoidance may be that individuals have difficulty identifying their personal triggers. In patients with asthma, agreement between reported asthma triggers and actual tests of physical or psychological trigger impact is only moderate [9]. Although this discrepancy could be due to a lack of accuracy of the allergy test, this finding suggests that patients may be unaware of all or some of their triggers, which may leave them uncertain about what the exacerbating factors of their disease are and about which specific triggers to avoid, and could leave them exposed to critical triggers repeatedly without protection. This way, they are presented with recurrent aversive somatic experiences that appear unpredictable and uncontrollable [10, 11]. Alternatively, patients with asthma may attribute their respiratory symptoms to a specific trigger despite the absence of a relationship between the trigger and actual airway obstruction. In other allergic conditions, such as food allergy, the discordance between perceived allergic triggers and triggers identified by atopy tests or provocation tests is even worse [12, 13]. Misidentification of asthma triggers can lead to unnecessary avoidance of perceived triggers and thus restrictions in daily functioning and impairments in quality of life. In asthma, a discrepancy between the perception of symptoms and actual lung function effects of benign daily life physical activity [14] could be associated with the long term risk of forgoing the protective effect of exercise.
In this paper, we will review the empirical evidence on trigger perception in asthma, outline psychological mechanisms that are involved in the identification of allergens or asthma triggers, and identify potential sources of errors in trigger identification. We will conclude the review with potential targets for interventions that aim to improve accurate trigger identification and thus enhance patients’ perception of their disease activity.
Perceived Triggers of Asthma: Measurement, Structure, and Association with Asthma Outcomes
Despite the importance of trigger perception in asthma management, only few studies have investigated patients’ self-report of asthma triggers. These studies have been carried out in a variety of settings and differ in methodology, making it difficult to compare their results. In absence of a more established research base, we will try to distill some general findings from these studies1.
The number of asthma triggers that is reported by patients in different studies varies widely, ranging from 4–12 [15–17]. Differences in the number of triggers reported across studies may be a function of the questions that are used to elicit personal trigger reports, with the number of triggers being evaluated ranging from 9–32 [9, 15–17]. In order to assess asthma triggers in a standardized form, studies have attempted to develop measures to probe patients’ trigger perceptions. An earlier instrument, the Asthma Trigger Index, [18] was specifically developed to evaluate emotional triggers of asthma. It consisted of a list of potential triggers and a series of situation vignettes linked to emotional experiences.. Although the instrument was reported to have a high test-retest reliability and good content validity, results of a psychometric evaluation have never been published. More recently, the Asthma Trigger Inventory (ATI) [9] has been developed to assess a broad spectrum of asthma triggers in a standardized way. It is a 32-item questionnaire, consisting of 7 subscales measuring trigger domains of pollen allergens, animal allergens, physical activity, air pollution/irritants, infections, and psychological factors. All domain scales have a high internal consistency and test-retest reliability.
Despite the variation in the assessment of self-reported asthma triggers, some general findings can be drawn from this literature. Self-reported asthma triggers are associated with disease severity and impact. Patients with a higher number of asthma triggers report less quality of life [16]. Furthermore, a higher number of self-reported asthma triggers is correlated with physician ratings of more severe asthma [9, 17, 19], more exacerbations, and a higher frequency of oral corticosteroid use [9, 16]. Self-reported asthma triggers, are also associated with more primary care visits and emergency room visits, as well as a higher rate of hospitalization [9, 16]. Moreover, patients with a higher number of asthma triggers had a greater chance of relapse defined as urgent or unscheduled physician visits in the two weeks after an emergency room visit [15].
Demographic variables appear to moderate asthma trigger reports. Consistently, female patients report more asthma triggers than male asthma patients [16, 17, 19]. Higher education levels are associated with the report of fewer asthma triggers [9, 19], although the latter association is not always found [16]. Similarly, evidence for a relationship with race or ethnicity of participants is still equivocal [9, 16].
Studies that have examined the association of different types of triggers with asthma and other health outcomes found that self-report of animals as asthma triggers is related a lower age of asthma onset [9, 16, 19]. Self-report of exercise as an asthma trigger is associated with obesity [16, 20]. Emotional triggers (e.g. stress, intense emotions) are associated with more severe asthma, occurrence of nighttime symptoms, and oral corticosteroid use [9, 17, 19]. Furthermore, emotional triggers are linked to a decrease in quality of life and increased anxiety and depression [16, 19]. In general, trigger domains of allergic versus non-allergic triggers appear to be relatively independent from each other and are associated differentially with demographics, asthma manifestations, and outcomes [9, 19].
When comparing two studies that used the ATI to investigate asthma triggers in different countries, Britain [9] and Germany [19], we noticed some differences in the relationship between ATI subscales and specific demographics or disease-related variables (e.g., gender differences in the report of air pollution and infections as asthma triggers). On the other hand, consistent associations with a standardized asthma symptom exacerbation measure, the Asthma Symptom Checklist [21] were observed, in that psychological asthma triggers were linked to hyperventilation symptoms [22]. In terms of the structure of trigger report, remarkable consistency was uncovered with British [9] and German [19] adult asthma patients as well as children with asthma in the United States [23]. The ATI subdomains of trigger perception were also readily identified in an Indonesian sample of adult asthma patients using the 32-item set of the ATI, but discrepancies appeared when the item pool was expanded and factors associated with regional specifics, such as weather conditions and specific aeroallergens, emerged [24]. These findings suggest both cross-cultural consistencies and variations in the perception of asthma triggers. More inconsistencies exist in form of culturally idiosyncratic trigger beliefs, such as exposure to cold foods in south Asian cultures, or imbalances in hot and cold elements often endorsed in Guatemala and Mexico, which are often tied to specific traditional remedies for asthma that are thought to alleviate these effects [25, 26].
The variation in self-reported asthma triggers and association of different asthma triggers with different demographic variables and disease outcome require further study. Although some of this variation could be explained by the existence of different asthma phenotypes (e.g., an adult-onset non-allergic phenotype vs. early onset atopic asthma, [27], other parts of this variation may be clarified by examining the psychological processes involved in asthma trigger identification.
Mechanisms of asthma trigger identification
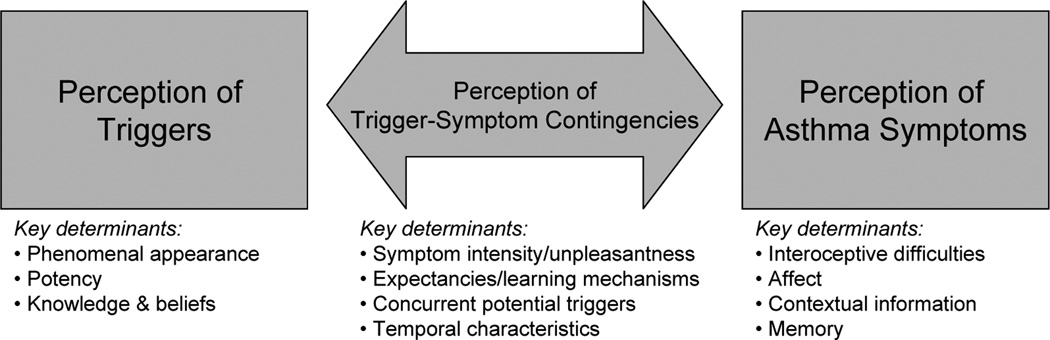
Asthma trigger identification is a complex task. It requires perception of asthma symptoms, perception of potential asthma triggers, and perception of a contingency or causal relationship between potential asthma triggers and symptoms (cf. Figure 1). Each of these components of trigger identification is associated with specific challenges. Furthermore, the components are not fully independent, as each of the components exerts an influence on the other. In pediatric asthma, the task of trigger identification may be further complicated by potential parent-child discordances on each of these components [28, 29].
Figure 1.
Key determinants of asthma trigger identification
Perception of Triggers and their Identification as Asthma-Relevant
Many potential asthma triggers, such as pollen, house dust mite, mold, small particulate matter, or respiratory viruses, do not have a phenomenal appearance that is easy to perceive. Therefore, presence of these triggers is often inferred from the occurrence of cues that are associated with these triggers, such as trees in summer, dust, damp indoor spaces, diesel smell, or the occurrence physical symptoms that are indicative of upper respiratory infections. This difficulty reflects for example on the association between perceived allergic triggers and allergy skin test results. The association between perceived allergens and skin prick test wheal sizes is sometimes stronger for triggers with a clearer phenomenal presentation, such as cats and dogs, than for less distinct triggers, such as pollen or molds [9, 30]. Similarly, the availability of a potent trigger that is easy to perceive may explain why smokers report fewer asthma triggers, especially allergic asthma triggers, compared to non-smokers [9, 16].
Prior knowledge and beliefs about potential asthma triggers and their occurrence may help identify asthma triggers that are hard to perceive, whereas a lack of knowledge about potential asthma triggers may hinder perception of triggers. Patients with greater knowledge about asthma report a higher number of asthma triggers [16], whereas lack of information about the role of mold or cockroaches as asthma triggers may hinder their perception as personally relevant asthma triggers [30–32]. Furthermore, given the large inter-individual variability of asthma triggers and allergic triggers [9, 33], knowledge and beliefs about common asthma triggers may both help and hinder identification of personal asthma triggers, depending on whether or not general knowledge of asthma triggers matches personal susceptibilities.
Perception of asthma symptoms
The perception of asthma symptoms occurs when changes in somatosensory information are detected and matched to mental models of asthma symptoms [34, 35]. In this process, several factors have been identified that may cause a divergence between the level of bronchoconstriction and the level of perceived asthma symptoms. For example, a person may be unable to detect changes in respiratory resistance, and the resulting absence of asthma symptoms increases the risk of near-fatal asthma [36] and may also hinder a person to perceive a contingency between airway obstruction and environmental factors that triggers the obstruction. Furthermore, concurrent affect or contextual information may also interfere with the accurate perception of asthma symptoms [37–41]. Experimental studies have shown that contextual information that is related to previous experience with asthma triggers can lead to the perception of asthma symptoms in absence of the original asthma trigger [42], whereas being in a situation that is perceived as unrelated to asthma may also reduce the perception of asthma symptoms [43]. Also, other factors such as memory, personality, gender, and cultural norms have been shown to influence perception and report of asthma symptoms [35, 44, 45]. Similar to perception of asthma symptoms, perception of other physical symptom, such as symptoms linked to upper respiratory tract infections, is also correlated with personality characteristics and may also lack accordance with objective disease criteria [46, 47]. However, information about the role of perception of upper respiratory tract infections in asthma management is currently lacking.
These examples show that perception of respiratory symptoms is a complex process that is prone to inaccuracies. It is therefore unsurprising that inaccurate perception of asthma symptoms is a widespread problem, with an estimated prevalence of 15 to 60%, depending on the methodology that is used to assess it [35]. As accurate perception of asthma symptoms is a prerequisite for accurate identification of asthma triggers, this may further complicate the identification of asthma triggers.
Perception of Contingencies Between Triggers and Symptoms
Accurate identification of asthma triggers is dependent upon the accurate perception of a contingency between the trigger and asthma symptoms. Humans and animals can be very adept at identifying contingencies and rely on this ability to reduce uncertainty and unpredictability in their environment [48]. In asthma and allergies, contingency perception is used to predict and avoid onset of asthma symptoms. However, contingency perception is often biased. Several studies have shown that perceived intensity or unpleasantness of an event is associated with an overestimation of the contingency between this event and preceding cues [49–51]. Because perceived unpleasantness and contingency perception are associated, this may also explain the discrepancy between self-reported allergy symptoms versus their diagnosis and treatment [52]. Indeed, in a survey on underdiagnosis and undertreatment of allergic rhinitis, patients reported that they have had symptoms for quite some time, but only started seeking diagnosis and treatment when their symptoms became intolerable [53]. If individuals with rhinitis perceive their symptoms to be present yet of little importance, this may not only lead to underdiagnosis and undertreatment, but may also leave these individuals less inclined to identify and avoid triggers of their symptoms.
Prior expectancies or beliefs can also have an influence on the perceived relationship between a potential trigger and respiratory symptoms. In laboratory experiments, prior expectancies have been shown to guide perceived contingencies and symptom reports, even when an objective contingency between the potential trigger and respiratory symptoms was absent [54]. Related research on anxiety disorders suggests that the effect of prior expectancies may be dependent upon fear levels. Whereas individuals show an increased expectancy of a negative event following fear-relevant (e.g. pictures of spiders) vs. fear-irrelevant (e.g. pictures of mushrooms) pictures, only in high fearful individuals, this expectancy persists after repeated confrontation with situations wherein presentation of these pictures is noncontingent with the aversive outcome [55]. Fear and anxiety also promote other inaccurate perceptions of contingencies that may be especially relevant for asthma trigger identification. For example, individuals with panic disorder show an overgeneralized fear response when confronted with cues that share perceptual similarity with a known fear cue [56]. Furthermore, after confrontation with a contingency between a cue A and an unpleasant outcome, and confrontation with a contingency between a compound cue AB and an unpleasant outcome, anxious individuals show sustained fear to element B, even though confrontation with cue A and compound cue AB resulted in the same aversive outcome. [57]. Thus, anxious individuals do not learn that one of the elements of the compound cue AB, the cue B, is unrelated to the aversive outcome. This observation is particularly relevant to the perception of asthma triggers as many asthma triggers may co-occur in complex stimulus configurations, which may lead individuals to experience asthma symptoms when confronted with individual elements of the compound stimulus. For example, confrontation with asthma symptoms in response to physical exercise may cause high anxious individuals to perceive co-occurring cues, such as particular aspects of the environment in which the exercise takes place, as an asthma trigger, even if physical exercise alone (e.g., on a treadmill) would cause the same amount of asthma symptoms. In low-anxious individuals, the perception of physical exercise as a trigger would ‘block’ the perception of the exercise context as an asthma trigger.
These examples suggest that confrontation with an aversive outcome may lead to the adoption of a “better safe than sorry” approach, especially in persons with asthma that are highly fearful or have comorbid anxiety disorders. The prevalence of anxiety disorders is higher in persons with asthma compared to the general population [58].
Other biases may occur in depressed individuals with asthma. Research on cognitive bias in depression shows that depressed individuals are less prone to inflating contingencies when there is no objective contingency between a cue and an outcome [59]. However, depressed individuals do exhibit an underestimation of contingency, especially in situations where control is important [60], which could lead to a reduction in the ability to detect asthma-trigger contingencies in individuals with asthma and comorbid depression. Experimental inductions of non-contingency or lack of control (learned helplessness) have similar effects on subsequent contingency ratings and task performance [61], and individuals with asthma are more susceptible to these effects (impaired problem solving after a learned helplessness induction) compared to matched controls [62].
Beyond cognitive biases, actual bronchoconstriction due to emotional states [63] may be present in anxiety disorders and depression, which might enhance the perception of contingencies between psychological triggers and resulting symptoms, or contribute to bronchoconstriction elicited by other triggers and therefore enhance their perception.
Temporal characteristics of the allergic response may further complicate the accurate perception of trigger-symptom contingencies. Allergic reactions consist of both an acute (within minutes after exposure) as well as a late phase response (4–24 hours after initial exposure)[64], which means that by the time the late response occurs it may not be easy to determine what triggered the response originally. Indeed, a decrease in lung function during the late phase response is perceived as less intense compared to a similar decrease in lung function during the acute phase [65]. In contrast, the airway constriction to emotional triggers happens while exposed to the trigger, thus providing a much better condition for perceiving trigger-symptom contingencies [66]. Consequently, reports of psychological asthma triggers in daily life have been linked to stronger bronchoconstriction to emotionally aversive laboratory stimuli [9, 66, 67] Furthermore, allergic reactions to specific triggers have been known to change during the lifetime response in later life [68]. This implies that previous knowledge about individually relevant asthma triggers may become inaccurate when the sensitivity to specific triggers changes.
Activation of the immune system may be involved in the perception of trigger-symptom contingencies. Although no study has directly investigated the effects of inflammation on contingency perception, stronger inflammatory responses to allergen provocation have been found in periods of stress [69], which could make it easier to perceive trigger-symptom contingencies. Furthermore, increased immune activation is also associated with attention for and avoidance of disease-related cues [70]. Asthma patients also show a specific association of airway inflammation and attention for asthma-specific cues, but not for general negative cues [71]. This increased attention to environmental asthma-specific cues may help identification of asthma triggers, but there is also a risk of biased contingency perception if inflammation directs attention to asthma-specific cues that are present but not involved in the airway response. Furthermore, prior beliefs may interact with the effect of inflammation on attention to potential triggers.
Bronchoconstriction due to trigger perception
Perception of an environmental agent as an asthma trigger or suggestion that an pharmacological agent is an asthma trigger not only can elicit the perception of asthma symptoms but also can lead to an increase in bronchoconstriction, as the large literature on suggestion-induced bronchoconstriction shows [72]. Although the effect of suggestion on bronchoconstriction and symptom perception appear to occur independently [73], both effects can be conceptualized as feed-forward mechanisms, motivating a person to get away from this context before more damage occurs. These feed-forward mechanisms may be involved in the maintenance of perceived trigger-symptom contingencies, which in turn may have an impact on asthma-related quality of life and may interfere with adequate self-management of asthma. Furthermore, there are large individual differences in the effect of suggestions on symptom experience and bronchoconstriction [72]. Further investigation of these individual differences may also aide our understanding of asthma trigger perception.
Potential interventions
The effects of suggestion on bronchoconstriction and symptom perception, as well as the role of contextual information and trigger beliefs on symptom perception, suggest that interventions that try to modify trigger information or trigger beliefs can be used to correct inaccurate perception of asthma symptoms [35]. Education about potential triggers (e.g. allergens, irritants, respiratory infections) is an essential part of asthma management (GINA, 2010). Asthma education programs, which include education about asthma triggers and environmental control measures have been shown to improve clinical outcomes (hospitalizations, emergency room visits, unscheduled doctor visits) and quality of life [74],with newer programs tailoring information to patient needs [75, 76].
However, in day to day allergy care, problems may occur that can interfere with optimal asthma trigger management. Although clinicians often inquire about potential asthma triggers during appointments, education about triggers and environmental control measures is less frequent [77]. In addition,, patients often have difficulty carrying out environmental control measures, or may be hindered by the cost of some of these measures [78]. More problematic is that about half of the environmental control measures that are carried out by patients are unlikely to be beneficial on the basis of current guidelines [79].
A further potential problem with trigger education and inquiry is that alerting participants to potential asthma triggers may promote unwarranted generalization of asthma triggers. Indeed, knowledge about asthma is associated with a larger number of self-reported asthma triggers [16] and experimental research has shown that informing participants about the danger of environmental agents promotes learned symptom responses [80]. A thorough evaluation of potential asthma triggers, including objective allergen tests may therefore help to identify asthma triggers that the patient was previously unaware of and correct erroneous trigger beliefs that erroneous symptom-trigger associations. For non-allergic triggers, monitoring of triggers, peak expiratory flow and asthma symptoms in daily life may help to detect previously unidentified and misidentified asthma triggers [81]. In order to improve participation in physical exercise, it may be important to provide patients with a clear plan that includes type of exercise and measures patients can take to reduce the occurrence of asthma symptoms [82].
The improvement of asthma trigger management in routine clinical care also remains a challenge. One descriptive study of urban pediatric clinic visits suggested that less than half of the parents received advice about environmental triggers and control measures [83]. Based on this and other shortcomings reported by clinicians and patients [77–79], it would be advisable for clinicians to routinely inquire about potential triggers and tailor information about triggers and environmental control methods to patient needs. Non-adherence to environmental control measures may be avoided when they are easy to implement and follow up (e.g. simple steps as part of an asthma action plan). Furthermore, clinicians would need to be mindful about inaccurate trigger beliefs and use of ineffective trigger control methods.
One way to tailor information to patient needs is the combination of allergy skin prick tests with trigger evaluation and education, which has been shown to result in the identification of discrepancies between asthma trigger beliefs and allergic response, an increase in the relationship between specific allergic sensitization and trigger-specific avoidance measures and improvement of lung function, compared to a limited intervention control group [30]. Furthermore, patients receiving a trigger evaluation intervention who did not report animal triggers among their top triggers at baseline, showed an increase in perceived animal-related triggers at follow-up, suggesting that this type of asthma trigger education may be especially beneficial for persons that were previously unaware of crucial asthma triggers [30]. However, given the limited data that is available, more research on trigger identification interventions and integration of these methods in routine clinical care is definitely needed.
Interventions that are aimed at increasing accuracy of trigger identification may be especially beneficial for certain subgroups of individuals with asthma. For example, individuals that are susceptible to respiratory infections may benefit more due to the synergistic effects of allergen exposure and respiratory infections on asthma exacerbations [84]. Moreover, persons with comorbid asthma and panic disorder may benefit from interventions aimed at correcting inaccurate asthma trigger beliefs, as these persons may fail to differentiate between triggers of asthma symptoms and triggers of panic [85]. Information about differences between asthma and panic symptoms has been included in a pilot trial for treatment of asthma and panic disorder that has shown promising results [86]. Furthermore, misidentification of asthma triggers that is associated with anxiety of fear about asthma triggers may benefit from treatment strategies that are adapted from the treatment of anxiety and fear. In anxiety disorders, the success of exposure therapy has shown that repeated exposure to fear-eliciting cues during treatment is one of the most effective ways to reduce fear [87]. In a similar fashion, we expect that a treatment that exposes patients to misidentified asthma triggers may result in a reduction of potential anxieties that are associated with these triggers and in a reduction of asthma symptoms that are associated with these misidentified triggers. Ideally, such a treatment would be preceded by a thorough identification of perceived asthma triggers using monitoring in daily life, lung function testing and allergy diagnosis, but due to practical limitations (e.g. the availability of trigger challenge chambers) more limited interventions will probably have a better chance at being implemented in routine clinical care.
Conclusion
Accurate identification of asthma triggers often is a prerequisite for adequate asthma management. However, so far, research on the identification of asthma triggers has not received sufficient attention. Problems with the perception of asthma symptoms and asthma triggers as well as difficulties perceiving contingencies between triggers and symptoms may hinder accurate identification of asthma triggers. Beliefs about asthma triggers can both help and hinder the identification of trigger-symptom contingencies. Lack of knowledge about an important asthma trigger may lead to a failure to identify this trigger as a personally relevant asthma trigger. Beliefs about asthma triggers can also lead to misidentification of asthma triggers, although further research into the role of anxiety and fear in this misidentification is needed. Finally, perceived asthma triggers can elicit the perception of asthma symptoms and/or elicit bronchoconstriction, which may further complicate the accurate identification of asthma triggers. The role of beliefs about asthma triggers in asthma trigger identification makes them a key target for interventions that are aimed at improving identification of asthma triggers. We have identified educational interventions, daily life monitoring, and exposure to perceived asthma triggers as interventions that may change trigger beliefs, but further research is needed to evaluate these interventions and study the ways in which they can improve asthma control.
Acknowledgements
Dr. Janssens is supported by grant PDMK/11/062 of the KU Leuven Research Fund and a travel grant of the Research Foundation - Flanders (FWO). Preparation of this manuscript was partly funded by a National Institutes of Health/National Heart, Lung and Blood Institute grant, R01 HL-089761 to Dr. Ritz.
Footnotes
The search for articles on trigger perception was conducted in Google Scholar, using the search term asthma trigger perception OR identification, and by identifying papers that cited to key articles identified by our search
Conflict of interest
The authors declare that no conflict of interest exists.
References
- 1.Brogger J, Bakke P, Eide GE, Johansen B, Andersen A, Gulsvik A. Long-term changes in adult asthma prevalence. Eur Respir J. 2003;21:468–472. doi: 10.1183/09031936.03.00056103. [DOI] [PubMed] [Google Scholar]
- 2.Brown CW, Hawkins L. Allergy prevalence and causal factors in the domestic environment: results of a random population survey in the United Kingdom. Annals of Allergy, Asthma & Immunology. 1999;83:240–244. doi: 10.1016/S1081-1206(10)62647-6. [DOI] [PubMed] [Google Scholar]
- 3.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, mortality: United States, 2005–2009. Hyattsville, MD: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. 2010 [Google Scholar]
- 5.Van Cauwenberge P, Bachert C, Passalacqua G, Bousquet J, Canonica GW, Durham SR, Fokkens WJ, Howarth PH, Lund V, Malling HJ, Mygind N, Passali D, Scadding GK, Wang DY. Consensus statement on the treatment of allergic rhinitis. Allergy. 2000;55:116–134. doi: 10.1034/j.1398-9995.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- 6.Gøtzsche PC, Johansen HK. House dust mite control measures for asthma. Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD001187.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Custovic A, van Wijk RG. The effectiveness of measures to change the indoor environment in the treatment of allergic rhinitis and asthma: ARIA update (in collaboration with GA2LEN) Allergy. 2005;60:1112–1115. doi: 10.1111/j.1398-9995.2005.00934.x. [DOI] [PubMed] [Google Scholar]
- 8.Platts-Mills TAE. Allergen avoidance in the treatment of asthma: Problems with the meta-analyses. J Allergy Clin Immunol. 2008;122:694–696. doi: 10.1016/j.jaci.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 9.Ritz T, Steptoe A, Bobb C, Harris AHS, Edwards M. The Asthma Trigger Inventory: Validation of a Questionnaire for Perceived Triggers of Asthma. Psychosom Med. 2006;68:956–965. doi: 10.1097/01.psy.0000248898.59557.74. [DOI] [PubMed] [Google Scholar]
- 10.Gillissen A. Managing asthma in the real world. Int J Clin Pract. 2004;58:592–603. doi: 10.1111/j.1368-5031.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 11.Caress A-L, Luker K, Woodcock A, Beaver K. An exploratory study of priority information needs in adult asthma patients. Patient Educ Couns. 2002;47:319–327. doi: 10.1016/s0738-3991(02)00005-8. [DOI] [PubMed] [Google Scholar]
- 12.Niestijl Jansen JJ, Kardinaal AFM, Huijbers G, Vlieg-Boerstra BJ, Martens BPM, Ockhuizen T. Prevalence of food allergy and intolerance in the adult Dutch population. J Allergy Clin Immunol. 1994;93:446–456. doi: 10.1016/0091-6749(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 13.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, Sigurdardottir ST, Lindner T, Goldhahn K, Dahlstrom J, McBride D, Madsen C. The prevalence of food allergy: A meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Ritz T, Rosenfield D, Steptoe A. Physical Activity, Lung Function, and Shortness of Breath in the Daily Life of Individuals With Asthma. Chest. 2010;138:913–918. doi: 10.1378/chest.08-3073. [DOI] [PubMed] [Google Scholar]
- 15.Emerman CL, Woodruff PG, Cydulka RK, Gibbs MA, Pollack CV, Camargo CA. Prospective Multicenter Study of Relapse Following Treatment for Acute Asthma Among Adults Presenting to the Emergency Department. Chest. 1999;115:919–927. doi: 10.1378/chest.115.4.919. [DOI] [PubMed] [Google Scholar]
- 16.Peterson MGE, Gaeta TJ, Birkhahn RH, Fernandez JL, Mancuso CA. History of Symptom Triggers in Patients Presenting to the Emergency Department for Asthma. J Asthma. doi: 10.3109/02770903.2012.690480. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Göksel Ö, Çelik GE, Öner Erkekol F, Güllü E, Mungan D, Mısırlıgil Z. Triggers in adult asthma: are patients aware of triggers and doing right? Allergol Immunopathol (Madr) 2009;37:122–128. doi: 10.1016/S0301-0546(09)71723-9. [DOI] [PubMed] [Google Scholar]
- 18.Janson-Bjerklie S, Boushey HA, Carrieri VK, Lindsey AM. Emotionally triggered asthma as a predictor of airway response to suggestion. Res Nurs Health. 1986;9:163–170. doi: 10.1002/nur.4770090212. [DOI] [PubMed] [Google Scholar]
- 19.Ritz T, Kullowatz A, Kanniess F, Dahme B, Magnussen H. Perceived triggers of asthma: Evaluation of a German version of the Asthma Trigger Inventory. Respir Med. 2008;102:390–398. doi: 10.1016/j.rmed.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Wright A, Lavoie KL, Jacob A, Rizk A, Bacon SL. Effect of Body Mass Index on Self-Reported Exercise-Triggered Asthma. The Physician and Sportsmedicine. 2010;38:61–66. doi: 10.3810/psm.2010.12.1826. [DOI] [PubMed] [Google Scholar]
- 21.Kinsman RA, Luparello T, O'Banion K, Spector S. Multidimensional Analysis of the Subjective Symptomatology of Asthma. Psychosom Med. 1973;35:250–267. doi: 10.1097/00006842-197305000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Ritz T, Kullowatz A, Bobb C, Dahme B, Magnussen H, Kanniess F, Steptoe A. Psychological triggers and hyperventilation symptoms in asthma. Annals of Allergy, Asthma & Immunology. 2008;100:426–432. doi: 10.1016/S1081-1206(10)60466-8. [DOI] [PubMed] [Google Scholar]
- 23.Wood BL, Cheah PA, Lim J, Ritz T, Miller BD, Stern T, Ballow M. Reliability and Validity of the Asthma Trigger Inventory Applied to a Pediatric Population. J Pediatr Psychol. 2007;32:552–560. doi: 10.1093/jpepsy/jsl043. [DOI] [PubMed] [Google Scholar]
- 24.Zeni SG, Yuniarti KW, von Leupoldt A, Dahme B, Ritz T. Structure and psychometric properties of an Indonesian version of the Asthma Trigger Inventory67th Annual Scientific Meeting of the American Psychosomatic Society. Chicago, IL: Psychosomatic Medicine; 2009. p. A-51. [Google Scholar]
- 25.Griffiths C, Kaur G, Gantley M, Feder G, Hillier S, Goddard J, Packe G. Influences on hospital admission for asthma in south Asian and white adults: qualitative interview study. BMJ. 2001;323:962. doi: 10.1136/bmj.323.7319.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pachter LM, Weller SC, Baer RD, Garcia de Alba Garcia JE, Trotter RT, Glazer M, Klein R. Variation in Asthma Beliefs and Practices Among Mainland Puerto Ricans, Mexican-Americans, Mexicans, and Guatemalans. J Asthma. 2002;39:119–134. doi: 10.1081/jas-120002193. [DOI] [PubMed] [Google Scholar]
- 27.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster Analysis and Clinical Asthma Phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lara M, Duan N, Sherbourne C, Lewis MA, Landon C, Halfon N, Brook RH. Differences Between Child and Parent Reports of Symptoms Among Latino Children With Asthma. Pediatrics. 1998;102:e68. doi: 10.1542/peds.102.6.e68. [DOI] [PubMed] [Google Scholar]
- 29.Yoos HL, Kitzman H, McMullen A, Sidora K. Symptom Perception in Childhood Asthmas: How Accurate Are Children and Their Parents? J Asthma. 2003;40:27–39. doi: 10.1081/jas-120017204. [DOI] [PubMed] [Google Scholar]
- 30.Bobb C, Ritz T, Rowlands G, Griffiths C. Effects of allergen and trigger factor avoidance advice in primary care on asthma control: a randomized-controlled trial. Clin Exp Allergy. 2010;40:143–152. doi: 10.1111/j.1365-2222.2009.03350.x. [DOI] [PubMed] [Google Scholar]
- 31.Li JTC, Andrist D, Bamlet WR, Wolter TD. Accuracy of patient prediction of allergy skin test results. Annals of Allergy, Asthma & Immunology. 2000;85:382–384. doi: 10.1016/S1081-1206(10)62550-1. [DOI] [PubMed] [Google Scholar]
- 32.Saengpanich S, Chochaipanitnon L, Auemjaturapat S, Supiyaphun P. Accuracy of patients' prediction in perennial allergic rhinitis. Chula Med J. 2004;48:531–538. [Google Scholar]
- 33.Heinzerling LM, Burbach GJ, Edenharter G, Bachert C, Bindslev-Jensen C, Bonini S, Bousquet J, Bousquet-Rouanet L, Bousquet PJ, Bresciani M, Bruno A, Burney P, Canonica GW, Darsow U, Demoly P, Durham S, Fokkens WJ, Giavi S, Gjomarkaj M, Gramiccioni C, Haahtela T, Kowalski ML, Magyar P, Muraközi G, Orosz M, Papadopoulos NG, Röhnelt C, Stingl G, Todo-Bom A, Von Mutius E, Wiesner A, Wöhrl S, Zuberbier T. GA2LEN skin test study I: GA2LEN harmonization of skin prick testing: novel sensitization patterns for inhalant allergens in Europe. Allergy. 2009;64:1498–1506. doi: 10.1111/j.1398-9995.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- 34.Brown RJ. Psychological Mechanisms of Medically Unexplained Symptoms: An Integrative Conceptual Model. Psychol Bull. 2004;130:793–812. doi: 10.1037/0033-2909.130.5.793. [DOI] [PubMed] [Google Scholar]
- 35.Janssens T, Verleden G, De Peuter S, Van Diest I, Van den Bergh O. Inaccurate perception of asthma symptoms: A cognitive-affective framework and implications for asthma treatment. Clin Psychol Rev. 2009;8:211–219. doi: 10.1016/j.cpr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Davenport PW, Cruz M, Stecenko AA, Kifle Y. Respiratory-related Evoked Potentials in Children with Life-threatening Asthma. Am J Respir Crit Care Med. 2000;161:1830–1835. doi: 10.1164/ajrccm.161.6.9903077. [DOI] [PubMed] [Google Scholar]
- 37.Affleck G, Apter A, Tennen H, Reisine S, Barrows E, Willard A, Unger J, ZuWallack R. Mood States Associated With Transitory Changes in Asthma Symptoms and Peak Expiratory Flow. Psychosom Med. 2000;62:61–68. doi: 10.1097/00006842-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Meek PM. Influence of Attention and Judgment on Perception of Breathlessness in Healthy Individuals and Patients With Chronic Obstructive Pulmonary Disease. Nurs Res. 2000;49:11–19. doi: 10.1097/00006199-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Wilson RC, Jones PW. Influence of prior ventilatory experience on the estimation of breathlessness during exercise. Clin Sci. 1990;78:149–153. doi: 10.1042/cs0780149. [DOI] [PubMed] [Google Scholar]
- 40.Main J, Moss-Morris R, Booth R, Kaptein AA, Kolbe J. The Use of Reliever Medication in Asthma: The Role of Negative Mood and Symptom Reports. J Asthma. 2003;40:357–365. doi: 10.1081/jas-120018635. [DOI] [PubMed] [Google Scholar]
- 41.von Leupoldt A, Riedel F, Dahme B. The impact of emotions on the perception of dyspnea in pediatric asthma. Psychophysiology. 2006;43:641–644. doi: 10.1111/j.1469-8986.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 42.De Peuter S, Put C, Lemaigre V, Demedts M, Verleden G, Van den Bergh O. Context-evoked overperception in asthma. Psychology & Health. 2007;22:737–748. [Google Scholar]
- 43.Rietveld S, van Beest I. Rollercoaster asthma: When positive emotional stress interferes with dyspnea perception. Behav Res Ther. 2006;45:977–987. doi: 10.1016/j.brat.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Fritz GK, McQuaid EL, Kopel SJ, Seifer R, Klein RB, Mitchell DK, Esteban CA, Rodriguez-Santana J, Colon A, Alvarez M, Canino G. Ethnic Differences in Perception of Lung Function: A Factor in Pediatric Asthma Disparities? Am J Respir Crit Care Med. 2010;182:12–18. doi: 10.1164/rccm.200906-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson D, Pennebaker JW. Health complaints, stress, and distress: Exploring the central role of negative affectivity. Psychol Rev. 1989;96:234–254. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- 46.Van Diest I, De Peuter S, Eertmans A, Bogaerts K, Victoir A, Van den Bergh O. Negative affectivity and enhanced symptom reports: Differentiating between symptoms in men and women. Soc Sci Med. 2005;61:1835–1845. doi: 10.1016/j.socscimed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 47.Cohen S, Doyle WJ, Skoner DP, Fireman P, Gwaltney JM, Newsom JT. State and Trait Negative Affect as Predictors of Objective and Subjective Symptoms of Respiratory Viral Infections. J Pers Soc Psychol. 1995:68. doi: 10.1037//0022-3514.68.1.159. [DOI] [PubMed] [Google Scholar]
- 48.Alloy LB, Tabachnik N. Assessment of covariation by humans and animals: The joint influence of prior expectations and current situational information. Psychol Rev. 1984;91:112–149. [PubMed] [Google Scholar]
- 49.Grupe DW, Nitschke JB. Uncertainty Is Associated With Biased Expectancies and Heightened Responses to Aversion. Emotion. 2011;11:413–424. doi: 10.1037/a0022583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janssens T, Van den Bergh O. Perceived Symptom Intensity Is Associated With Perceived Predictability of Respiratory Symptoms24th Annual Convention of the Association for Psychological Science. Chicago, IL: 2012. [Google Scholar]
- 51.Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB. Uncertainty during Anticipation Modulates Neural Responses to Aversion in Human Insula and Amygdala. Cereb Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nolte H, Nepper-Christensen S, Backer V. Unawareness and undertreatment of asthma and allergic rhinitis in a general population. Respir Med. 2006;100:354–362. doi: 10.1016/j.rmed.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Maurer M, Zuberbier T. Undertreatment of rhinitis symptoms in Europe: findings from a cross-sectional questionnaire survey. Allergy. 2007;62:1057–1063. doi: 10.1111/j.1398-9995.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 54.Devriese S, Winters W, Diest I, Peuter S, Vos G, Van de Woestijne K, Van den Bergh O. Perceived relation between odors and a negative event determines learning of symptoms in response to chemicals. Int Arch Occup Environ Health. 2004;77:200–204. doi: 10.1007/s00420-003-0488-8. [DOI] [PubMed] [Google Scholar]
- 55.Davey GCL. Preparedness and phobias: Specific evolved associations or a generalized expectancy bias. Behav Brain Sci. 1995;18:289–325. [Google Scholar]
- 56.Lissek S, Rabin S, Heller R, Lukenbaugh D, Geraci M, Pine DS, Grillon C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boddez Y, Vervliet B, Baeyens F, Lauwers S, Hermans D, Beckers T. Expectancy bias in a selective conditioning procedure: Trait anxiety increases the threat value of a blocked stimulus. J Behav Ther Exp Psychiatry. 2012;43:832–837. doi: 10.1016/j.jbtep.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Weiser E. The Prevalence of Anxiety Disorders Among Adults with Asthma: A Meta-Analytic Review. Journal of Clinical Psychology in Medical Settings. 2007;14:297–307. 307. [Google Scholar]
- 59.Alloy LB, Abramson LY. Judgment of contingency in depressed and nondepressed students: Sadder but wiser? J Exp Psychol Gen. 1979;108:441–485. doi: 10.1037//0096-3445.108.4.441. [DOI] [PubMed] [Google Scholar]
- 60.Abramson LY, Alloy LB, Rosoff R. Depression and the generation of complex hypotheses in the judgment of contingency. Behav Res Ther. 1981;19:35–45. doi: 10.1016/0005-7967(81)90110-8. [DOI] [PubMed] [Google Scholar]
- 61.Abramson LY, Seligman ME, Teasdale JD. Learned helplessness in humans: Critique and reformulation. J Abnorm Psychol. 1978;87:49–74. [PubMed] [Google Scholar]
- 62.Chaney JM, Mullins LL, Uretsky DL, Pace TM, Werden D, Hartman VL. An experimental examination of learned helplessness in older adolescents and young adults with long-standing asthma. J Pediatr Psychol. 1999;24:259–270. doi: 10.1093/jpepsy/24.3.259. [DOI] [PubMed] [Google Scholar]
- 63.Ritz T. Airway Responsiveness to Psychological Processes in Asthma and Health. Frontiers in Physiology. 2012;3:343. doi: 10.3389/fphys.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skoner DP. Allergic rhinitis: Definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2–S8. doi: 10.1067/mai.2001.115569. [DOI] [PubMed] [Google Scholar]
- 65.Turcotte H, Boulet L-P H. Perception of Breathlessness during Early and Late Asthmatic Responses. Am J Respir Crit Care Med. 1993;148:514–518. doi: 10.1164/ajrccm/148.2.514. [DOI] [PubMed] [Google Scholar]
- 66.Ritz T, Rosenfield D, Wilhelm FH, Roth WT. Airway constriction in asthma during sustained emotional stimulation with films. Biol Psychol. doi: 10.1016/j.biopsycho.2012.03.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ritz T, Kullowatz A, Goldman MD, Smith H, Jr, Kanniess F, Dahme B, Magnussen H. Airway response to emotional stimuli in asthma: the role of the cholinergic pathway. J Appl Physiol. 2010;108:1542–1549. doi: 10.1152/japplphysiol.00818.2009. [DOI] [PubMed] [Google Scholar]
- 68.Scichilone N, Callari A, Augugliaro G, Marchese M, Togias A, Bellia V. The impact of age on prevalence of positive skin prick tests and specific IgE tests. Respir Med. 2011;105:651–658. doi: 10.1016/j.rmed.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 69.Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School Examinations Enhance Airway Inflammation to Antigen Challenge. Am J Respir Crit Care Med. 2002;165:1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- 70.Miller SL, Maner JK. Sick Body, Vigilant Mind. Psychological Science. 2011;22:1467–1471. doi: 10.1177/0956797611420166. [DOI] [PubMed] [Google Scholar]
- 71.Rosenkranz MA, Busse WW, Johnstone T, Swenson CA, Crisafi GM, Jackson MM, Bosch JA, Sheridan JF, Davidson RJ. Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proc Natl Acad Sci U S A. 2005;102:13319–13324. doi: 10.1073/pnas.0504365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Isenberg SA, Lehrer PM, Hochron S. The effects of suggestion and emotional arousal on pulmonary function in asthma: a review and a hypothesis regarding vagal mediation. Psychosom Med. 1992;54:192–216. doi: 10.1097/00006842-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Put C, Van den Bergh O, Van Ongeval E, De Peuter S, Demedts M, Verleden G. Negative affectivity and the influence of suggestion on asthma symptoms. J Psychosom Res. 2004;57:249–255. doi: 10.1016/S0022-3999(03)00541-5. [DOI] [PubMed] [Google Scholar]
- 74.Gibson PG, Powell H, Wilson A, Abramson MJ, Haywood P, Bauman A, Hensley MJ, Walters EH, Roberts JJL. Self-management education and regular practitioner review for adults with asthma. Cochrane Database of Systematic Reviews. 2002 doi: 10.1002/14651858.CD001117. [DOI] [PubMed] [Google Scholar]
- 75.Sundberg R, Tunsäter A, Palmqvist M, Ellbjär S, Löwhagen O, Torén K. A randomized controlled study of a computerized limited education program among young adults with asthma. Respir Med. 2005;99:321–328. doi: 10.1016/j.rmed.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 76.Thoonen BPA, Schermer TRJ, van den Boom G, Molema J, Folgering H, Akkermans RP, Grol R, van Weel C, van Schayck CP. Self-management of asthma in general practice, asthma control and quality of life: a randomised controlled trial. Thorax. 2003;58:30–36. doi: 10.1136/thorax.58.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rank MA, Wollan P, Li JT, Yawn BP. Trigger recognition and management in poorly controlled asthmatics. Allergy Asthma Proc. 2010;31:99–105. doi: 10.2500/aap.2010.31.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brandt DM, Levin L, Matsui E, Phipatanakul W, Smith AM, Bernstein JA. Allergists' attitudes toward environmental control: Insights into its current application in clinical practice. The Journal of allergy and clinical immunology. 2008;121:1053–1054. doi: 10.1016/j.jaci.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cabana MD, Slish KK, Lewis TC, Brown RW, Nan B, Lin X, Clark NM. Parental management of asthma triggers within a child's environment. J Allergy Clin Immunol. 2004;114:352–357. doi: 10.1016/j.jaci.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 80.Winters W, Devriese S, Van Diest I, Nemery B, Veulemans H, Eelen P, Van de Woestijne K, Van den Bergh O. Media Warnings About Environmental Pollution Facilitate the Acquisition of Symptoms in Response to Chemical Substances. Psychosom Med. 2003;65:332–338. doi: 10.1097/01.psy.0000041468.75064.be. [DOI] [PubMed] [Google Scholar]
- 81.Dahl J. A Behavioural Medicine Approach to the Analysis and Treatment of Childhood Asthma. Scandinavian Journal of Behaviour Therapy. 1998;27:30–41. [Google Scholar]
- 82.Lucas SR, Platts-Mills TAE. Physical activity and exercise in asthma: Relevance to etiology and treatment. The Journal of allergy and clinical immunology. 2005;115:928–934. doi: 10.1016/j.jaci.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 83.Halterman JS, Kitzman H, McMullen A, Lynch K, Fagnano M, Conn KM, Yoos HL. Quantifying Preventive Asthma Care Delivered at Office Visits: The Preventive Asthma Care - Composite Index (PAC-CI) J Asthma. 2006;43:559–564. doi: 10.1080/02770900600859172. [DOI] [PubMed] [Google Scholar]
- 84.Bush A. Coughs and wheezes spread diseases: but what about the environment? Thorax. 2006;61:367–369. doi: 10.1136/thx.2005.048389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deshmukh VM, Toelle BG, Usherwood T, O'Grady B, Jenkins CR. Anxiety, panic and adult asthma: A cognitive-behavioral perspective. Respir Med. 2007;101:194–202. doi: 10.1016/j.rmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Lehrer PM, Karavidas MK, Lu SE, Feldman J, Kranitz L, Abraham S, Sanderson W, Reynolds R. Psychological treatment of comorbid asthma and panic disorder: A pilot study. J Anxiety Disord. 2008;22:671–683. doi: 10.1016/j.janxdis.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Emmelkamp PMG. Behavior therapy with adults. In: Lambert MJ, editor. Bergin and Garfield's Handbook of Psychotherapy and Behavior Change. New York: Wiley; 2003. pp. 393–446. [Google Scholar]