Abstract
Extracellular K+ homeostasis has been explained by feedback mechanisms in which changes in extracellular K+ concentration drive renal K+ excretion directly or indirectly via stimulating aldosterone secretion. However, this cannot explain meal-induced kaliuresis that often occurs without increases in plasma K+ or aldosterone concentration. Recent studies have produced evidence supporting a feedforward control in which gut sensing of dietary K+ increases renal K+ excretion (and extrarenal K+ uptake) independent of plasma K+ concentrations, namely, a “gut factor”. This review focuses on these new findings and discusses the role of gut factor in acute and chronic regulation of extracellular K+ as well as in the beneficial effects of high K+ intake on the cardiovascular system.
Keywords: potassium excretion, potassium balance, potassium adaptation, feedback control, feedforward control
INTRODUCTION
Extracellular K+ concentration ([K+]) is tightly regulated in mammals, and this is critical for normal membrane potential and cell functions (1, 2). Extracellular K+ homeostasis depends on the maintenance of total body K+ content as well as distribution of K+ between intracellular (ICF) and extracellular fluid (ECF). Total body K+ content is maintained by a continuous balance between dietary intake and excretion of K+. The kidneys are responsible for approximately 90% of K+ excretion and have a remarkable capacity to regulate K+ excretion to match K+ intake (1, 3). Thus, the kidneys play a predominant role in the maintenance of chronic K+ balance. In addition, extrarenal tissues, mainly skeletal muscle and liver, provide K+ buffering by shifting K+ between ICF and ECF, which is critically important in the acute regulation of extracellular K+ (1, 2, 4). Long-term effects of altered dietary K+ intake on K+ handling and molecular changes in the kidney and skeletal muscle have been extensively studied and reviewed (5–7). However, less attention has been focused on the mechanisms by which renal and extrarenal K+ handling is acutely regulated during dietary K+ intake. This review highlights the recent progress in understanding the acute regulation of extracellular [K+], discussing the concepts of feedforward control and evidence for a “gut factor” that is activated during dietary K+ intake to increase renal K+ excretion and extrarenal K+ uptake (8, 9).
ACUTE REGULATION OF EXTRACELLULAR POTASSIUM
Challenges in K+ homeostasis
Compared to other major electrolytes, K+ has a higher ratio of dietary intake to extracellular pool size (i.e., turnover), representing a significant homeostatic challenge. This is, in part, due to a small extracellular K+ pool; only 2% of the total body K+ is distributed in ECF and the predominant rest in ICF. Therefore, a K+-rich meal would substantially increase extracellular [K+] in the absence of appropriate regulation, causing a risk of hyperkalemia. To meet this challenge, the K+ homeostatic system is very efficient at clearing plasma K+ during dietary K+ intake. The kidneys increase K+ excretion with an increase in K+ intake. However, during the first 4–6 h after an acute K+ load, only half of the administered dose appears in the urine, suggesting that extrarenal tissues also play an important role in the disposal of an acute extracellular K+ load (10). Considering the challenges to K+ homeostasis, it may not be by chance that insulin, which is secreted by ingested nutrients after a meal (i.e., when dietary K+ is absorbed), promotes K+ uptake by extrarenal tissues and thereby prevents an excessive rise of plasma [K+].
Feedback control of K+ homeostasis
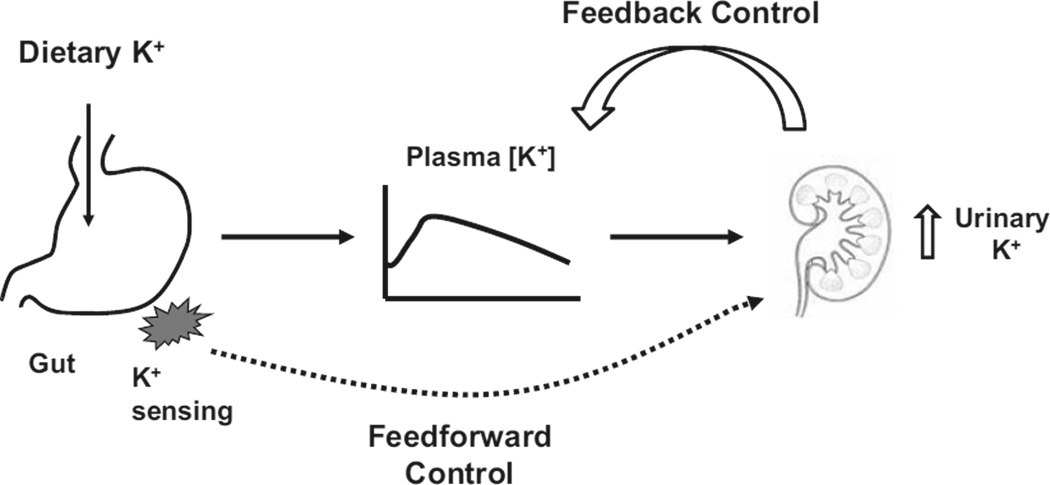
According to the traditional view, extracellular [K+] is the major factor in the regulation of renal K+ excretion (11, 12). Extracellular [K+] increases during dietary K+ intake, and this increase stimulates renal K+ excretion (Figure 1) by its direct action on K+ secretion in the collecting duct (11). In addition, increased extracellular [K+] stimulates aldosterone secretion, further stimulating renal K+ secretion and excretion. Increased renal K+ excretion will then tend to restore extracellular [K+]. Conversely, during K+ restriction, decreased plasma [K+] decreases renal K+ excretion, which will then serve to maintain extracellular [K+] within its normal range. Thus, the maintenance of K+ homeostasis has been traditionally understood based on the concept of a negative feedback control.
Figure 1.
A schematic diagram illustrating feedback vs. feedforward control of K+ homeostasis.
Feedforward control of K+ homeostasis
The traditional view was challenged by Rabinowitz who reviewed available data from a quantitative stance (13). He noted that plasma [K+] can stimulate renal K+ excretion, but this pathway required an increase of plasma [K+] above its normal range (14, 15). He also noted that aldosterone stimulates renal K+ excretion at supraphysiological levels but not in its physiological range (13, 15). In his studies in sheep (16), meal-induced kaliuresis was accompanied by no significant change in plasma aldosterone concentration. Plasma [K+] increased during meal intake, but the increase was too small, when reproduced by intravenous K+ infusion, to account for the meal-induced kaliuresis (15). Thus, meal-induced increases in renal K+ excretion could not be fully explained by changes in plasma K+ or aldosterone concentration. Rabinowitz proposed a kaliuretic reflex arising from K+ sensors in the splanchnic bed. According to this proposal, renal K+ excretion is increased, without (or before) increases in extracellular [K+] (or aldosterone), by a mechanism controlled by sensing of K+ intake in the splanchnic area (Figure 1). Thus, a concept of feedforward control was proposed. The idea of K+ sensing in the splanchnic area was supported by the studies of Morita and colleagues (17, 18) in which intraportal KCl infusion in anesthetized rats increased hepatic afferent nerve activity and urinary K+ excretion in the absence of increases in plasma [K+]. These effects were attenuated by bumetanide, suggesting that Na+-K+-2 Cl− cotransporter was involved in the sensing of portal [K+]. This research group suggested that Na+-K+ -2 Cl− cotransporter serves as both Na+ and K+ sensor in the portal vein and is downregulated by high Na+ and K+ intake (19).
Feedback vs. feedforward control
Most homeostatic regulation in the body is under feedback control because it allows a robust (or fine) control (4). However, this type of control can be slow in responding to an external challenge and inevitably results in an error signal, that is, a significant disturbance of the system. In contrast, feedforward control offers a quick control of output function (e.g., renal K+ excretion) in anticipation of a rise in the controlled variable (e.g., plasma [K+] during K+ intake). This type of control may add speed to the control and help minimize the disturbance of the system (at the expense of robustness). A combination of both feedback and feedforward control mechanisms may provide robust and speedy control with minimal disturbance. Systemic disturbances could be small even with a negative feedback control alone, if the feedback control is very strong. For example, if renal K+ excretion is exquisitely sensitive to an increase in plasma [K+], increases in plasma [K+] after an acute K+ load would be small. However, this may not always be optimal, e.g., during exercise when there is a K+ shift from ICF to ECF and an increase in plasma [K+] (without K+ intake or an increase in total body K+), a strong and sensitive feedback control would increase K+ excretion and reduce the body’s K+ pool (8). In contrast, a feedforward control activated in response to a meal (i.e., dietary K+ intake) to increase renal K+ excretion would not be activated during exercise, which would better fit the role of the kidneys in chronic K+ balance.
Feed forward control in glucose homeostatic system
Of the many homeostatic systems of the body, the K+ and the glucose homeostatic systems are unique because they share acute regulation by insulin. In addition, the two systems resemble each other in many aspects: both glucose and K+ stimulate pancreatic secretion of insulin (20); insulin stimulates transport of both glucose and K+ into insulin-sensitive tissues (21); transport of glucose and K+ is mediated by specific carriers—GLUT4 and Na-K-ATPase, respectively (22, 23)-and in both systems, liver and skeletal muscles are the major sites of disposal/storage following a meal. As for control mechanisms, blood glucose, like plasma [K+], is controlled by strong feedback mechanisms, involving insulin to promote skeletal muscle glucose uptake and inhibit hepatic glucose production after a meal and counterregulatory hormones to increase hepatic glucose production when blood glucose is low. In addition, gut hormones, such as glucagon-like peptide-1 (GLP-1) or gastric inhibitory peptide (GIP) may contribute to a feedforward system; these hormones are secreted into the blood by intestinal cells during ingestion of nutrients (24) and enhance insulin secretion and action after a meal independent of blood glucose (“gut factor” [25]). Considering the parallels between the two systems these findings suggest the possible existence of a gut factor(s) for K+ homeostasis.
FEED FORWARD CONTROL IN POTASSIUM HOMEOSTASIS
Feedforward control by insulin
After a meal, insulin secretion by the pancreatic β-cells is stimulated by meal nutrients, and the increase in plasma insulin concentration plays a critical role in the cellular disposal of ingested nutrients. Because insulin also stimulates cellular K+ uptake, meal ingestion also leads to a rapid transfer of K+ from ECF to ICF of insulin-sensitive cells, preventing excessive increases in [K+] due to dietary K+. In fact, intravenous K+ infusion results in lower plasma [K+] profile in rats simultaneously fed a meal than in fasted rats (8). In addition, raising blood glucose alone with an oral load has been shown to enhance extrarenal disposal of a potassium load by stimulating insulin secretion (26). This physiologic action of insulin and glucose is used as a therapeutic intervention to lower serum [K+] in patients with dangerously high levels of plasma [K+] which is frequently associated with acute or chronic renal failure, demonstrating the importance of insulin to transfer K+ from ECF to ICF. Although not necessarily the preferred approach, some studies have shown that endogenous insulin can also correct hyperkalemia (27). Thus, in both normal and pathological conditions, there is feedforward control by insulin of extracellular K+ homeostasis which is independent of plasma [K+]after a meal and dietary K+ intake.
Feedforward control activated by dietary K+
Lee et al. (8) tested the hypothesis that K+ intake is sensed by putative K+ sensors in the splanchnic bed, and that renal K+ excretion is regulated by this signal. To achieve this aim, K+ was infused for 2 h into either the stomach, the hepatic portal vein, or a systemic vein of overnight-fasted rats, and plasma [K+] and renal K+ excretion were measured before, during, and after the K+ infusion. The K+ infusions via the different routes resulted in very similar profiles of plasma [K+] and renal K+ excretion. These data do not provide evidence for a portal or gut sensing of K+ intake. However, they also tested the idea that a signal may be stimulated by ingesting a meal with K+, which is the normal route of K+ intake. Therefore, the K+ infusion experiments were repeated during simultaneous feeding with a K+ deficient diet. Interestingly, the route of the K+ infusion had a differential effect on plasma [K+] during the K+ free meal: intraportal and systemic infusions resulted in similar increases in plasma [K+], whereas intragastric K+ infusion did not significantly increase plasma [K+] despite near complete absorption of an equivalent K+ load. These data indicate that plasma K+ clearance is markedly increased when intragastric K+ infusion was combined with a meal, which is the normal route of K+ intake. The blunting of the rise in plasma [K+] was accompanied by enhancement of renal K+ excretion and extrarenal K+ uptake, which is consistent with a gut factor1 or sensor mechanism that enhances both renal and extrarenal K+ handling during meal (or dietary K+) intake, as a component of the feedforward control of K+ homeostasis.
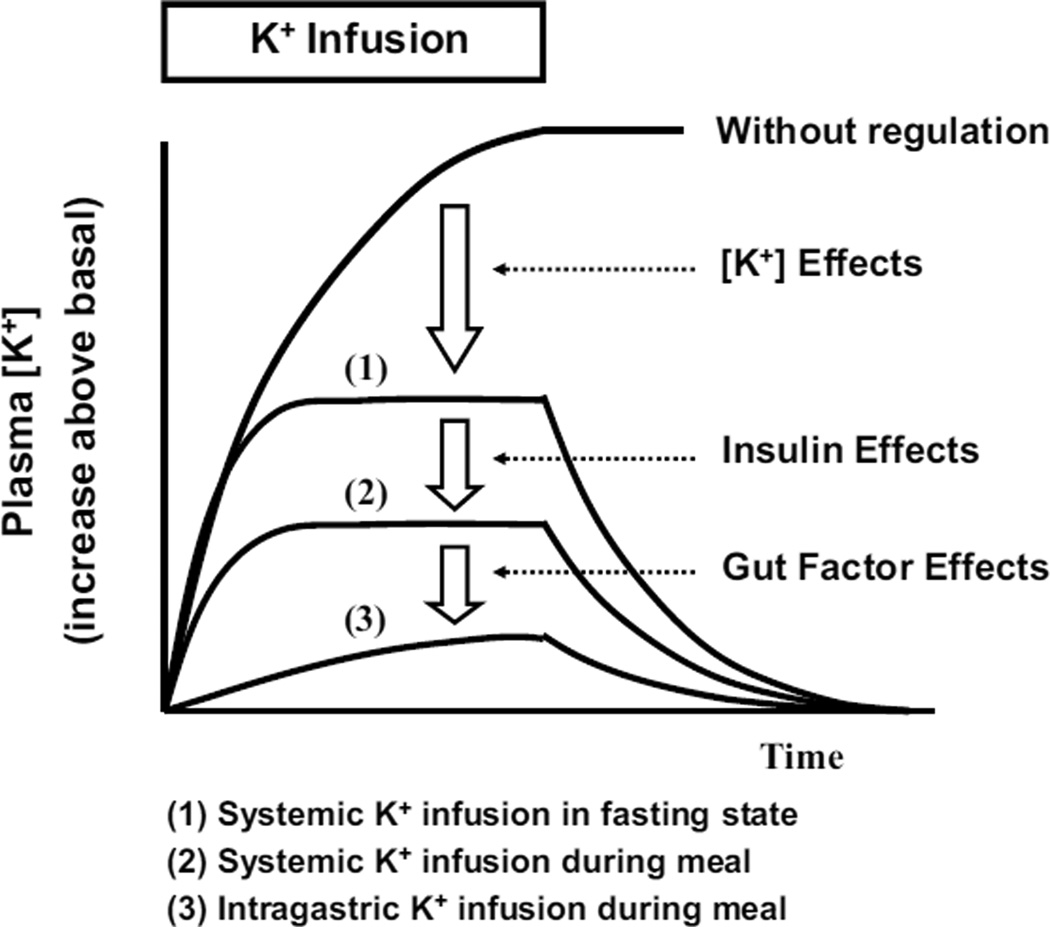
The study by Lee et al. (8) provided significant insight into the acute regulation of K+ homeostasis. In the fasting state, the systemic K+ infusion increased plasma [K+], increased renal K+ excretion, and also possibly cellular K+ uptake. These effects of plasma [K+] would be important to limit the rise in plasma [K+] during K+ intake, and normalize plasma [K+] when K+ intake ceases (Figure 2). However, the increases in plasma [K+] during the K+ infusion were large (i.e., 1.3 mM with an infusion rate of 100 mg/kg/h) in the fasting state. During a meal (i.e., dietary K+ intake), insulin helps prevent an excessive rise in plasma [K+] by stimulating extrarenal cellular K+ uptake (1, 28): the rise in plasma [K+] with systemic K+ infusion during a meal was decreased to ~0.6 mM, indicating meal and/or insulin effects. Finally, when K+ was given intragastrically with a meal, there was evidence of a gut factor(s) that further increases plasma K+ clearance, resulting in an insignificant rise in plasma [K+].
Figure 2.
A schematic figure illustrating the effects of plasma [K+] per seinsulin, and the hypothetical gut factor on the rise of plasma [K+] during K+ intake.
Gut sensing of K+ stimulates kaliuresis after a meal
Dietary K+ intake may increase renal K+ excretion either by increasing plasma [K+] (traditional view) or by activating a mechanism independent of plasma [K+], or by both mechanisms. Oh et al. (9) evaluated these mechanisms during normal dietary K+ intake in rats. After an overnight fast, rats were fed a small amount (~3 g or ~15% daily consumption) of diet containing either 0% or normal 1% K+, and plasma [K+] and renal K+ excretion were measured. In a third group, rats were fed the 0% K+ diet, and KCl was infused to match the plasma [K+] profile to that of the 1% K+ group. The 1% K+ feeding significantly increased renal K+ excretion, associated with slight increases in plasma [K+] (~0.2 mM). In the KCl-infused 0% K+ diet group, renal K+ excretion was significantly less than that in the 1% K+ group despite matched plasma [K+] profiles. This study indicates that postprandial increases in renal K+ excretion cannot be fully accounted for by changes in plasma [K+], and gut sensing of dietary K+ was a major component for regulation of renal K+ excretion. Plasma aldosterone levels were not altered by either meal feeding or dietary K+ content. Thus, there was an increase in renal K+ excretion during dietary K+ intake independent of plasma levels of K+ or aldosterone, two factors that have been viewed as classic regulators of renal K+ excretion. These findings are consistent with the concept of gut-factor effects or a feedforward control of extracellular K+ homeostasis.
MECHANISMS OF THE GUT-FACTOR EFFECTS
Are gut peptides involved?
The exact mechanisms underlying the gut-factor effects are unknown. One simple idea is that the gut may increase (or decrease) the secretion of a humoral factor upon sensing dietary K+ to increase renal K+ excretion. There is ample evidence for interactions between the gastrointestinal tract and the kidney; there are many gut peptides and hormones released in response to intake of dietary nutrients and ions that affect renal functions (29). Recent studies have suggested a gut-renal axis in the regulation of renal excretion of phosphate (30, 31). Like K+, phosphate is an electrolyte with a high ratio of dietary intake to extracellular pool size (i.e., a large turnover rate). Although the plasma phosphate level is not as tightly controlled as [K+] after a meal, there is evidence that a phosphaturic substance exists in the intestine, which may be released to increase renal phosphate excretion during dietary phosphate intake (30). Thus, analogous mechanisms may be involved in the regulation of both of these two major electrolytes with high turnover (and homeostatic challenge).
Oh et al. (9) examined whether there are kaliuretic hormones released from the gut in response to dietary K+. One necessary condition for a humoral mediator of the gut-factor effects is that its plasma level must be increased (or decreased) during dietary K+ intake. Because the putative gut factor is activated only after a meal (8), they first examined the incretin hormones GIP and GLP-1, gut hormones known to be secreted in response to meal nutrients. GLP-1 has been shown to have effects on renal sodium excretion and urinary flow (32, 33), and the GLP-1 receptor is expressed in glomerulus and proximal convoluted tubule cells (33). They found that plasma GIP or GLP-1 profiles were not altered by dietary K+, suggesting that these incretin hormones may not mediate the gut-factor effects. They further tested guanylin peptides, uroguanylin and guanylin, as these peptides are secreted by the intestine and have natriuretic and kaliuretic effects (34–36). Plasma levels of uroguanylin and guanylin were unaltered by either a meal or by dietary K+ content, indicating that these peptide hormones may not be involved in meal-induced kaliuresis. There are many other gut peptide hormones not examined by Oh et al., and it remains to be determined whether any of them has a previously unrecognized function of stimulating K+ excretion during dietary K+ intake.
Is the brain involved?
An alternative hypothesis is that gut sensing of K+ intake is transmitted to the brain, and the brain regulates renal and/or extrarenal K+ handling. There is significant evidence that the brain is involved in K+ homeostasis, as excellently reviewed by Rabinowitz (37). Removal of one kidney (i.e., uninephrectomy) in anesthetized rats causes ~2-fold increases in Na+ and K+ excretion in the remaining kidney. A series of elegant studies by Humphreys and colleagues (38–40) demonstrated that these effects are mediated by γ-melanocyte-stimulating hormone (MSH), whose secretion from the pituitary is stimulated after uninephrectomy. Removal of renal afferent nerve input prevented increases in γ-MSH after uninephrectomy (38), which indicates an important role of an afferent neural pathway from the kidney. Unilateral ureteral occlusion, like unilateral nephrectomy, resulted in large increases in Na+ and K+ excretion by the contralateral kidney (40). These data suggest that the brain continuously monitors the status of renal Na+ and/or K+ excretion2 and makes appropriate adjustments as necessary. In addition, the brain may monitor dietary K+ intake (input) and make adjustments in renal K+ excretion (output) to maintain K+ balance. Thus, the hypothalamus may monitor both K+ input and output and coordinate these signals to regulate renal (and/or extrarenal) K+ handling via pituitary hormones (9). Our preliminary study (Oh YT, Youn JH; unpublished data) showed that K+ adaptation to altered K+ intake was impaired in hypophysectomized rats, consistent with the idea that the brain is involved in the sensing of K+ intake.
Oh et al. (9) examined whether plasma γ-MSH levels are altered during dietary K+ intake, a prerequisite for a mediator of gut-factor effects, as discussed above. Plasma levels of γ-MSH (or γ-MSH) were not altered either by a meal or dietary K+ content, providing no support for a role of γ-MSH in gut-factor effects. In this study, the MSH peptide studied was γ2-MSH, as it was measured or administered in the studies of Humphreys and colleagues. Based on the gene sequence of POMC and potential dibasic residue cleavage sites, three MSH peptides (γ1, γ2, and γ3-MSH) were predicted (41). However, whereas γ1 and γ3-MSH have been isolated from rodent pituitary cells (42, 43), γ2-MSH has not, leading to the suggestion that γ2-MSH may not exist in rodents (44). Therefore, the potential role of γ-MSH as a mediator of gut-factor effects will need to be further explored and/or extended to studies with γ1- and γ3-MSH. In addition, other pituitary peptides may be involved in the regulation of renal K+ excretion by dietary K+.
Potential targets of a gut factor in the kidney
Tissue kallikrein (TK) is a serine protease that cleaves kininogens to form vasodilator peptides kinins. TK is synthesized in many organs, including the kidney. In the kidney, TK is produced by connecting tubule cells and is secreted into the tubular fluid. Studies on the role of the renal kallikrein-kinin system have shown that high K+ diets or acute K+ loading increases urinary excretion of TK (45, 46). El Moghrabi et al. (47) recently showed that TK is also kaliuretic, and this effect is mediated by the effects of TK to activate the epithelial Na+ channel (ENaC) to increase K+ secretion in principal cells and to inhibit the colonic H+, K+-ATPase to decrease K+ reabsorption in intercalated cells. They further demonstrated that renal K+ and TK excretion increased in parallel after a single meal in mice. These responses occurred in normal mice independent of aldosterone secretion and without increasing plasma [K+], when measured 5 hours after the initiation of the meal. In contrast, in TK knockout mice, plasma [K+] increased significantly after a meal, indicating that TK is responsible for kaliuretic responses after a meal. Thus, meal-induced kaliuresis may be due in part to TK synthesis and action in the kidney, which appear to occur independent of plasma aldosterone and [K+]. These data suggest that renal TK may be a target of the putative gut factor activated during dietary K+ intake.
Wang and colleagues have demonstrated that K+ depletion increases protein tyrosine kinase-mediated phosphorylation of ROMK, which causes retraction of the K+ channels out of the apical membrane to reduce K+ secretion and excretion, and these effects are mediated by increases in NADPH oxidase and superoxide anion production (5, 48). A recent study by this group further demonstrated that all of these changes occurred with normal plasma [K+] in rats and mice maintained on a low (0.1%) K+ diet (49). Our previous study also showed that modest K+ deprivation increases PTK expression and ROMK phosphorylation in the absence of a fall in plasma K+ or aldosterone levels (50). On the other hand, consuming a high K+ diet for 1–3 days decreased PTK-mediated ROMK phosphorylation (51). Taken together, these data suggest that NADPH oxidase in the kidney may be another potential target of a gut factor. If this is true, NADPH oxidase activity and superoxide anion production in the kidney could be acutely altered after a meal to account for rapid increases in renal K+ excretion.
ROLE OF GUT FACTOR IN CHRONIC K+ ADAPTATION
Renal K+ adaptation
When dietary K+ intake is increased or decreased, the kidneys respond by appropriately increasing or decreasing K+ excretion, respectively (11, 52). This so-called "K+ adaptation" is critical for chronic K+ balance and has been well recognized for several decades. In the kidney K+ is filtered at the glomerulus, and the filtered K+ load is almost completely reabsorbed in the proximal nephron. Under normal or high K+ intake, K+ is secreted in the cortical collecting duct (CCD), accounting for the majority of excreted K+. Under a low K+ diet, there is net K+ reabsorption in the CCD. Thus, K+ secretion in the CCD is highly adaptable to dietary K+ intake and is the major site of regulation of K+ excretion (52). Molecular mechanisms underlying renal K+ adaptation have been extensively studied, as reviewed by Paul Welling in this issue of the Journal. Thus, the effector system is well characterized, although the signal for renal K+ adaptation (or how the body senses dietary K+ intake) is unclear (13).
Adaptation of extrarenal tissues to low K+ intake
Some extrarenal tissues, such as skeletal muscle and liver, increase K+ uptake in response to insulin (1, 28), constituting an important mechanism of extracellular K+ homeostasis after a meal and K+ intake. Some years ago, Choi et al. introduced a novel technique for the quantification of in vivoinsulin action on cellular K+ uptake, termed the “K+ clamp” (53, 54). In this method plasma K+ is maintained constant during insulin infusion by varying exogenous K+ infusion. The K+ infusion rate required to clamp plasma K+ is a measure of insulin action to increase the rate of cellular K+ uptake (54). Using this technique, we have demonstrated in rats that insulin-stimulated cellular K+ uptake, like renal K+ excretion, is profoundly suppressed during K+ deprivation (50, 53, 54), indicating the body’s concerted efforts to conserve extracellular K+. Thus, K+ adaptation occurs both in the kidneys and extrarenal tissues in response to changes in K+ intake.
A common signal for renal and extrarenal adaptations?
Interestingly, Choi et al. found a strong correlation between the degree of change in renal K+ excretion and extrarenal K+ uptake during K+ deprivation (54), suggesting a common signal or factor for these changes. Chen et al. demonstrated that these changes in renal and extrarenal K+ handling occurred in the absence of changes in plasma K+ or aldosterone concentration when K+ intake was reduced to 1/3 of control (50). Thus, it seems that the body was able to sense reduced K+ intake, even without changes in extracellular [K+], and decrease renal and extrarenal K+ handling for extracellular K+ conservation. These data support the role of a gut factor in renal and extrarenal K+ adaptation (see below).
Gut factor may explain rapid induction and reversal of a K+ conservation state
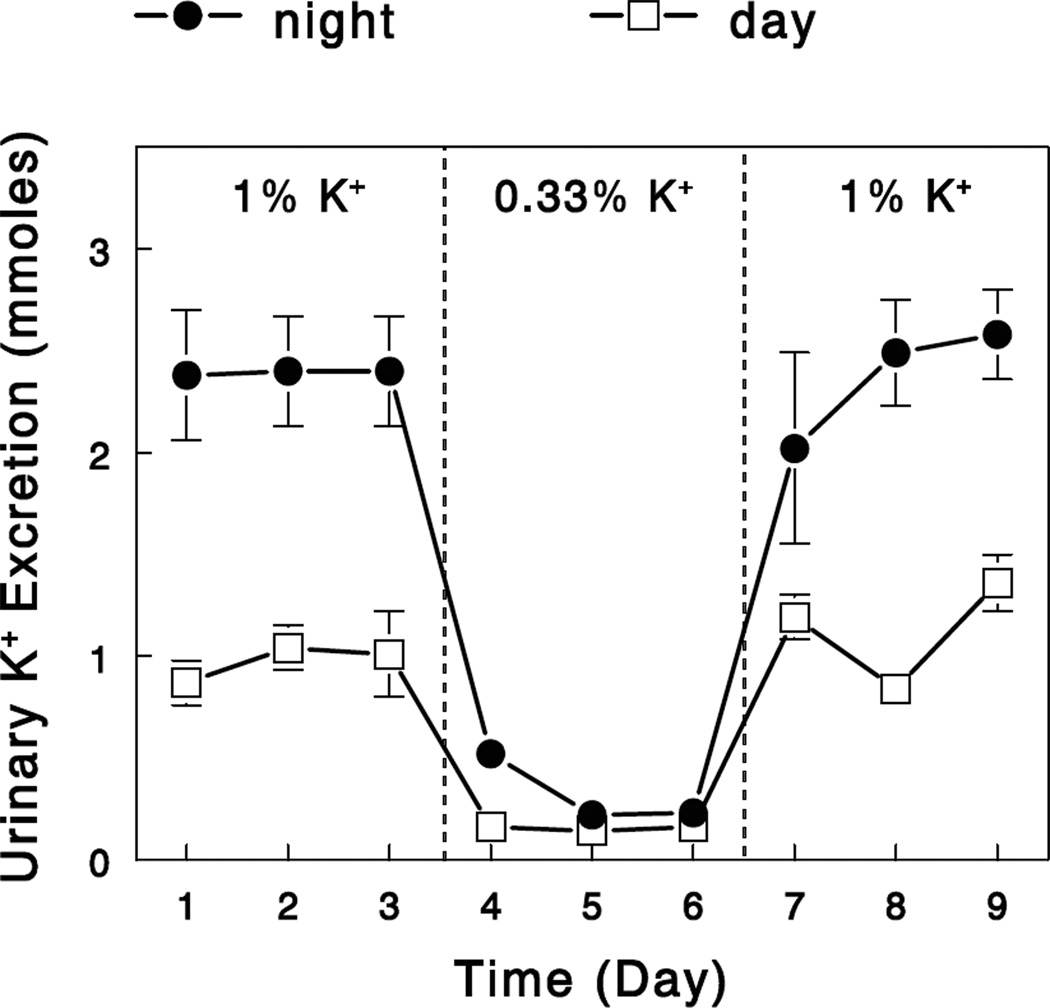
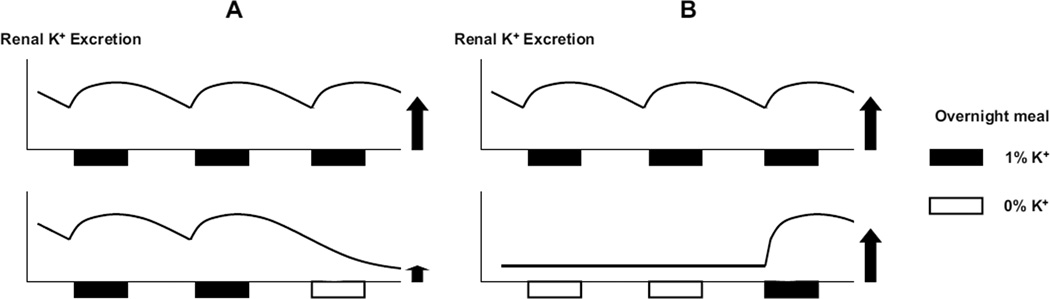
Previous studies showed that renal K+ adaptation occurs in rats within a day of altered K+ intake (55). Our preliminary study in rats demonstrated that an overnight, modest K+ restriction (that does not decrease plasma [K+]) was sufficient to trigger renal K+ conservation, and this was completely reversed by an overnight feeding with a normal diet (Figure 3). If a gut factor is activated during dietary K+ intake to enhance renal and extrarenal K+ handling, renal and extrarenal K+ handling would be efficient during normal K+ intake due to gut-factor effects but would be reduced during K+ deprivation due to a reduced gut-factor effects (Figure 4A). Although these changes (i.e., decreased renal K+ excretion and extrarenal K+ uptake during K+ deprivation) are important for extracellular K+ conservation, this may impose a risk for life-threatening hyperkalemia with sudden K+ intake. The gut factor may allow a rapid reversal of a K+-conservation state with increased K+ intake by sensing K+ and rapidly normalizing renal K+ excretion and extrarenal K+ uptake (i.c; Figure 4B). This would provide protection from life-threatening hyperkalemia. Thus, the concept of the gut factor (or feedforward control) provides novel mechanistic insights into the renal and extrarenal adaptations to altered K+ intake.
Figure 3.
Changes in urinary K+ excretion with altered K+ intake. Rats were maintained on a control 1.0% K+ diet for 3 days, a modest K+-restricted (0.33% K+) diet for the following 3 days, and again the control diet for the final 3 days. Animals were individually housed and had free access to food only at night (6 PM – 6 AM). Urinary K+ excretion was determined for the absorptive (6 PM – 6 AM, night) and the postabsorptive (6 AM – 6 PM, day) periods. Data are means ± SE (n=4).
Figure 4.
Schematic diagrams illustrating a decrease in renal K+ excretion after an overnight K+ deprivation (A) or its rapid reversal following an overnight feeding with a normal 1% K+ diet (B). In Athe gut factor effect (with 1% K+ meal) to enhance renal K+ excretion from previous feeding wanes over time, resulting in a profound decrease in K+ excretion after an overnight K+ restriction (0% K+ meal; i.e., without gut factor activation).
Two phases of K+ adaptation
During K+ deprivation in rodents, plasma [K+] does not fall during the initial few days (e.g., 2–6 days [53, 56]), even with total K+ deprivation (i.e., no K+ in the diet), due to the concerted efforts of the kidneys to decrease K+ excretion and extrarenal tissues to release K+ into ECF. Thus, in this early phase of adaptation to K+ deprivation, the efforts for extracellular potassium conservation are effective to maintain normal plasma [K+]. However, even with profound decreases in renal K+ excretion, K+ loss continues through the feces and sweat, slowly decreasing total body K+ content. Plasma [K+] can be still maintained as normal because of the donation of K+ from skeletal muscle to compensate for this loss. However, because there is apparently a limit to how much K+ can be donated from skeletal muscle, plasma [K+] starts to fall after a few days. After 1–2 weeks of total K+ deprivation, plasma [K+] decreases to very low levels (48, 53, 56). Thus, during total K+ deprivation, there may be two phases of adaptation; first or early adaptations occur in the absence of changes in plasma [K+], and second or late adaptations may occur in response to a fall in plasma [K+]. It is reasonable to assume that these adaptations (early vs. late) may be brought about by independent mechanisms. With partial K+ deprivation, plasma [K+] may not fall even after an extended period of time, if dietary K+ intake, although reduced, is sufficient to replenish the K+ loss. Plasma [K+] was normal in rats after 30 days of reducing K+ intake to 1/3 of normal (50) or in rats and mice after a week on a 0.1% K+ diet (49). In these cases, the second phase of adaptation may not occur.
Most previous animal studies on molecular changes associated with K+ deprivation employed weeks of total K+ deprivation. Many changes observed in these studies may represent those resulting from hypokalemia, possibly different from those responsible for early adaptations that occur without changes in plasma [K+]. Because K+ adaptations can occur within a day of altered K+ intake and in the absence of plasma [K+], it is important to study molecular changes in the kidneys (and extrarenal tissues) under conditions of normal [K+], either early during total K+ deprivation or with partial K+ deprivation. These changes would reveal molecular mechanisms involved in gut-factor effects and help identify the signal for dietary K+ intake.
BENEFICIAL EFFECTS OF DIETARY POTASSIUM
The effects of high K+ diets to lower blood pressure have long been recognized (57, 58). In addition, there is ample evidence that high dietary K+ intake has beneficial effects on stroke and cardiovascular disease (57, 58), but the underlying molecular mechanisms are not well understood. Young and colleagues proposed the hypothesis that beneficial effects of increased dietary K+ intake are brought about by increased extracellular K+ concentration ([K+]), based on in vitro observations that high K+ levels exert cellular effects to inhibit free-radical formation, smooth-muscle proliferation, and thrombus formation (58). However, beneficial effects of K+ supplementation have been observed in clinical studies without an increase in plasma [K+] (59). For example, potassium supplementation (60 mmoles KCl/70 kg body weight) for 3 days did not change serum [K+] but significantly diminished platelet reactivity in healthy men and women, providing a potential link between K+ intake and occlusive stroke (60). In another study, increasing dietary potassium (30 mmoles per day) significantly lowered blood pressure in normotensive volunteers without changing plasma [K+] (61). These findings suggest that the beneficial effects of dietary K+ intake may be mediated by a mechanism independent of plasma [K+] (62).
The above considerations support the intriguing possibility that dietary K+ intake stimulates the production of an unknown mediator(s) of beneficial effects of a high K+ diet. Alternatively, there is the possibility that a high K+ diet inhibits the production of a factor that plays a role in K+ conservation during K+ deprivation but has long-term detrimental effects on the cardiovascular system. Regarding this alternative, a strong candidate for such a factor is reactive oxygen species (ROS) including superoxide anions. As discussed above, Wang and colleagues have suggested that renal K+ adaptation is mediated by changes in superoxide anion production in the kidneys, which occurred even with normal plasma [K+] (48, 49). On the other hand, recent studies have suggested that ROS is involved in detrimental effects of low K+ diet and protective effects of high K+ diet on the cardiovascular system (63, 64). Thus, there may be a factor activated during K+ deprivation to increase NADPH oxidase expression in various tissues, which would decrease renal K+ excretion but exert detrimental effects on the cardiovascular system. Whereas evidence accumulates that high K+ intake can decrease superoxide anion production to reduce cardiovascular injury and dysfunction, it remains to be determined how dietary K+ intake is connected to the changes in NADPH expression and activity. Additional studies are warranted to examine whether gut sensing of K+ intake is critically involved in this connection.
Acknowledgments
Supported in part by NIH DK090749
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The term “gut factor” is used in this article to generally refer to a mechanism activated by gut sensing of K+ intake that enhances renal K+ excretion and extrarenal K+ uptake, independent of plasma [K+]. The exact nature of this mechanism is currently unknown; the factor may involve neural, not necessarily humoral regulation, as the name may imply.
The studies of Humphreys and colleagues have largely focused on the role of γ-MSH in the regulation of Na+ excretion, and therefore the role of γ-MSH in the regulation of K+ excretion is less clear.
REFERENCES
- 1.Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol. 2009;71:381–401. doi: 10.1146/annurev.physiol.010908.163241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng C-J, Kuo E, Huang C-L. Extracellular Potassium Homeostasis: Insights from Hypokalemic Periodic Paralysis. Seminars Nephrology. 2012 doi: 10.1016/j.semnephrol.2013.04.004. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stokes JB. Potassium intoxication: pathogenesis and treatment. In: Seldin DW, Giebisch G, editors. The regulation of potassium balance. New York: Raven; 1989. [Google Scholar]
- 4.McDonough AA, Thompson CB, Youn JH. Skeletal muscle regulates extracellular potassium. Am J Physiol Renal Physiol. 2002;282:F967–F974. doi: 10.1152/ajprenal.00360.2001. [DOI] [PubMed] [Google Scholar]
- 5.Wang W. Regulation of renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:547–569. doi: 10.1146/annurev.physiol.66.032102.112025. [DOI] [PubMed] [Google Scholar]
- 6.Welling PA, Ho K. A comprehensive guide to the ROMK potassium channel: form and function in health and disease. Am J Physiol Renal Physiol. 2009;297:F849–F863. doi: 10.1152/ajprenal.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodan AR, Cheng CJ, Huang CL. Recent advances in distal tubular potassium handling. Am J Physiol Renal Physiol. 2011;300:F821–F827. doi: 10.1152/ajprenal.00742.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee FN, Oh G, McDonough AA, Youn JH. Evidence for gut factor in K+ homeostasis. Am J Physiol Renal Physiol. 2007;293:F541–F547. doi: 10.1152/ajprenal.00427.2006. [DOI] [PubMed] [Google Scholar]
- 9.Oh KS, Oh YT, Kim SW, Kita T, Kang I, Youn JH. Gut sensing of dietary K+intake increases renal K+excretion. Am J Physiol Regul Integr Comp Physiol. 2011;301:R421–R429. doi: 10.1152/ajpregu.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bia MJ, DeFronzo RA. Extrarenal potassium homeostasis. Am J Physiol. 1981;240:F257–F268. doi: 10.1152/ajprenal.1981.240.4.F257. [DOI] [PubMed] [Google Scholar]
- 11.Gennari FJ, Segal AS. Hyperkalemia: An adaptive response in chronic renal insufficiency. Kidney Int. 2002;62:1–9. doi: 10.1046/j.1523-1755.2002.00350.x. [DOI] [PubMed] [Google Scholar]
- 12.Giebisch G, Windhager E. Transport of potassium. In: Boron WF, Boulpaep EL, editors. Medical Physiology. Philadelphia: Elsevier Science; 2003. pp. 814–827. [Google Scholar]
- 13.Rabinowitz L. Aldosterone and potassium homeostasis. Kidney Int. 1996;49:1738–1742. doi: 10.1038/ki.1996.258. [DOI] [PubMed] [Google Scholar]
- 14.Calo L, Borsatti A, Favaro S, Rabinowitz L. Kaliuresis in normal subjects following oral potassium citrate intake without increased plasma potassium concentration. Nephron. 1995;69:253–258. doi: 10.1159/000188466. [DOI] [PubMed] [Google Scholar]
- 15.Rabinowitz L, Sarason RL, Yamauchi H. Effects of KCl infusion on potassium excretion in sheep. Am J Physiol. 1985;249:F263–F271. doi: 10.1152/ajprenal.1985.249.2.F263. [DOI] [PubMed] [Google Scholar]
- 16.Rabinowitz L, Green DM, Sarason RL, Yamauchi H. Homeostatic potassium excretion in fed and fasted sheep. Am J Physiol. 1988;254:R357–R380. doi: 10.1152/ajpregu.1988.254.2.R357. [DOI] [PubMed] [Google Scholar]
- 17.Morita H, Fujiki N, Hagiike M, Yamaguchi O, Lee K. Functional evidence for involvement of bumetanide-sensitive Na+K+2CI- cotransport in the hepatoportal Na+ receptor of the Sprague-Dawley rat. Neurosci Lett. 1999;264:65–68. doi: 10.1016/s0304-3940(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 18.Morita H, Fujiki N, Miyahara T, Lee K, Tanaka K. Hepatoportal bumetanide-sensitive K(+)-sensor mechanism controls urinary K(+) excretion. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1134–R1139. doi: 10.1152/ajpregu.2000.278.5.R1134. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiya Y, Nakashima S, Banno Y, Suzuki Y, Morita H. Effect of high-NaCl or high-KCl diet on hepatic Na+- and K+-receptor sensitivity and NKCC1 expression in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R591–R596. doi: 10.1152/ajpregu.00559.2003. [DOI] [PubMed] [Google Scholar]
- 20.Hiatt N, Davidson MB, Bonorris G. The effect of potassium chloride infusion on insulin secretion in vivo. Horm Metab Res. 1972;4:64–68. doi: 10.1055/s-0028-1094101. [DOI] [PubMed] [Google Scholar]
- 21.Ferrannini E, Taddei S, Santoro D, Natali A, Boni C, Del Chiaro D, Buzzigoli G. Independent stimulation of glucose metabolism and Na+-K+ exchange by insulin in the human forearm. Am J Physiol. 1988;255:E953–E958. doi: 10.1152/ajpendo.1988.255.6.E953. [DOI] [PubMed] [Google Scholar]
- 22.James DE, Brown R, Navarro J, Pilch PF. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988;333:183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- 23.Clausen T, Everts ME. Regulation of the Na,K-pump in skeletal muscle. Kidney Int. 1989;35:1–13. doi: 10.1038/ki.1989.1. [DOI] [PubMed] [Google Scholar]
- 24.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 25.Bergman RN, Beir JR, Hourigan PM. Intraportal glucose infusion matched to oral glucose absorption. Lack of evidence for "gut factor" involvement in hepatic glucose storage. Diabetes. 1982;31:27–35. doi: 10.2337/diab.31.1.27. [DOI] [PubMed] [Google Scholar]
- 26.Allon M, Dansby L, Shanklin N. Glucose modulation of the disposal of an acute potassium load in patients with end-stage renal disease. Am J Med. 1993;94:475–482. doi: 10.1016/0002-9343(93)90081-Y. [DOI] [PubMed] [Google Scholar]
- 27.Muto S, Sebata K, Watanabe H, Shoji F, Yamamoto Y, Obashi M, et al. Effect of oral glucose administration on serum potassium concentration in hemodialysis patients. Am J Kidney Dis. 2005;46:697–705. doi: 10.1053/j.ajkd.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 28.DeFronzo RA, Felig P, Ferrannini E, Wahren J. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am J Physiol. 1980;238:E421–E429. doi: 10.1152/ajpendo.1980.238.5.E421. [DOI] [PubMed] [Google Scholar]
- 29.Michell AR, Debnam ES, Unwin RJ. Regulation of Renal Function by the Gastrointestinal Tract: Potential Role of Gut-Derived Peptides and Hormones. Annu Rev Physiol. 2008;70:379–403. doi: 10.1146/annurev.physiol.69.040705.141330. [DOI] [PubMed] [Google Scholar]
- 30.Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ, et al. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci U S A. 2007;104:11085–11090. doi: 10.1073/pnas.0704446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar R. Phosphate sensing. Curr Opin Nephrol Hypertens. 2009;18:281–284. doi: 10.1097/MNH.0b013e32832b5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahren B. GLP-1 and extra-islet effects. Horm Metab Res. 2004;36:842–845. doi: 10.1055/s-2004-826173. [DOI] [PubMed] [Google Scholar]
- 33.Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, et al. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol. 2011;301:F355–F363. doi: 10.1152/ajprenal.00729.2010. [DOI] [PubMed] [Google Scholar]
- 34.Forte LR, Fan X, Hamra FK. Salt and water homeostasis: uroguanylin is a circulating peptide hormone with natriuretic activity. Am J Kidney Dis. 1996;28:296–304. doi: 10.1016/s0272-6386(96)90318-2. [DOI] [PubMed] [Google Scholar]
- 35.Fukae H, Kinoshita H, Fujimoto S, Kita T, Nakazato M, Eto T. Changes in urinary levels and renal expression of uroguanylin on low or high salt diets in rats. Nephron. 2002;92:373–378. doi: 10.1159/000063311. [DOI] [PubMed] [Google Scholar]
- 36.Nakazato M. Guanylin family: new intestinal peptides regulating electrolyte and water homeostasis. J Gastroenterol. 2001;36:219–225. doi: 10.1007/s005350170106. [DOI] [PubMed] [Google Scholar]
- 37.Rabinowitz L, Aizman RI. The central nervous system in potassium homeostasis. Frontiers in Neuroendocrinology. 1993;14:1–26. doi: 10.1006/frne.1993.1001. [DOI] [PubMed] [Google Scholar]
- 38.Humphreys MH, Lin SY, Wiedemann E. Renal nerves and the natriuresis following unilateral renal exclusion in the rat. Kidney Int. 1991;39:63–70. doi: 10.1038/ki.1991.8. [DOI] [PubMed] [Google Scholar]
- 39.Lin SY, Chaves C, Wiedemann E, Humphreys MH. A gamma-melanocyte stimulating hormone-like peptide causes reflex natriuresis after acute unilateral nephrectomy. Hypertension. 1987;10:619–627. doi: 10.1161/01.hyp.10.6.619. [DOI] [PubMed] [Google Scholar]
- 40.Ribstein J, Humphreys MH. Renal nerves and cation excretion after acute reduction in functioning renal mass in the rat. Am J Physiol. 1984;246:F260–F265. doi: 10.1152/ajprenal.1984.246.3.F260. [DOI] [PubMed] [Google Scholar]
- 41.Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, et al. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979;278:423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- 42.Zhou A, Bloomquist BT, Mains RE. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J Biol Chem. 1993;268:1763–1769. [PubMed] [Google Scholar]
- 43.Breen TL, Conwell IM, Wardlaw SL. Effects of fasting, leptin, and insulin on AGRP and POMC peptide release in the hypothalamus. Brain Res. 2005;1032:141–148. doi: 10.1016/j.brainres.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Harmer SC, Pepper DJ, Cooke K, Bennett HP, Bicknell AB. Evidence of a possible role for Lys-gamma3-MSH in the regulation of adipocyte function. J Endocrinol. 2008;196:149–158. doi: 10.1677/JOE-07-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vío CP, Figueroa CD. Evidence for a stimulatory effect of high potassium diet on renal kallikrein. Kidney Int. 1987;31:1327–1334. doi: 10.1038/ki.1987.146. [DOI] [PubMed] [Google Scholar]
- 46.Murakami E, Hiwada K, Kokubu T, Imamura Y. Effect of oral potassium on urinary kallikrein excretion in essential hypertension. Adv Exp Med Biol. 1989;247B:133–137. doi: 10.1007/978-1-4615-9546-5_22. [DOI] [PubMed] [Google Scholar]
- 47.El Moghrabi S, Houillier P, Picard N, Sohet F, Wootla B, Bloch-Faure M, et al. Tissue kallikrein permits early renal adaptation to potassium load. Proc Natl Acad Sci U S A. 2010;107:13526–13531. doi: 10.1073/pnas.0913070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babilonia E, Lin D, Zhang Y, Wei Y, Yue P, Wang WH. Role of gp91phox -containing NADPH oxidase in mediating the effect of K restriction on ROMK channels and renal K excretion. J Am Soc Nephrol. 2007;18:2037–2045. doi: 10.1681/ASN.2006121333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang ZJ, Sun P, Xing W, Pan C, Lin DH, Wang WH. Decrease in dietary K intake stimulates the generation of superoxide anions in the kidney and inhibits K secretory channels in the CCD. Am J Physiol Renal Physiol. 2010;298:F1515–F1522. doi: 10.1152/ajprenal.00502.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen P, Guzman JP, Leong PK, Yang LE, Perianayagam A, Babilonia E, et al. Modest dietary K+ restriction provokes insulin resistance of cellular K+ uptake and phosphorylation of renal outer medulla K+ channel without fall in plasma K+ concentration. Am J Physiol Cell Physiol. 2006;290:C1355–C1363. doi: 10.1152/ajpcell.00501.2005. [DOI] [PubMed] [Google Scholar]
- 51.Wei Y, Bloom P, Lin D, Gu R, Wang WH. Effect of dietary K intake on apical smallconductance K channel in CCD: role of protein tyrosine kinase. Am J Physiol Renal Physiol. 2001;281:F206–F212. doi: 10.1152/ajprenal.2001.281.2.F206. [DOI] [PubMed] [Google Scholar]
- 52.Laroche-Joubert N, Doucet A. Collecting duct adaptation to potassium depletion. Semin Nephrol. 1999;19:390–398. [PubMed] [Google Scholar]
- 53.Choi CS, Thompson CB, Leong PK, McDonough AA, Youn JH. Short-term K(+) deprivation provokes insulin resistance of cellular K(+) uptake revealed with the K(+) clamp. Am J Physiol Renal Physiol. 2001;280:F95–F102. doi: 10.1152/ajprenal.2001.280.1.F95. [DOI] [PubMed] [Google Scholar]
- 54.Choi CS, Lee FN, McDonough AA, Youn JH. Independent regulation of in vivo insulin action on glucose versus K(+) uptake by dietary fat and K(+) content. Diabetes. 2002;51:915–920. doi: 10.2337/diabetes.51.4.915. [DOI] [PubMed] [Google Scholar]
- 55.Rabinowitz L, Sarason RL, Yamauchi H, Yamanaka KK, Tzendzalian PA. Time course of adaptation to altered K intake in rats and sheep. Am J Physiol. 1984;247:F607–F617. doi: 10.1152/ajprenal.1984.247.4.F607. [DOI] [PubMed] [Google Scholar]
- 56.Amlal H, Wang Z, Soleimani M. Potassium depletion downregulates chloride-absorbing transporters in rat kidney. J Clin Invest. 1998;101:1045–1054. doi: 10.1172/JCI686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 58.Young DB, Lin H, McCabe RD. Potassium's cardiovascular protective mechanisms. Am J Physiol. 1995;268:R825–R837. doi: 10.1152/ajpregu.1995.268.4.R825. [DOI] [PubMed] [Google Scholar]
- 59.Barri YM, Wingo CS. The Effects of Potassium Depletion and Supplementation on Blood Pressure: A Clinical Review. Am J Med Sci. 1997;314:37–40. doi: 10.1097/00000441-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Kimura M, Lu X, Skurnick J, Awad G, Bogden J, Kemp F, et al. Potassium chloride supplementation diminishes platelet reactivity in humans. Hypertension. 2004;44:969–973. doi: 10.1161/01.HYP.0000147660.58694.6f. [DOI] [PubMed] [Google Scholar]
- 61.Braschi A, Naismith DJ. The effect of a dietary supplement of potassium chloride or potassium citrate on blood pressure in predominantly normotensive volunteers. Br J Nutr. 2008;99:1284–1292. doi: 10.1017/S0007114507864853. [DOI] [PubMed] [Google Scholar]
- 62.Sica DA, Struthers AD, Cushman WC, Wood M, Banas JS, Jr, Epstein M. Importance of potassium in cardiovascular disease. J Clin Hypertens (Greenwich) 2002;4:198–206. doi: 10.1111/j.1524-6175.2002.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang BC, Li DY, Weng YF, Lynch J, Wingo CS, Mehta JL. Increased superoxide anion generation and altered vasoreactivity in rabbits on low-potassium diet. Am J Physiol. 1998;274:H1955–H1961. doi: 10.1152/ajpheart.1998.274.6.H1955. [DOI] [PubMed] [Google Scholar]
- 64.Kido M, Ando K, Onozato ML, Tojo A, Yoshikawa M, Ogita T, et al. Protective effect of dietary potassium against vascular injury in salt-sensitive hypertension. Hypertension. 2008;51:225–231. doi: 10.1161/HYPERTENSIONAHA.107.098251. [DOI] [PubMed] [Google Scholar]