Abstract
The majority of the approximately 1.7 million civilians in the United States who seek emergency care for traumatic brain injury (TBI) are classified as mild (MTBI). Premorbid and comorbid conditions that commonly accompany MTBI may influence neurocognitive and functional recovery. This study assessed the influence of chronic smoking and hazardous alcohol consumption on neurocognitive recovery after MTBI. A comprehensive neurocognitive battery was administered to 25 non-smoking MTBI participants (nsMTBI), 19 smoking MTBI (sMTBI) 38±22 days (assessment point 1: AP1) and 230±36 (assessment point 2: AP2) days after injury. Twenty non-smoking light drinkers served as controls (CON). At AP1, nsMTBI and sMTBI were inferior to CON on measures of auditory-verbal learning and memory; nsMTBI performed more poorly than CON on processing speed and global neurocognition, and sMTBI performed worse than CON on working memory measures; nsMTBI were inferior to sMTBI on visuospatial memory. Over the AP1-AP2 interval, nsMTBI showed significantly greater improvement than sMTBI on measures of processing speed, visuospatial learning and memory, visuospatial skills, and global neurocognition, whereas sMTBI only showed significant improvement on executive skills. At AP2, sMTBI remained inferior to CON on auditory-verbal learning and auditory-verbal memory; there were no significant differences between nsMTBI and CON or among nsMTBI and sMTBI on any domain at AP2. Hazardous alcohol consumption was not significantly associated with change in any neurocognitive domain. For sMTBI, over the AP1-AP2 interval, greater lifetime duration of smoking and pack-years were related to significantly less improvement on multiple domains. Results suggest consideration of the effects of chronic cigarette smoking is necessary to understand the potential factors influencing neurocognitive recovery after MTBI.
Key words: cigarette smoking, cognition, mild traumatic brain injury, recovery
Introduction
Annually, approximately 1.7 million civilians in the United States seek emergency care for traumatic brain injury (TBI) secondary to motor vehicle accidents, falls, assaults, sports-related events, and other mechanisms.1 The majority (70–90%) of these TBI cases are classified as mild.1,2 In addition, 10–25% of the 2.3 million military personnel deployed to Iraq and Afghanistan since 2001 have sustained a mild traumatic brain injury (MTBI) during their service.3
Within the first month after MTBI, variable levels of dysfunction on measures of attention, learning, and memory, information processing speed, and working memory are frequently observed. In the majority of cases, the neurocognitive abnormalities demonstrated following MTBI are principally resolved within approximately 3 months post-injury.4 There is still considerable controversy, however, regarding the influence of premorbid and comorbid factors on the expected rate and extent of recovery from the acute neurocognitive sequelae associated with MTBI.4,5 Specifically, it has long been recognized that premorbid and comorbid conditions that commonly accompany MTBI (e.g., previous MTBI, alcohol/substance abuse, post-traumatic stress disorder [PTSD], litigation) may influence neurocognitive and functional outcome.6–9 Many previous studies, however, either excluded such premorbid/comorbid conditions to study the “pure” or “uncomplicated” consequences of MTBI, or did not consider the potential effects of these co-occurring conditions.
Excluding patients with premorbid and comorbid conditions that commonly accompany MTBI, such as alcohol and substance use disorders, may result in a selection bias that creates study cohorts that are not representative of the majority seeking medical treatment/neuropsychological assessment for MTBI. Such selection bias may significantly limit the clinical relevance and generalizability of the reported findings. Alternately, failure to specifically evaluate for, or consider the influence of, prevalent comorbid conditions may lead to spurious conclusions about the mechanisms promoting neurobiological and neurocognitive dysfunction secondary to MTBI.
Alcohol and substance use disorders are the most prevalent comorbid conditions across the TBI severity spectrum.10–12 Independent of TBI, chronic hazardous alcohol consumption (i.e., ≥5 drink equivalents/day for males, ≥4 females13,14) and alcohol use disorders are associated with multiple neurobiological and neurocognitive abnormalities.15,16 Nevertheless, few studies have specifically evaluated the influence of pre-injury hazardous alcohol consumption or alcohol abuse/dependence on post-TBI neurocognition or neurocognitive recovery.
In a study of TBI severity ranging from mild to severe, higher scores on the Short Michigan Alcohol Screening Test were associated with lower verbal intelligence and performance on a measure of set-shifting and visuomotor scanning 1 month post-injury and with lower verbal intelligence at 1 year post-injury.17 In another study of mild-to-severe TBI, no significant differences in neurocognition were observed 1 month post-injury between those who demonstrated hazardous pre-injury alcohol consumption or alcohol abuse/dependence and those without an alcohol use disorder.10 Given the mixed severities of patients with TBI of these studies, the effects of hazardous alcohol consumption on neurocognitive recovery following MTBI is essentially unknown.
Chronic cigarette smoking is also robustly associated with significant neurobiological and neurocognitive abnormalities in non-clinical cohorts and those with various neuropsychiatric conditions.16,18–20 In contrast to hazardous alcohol consumption, the prevalence of cigarette smoking in MTBI is unclear, and there are no published studies that have specifically assessed the potential effects of chronic smoking on neurocognitive recovery in MTBI.
The primary goal of this study was, therefore, to assess the influence of chronic smoking and hazardous alcohol consumption on changes of neurocognitive function over approximately 7 months after MTBI. We predicted that:
1. At baseline (approximately 5 weeks post-injury) and follow-up assessments (approximately 7 months post-injury), smoking persons with MTBI (sMTBI) perform worse than non-smoking persons with MTBI (nsMTBI) and non-smoking controls (CON) on the domains of executive skills, learning, and memory, processing speed, and working memory.
2. Between baseline and 7-month follow-up assessments, sMTBI show significantly lower rates of improvement on the above neurocognitive domains than nsMTBI.
3. In MTBI, greater smoking severity (i.e., pack-years and lifetime years of smoking) and greater drinking severity are related to poorer performance on the above neurocognitive domains at baseline and follow-up assessment, and to diminished recovery on these domains.
Methods
Participants
MTBI was defined as closed-head trauma with witnessed loss of consciousness (LOC) of less than 30 min, an initial Glasgow Coma Scale (GCS) score of 13–15 in the Emergency Department (ED), post-traumatic amnesia of less than 24 h, and no radiologic evidence of depressed skull fracture. Forty-four persons with MTBI (35±12 years of age) were enrolled prospectively (Table 1) from the ED at San Francisco General Hospital. Injury mechanisms involved bicycle/skateboard accidents (n=22, 50%), motor-vehicle-related accidents (n=6, 13.6%), falls (n=6, 13.6%), assaults (n=6, 13.6%), and pedestrian versus motor vehicle (n=4, 9%). Baseline assessment point (AP1) for MTBI participants (25 nsMTBI; 19 sMTBI) was 38±22 days after injury, and follow-up assessment point (AP2) was 230±36 days post-injury (22 nsMTBI, 17 sMTBI).
Table 1.
Group Demographic and Clinical Variables
| Variable | Controls (n=20) | Non-smoking MTBI (n=25) | Smoking MTBI (n=19) |
|---|---|---|---|
| Age (years) | 40.2 (9.4) | 34.6 (12.1) | 35.7 (10.9) |
| Education (years) | 16.0 (2.0) | 15.8 (2.4) | 15.2 (2.2) |
| Caucasian (%) | 70% | 72% | 74% |
| Male (%) | 74 | 76 | 72 |
| GCS 13/14/15 (%) | NA | 4/28/68 | 6/26/68 |
| Interval from injury to AP1 (days) | NA | 40 (22) | 36 (22) |
| Interval from injury to AP2 (days) | NA | 230 (37) | 231 (35) |
| Positive AP1 radiological findings (%) | NA | 52 | 63 |
| AMNART | 119 (8) | 119 (10) | 119 (7) |
| Beck Depression Inventory | 3.5 (3.4) | 7.0 (7.9) | 9.6 (6.7) |
| AUDIT-past-30-days | NA | 4.7 (4.7) | 8.7 (6.2) |
| AUDIT | 2.2 (1.0) | 8.0 (6.5) | 13.4 (7.2) |
| STAI-State | 30.0 (8.0) | 33.5 (9.7) | 35.2 (7.9) |
| STAI-Trait | 35.1 (8.2) | 36.4 (11.3) | 38.6 (8.5) |
| FTND | NA | NA | 3.5 (1.4) |
| Cigarettes/day | NA | NA | 11 (6) |
| Total lifetime years of smoking | NA | NA | 11.4 (7.5) |
| Pack years | NA | NA | 7.0 (7.4) |
MTBI, mild traumatic brain injury; GCS, Glasgow Coma Scale; AP1, assessment point 1; AP2, assessment point 2; AMNART, American National Adult Reading Test; AUDIT, Alcohol Use Disorders Identification Test; STAI, State-Trait Anxiety Inventory; FTND, Fagerstrom Test for Nicotine Dependence; Mean (standard deviation).
Twenty never-smoking CON (40±9 years of age) were recruited from the community. All CON were free of any biomedical or psychiatric conditions known to influence our dependent measures and were compared with nsMTBI and sMTBI on neurocognitive and clinical variables. Ten CON were reassessed on neurocognitive measures 255±55 days after their baseline assessment.
Two nsMTBI reported a single previous TBI with LOC (less than 5 min) that occurred approximately 20 years before the study. One nsMTBI reported daily marijuana use for approximately 10 consecutive years, but no marijuana or other substance use in the year before the study. One sMTBI reported almost daily methamphetamine use for approximately 3 consecutive years, which ended 5 years before the study, and no other substance use. All nsMTBI reported they never smoked cigarettes or did not smoke consistently during their lifetime (i.e., <50 cigarettes over lifetime and no tobacco use within 10 years of study); all sMTBI were actively smoking at AP1 and AP2, and their cigarette consumption was unchanged between assessment points. The MTBI participants reported no other biomedical or psychiatric conditions and no participant was involved in litigation, or had litigation pending, at any assessment point.
Medical, psychiatric, alcohol and substance use history
AP1
MTBI medical history was obtained from self-report and confirmed via available medical records. All participants were screened for mood, anxiety, and psychotic disorders via an adapted version of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-IV Axis I disorders, Patient Edition, Version 2.0 [SCID-I/P; 21]. All participants completed the Alcohol Use Disorder Identification Test (AUDIT),22 a version of the AUDIT modified to assess the past 30 days of alcohol consumption (AUDIT-past-30-days), and an in-house questionnaire assessing type, quantity, and frequency of substance use.23 The AUDIT is a widely used, cross-national, clinical and research measure used to identify hazardous/harmful alcohol use. AUDIT scores ≥8 are indicative of hazardous and harmful levels of alcohol consumption.22
All participants completed self-report measures of depressive (Beck Depression Inventory, BDI24) and anxiety symptomatology (State-Trait Anxiety Inventory, form Y-2, STAI 25) as well as nicotine dependence (Fagerstrom Tolerance Test for Nicotine Dependency [FTND]).26 The total number of cigarettes currently smoked per day and the number of years of smoking at the current level were also recorded, and pack years (i.e., number of cigarettes per day/20×number of years of smoking) were calculated for sMTBI (Table 1). All participants were reassessed with the AUDIT, AUDIT-past-30-days, BDI, STAI, and FTND again at AP2.
Neurocognitive assessment
At both assessment points, participants completed a comprehensive neurocognitive battery (approximately 2 h), which evaluated neurocognitive functions known to be adversely affected by MTBI,27 alcohol use disorders,28 and chronic cigarette smoking.29 sMTBI were allowed to smoke ad libitum before assessment and to take smoke breaks during assessments, if requested. At AP1 and AP2, the domains evaluated and the constituent measures were as follows: Executive skills: Short Categories Test,30 Stroop Test: Color-Word,31 Trail Making Test part B,32 Wechsler Adult Intelligence Scale 3rd Edition (WAIS-III) Similarities,33 Wisconsin Card Sorting Test-64: Computer Version 2-Research Edition (non-perseverative errors, perseverative errors, and perseverative responses). Learning and memory: Auditory-verbal: California Verbal Learning Test-II (CVLT-II),34 Immediate Recall trials 1–5 (learning), Short and Long Delay Free Recall (memory). Visuospatial: Brief Visuospatial Memory Test-Revised (BVMT-R),35 Total recall (learning) and delayed Recall (memory). Processing speed: WAIS-III Digit Symbol, Stroop Test-Color & Word,31 WAIS-III Symbol Search,33 Trail Making Test-A.32 Visuospatial skills: WAIS-III Block Design, Luria-Nebraska Item 99.36 Estimated premorbid verbal intelligence: American National Adult Reading Test (AMNART).37 Visuospatial skills: WAIS-III Block Design; Luria-Nebraska Item 99.36 Working memory: WAIS-III Arithmetic, WAIS-III Digit Span. Premorbid verbal intelligence was estimated with the AMNART.37
Raw scores for the BVMT-R, CVLT-II, Short Categories Test, Stroop Color–Word Test, and WAIS-III subtests were converted to age-adjusted standardized scores via the normative data accompanying each test. WCST-64 variables and Trails A and B were converted to age-and-education adjusted standardized scores via Heaton Compendium Norms.38 Standardized scores were then converted to z-scores for all measures. The score of domains with multiple measures represents the arithmetic mean of the individual z-scores of the constituent measures. A global neurocognitive score was calculated from the arithmetic mean of z-scores for all of the individual domains.
Magnetic resonance imaging
As part of this longitudinal study, all MTBI participants completed structural magnetic resonance imaging involving three-dimensional T1- and T2-weighted imaging and fluid attenuated inversion recovery (FLAIR) sequences. For this report, any MTBI participant, who demonstrated evidence of contusion or diffuse axonal injury on FLAIR at AP1 (defined as focal signal abnormalities in the cortex, white matter or gray/white matter boundary), was considered to have a positive radiological finding. FLAIR images were independently evaluated by two of the authors (GEG and TCD) with formal training and extensive experience in reading clinical and research MRIs of MTBI; there was 100% agreement between raters on the presence or absence of focal signal hyperintensities. The purpose of indentifying those with positive radiological findings was to determine if nsMTBI and sMTBI differed on the frequency of macroscopic structural indicators of brain trauma (e.g., contusion). Previous studies reported that those with positive neuroradiological findings in the acute/sub-acute stage (i.e., “complicated” MTBI) may have an increased risk for delayed or incomplete recovery after MTBI.9 Results from morphological comparisons of these groups will be detailed elsewhere.
Data analyses
Cross-sectional
The Fisher exact test or pairwise t tests, where appropriate, assessed for group differences on demographic and clinical variables. Multivariate analyses of covariance (MANCOVA) examined effects of group (CON, non-smoking MTBI, and smoking MTBI) at AP1 and AP2 on the nine domains of neurocognition (Table 2); education, AMNART, and AUDIT scores served as primary covariates in cross-sectional analyses at AP1 and AP2. BDI, STAI were entered as covariates in secondary analyses. AP1 domain scores for CON were used in group comparisons at AP2, because only 10 CON were reassessed AP2, and CON showed no significant changes in any domain between AP1 and AP2. There were no significant differences among nsMTBI, sMTBI, and CON on education or AMNART. Nevertheless, because AMNART was a significant predictor of several neurocognitive domains in our previous work examining the influence of chronic smoking on neurocognition in alcohol use disorders,39,40 we specifically examined if years of education and estimated premorbid verbal intellectual ability were significant predictors of performance in all group comparisons.
Table 2.
Group Performance on Neurocognitive Domains
| Domain | Group | AP1 mean (SD) | AP2 mean (SD) | AP1-AP2 % change | p value AP1-AP2 change | AP1 effect size (Cohen's d) | AP2 effect size (Cohen's d) |
|---|---|---|---|---|---|---|---|
| Auditory-verbal learning | CON | 0.98 (0.96) | 1.02 (1.02) | 3.6 | NS | CON vs. nsMTBI: 0.80 | CON vs. nsMTBI: 0.34 |
| nsMTBI | 0.23 (0.92) | 0.64 (0.96) | 7.8 | 0.04 | CON vs. sMTBI: 0.69 | CON vs. sMTBI: 0.74 | |
| sMTBI | 0.32 (0.96) | 0.28 (0.93) | −0.8 | NS | nsMTBI vs. sMTBI: 0.10 | nsMTBI vs. sMTBI: 0.39 | |
| Auditory-verbal memory | CON | 0.60 (0.89) | 0.63 (0.95) | 4.9 | NS | CON vs. nsMTBI: 0.89 | CON vs. nsMTBI: 0.36 |
| nsMTBI | −0.20 (0.92) | 0.27 (0.97) | 9.8 | 0.03 | CON vs. sMTBI: 0.82 | CON vs. sMTBI: 0.70 | |
| sMTBI | −0.15 (0.92) | −0.08 (0.97) | 1.5 | NS | nsMTBI vs. sMTBI: 0.10 | nsMTBI vs. sMTBI: 0.36 | |
| Executive skills | CON | 0.07 (0.67) | 0.07 (0.72) | 3.3 | NS | CON vs. nsMTBI: 0.15 | CON vs. nsMTBI: 0.12 |
| nsMTBI | −0.03 (0.68) | 0.15 (0.63) | 3.6 | 0.08 | CON vs. sMTBI: 0.53 | CON vs. sMTBI: 0.02 | |
| sMTBI | −0.28 (0.67) | 0.08 (0.62) | 7.8 | 0.01 | nsMTBI vs. sMTBI: 0.38 | nsMTBI vs. sMTBI: 0.11 | |
| Processing speed | CON | 0.17 (0.63) | 0.17 (0.69) | 0.3 | NS | CON vs. nsMTBI: 0.66 | CON vs. nsMTBI: 0.05 |
| nsMTBI | −0.25 (0.65) | 0.14 (0.67) | 8.2 | <0.01 | CON vs. sMTBI: 0.47 | CON vs. sMTBI: 0.33 | |
| sMTBI | −0.13 (0.63) | −0.05 (0.67) | 1.6 | NS | nsMTBI vs. sMTBI: 0.19 | nsMTBI vs. sMTBI: 0.28 | |
| Visuospatial learning | CON | 0.04 (0.94) | 0.04 (0.99) | 1.3 | NS | CON vs. nsMTBI: 0.44 | CON vs. nsMTBI: 0.36 |
| nsMTBI | −0.40 (1.01) | 0.48 (0.74) | 19.2 | <0.01 | CON vs. sMTBI: 0.02 | CON vs. sMTBI: 0.01 | |
| sMTBI | 0.06 (1.01) | 0.05 (0.74) | −0.3 | NS | nsMTBI vs. sMTBI: 0.46 | nsMTBI vs. sMTBI: 0.59 | |
| Visuospatial memory | CON | 0.15 (1.07) | 0.15 (1.12) | 1.9 | NS | CON vs. nsMTBI: 0.49 | CON vs. nsMTBI: 0.32 |
| nsMTBI | −0.39 (1.08) | 0.43 (0.72) | 18.0 | 0.01 | CON vs. sMTBI: 0.23 | CON vs. sMTBI: 0.31 | |
| sMTBI | 0.40 (1.09) | 0.38 (0.72) | −0.4 | NS | nsMTBI vs. sMTBI: 0.72 | nsMTBI vs. sMTBI: 0.08 | |
| Visuospatial skills | CON | 0.28 (0.94) | 0.29 (1.00) | 4.5 | NS | CON vs. nsMTBI: 0.23 | CON vs. nsMTBI: 0.05 |
| nsMTBI | 0.06 (0.94) | 0.44 (0.94) | 7.4 | 0.05 | CON vs. sMTBI: 0.21 | CON vs. sMTBI: 0.33 | |
| sMTBI | 0.47 (0.95) | 0.44 (0.95) | −0.6 | NS | nsMTBI vs. sMTBI: 0.44 | nsMTBI vs. sMTBI: 0.28 | |
| Working memory | CON | 0.53 (0.74) | 0.53 (0.79) | 2.0 | NS | CON vs. nsMTBI: 0.37 | CON vs. nsMTBI: 0.05 |
| nsMTBI | 0.25 (0.73) | 0.49 (0.75) | 4.6 | NS | CON vs. sMTBI: 0.71 | CON vs. sMTBI: 0.33 | |
| sMTBI | 0.00 (0.74) | 0.15 (0.75) | 2.9 | NS | nsMTBI vs. sMTBI: 0.34 | nsMTBI vs. sMTBI: 0.28 | |
| Global Neuro-cognition | CON | 0.35 (0.56) | 0.36 (0.59) | 1.9 | NS | CON vs. nsMTBI: 0.85 | CON vs. nsMTBI: 0.12 |
| nsMTBI | −0.12 (0.55) | 0.39 (0.46) | 11.0 | <0.01 | CON vs. sMTBI: 0.47 | CON vs. sMTBI: 0.42 | |
| sMTBI | 0.09 (0.54) | 0.16 (0.46) | 1.3 | NS | nsMTBI vs. sMTBI: 0.37 | nsMTBI vs. sMTBI: 0.55 |
AP1, assessment point 1; AP2, assessment point 2; CON, non-smoking controls; nsMTBI, non-smoking mild traumatic brain-injured participants; sMTBI, non-smoking mild traumatic brain-injured participants; p values>0.10 listed as not significant (NS).
Significant MANCOVA omnibus effects (p<0.05) for group were followed up with pairwise t tests among nsMTBI, sMTBI, and CON. Although we had specific a priori predictions, all group pairwise comparisons at AP1 and AP2 were corrected for multiplicity of tests. Significance levels (p=0.05) for pairwise comparisons for each neurocognitive domain were adjusted according to the number of neurocognitive domains (i.e., nine) and the average intercorrelation among the domains for all groups (i.e., r=0.54), resulting in a corrected p value=0.019.41 Effect sizes (ES) for pairwise comparisons at each AP were calculated via Cohen's d.42 Associations between AUDIT and AUDIT-past-30-days were evaluated with bivariate correlations (Spearman Rho). For sMTBI, associations between the nine neurocognitive domains, FTND score, cigarettes smoked per day, pack-years, and lifetime years of smoking were examined with multiple linear regression (semi-partial coefficients are reported), controlling for AUDIT or AUDIT-past-30-days.
Longitudinal
Comparisons of longitudinal change between nsMTBI and sMTBI on neurocognitive domains across the AP1-AP2 interval were conducted with linear mixed modeling. Education, AMNART, and AUDIT scores served as primary covariates. BDI and STAI scores were entered as covariates in secondary analyses. Main effects and interactions were considered to be statistically significant at p<0.05. Paired t tests for nsMTBI and sMTBI were corrected for multiple comparisons as described in the Cross-Sectional section. Changes between AP1 and AP2 in AUDIT, AUDIT-past-30-days, BDI, and STAI for nsMTBI and sMTBI were also evaluated with linear mixed models. Ten CON were reassessed on neurocognitive measures 255±55 days after their baseline assessment. Paired t tests for CON were corrected for multiple comparisons as described in the above Cross-Sectional section. Statistical analyses were completed with SPSS v 19.0.
Results
Participant demographic and clinical variables
Forty-three percent of MTBI participants were chronic smokers. sMTBI and nsMTBI participants were younger than CON (both p<0.05). At AP1, sMTBI had higher BDI than CON. sMTBI and nsMTBI showed higher AUDIT than CON; sMTBI had a higher AUDIT-past-30-days than nsMTBI. Groups were equivalent on education, AMNART score, ethnic frequency, and STAI-state-and-trait scores. The foregoing findings were identical at AP2. nsMTBI and sMTBI were not significantly different on the interval from injury to AP1 or AP2, on the frequency of GCS scores (13/14/15), or on the proportion of positive radiological findings at AP1 (Table 1).
AP1 Cross-sectional comparisons
MANCOVA yielded a significant effect for group on the following measures: visuospatial memory (F[2, 60]=3.20, p=0.049), auditory-verbal learning (F[2, 60]=3.63, p=0.033), auditory-verbal memory (F[2, 60]=5.00, p=0.010), and global neurocognition (F[2, 60]=3.99, p=0.024), with trends for processing speed (p=0.081), and working memory (p=0.096). Planned pairwise comparisons indicated that nsMTBI and sMTBI were inferior to CON on auditory-verbal learning and auditory-verbal memory (both p<0.019). nsMTBI performed more poorly than CON on processing speed and global neurocognition (both p≤.0019), and worse than sMTBI on visuospatial memory (p=0.019). sMTBI performed worse than CON on working memory (p<0.019). AMNART was a significant predictor of all domains (all p<0.024), where higher AMNART scores were associated with better performance. Education, AUDIT, AUDIT-past-30-days, and measures of depressive (i.e., BDI) and anxiety (i.e., STAI State and Trait) symptomatology were not significant predictors of any domain at AP1, and did not alter the differences among CON, nsMTBI, and sMTBI reported above. The performance of persons with previous MTBI and premorbid heavy substance use were within±0.5 standard deviation of the MTBI group mean on all domains.
Longitudinal comparison
nsMTBI vs. sMTBI
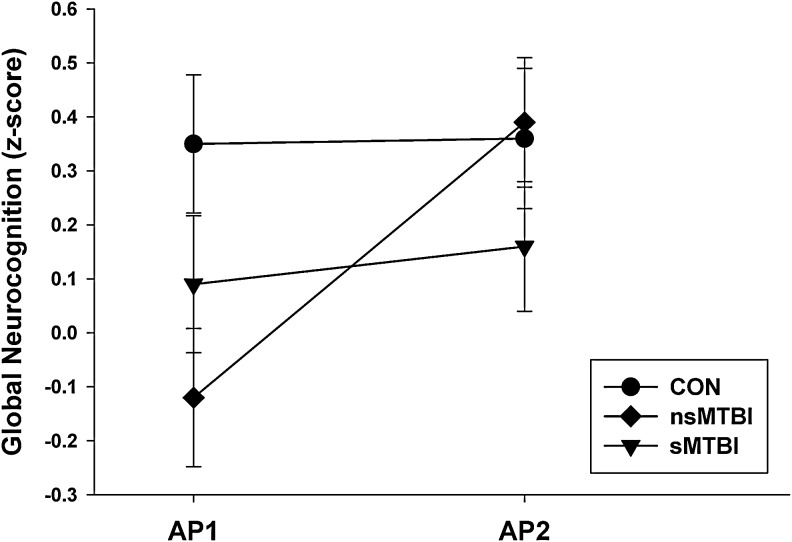
Significant group (nsMTBI, sMTBI)×AP (AP1, AP2) interactions were observed for the following domains: processing speed (F[1, 34]=5.66, p=0.023), visuospatial learning (F[1, 37]=6.55, p=0.015), visuospatial memory (F[1, 34]=4.23, p=0.048), visuospatial skills (F[1, 48]=4.81, p=0.033), and global neurocognition (F[1, 32]=5.47, p=0.026). nsMTBI showed significant improvement on processing speed, visuospatial learning, visuospatial memory, and global neurocognition over the AP1-AP2 interval (all p<0.019), with trends for improved visuospatial skills (p=0.044). sMTBI showed no significant improvement on any of the above domains (all p>0.10). These findings indicate the group×AP interactions were driven by significant improvements in nsMTBI. See Figure 1 for the general pattern of change demonstrated by groups.
FIG. 1.
Longitudinal change in global neurocognition. AP1, assessment point 1; AP2, assessment point 2; CON, controls; nsMTBI, non-smoking mild traumatic brain-injured participants; sMTBI, smoking mild traumatic brain-injured participants.
Main effects for AP were seen for executive skills (p<0.019), where sMTBI showed significant improvement (p<0.019) and nsMTBI demonstrated a trend for improvement (p=0.05). No main effects for AP were apparent for auditory-verbal learning, auditory-verbal memory, or working memory, indicating the MTBI group, as a whole, did not show statistically significant recovery on these domains over the AP1-AP2 interval. nsMTBI, however, showed trends for improvement on auditory-verbal learning (p=0.04) and auditory-verbal memory (p=0.03). AMNART was a significant predictor of all domains (all p<0.019), where higher AMNART scores were associated with greater improvement in performance. AUDIT-past-30-days showed trends for associations with executive skills (p=0.056) and auditory-verbal learning (both p=0.071), where higher scores were related to less improvement on these domains. AUDIT and measures of depressive and anxiety symptomatology were not significant predictors of change for any neurocognitive domain over the AP1-AP2 interval.
There were no significant changes observed in the MTBI groups for AUDIT, AUDIT-past-30-days, BDI, and STAI (State and Trait). The FTND and cigarettes smoked per day in sMTBI did not change significantly from AP1 to AP2.
CON
No significant changes were observed for CON (n=10) on any domain (all p>0.20), reflecting generally stable neurocognitive performance in this small subsample across the AP1-AP2 interval (see Table 2).
AP2 Cross-sectional comparisons
MANCOVA yielded trends for group main effect for auditory-verbal learning (F[1, 50]=2.44, p=0.097] and auditory-verbal memory (F[1, 50]=2.43, p=0.097]. Planned pairwise comparisons showed sMTBI were inferior to CON on auditory-verbal learning and auditory-verbal memory (both p<0.019). There were no significant differences between nsMTBI and CON or among nsMTBI and sMTBI on any domain at AP2. AMNART was a significant predictor of all domains (all p<0.036), where higher AMNART scores were related to better performance. AUDIT and AUDIT-past-30-days depressive (i.e., BDI) and anxiety symptomatology (i.e., STAI State and Trait) were not significant predictors of any domain at AP2.
Associations of hazardous/harmful alcohol consumption, and smoking severity with cross-sectional and longitudinal neurocognitive measures
Cross-sectional
At AP1, higher AUDIT-past-30-days score was related to poorer executive skills (Rho=−0.39; p=0.009) in the whole MTBI cohort (i.e., nsMTBI and sMTBI combined); nsMTBI and sMTBI individually showed a similar magnitude of correlation. At AP2, AUDIT scores did not correlate significantly with neurocognition. For sMTBI, at AP1 and AP2, greater lifetime duration of smoking and pack-years (controlled for AUDIT or AUDIT-past-30-days score) were associated with poorer performance on visuospatial learning, visuospatial memory, visuospatial skills, working memory, and global neurocognition; these relationships were moderate to strong in magnitude (Table 3). Level of nicotine dependence (i.e., FTND score) at AP1 or AP2 was not significantly related to neurocognition.
Table 3.
Relationships Between Measures of Smoking Severity and Performance on Neurocognitive Domains for sMTBI at Assessment Point 1 and Assessment Point 2
| Smoking severity measure | Domain | AP | r* | p value |
|---|---|---|---|---|
| Lifetime duration of smoking (years) | Visuospatial learning | 1 | −0.71 | <0.01 |
| 2 | −0.50 | 0.02 | ||
| Visuospatial memory | 1 | −0.52 | 0.01 | |
| 2 | −0.44 | 0.04 | ||
| Visuospatial skills | 1 | −0.55 | 0.01 | |
| 2 | −0.53 | 0.02 | ||
| Working memory | 1 | −0.48 | 0.02 | |
| 2 | −0.34 | 0.10 | ||
| Global neurocognition | 1 | −0.51 | 0.02 | |
| 2 | −0.59 | <0.01 | ||
| Pack-years | Visuospatial learning | 1 | −0.55 | <0.01 |
| 2 | −0.55 | 0.01 | ||
| Visuospatial Memory | 1 | −0.37 | 0.06 | |
| 2 | −0.46 | 0.04 | ||
| Visuospatial skills | 1 | −0.44 | 0.03 | |
| 2 | −0.43 | 0.04 | ||
| Working memory | 1 | −0.51 | 0.02 | |
| 2 | −0.43 | 0.04 | ||
| Global neurocognition | 1 | −0.37 | 0.06 | |
| 2 | −0.56 | 0.01 | ||
| Cigarettes smoked/day | Working memory | 1 | −0.48 | 0.02 |
| 2 | −0.47 | 0.03 |
Semi-partial correlation coefficients controlling for AUDIT; AP: assessment point; p<.05 is considered statistically significant.
Longitudinal
In sMTBI, over the 7-month interval, greater lifetime duration of smoking (controlled for AUDIT scores) was related to significantly less improvement on visuospatial learning (β=− 0.10, standard error (SE)=0.02, p<0.001), visuospatial memory (β=− 0.07, SE=0.02, p<0.001), working memory (β=−0.08, SE=0.03, p<0.01), visuospatial skills (β=−0.08, SE=0.03, p<0.001), and global cognition (β=−0.05, SE=0.02, p<0.016). Higher pack years (controlled for AUDIT score) was related to less improvement on visuospatial learning (β=−0.09, SE=0.03, p<0.004), and visuospatial memory (β=−0.05, SE=0.03, p<0.033). Virtually identical results were observed when these associations were controlled for AUDIT-past-30-days. Again, level of nicotine dependence was not related to change on any neurocognitive domain.
Discussion
The primary findings in this longitudinal study of neurocognitive changes over approximately 7 months in MTBI persons were as follows: (1) At AP1 (approximately 38 days post-injury), nsMTBI and sMTBI were inferior to CON on measures of auditory-verbal learning and auditory-verbal memory; nsMTBI performed more poorly than CON on processing speed and global neurocognition and sMTBI performed poorer than CON on working memory; nsMTBI were inferior to sMTBI on visuospatial memory. Moderate to strong effect sizes were apparent for the above group differences. (2) Over the AP1-AP2 interval, nsMTBI showed significantly greater improvement than sMTBI on measures of processing speed, visuospatial learning and memory, visuospatial skills, and global neurocognition, whereas CON (n=10) showed no significant changes on any neurocognitive domain over the AP1-AP2 interval. (3) At AP2 (approximately 7 months post-injury), sMTBI remained inferior to CON on auditory-verbal learning and auditory-verbal memory, and these group differences showed moderate effect sizes; there were no significant differences between nsMTBI and CON or among nsMTBI or sMTBI on any domain at AP2. (4) For sMTBI, greater lifetime duration of smoking and pack-years were related to significantly less improvement on multiple domains over the AP1-AP2 interval; similarly, at AP1 and AP2, greater lifetime duration of smoking and pack-years were associated with poorer performance on multiple neurocognitive domains. (5) Forty-three percent of the MTBI sample were chronic smokers; 59% of MTBI participants demonstrated hazardous/harmful levels of alcohol consumption at AP1, and 62% at AP2.
The most clinically relevant findings were the significantly greater recovery shown by nsMTBI compared with sMTBI on measures of processing speed, visuospatial learning and memory, visuospatial skills, and global neurocognition. The differential recovery demonstrated by these groups was not mediated by education, estimated premorbid verbal intelligence (i.e., AMNART), hazardous alcohol consumption (i.e., AUDIT scores), or depressive (i.e., BDI) and anxiety (i.e., STAI) symptomatology. Further, nsMTBI and sMTBI did not differ on the frequency of positive radiological findings or GCS scores at AP1, indicating the greater improvements shown by nsMTBI across the AP1-AP2 interval were not attributable to baseline group differences in macrostructural indicators of injury severity. nsMTBI showed steep improvement on processing speed, visuospatial learning and memory, visuospatial skills, and global neurocognition, with trends for improvement on auditory-verbal learning and memory. The percent change on these domains demonstrated by nsMTBI were substantially larger than CON, suggesting the increases exhibited by nsMTBI were beyond measurement error and/or practice effects. The modest-sized group of CON with follow-up assessment evidenced no significant changes in any domain over the AP1-AP2 interval. sMTBI, essentially showed a flat trajectory over the 7-month interval for all domains, except for improved executive skills. The overall pattern exhibited by nsMTBI and sMTBI over the AP1-AP2 interval indicates that chronic smoking in this cohort of MTBI was associated with significantly diminished recovery in multiple neurocognitive domains over approximately 7 months post-injury.
The group differences observed at AP1 and AP2 were not influenced by education, estimated premorbid intelligence, hazardous alcohol consumption, or depressive and anxiety symptomatology. Although, nsMTBI and sMTBI performed worse than CON on the auditory-verbal learning and auditory-verbal memory domains at AP1, the performance of the MTBI groups was in the average range of function (42nd–58th percentile). Similarly, the performance of nsMTBI and sMTBI on executive skills, processing speed, visuospatial learning and memory, working memory, and global neurocognition at AP1 were in the average range (34th–66th percentile). CON performed in the average range on all domains (50th–73rd percentile), except auditory-verbal learning, which was in the high average (84th percentile) range of function. At AP2, sMTBI continued to perform significantly worse than CON on auditory-verbal learning and memory. Alternately, auditory-verbal learning and memory in nsMTBI improved over the AP1-AP2 interval, and nsMTBI showed no significant differences from CON on any domain at AP2. There were no significant differences between nsMTBI and sMTBI on any domain at AP2, and, similar to AP1, the performance of both these groups was in the average range of functioning (46th–74th percentile) on all domains at AP2. The overall results for MTBI at AP2 are consistent with previous research indicating that the acute/sub-acute neurocognitive sequelae of MTBI are largely resolved after approximately 3 months post-injury.4
For sMTBI, greater lifetime duration of smoking was related to less improvement on the domains of visuospatial learning and visuospatial memory, visuospatial skills, working memory, and global neurocognition, and greater pack-years was inversely associated with improvement on visuospatial learning and memory and visuospatial skills. At AP1 and AP2, greater lifetime duration of smoking and pack-years were moderately-to-strongly related to poorer performance on the domains of visuospatial learning and visuospatial memory, visuospatial skills, working memory, and global neurocognition. These findings suggest that greater smoking chronicity over lifetime in sMTBI, but not level of nicotine dependence (as measured with the FTND), was robustly associated with poorer neurocognitive recovery and lower performance on multiple measures at AP1 and AP2.
Previous research with normal controls showed greater duration of smoking over lifetime and/or dose-duration (i.e., pack-years) were inversely related to multiple domains of neurocognition in adults across a wide age range.29 The current findings also show remarkable similarity to our studies with smoking alcohol dependent persons, where longer duration of smoking over lifetime was associated with poorer performance on measures of processing speed, visuospatial skills, and visuospatial learning and memory,39 as well as with decreased neurocognitive recovery over approximately 8 months of abstinence from alcohol.43
The elevated AUDIT scores in the MTBI cohorts underscore the importance of assessing for pre-injury hazardous/harmful alcohol consumption given that across the spectrum of TBI severity, 50–70% of cases have a history of heavy alcohol use or abuse/dependence.12 Pre-injury alcohol consumption and related problems are strongly associated with post-injury use and problems,44 and persons with lower severity TBI tend to show similar levels of alcohol consumption over 1 year post-injury,6 which is consistent with the findings from this report. In addition, chronic cigarette smoking is associated with significantly higher quantity and frequency of alcohol consumption.45 It is important to note that, despite mean AUDIT scores for both nsMTBI and sMTBI that were indicative of a hazardous level of alcohol consumption, none of the participants in the current study reported pre- or-post-injury treatment for an alcohol use disorder.
There are several potential mechanisms that may operate independently or concurrently to promote the diminished neurocognitive improvement and the associations of smoking severity measures and neurocognition in sMTBI. Nicotine is only one of more than 4000 compounds composing the particulate and gas phases of cigarette smoke.46–48 While nicotine underlies the addictive properties of tobacco,49–53 the adverse effects of chronic smoking appear to be related to the persistent exposure of multiple peripheral organ systems and the brain to the toxic combustion products in cigarette smoke.16, 54–57 The multitude of potentially cytotoxic compounds in cigarette smoke (e.g., carbon monoxide, aldehydes, ketones, nitrosamines, dihydroxybenzenes)56 may directly compromise neuronal and cellular membrane function of cerebral tissue. The gas and particulate phases of cigarette smoke contain high levels of reactive oxygen species (ROS), reactive nitrogen species (RNS) and oxidizing agents (e.g., hydrogen peroxide), and these compounds in the particulate phase are long-lived (hours to weeks).48,58
Smoking increases plasma carbon monoxide levels59 and alters mitochondrial respiratory chain function,60 both of which increase oxidative stress. Chronic smoking induces nuclear factor-κB (NF-κB), resulting in amplified cerebral proinflammatory cytokine levels and radical generation by peripheral and central nervous system glial cells.61 In vivo chronic cigarette smoke exposure is also associated with decreased concentrations of enzyme-based free radical scavengers (e.g., superoxide dismutase, catalase, glutathione reductase) and non-enzyme–based radical scavengers (e.g., glutathione and vitamins A, C, and E) concentrations in animal models 62,63 and humans.64–69 This may render brain tissue more vulnerable to oxidative damage by radical species generated by normal cellular metabolism or other exogenous sources (e.g., increased oxidative stress secondary to TBI). The brain, in general, is highly susceptible to oxidative damage because of high levels of unsaturated fatty acids in the composition of cell membranes and myelin. Thus, chronic smoking may promote neuronal/glial injury and neurodegeneration in the human brain via increased oxidative stress. The entire range of TBI severity is associated with increased oxidative stress during the acute and sub-acute post-injury phases.3,70,71
We postulate that smoking in MTBI provides a significant and sustained direct source of exogenous free radical species and other oxidizing agents. Therefore, continued smoking after injury will increase cerebral oxidative stress, which may hinder neurobiological recovery, and, by extension, adversely affect neurocognitive recovery in sMTBI. For further discussion of potential biological mechanisms contributing to smoking-related neurocognitive dysfunction, see articles by Durazzo and colleagues.16,29
This study has limitations that may affect the generalizability of the findings. The sample size for this report was modest and did not provide sufficient numbers of participants to evaluate for sex differences. TBI participants were assessed 38±22 after injury at AP1. Given that the greatest neurocognitive dysfunction is typically apparent during the initial 14 days after injury in MTBI,9 a significant amount of recovery across domains may have occurred by AP1. Consequently, the actual magnitudes of change since the time of injury observed at AP2 may be underestimated for both TBI groups. A smoking control group was not included; therefore, it is not known if chronic smoking was independently associated with neurocognition in cross-sectional analyses and longitudinal analyses. Although all MTBI participants were screened for major psychiatric syndromes, and measures of mood and anxiety symptomatology were not significantly different between nsMTBI and sMTBI, participants did not complete a full standardized structured diagnostic interview for DSM-IV Axis I disorders. In addition, we did not assess for potential group differences in nutrition, exercise, and genetic predispositions, which may have influenced performance on the neurocognitive domains evaluated in this study.
Conclusions
Chronic cigarette smoking in this cohort of MTBI participants, but not hazardous/harmful alcohol consumption, was associated with diminished recovery on multiple neurocognitive domains, and persistently poorer performance on measures of auditory-verbal leaning and auditory-verbal memory at 7 months post-injury. In addition, greater smoking severity in sMTBI was related to both poorer recovery and performance at baseline and follow-up on multiple domains. It is recommended that future studies include a group of smoking CON to specifically assess for the additive and/or synergistic effects of smoking status on longitudinal recovery in MTBI. In addition, inclusion of alcohol quantity and frequency measures (e.g., Lifetime Drinking History72) is necessary to better understand the influence of hazardous/harmful alcohol consumption on neurocognitive recovery in MTBI.
Comparisons of nsMTBI and sMTBI (with the appropriate non-smoking and smoking CON) on diffusion tensor imaging measures, susceptibility-weighted imaging, regional brain metabolite makers of oxidative stress and neuronal integrity, and cerebral perfusion can assist with identification of the neurobiological correlates of the smoking-related neurocognitive findings observed in this study.
Chronic smoking and hazardous alcohol consumption are modifiable health risks, representing the first and third leading causes, respectively, of preventable mortality in the United States.73,74 A better understanding of their potential influence on neurocognitive and neurobiological recovery from MTBI may inform the design of targeted pharmacological (prophylaxis and/or acute treatment with antioxidants and anti-inflammatory agents) and behavioral (i.e., smoking cessation programs, treatment of hazardous drinking levels) interventions to facilitate maximum rate and magnitude of recovery after MTBI.
Future research to assess the effects of smoking cessation on neurocognitive recovery after MTBI is clearly warranted. A greater understanding of the factors that influence the neurobiological and neurocognitive recovery after MTBI is also of critical importance to the U.S. Armed Services to support informed decisions in theater regarding the optimal timing for return to duty after MTBI, particularly for persons involved in combat operations.
Acknowledgments
This material is the result of work supported by the Department of Defense (W81XWH-05-2-0094 to TCD and GEG), National Institute on Drug Abuse (DA24136 to TCD), National Institute on Alcohol and Alcoholism (AA10788 to Dieter J. Meyerhoff), and with resources and the use of facilities at the San Francisco Veterans Affairs Medical Center, San Francisco, CA. We thank Dr. Geoffrey Manley and Michelle Meeker for invaluable assistance in recruitment, Hana Lee for assistance with neurocognitive assessment, and Dr. Dieter J. Meyerhoff for providing a portion of the neurocognitive data for control participants. We also wish to extend our gratitude to the study participants, who made this research possible.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Laker S.R. Epidemiology of concussion and mild traumatic brain injury. PM R. 2011;3(Suppl 2):S354–S358. doi: 10.1016/j.pmrj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy J.D. Carroll L.J. Peloso P.M. Borg J. von Holst H. Holm L. Kraus J. Coronado V.G. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004;(Suppl 43):28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- 3.Petraglia A.L. Maroon J.C. Bailes J.E. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70:1520–1533. doi: 10.1227/NEU.0b013e31824cebe8. [DOI] [PubMed] [Google Scholar]
- 4.Carroll L.J. Cassidy J.D. Peloso P.M. Borg J. von Holst H. Holm L. Paniak C. Pepin M. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004;(Suppl 43):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 5.Dikmen S.S. Corrigan J.D. Levin H.S. Machamer J. Stiers W. Weisskopf M.G. Cognitive outcome following traumatic brain injury. J. Head Trauma Rehabil. 2009;24:430–438. doi: 10.1097/HTR.0b013e3181c133e9. [DOI] [PubMed] [Google Scholar]
- 6.Dikmen S.S. Machamer J.E. Donovan D.M. Winn H.R. Temkin N.R. Alcohol use before and after traumatic head injury. Ann. Emerg. Med. 1995;26:167–176. doi: 10.1016/s0196-0644(95)70147-8. [DOI] [PubMed] [Google Scholar]
- 7.Robertson E., Jr. Rath B. Fournet G. Zelhart P. Estes R. Assessment of mild brain trauma: a preliminary study of the influence of premorbid factors. Lisse, PAYS-BAS; Swets & Zeitlinger: 1994. [Google Scholar]
- 8.Kay T. Newman B. Cavallo M. Ezrachi O. Resnick M. Toward a neuropsychological model of functional disability after mild traumatic brain injury. Neuropsychology. 1992;6:371–384. [Google Scholar]
- 9.McCrea M. Iverson G.L. McAllister T.W. Hammeke T.A. Powell M.R. Barr W.B. Kelly J.P. An integrated review of recovery after mild traumatic brain injury (MTBI): implications for clinical management. Clin. Neuropsychol. 2009;23:1368–1390. doi: 10.1080/13854040903074652. [DOI] [PubMed] [Google Scholar]
- 10.Jorge R.E. Starkstein S.E. Arndt S. Moser D. Crespo-Facorro B. Robinson R.G. Alcohol misuse and mood disorders following traumatic brain injury. Arch. Gen. Psychiatry. 2005;62:742–749. doi: 10.1001/archpsyc.62.7.742. [DOI] [PubMed] [Google Scholar]
- 11.Graham D.P. Cardon A.L. An update on substance use and treatment following traumatic brain injury. Ann. N. Y. Acad. Sci. 2008;1141:148–162. doi: 10.1196/annals.1441.029. [DOI] [PubMed] [Google Scholar]
- 12.Vickery C.D. Sherer M. Nick T.G. Nakase-Richardson R. Corrigan J.D. Hammond F. Macciocchi S. Ripley D.L. Sander A. Relationships among premorbid alcohol use, acute intoxication, and early functional status after traumatic brain injury. Arch. Phys. Med. Rehabil. 2008;89:48–55. doi: 10.1016/j.apmr.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 13.Mertens J.R. Weisner C. Ray G.T. Fireman B. Walsh K. Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol Clin. Exp. Res. 2005;29:989–998. doi: 10.1097/01.alc.0000167958.68586.3d. [DOI] [PubMed] [Google Scholar]
- 14.McKee S.A. Falba T. O'Malley S.S. Sindelar J. O'Connor P.G. Smoking status as a clinical indicator for alcohol misuse in US adults. Arch. Intern. Med. 2007;167:716–721. doi: 10.1001/archinte.167.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyerhoff D.J. Durazzo T.C. Ende G. Chronic alcohol consumption, abstinence and relapse: brain proton magnetic resonance spectroscopy studies in animals and humans. Curr. Top. Behav. Neurosci. 2013;13:511–540. doi: 10.1007/7854_2011_131. [DOI] [PubMed] [Google Scholar]
- 16.Durazzo T.C. Meyerhoff D.J. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front. Biosci. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- 17.Dikmen S.S. Donovan D.M. Loberg T. Machamer J.E. Temkin N.R. Alcohol use and its effects on neuropsychological outcome in head injury. Neuropsychology. 1993;7:296–305. [Google Scholar]
- 18.Durazzo T.C. Meyerhoff D.J. Nixon S.J. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 2012;122:105–111. doi: 10.1016/j.drugalcdep.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durazzo T. Insel P.S. Weiner M.W the Alzheimer Disease Neuroimaging Initiative, A. Greater regional brain atrophy rate in healthy elderly subjects with a history of cigarette smoking. Alzheimers Dement. 2012;8:513–519. doi: 10.1016/j.jalz.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azizian A. Monterosso J. O'Neill J. London E.D. Magnetic resonance imaging studies of cigarette smoking, in: Nicotine Psychopharmacology. Springer-Verlag Berlin Heidelberg. 2009:113–143. doi: 10.1007/978-3-540-69248-5_5. [DOI] [PubMed] [Google Scholar]
- 21.First M.B. Spitzer R.L. Gibbon M. Williams J.B. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0, 8/98 revision) Biometrics Research Department; New York: 1998. [Google Scholar]
- 22.Babor T.F. Higgins-Biddle J.C. Saunders J.B. Monteiro M.G. The Alcohol Use Disorders Identification Test (AUDIT): Guidelines for Use in Primary Care. 2nd. World Health Organization; Geneva: 2001. 2001. [Google Scholar]
- 23.Durazzo T.C. Gazdzinski S. Banys P. Meyerhoff D.J. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin. Exp. Res. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT. Depression Inventory. Center for Cognitive Therapy; Philadelphia: 1978. [Google Scholar]
- 25.Spielberger C.D. Gorsuch R.L. Lushene R. Vagg P.R. Jacobs G.A. Self-Evaluation Questionnaire. Consulting Psychologists Press; Palo Alto,CA: 1977. [Google Scholar]
- 26.Fagerström K.O. Heatherton T.F. Kozlowski L.T. Nicotine addiction and its assessment. Ear Nose Throat J. 1991;69:763–765. [PubMed] [Google Scholar]
- 27.Wilde E.A. Whiteneck G.G. Bogner J. Bushnik T. Cifu D.X. Dikmen S. French L. Giacino J.T. Hart T. Malec J.F. Millis S.R. Novack T.A. Sherer M. Tulsky D.S. Vanderploeg R.D. von Steinbuechel N. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehabil. 2010;91:1650–1660. doi: 10.1016/j.apmr.2010.06.033. .e17. [DOI] [PubMed] [Google Scholar]
- 28.Rourke S.B. Grant I. The neurobehavior correlates of alcoholism, in: Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders. 3rd. Oxford University Press; New York: 2009. pp. 398–454. [Google Scholar]
- 29.Durazzo T.C. Meyerhoff D.J. Nixon S.J. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int. J. Environ. Res. Public Health. 2010;7:3760–3791. doi: 10.3390/ijerph7103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wetzel L. Boll T.J. Short Category Test, Booklet Format. Western Psychological Services; Los Angeles: 1987. [Google Scholar]
- 31.Golden C.J. Stroop Color and Word Test. Stoelting Company; Chicago: 1978. [Google Scholar]
- 32.Reitan RM. Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Interpetation. Neuropsychological Press; Tucson: 1985. [Google Scholar]
- 33.Wechsler D. The Wechsler Adult Intelligence Scale-Third Edition. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- 34.Delis D.C. Kramer J.H. Kaplan E. Ober B.A. California Verbal Learning Test. 2nd. The Psychological Corporation; San Antonio: 2000. [Google Scholar]
- 35.Benedict R. Brief Visuospatial Memory Test - Revised: Professional Manual. Psychological Assessment Resources, Inc.; Odessa, FL: 1997. [Google Scholar]
- 36.Golden C.J. Hammeke T.A. Purisch A.D. Diagnostic validity of a standardized neuropsychological battery derived from Luria's neuropsychological tests. J. Consult. Clin. Psychol. 1978;46:1258–1265. [PubMed] [Google Scholar]
- 37.Grober E. Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J. Clin. Exp. Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 38.Heaton RK. Grant I. Matthews CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources, Inc.; Odessa, FL: 1991. [Google Scholar]
- 39.Durazzo T.C. Fryer S.L. Rothlind J.C. Vertinski M. Gazdzinski S. Mon A. Meyerhoff D.J. Measures of learning, memory and processing speed accurately predict smoking status in short-term abstinent treatment-seeking alcohol-dependent individuals. Alcohol Alcohol. 2010;45:507–513. doi: 10.1093/alcalc/agq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durazzo T.C. Rothlind J.C. Gazdzinski S. Meyerhoff D.J. The relationships of sociodemographic factors, medical, psychiatric, and substance-misuse co-morbidities to neurocognition in short-term abstinent alcohol-dependent individuals. Alcohol. 2008;42:439–449. doi: 10.1016/j.alcohol.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sankoh A.J. Huque M.F. Dubey S.D. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat. Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 42.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 43.Durazzo T.C. Rothlind J.C. Gazdzinski S. Banys P. Meyerhoff D.J. Chronic smoking is associated with differential neurocognitive recovery in abstinent alcoholic patients: a preliminary investigation. Alcohol Clin. Exp. Res. 2007;31:1114–1127. doi: 10.1111/j.1530-0277.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- 44.Bombardier C.H. Temkin N.R. Machamer J. Dikmen S.S. The natural history of drinking and alcohol-related problems after traumatic brain injury. Arch. Phys. Med. Rehabil. 2003;84:185–191. doi: 10.1053/apmr.2003.50002. [DOI] [PubMed] [Google Scholar]
- 45.John U. Meyer C. Rumpf H.J. Schumann A. Thyrian J.R. Hapke U. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol. 2003;38:606–612. doi: 10.1093/alcalc/agg122. [DOI] [PubMed] [Google Scholar]
- 46.Bates C. Jarvis M. Connolly G. Tobacco Additives: Cigarette Engineering and Nicotine Addiction. Massachusetts Tobacco Control Program; Boston: 1999. Jul 14, 1999. [Google Scholar]
- 47.Bartal M. Health effects of tobacco use and exposure. Monaldi Arch. Chest Dis. 2001;56:545–554. [PubMed] [Google Scholar]
- 48.Ambrose J.A. Barua R.S. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J. Am. Coll. Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 49.Dani J.A. De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol. Biochem. Behav. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- 50.Mansvelder H.D. De Rover M. McGehee D.S. Brussaard A.B. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. Eur. J. Pharmacol. 2003;480:117–123. doi: 10.1016/j.ejphar.2003.08.099. [DOI] [PubMed] [Google Scholar]
- 51.Mansvelder H.D. van Aerde K.I. Couey J.J. Brussaard A.B. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology (Berl) 2006;184:292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 52.Vallejo Y.F. Buisson B. Bertrand D. Green W.N. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J. Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volkow N.D. Fowler J.S. Ding Y.S. Wang G.J. Gatley S.J. Imaging the neurochemistry of nicotine actions: studies with positron emission tomography. Nicotine Tob. Res. 1999;1(Suppl 2):S127–S132. doi: 10.1080/14622299050011941. S139–S140. [DOI] [PubMed] [Google Scholar]
- 54.Haustein K.O. Smoking tobacco, microcirculatory changes and the role of nicotine. Int. J. Clin. Pharmacol. Ther. 1999;37:76–85. [PubMed] [Google Scholar]
- 55.Swan G.E. Lessov-Schlaggar C.N. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol. Rev. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 56.Fowles J. Bates M. Noiton D. The Chemical Constituents in Cigarettes and Cigarette Smoke: Priorities for Harm Reduction. New Zealand: Epidemiology and Toxicology Group; New Zealand: 2000. [Google Scholar]
- 57.Yang Y.M. Liu G.T. Injury of mouse brain mitochondria induced by cigarette smoke extract and effect of vitamin C on it in vitro. Biomed. Environ. Sci. 2003;16:256–266. [PubMed] [Google Scholar]
- 58.Valavanidis A. Vlachogianni T. Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health. 2009;6:445–462. doi: 10.3390/ijerph6020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deveci S. Deveci F. Acik Y. Ozan A. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir. Med. 2004;98:551–556. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Alonso J.R. Cardellach F. Casademont J. Miro O. Reversible inhibition of mitochondrial complex IV activity in PBMC following acute smoking. Eur. Respir. J. 2004;23:214–218. doi: 10.1183/09031936.03.00038203. [DOI] [PubMed] [Google Scholar]
- 61.Mazzone P. Tierney W. Hossain M. Puvenna V. Janigro D. Cucullo L. Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: expanding the awareness of smoking toxicity in an underappreciated area. Int. J. Environ. Res. Public Health. 2010;7:4111–4126. doi: 10.3390/ijerph7124111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mendez-Alvarez E. Soto-Otero R. Sanchez-Sellero I. Lopez-Rivadulla Lamas M. In vitro inhibition of catalase activity by cigarette smoke: relevance for oxidative stress. J. Appl. Toxicol. 1998;18:443–448. doi: 10.1002/(sici)1099-1263(199811/12)18:6<443::aid-jat530>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 63.Anbarasi K. Vani G. Balakrishna K. Devi C.S. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci. 2006;78:1378–1384. doi: 10.1016/j.lfs.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 64.Panda K. Chattopadhyay R. Chattopadhyay D.J. Chatterjee I.B. Vitamin C prevents cigarette smoke-induced oxidative damage in vivo. Free Radic. Biol. Med. 2000;29:115–124. doi: 10.1016/s0891-5849(00)00297-5. [DOI] [PubMed] [Google Scholar]
- 65.Moriarty S.E. Shah J.H. Lynn M. Jiang S. Openo K. Jones D.P. Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic. Biol. Med. 2003;35:1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Bloomer R.J. Decreased blood antioxidant capacity and increased lipid peroxidation in young cigarette smokers compared to nonsmokers: Impact of dietary intake. Nutr. J. 2007;6:39. doi: 10.1186/1475-2891-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim S.H. Kim J.S. Shin H.S. Keen C.L. Influence of smoking on markers of oxidative stress and serum mineral concentrations in teenage girls in Korea. Nutrition. 2003;19:240–243. doi: 10.1016/s0899-9007(02)01002-x. [DOI] [PubMed] [Google Scholar]
- 68.Kim S.H. Ensunsa J.L. Zhu Q.Y. Kim J.S. Shin H.S. Keen C.L. An 18-month follow-up study on the influence of smoking on blood antioxidant status of teenage girls in comparison with adult male smokers in Korea. Nutrition. 2004;20:437–444. doi: 10.1016/j.nut.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Northrop-Clewes C.A. Thurnham D.I. Monitoring micronutrients in cigarette smokers. Clin. Chim. Acta. 2007;377:14–38. doi: 10.1016/j.cca.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 70.Bigler E.D. Maxwell W.L. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav. 2012;6:108–136. doi: 10.1007/s11682-011-9145-0. [DOI] [PubMed] [Google Scholar]
- 71.Tavazzi B. Signoretti S. Lazzarino G. Amorini A.M. Delfini R. Cimatti M. Marmarou A. Vagnozzi R. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery. 2005;56:582–589. doi: 10.1227/01.neu.0000156715.04900.e6. [DOI] [PubMed] [Google Scholar]
- 72.Skinner H.A. Sheu W.J. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J. Stud. Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- 73.Bouchery E.E. Harwood H.J. Sacks J.J. Simon C.J. Brewer R.D. Economic costs of excessive alcohol consumption in the U.S. Am. J. Prev. Med. 2011;2006;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 74.Dube S.R. McClave A. James C. Caraballo R. Kaufmann R. Pechacek T. Vital Signs: Current Cigarette Smoking Among Adults Aged ≥18 Years—United States. Centers for Disease Control and Prevention; Atlanta: 2010. 2009. [Google Scholar]