Abstract
Molecular methods have been proposed as an alternative tool for the diagnosis of visceral leishmaniasis (VL), but no data are available regarding use for monitoring clinical outcome. A prospective cohort study of human immunodeficiency virus-(HIV) and VL-coinfected patients was conducted in a university-affiliated hospital in Barcelona, Spain. Leishmania parasite load was monitored using a real-time polymerase chain reaction (PCR) at baseline and every 3 months. Cutoff values for PCR were determined using receiver operating characteristic (ROC) curves. Overall, 37 episodes were analyzed, and 25 of these episodes were considered as relapsing episodes. A significant decrease of parasite load measured 3 months after treatment could predict the clinical evolution of VL. A parasite load over 0.9 parasites/mL measured 12 months after treatment could predicts relapse with a sensitivity of 100% and a specificity of 90.9%. Monitoring parasite load by an ultrasensitive quantitative Leishmania PCR is useful to predict the risk of relapse after a VL episode in HIV-infected patients.
Introduction
Visceral leishmaniasis (VL) remains a diagnostic and therapeutic challenge among human immunodeficiency virus (HIV)-infected patients. The clinical course of VL in these patients is characterized by frequent relapses.1,2 Despite the availability of highly active antiretroviral therapy (HAART), the frequency of VL relapses in HIV-infected patients remains high.3,4 Because of this high risk of relapse despite appropriate treatment, secondary prophylaxis and close follow-up are mandatory until a satisfactory maintained immunity status is achieved.5
In countries where the disease is endemic, standard diagnosis of VL is based on direct observation and/or culture of the parasite in tissue samples (bone marrow, lymph node, liver, or spleen), which requires invasive procedures in most of cases.2–4 Diagnosing each relapsing episode with such invasive techniques can be unpleasant for patients. In recent years, there has been growing interest in the use of samples obtained through less-invasive procedures, such as peripheral blood (conventional culture, serologic tests, and microculture method among others), for both VL diagnosis and follow-up after treatment.6
Over the past decade, molecular diagnostic methods such as polymerase chain reaction (PCR) have been increasingly used for the diagnosis of VL, and PCR amplification has been proposed as an alternative tool to be incorporated in diagnostic algorithms. Data exist regarding its use in peripheral blood and bone marrow samples, with sensitivities ranging from 73% to 100% and specificity close to 100% for the diagnosis of the initial VL episode, mainly in HIV-infected patients.7–18 However, its value as a useful tool for monitoring VL in HIV-infected patients remains to be proven. Various published studies seem to correlate the presence of a high parasite load level in peripheral blood measured by PCR after an initial episode treated and cured with a higher risk of future clinical relapse (Table 1).19–25 However, it is well-known that, in Leishmania infantum-endemic areas, because of the natural exposure to Leishmania parasites, the seroprevalence ranges from 3% in the general population to 45% in former drug users.26–28 In these settings, PCR methods have revealed up to 30% of coinfection with Leishmania in asymptomatic HIV-infected patients.29
Table 1.
Summary of clinical studies of parasitological follow-up of VL in HIV-infected patients measured by PCR
| Authors | Year | Patients followed with PCR | Type of PCR | Follow-up time (months) | Results |
|---|---|---|---|---|---|
| Lachaud and others19 | 1999 | 19 | 18S SSU-rRNA | 0.5–36 | 6 PCR+: 6R (only 3 microbiologically confirmed) |
| Pizzuto and others11 | 2001 | 8 | SSU-rRNA | 7.5–27 | 3 died; 1 PCR−: 1R; 4 PCR+: 4R |
| Cruz and others13 | 2002 | 30 | SSU-rRNA nested PCR | 6–15 | 26 PCR+: 21R (17 confirmed); 4 PCR−: 2R |
| Fisa and others12 | 2002 | 8 | Genomic nested PCR | 2–10 | 8 PCR+: 4R |
| Bossolasco and others16 | 2003 | 5 | SSU-rRNA rt-PCR | 0–11 | 4 PCR+: 3R; 1 PCR−: 0R |
| Mary and others21 | 2004 | 4 | Kinetoplast DNA rt-PCR | 7–15 | 4 PCR+: 2R (1 not treated; 1 NS) |
| Riera and others22 | 2005 | 20 | Genomic nested PCR | 1–28 | Patients PCR+: 50% relapsed in 24 months; patients PCR−: 12% relapsed in 24 months |
| Mary and others23 | 2006 | 6 | Kinetoplast DNA rt-PCR | 6–24 | 4 PCR+: 3R (1 not treated); 2 PCR−: 0R |
| Antinori and others24 | 2007 | 17 | SSU-rRNA semiquantitative | 13 | 11 PCR+; 7 PCR persistently +: 6R |
| Bourgeois and others25 | 2008 | 27 | 18S SSU-rRNA | 51 (51–108) | 7 died: 2L; 18 PCR−: 7R; 9 PCR+: 29R |
| Antinori and others20 | 2009 | 6 | 18S SSU-rRNA rt-PCR | NS | NS |
L = lost to follow-up; NS = not shown; R = relapse.
The lack of knowledge on parasite kinetics, the scarce number of patients studied, and the short follow-up period are the main limitations of most published data on this topic.
The main objectives of our study were to understand the parasite kinetics and therefore, evaluate the usefulness of a real-time PCR (rt-PCR) alone during the follow-up of HIV-infected patients with VL.
Materials and Methods
A prospective cohort study of patients with VL and HIV coinfection was conducted from January of 1999 to September of 2010 in the Hospital Universitari Vall d'Hebron, Barcelona, Spain.
Patients.
Inclusion criteria for the study were (1) adult (≥ 18 years) patients with HIV infection with an initial episode of VL, (2) samples available to perform PCR for Leishmania detection at baseline and during follow-up, and (3) patients receiving HAART. If patients were not receiving HAART when VL was diagnosed, this treatment was initiated.
There were 3 females and 13 males. Thirteen (81.2%) patients were former drug users, and three (18.8%) patients acquired HIV infection by sexual transmission. The median age of the patients at the time of the first leishmaniasis episode was 36 years (range = 27–48). One patient was excluded from the study, because he was lost during follow-up after the first episode.
Ethics statement.
The study protocol was approved by the institutional review board of the hospital, and informed written consent was obtained from all patients.
Diagnosis of VL and relapse.
VL was diagnosed on the basis of Leishmania identification by direct bone marrow examination and/or isolation of culture in bone marrow or other tissue samples.
Four weeks after treatment of the acute episode, cure was documented using a combination of clinical and parasitological criteria.
Clinical criteria for cure were both (1) resolution of fever and (2) improvement of the hematological parameters according to our hospital's normality values. Parasitological criteria for cure were either an absence of parasites in bone marrow aspirate or negative peripheral blood mononuclear cell (PBMC) culture as previously described performed 1 month after completing treatment of the acute episode.22 Cure assessment by bone marrow aspiration was decided by the attending physician.
VL episodes.
VL episodes were classified as relapsing or non-relapsing episodes. A relapsing episode was defined when a subsequent episode occurred after initial cure. A non-relapsing episode was considered when there was no clinical and parasitological evidence of relapse by the end of the study.
Because of the possibility that some episodes at the end of the study could have short follow-up periods, those episodes classified as non-relapsing were required to have a minimum of 18 months follow-up.
Follow-up, assessment, and analytical methods.
For each episode, variables related to HIV infection, such as demography, CD4 cell count, and HIV viral load, were recorded every 3 months. The Leishmania PCR values at baseline and every 3 months were also recorded in addition to the duration of each episode. Patients were assessed every 4 weeks with physical examination; laboratory tests and adverse event assessment were carried out at each visit.
Treatment and prophylaxis.
Each VL episode was treated with either 4 mg/kg per day Liposomal Amphotericine B (L-AmB) intravenously for 5 consecutive days and one time per week thereafter for 5 more weeks (total of 10 doses = 40 mg/kg) or amphotericin B lipid complex 3 mg/kg per day for 10 days. After cure was determined, all patients received 5 mg/kg intravenous L-AmB every 3 weeks or 20 mg/kg intravenous meglumine antimoniate every 4 weeks as secondary prophylaxis.
PCR procedure.
PCR was performed on DNA extracted from PBMCs using the High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany) using the manufacturer's instructions. The detection and quantification of Leishmania DNA were analyzed by amplification of kinetoplast minicircle DNA sequence by a rt-PCR with some modifications.21 Each amplification was performed in triplicate in a 20 μL reaction mixture containing 1× iTaq supermix with Rox (Bio-Rad, Hercules, CA), 15 pmol direct primer (CTTTTCTGGTCCTCCGGGTAGG), 15 pmol reverse primer (CCACCCGGCCCTATTTTACACCAA), 50 pmol labeled TaqMan probe (FAM-TTTTCGCAGAACGCCCCTACCCGC-TAMRA), and 5 μL sample DNA. The ABI Prism 7700 System (Applied Biosystems, Foster City, CA) at 94°C and 55°C cycling over 45 cycles was used. The pre-developed reagent for the RNase P human gene (TaqMan Human RNase P detection reagent; Applied Biosystems) was included in the PCR reaction as an internal control of amplification. The thermal profile was identical to those profiles of kinetoplast DNA amplification. A non-template control was included in each run as the rt-PCR negative control. rt-PCR was considered positive for Leishmania when the threshold cycle (tC) was < 45. The tC for a given sample was the first cycle of the PCR reaction where fluorescence is detected above the baseline. Therefore, a higher parasite load implies a lower tC.
A 10-fold dilution series of standard DNA from promastigotes (MHOM/ES/04/BCN-61; L. infantum ZMON-1) was used as a calibrator (serial dilution from 105 parasites/mL to 10−3 parasites/mL), allowing for the plotting of a standard curve; each dilution was tested in triplicate. The rt-PCR protocol used in this study was able to detect very low amounts of Leishmania kDNA, with a detection limit of 5 × 10−5 parasites per PCR reaction tube.
Statistical analysis.
Each episode was analyzed separately. Continuous variables between relapsing and non-relapsing episodes were compared with the Mann–Whitney U test and expressed as the medians and ranges or interquartile ranges (IQRs). Categorical variables were compared with the χ2 or Fisher's test when the expected frequency was ≤ 5. Repeated-measures analysis of variance (ANOVA) was used to compare CD4 cell count with PCR values along time. A P value < 0.05 was considered to indicate statistical significance.
Cutoff values for PCR values and PCR increases that resulted in the best compromise between sensitivity and specificity for predicting relapse were determined every 3 months using receiver operating characteristic (ROC) curves. The positive and negative likelihood ratios (LRs) were calculated as a measure of the extent to which pre-test odds were altered by the test results. Low negative LR (< 0.1) and high positive LR (> 10) are considered useful for ruling out and ruling in decisions, respectively. Finally, the negative predictive value of a negative PCR was also calculated.
Statistical analysis was performed with the SPSS statistical package (version 15.0).
Results
Forty-seven episodes of VL in 16 HIV-infected patients were recorded. Of 47 episodes of VL, 10 episodes were not evaluated, because PCR determination was not performed during follow-up. Therefore, 37 episodes were finally analyzed, and 25 of these episodes were considered as relapsing episodes. The remaining 12 episodes were non-relapsing episodes at the end of the study. Median CD4 cell count was 100 cells/mL (IQR = 4–300) at the time of diagnosis. The CD4 lymphocyte count was lower than 200 cells/mL in 33 of 37 (89.2%) episodes, and plasma HIV RNA was lower than 50 copies/mL in 19 (51.3%) episodes. The median parasite load at the time of diagnosis was 50 parasites/mL (IQR = 4–130 p/mL). All 37 episodes were considered cured by clinical criteria and had negative PBMC cultures 4 weeks after treatment. In 17 of 37 (45.9%) episodes, parasitological cure was also documented by a negative bone marrow aspiration 1 month after treatment.
The main clinical and HIV characteristics in respect to both types of episodes are shown in Table 2. No significant differences regarding initial CD4 lymphocyte count and percentage, proportion of patients with viral load under 50 copies/mL, and parasite load were observed between either group at the time of diagnosis. In contrast, during follow-up, those episodes that finally relapsed presented a lower increase in the number and percentage of CD4 lymphocytes, showed a scarce variation in the parasite load, and showed less time free of relapse than those episodes that did not.
Table 2.
Comparison between relapsing and non-relapsing episodes with baseline characteristics of VL in HIV-infected patients
| Relapsing episodes (median [IQR]) | Non-relapsing episodes (median [IQR]) | P | |
|---|---|---|---|
| Number of episodes | 25 | 12 | – |
| Time free of relapse (relapsing episodes)/time to end of study (non-relapsing episodes; months) | 16 (9–18) | 67.5 (28.5–89) | < 0.001 |
| CD4 lymphocyte count/CD4 % | 101 (50–174)/11% (10–17) | 98 (68–108)/16% (11–17) | 0.64/0.49 |
| Patients with HIV viral load < 50 copies/mL (number/%) | 12/48% | 7/58% | 0.73 |
| Leishmania PCR at diagnosis of episode (parasites/mL) | 40 (4–50) | 10 (5–50) | 0.19 |
| ΔCD4 | 14 | 135.5 | < 0.001 |
| Δ% CD4 | 1 | 5.5 | 0.026 |
CD4 = CD4 increase is the median of the CD4 count at the end of the episode minus the CD4 count at diagnostic; % CD4 = CD4% increase is the median of the CD4% at the end of the episode minus the CD4% at diagnostic.
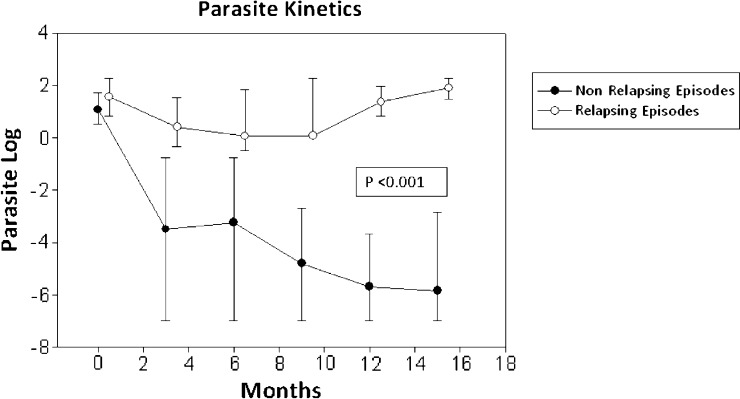
Concerning parasite kinetics measured by PCR, a significant decrease in parasite load was seen in those episodes considered as non-relapsing, mainly occurring from the third month on (P < 0.001) (Figure 1).
Figure 1.
Parasite kinetics (expressed in logarithm of parasite load) according to the type of episode. Black circles represent non-relapsing episodes. White circles represent relapsing episodes.
We analyzed the best PCR cutoff to predict relapse at different points in time (3, 6, 9, and 12 months after initial treatment) using an ROC curve. The best area under the curve (0, 983) was found at the point of 12 months. A cutoff value of 0.9 parasites/mL (−0.05 log) measured in this moment showed sensitivity of 100% (95% confidence interval [95% CI] = 0.77–1), specificity of 90.9% (95% CI = 0.60–0.98), a positive likelihood ratio of 10 (95% CI = 1.5–64), and a negative likelihood ratio of 0 (Table 3).
Table 3.
Accuracy of PCR to predict the outcome of VL episodes at 3 and 12 months using different cutoff values calculated with ROC curves
| Parasite load at 3rd month (cutoff value = 0.03 parasite/mL) | Parasite load at 12th month (cutoff value = 0.9 parasite/mL) | |
|---|---|---|
| Test is positive in relapsing group | 20 of 22 | 12 of 12 |
| Test is positive in non-relapsing group | 3 of 12 | 1 of 10 |
| Sensitivity | 90.9% | 100% |
| Specificity | 75% | 90% |
| Positive predictive value | 86.9% | 92.3% |
| Negative predictive value | 81.8% | 100% |
| Positive likelihood ratio | 2, 720 | 10 |
| Negative likelihood ratio | 0, 136 | 0 |
Interestingly, the parasite load variation observed in both groups during the first 3 months of follow-up was, at this point, different enough to allow prediction of who was not going to relapse. At the third month, a parasite load above 0.03 parasite/mL (−1.5 log) estimates (with 90% sensitivity and 75% specificity) the patients who are not going to relapse, with 82% negative predictive value and almost 87% positive predictive value, a positive likelihood ratio of 3.7 (95% CI = 1,353–9,775), and a negative likelihood ratio of 0.1 (95% CI = 0.03–0.47) (Table 3).
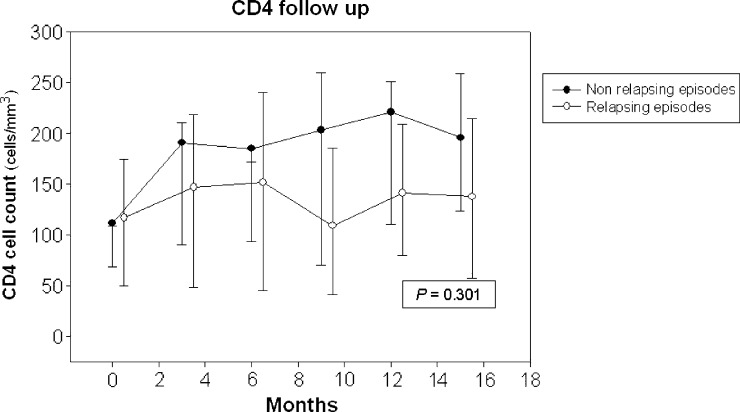
Although the CD4 progression showed an increasing trend in the non-relapsing group, no significant differences between the groups were observed (Figure 2).
Figure 2.
CD4 count cells follow-up according to the type of episode. Black circles represent non-relapsing episodes. White circles represent relapsing episodes.
When the non-relapsing episodes were analyzed, all had at least two consecutive negative PCR determinations, whereas in the relapsing episode, this condition occurred in only one case. Therefore, when considering two consecutive negative PCRs as parasitological cure condition, a negative predictive value of 92.3% is obtained. In our series, the only patient who had a relapsing episode despite having two consecutive negative PCR was one who interrupted the HIV treatment and suffered an important immunovirological impairment (CD4 cell count dropped from 120 to 0 cells/mm3 and viral load increased from undetectable level to 670.000 copies/mL).
After treatment of VL, patients required a median of 19.5 months (range = 3–57 months) to achieve two consecutive negative PCR.
Discussion
The chronic nature and frequent relapses of leishmaniasis in patients infected with HIV require close follow-up.
Conventional methods used to monitor these patients, such as serology, antigen detection in urine, and peripheral blood cultures, have not proven useful with HIV-infected patients in predicting recurrence because of either low sensitivity and specificity or the time needed to obtain a result.30,31
Although molecular biology techniques have proven valuable as diagnostic tools because of their relatively easy implementation and high sensitivity and specificity, their role in monitoring patients remains to be defined. The published studies listed in Table 1 showed that, after the initial episode is cured, a group of patients with detectable parasite burden would be at risk to develop new relapses. Thus, it could be thought that, despite receiving a treatment capable of controlling the clinical symptoms, a residual parasite load only detectable by sensitive techniques, such as PCR, could justify the subsequent recurrences. However, as mentioned in the literature, the PCR positivity alone could not be considered clinically relevant. This finding has been called active chronic VL or cryptic Leishmania infections by some authors.29,32,33 Therefore, interpretation of PCR in monitoring these patients must be taken with caution. It is for this reason that ultrasensitive quantitative PCR was incorporated into our study to understand the kinetics of the Leishmania parasite in HIV-coinfected patients and assess its usefulness in monitoring clinical outcome.
There are many factors determining the clinical expression of leishmaniasis in patients with residual parasitemia: some depending on the immune system of the host, others depending on virulence factors of the parasite, and others depending on the relation with the HIV itself.31,33 However, it seems logical that, independent of all these factors, a patient who is going to present a clinical event will suffer an increase in parasite load high enough to lead them to relapse.
After analyzing all VL episodes, we found that, regardless final outcome, they had similar baseline characteristics. There were no differences in the immune status at the time of diagnosis or the parasite load. After the acute process of the episode passed and after having been treated, two very different patterns are observed among the cases that resulted in relapse and cases that did not. In keeping with previous data, those episodes that, on receiving appropriate treatment, could not eliminate the parasite burden, showing a persistently positive PCR, finally developed a new clinical relapse, whereas in those episodes without relapse, the parasite load tends to be eliminated.
Considering that the parasite load itself may be a factor in determining recurrence, it would be very useful to find out whether there is a cutoff point at which, with the highest reliability, the clinical evolution could be predicted. As shown in Figure 1, the trend of parasite load seems to significantly differ beyond the third month. Using the ROC curve, it could be said that a parasite load above 0.03 parasite/mL at this time point estimates (with almost 90% probability) the risk of having a relapsing course. If the parasite load decreases below that cutoff, the clinical outcome will be favorable with 82% likelihood. However, for those episodes in which such a parasite clearance could not be achieved, a parasite load of 0.9 parasites/mL at 12 months showed the most favorable sensitivity and specificity (100% and 90%, respectively) for predicting recurrence.
Correspondingly, one would expect that those episodes achieving complete elimination of parasite load after treatment, defined as negative PCR performed on two consecutive occasions over a 6-month period, could be those episodes that cure. Indeed, all the episodes without relapse satisfy this condition. Only one of the relapsing episodes fulfils this premise. However, on analyzing this case individually, it corresponds to a patient who, after an episode of VL and achievement of a negative PCR, discontinued HAART two times, suffering a deep immunosuppression (0 CD4 cell/mL), which led the patient to a new clinical episode. The Leishmania strain identification in that particular case could not be done, therefore making it impossible to determine whether it was, in fact, a relapse or reinfection. Nevertheless, the determination of two consecutive negative PCRs in a 6-month period after appropriate treatment correlates well with no recurrence, with a negative predictive value of 92.3%. For this reason, because of its high negative predictive value, this condition could be used to withdraw chemoprophylaxis.
In our series, it took a median of 19 months to obtain a negative result for the parasite load. This result differs from some previous publications, where much less time was required to achieve a negative PCR: from 17.5 days (8 days to 21 weeks) in the article by Antinori and others24 to 3 months (2 weeks to 7 months) in the work by Lachaud and others19 to 6 months according to Bourgeois and others25 to between 6 and 21 weeks in the work by Pizzuto and others.11 The reason justifying this difference is precisely the type of PCR used. In our case, the kinetoplast DNA was used as a target of amplification, which has a much lower detection threshold than small subunit ribosal RNA (SSU-rRNA). Using an ultrasensitive quantitative PCR, such as the one used in our study, may not pose a great advantage in VL episode diagnosis given that the circulating parasite load is so high that it can easily be detected by another PCR that amplifies other targets as ribosomal DNA.20 However, if we intend to use PCR to determine whether the parasite load has been removed and predict the clinical outcome, an ultrasensitive technique such as the technique that we propose should be used.
It would have been expected that, in those episodes that did not relapse, the increase in the number of CD4 lymphocytes and therefore, the patient's immunity would play a decisive role. Although the trend of CD4 lymphocytes suggests that this hypothesis is correct, no statistically significant differences were found in either group, possibly because of sample size.
The antiretroviral therapy or secondary chemoprophylaxis has not been analyzed, because all patients where under treatment and received prophylaxis; however, the aim of the study was to analyze the parasite kinetics independently of these factors related to the treatment.
One of the main limitations presented by this study is the difficulty in cataloguing episodes into relapsing and non-relapsing at the time of analysis, because at this point, future recurrences cannot be determined. Hence, those episodes that, at the time of evaluation, had no reasonable monitoring period (minimum of 18 months) were excluded from the study. Likewise, we consider the differences in both duration of episodes (Table 2) and the parasite load kinetics (Figure 1) enough to acknowledge them as different episodes.
Another limitation presented by our study is the fact that documentation of parasitological cure by bone marrow aspiration was only performed in 45.9% (17 of 37 episodes) of cases. However, despite not having bone marrow confirmation, all those episodes considered cured had negative PBMC cultures, and all relapsing episodes had positive PBMC cultures.
Conclusion
In summary, we think that incorporating an ultrasensitive quantitative Leishmania PCR in the monitoring of HIV-infected patients suffering from VL may be useful.
During patient follow-up, if the parasite burden is significantly reduced during the first 3 months and medical conditions are maintained (HAART and correct follow-up), a good clinical evolution could be predicted, allowing the cessation of performing follow-up PCR. If this reduction is not achieved, a PCR follow-up is recommended. A parasite load equal to or higher than 0.9 parasites/mL 12 months after the episode requires a closer monitoring of the patient, because the next clinically compatible febrile episode will most likely correspond to a leishmaniasis relapse, at which point other invasive techniques could be avoided.
In addition, after an episode of treated and cured leishmaniasis, if the patient has two consecutive negative PCRs in a 6-month period, the patient may be considered disease-free with a probability above 90%, and the withdrawal of secondary prophylaxis could be considered. It would seem reasonable to add maintenance of a good immunovirological status to this last condition. However, this finding has not been entirely proven within this study. Additional larger studies are required to analyze classical factors of relapse in addition to new factors, such as quantification of parasite load.
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This study was partially supported by Red Temática de Investigación en Síndrome de Inmunodeficiencia Adquirida (SIDA) (RIS G03/173-RETIC RD06/0006/0039).
Authors' addresses: Israel Molina, Vicenç Falcó, Aleix Elizalde, Fernando Salvador, Manuel Crespo, Adrian Curran, Esteban Ribera, and Albert Pahissa, Infectious Disease Department, Hospital Universitari Vall d'Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain, E-mails: imolina@vhebron.net, vfalco@vhebron.net, ealeix@hotmail.com, medinano@yahoo.es, mcrespo@vhebron.net, acurran@vhebron.net, eribera@vhebron.net, and apahissa@vhebron.net. Roser Fisa, Cristina Riera, Paulo López-Chejade, and Silvia Tebar, Parasitology Department, Faculty of Pharmacy, Universitat de Barcelona, Barcelona, Spain, E-mails: rfisa@ub.edu.es, mcriera@ub.edu.es, pllch@yahoo.com, and stebar@ub.edu.es. Santiago Pérez-Hoyos, Unit of Methodologic Support in Biomedical Research, Vall d'Hebron Research Institute, Spain, E-mail: santi.perezhoyos@vhir.org.
References
- 1.Ribera E, Ocaña I, de Otero J, Cortes E, Gasser I, Pahissa A. Prophylaxis of visceral leishmaniasis in human immunodeficiency virus infected patients. Am J Med. 1996;10:496–501. doi: 10.1016/s0002-9343(97)89503-4. [DOI] [PubMed] [Google Scholar]
- 2.Ribera E, Cucurull E, Ocana I, Vallespí T, Gasser I, Juste C. Visceral leishmaniasis in patients with HIV infection. Enferm Infecc Microbiol Clin. 1995;13:73–79. [PubMed] [Google Scholar]
- 3.Mira JA, Corzo JE, Rivero A Macias J, De Leon FL, Torre-Cisneros J, Gómez-Mateos J, Jurado R, Pineda JA. Frequency of visceral leishmaniasis relapses in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy. Am J Trop Med Hyg. 2004;70:298–301. [PubMed] [Google Scholar]
- 4.Fernández-Cotarelo MJ, Martínez Abellan J, Vales Guerra JM, Martínez Sánchez P, Rodrigo Gómez De La Bárcena M, Salto Fernandez E. Effect of highly active antiretroviral therapy on the incidence and clinical manifestations of visceral leishmaniasis in human immunodeficiency virus-infected patients. Clin Infect Dis. 2003;37:973–977. doi: 10.1086/377607. [DOI] [PubMed] [Google Scholar]
- 5.Molina I, Falcó V, Crespo M, Riera C, Ribera E, Curran A, Carrio J, Diaz M, Villar del Saz S, Fisa R, López-Chejade P, Ocaña I, Pahissa A. Efficacy of liposomal amphotericin B for secondary prophylaxis of visceral leishmaniasis in HIV-infected patients. J Antimicrob Chemother. 2007;60:837–842. doi: 10.1093/jac/dkm294. [DOI] [PubMed] [Google Scholar]
- 6.Allahverdiyev AM, Bagirova M, Uzun S, Alabaz D, Aksaray N, Kocabas E, Koksal F. The value of a new microculture method for diagnosis of visceral leishmaniasis by using bone marrow and peripheral blood. Am J Trop Med Hyg. 2005;73:276–280. [PubMed] [Google Scholar]
- 7.Mathis A, Deplazes P. PCR and in vitro cultivation for detection of Leishmania spp. in diagnostic samples from human and dogs. J Clin Microbiol. 1995;33:1145–1149. doi: 10.1128/jcm.33.5.1145-1149.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piarroux R, Gambarelli F, Toga B, Dumon H, Fontes M, Dunan S, Quilici M. Interest and reliability of a polymerase chain reaction on bone marrow samples in the diagnosis of visceral leishmaniasis in AIDS. AIDS. 1996;10:452–453. doi: 10.1097/00002030-199604000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Costa JM, Durand R, Deniau M, Rivollet D, Izri M, Houin R, Vidaud M, Bretagne S. PCR enzyme-linked immunosorbent assay for diagnosis of leishmaniasis in human immunodeficiency virus–infected patients. J Clin Microbiol. 1996;34:1831–1833. doi: 10.1128/jcm.34.7.1831-1833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campino L, Cortes S, Pires R, Oskam L, Abranches P. Detection of Leishmania in immunocompromised patients using peripheral blood spots on filter paper and the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 2000;19:396–398. doi: 10.1007/s100960050503. [DOI] [PubMed] [Google Scholar]
- 11.Pizzuto M, Piazza M, Senese D, Scalamogna C, Calattini S, Corsico L, Persico T, Adriani B, Magni C, Guaraldi G, Gaiera G, Ludovisi A, Gramiccia M, Galli M, Moroni M, Corbellino M, Antinori S. Role of PCR in diagnosis and prognosis of visceral leishmaniasis in patients coinfected with human immunodeficiency virus type 1. J Clin Microbiol. 2001;39:357–361. doi: 10.1128/JCM.39.1.357-361.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisa R, Riera C, Ribera E, Gallego M, Portus M. A nested polymerase chain reaction for diagnosis and follow-up of human visceral leishmaniasis patients using blood samples. Trans R Soc Trop Med Hyg. 2002;96((Suppl 1)):S191–S194. doi: 10.1016/s0035-9203(02)90075-1. [DOI] [PubMed] [Google Scholar]
- 13.Cruz I, Canavate C, Rubio JM, Morales MA, Chicharro C, Laguna F, Jiménez-Mejías M, Sirera G, Videla S, Alvar J. A nested polymerase chain reaction (Ln-PCR) for diagnosing and monitoring Leishmania infantum in patients coinfected with human immunodeficiency virus. Trans R Soc Trop Med Hyg. 2002;96((Suppl 1)):S185–S189. doi: 10.1016/s0035-9203(02)90074-x. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Sanchez J, Pineda JA, Andreu-Lopez M, Delgado J, Macías J, De la Rosa L, Morillas-Márquez F. The high sensitivity of a PCR-ELISA in the diagnosis of cutaneous and visceral leishmaniasis caused by Leishmania infantum. Ann Trop Med Parasitol. 2002;96:669–677. doi: 10.1179/000349802125001906. [DOI] [PubMed] [Google Scholar]
- 15.Deniau M, Canavate C, Faraut-Gambarelli F, Marty P. The biological diagnosis of leishmaniasis in HIV-infected patients. Ann Trop Med Parasitol. 2003;97((Suppl 1)):S115–S133. doi: 10.1179/000349803225002598. [DOI] [PubMed] [Google Scholar]
- 16.Bossolasco S, Gaiera G, Olchini D, Gulletta M, Martello L, Bestetti A, Bossi L, Germagnoli L, Lazzarin A, Uberti-Foppa C, Cinque P. Real-time PCR assay for clinical management of human immunodeficiency virus–infected patients with visceral leishmaniasis. J Clin Microbiol. 2003;41:5080–5084. doi: 10.1128/JCM.41.11.5080-5084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fissore C, Delaunay P, Ferrua B, Rosenthal E, Del Giudice P, Aufeuvre JP, Le Fichoux Y, Marty P. Convenience of serum for visceral leishmaniasis diagnosis by PCR. J Clin Microbiol. 2004;42:5332–5333. doi: 10.1128/JCM.42.11.5332-5333.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatti S, Gramegna M, Klersy C, Madama S, Bruno A, Maserati R, Bernuzzi AM, Cevini C, Scaglia M. Diagnosis of visceral leishmaniasis: the sensitivities and specificities of traditional methods and a nested PCR assay. Ann Trop Med Parasitol. 2004;98:667–676. doi: 10.1179/000349804225011488. [DOI] [PubMed] [Google Scholar]
- 19.Lachaud L, Dereure J, Chabbert E, Reynes J, Mauboussin JM, Oziol E, Dedet JP, Bastien P. Optimized PCR using patient blood samples for diagnosis and follow-up of visceral Leishmaniasis, with special reference to AIDS patients. J Clin Microbiol. 2000;38:236–240. doi: 10.1128/jcm.38.1.236-240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antinori S, Calattini S, Piolini R, Longhi E, Bestetti G, Piolini R, Magni C, Orlando G, Gramiccia M, Acquaviva V, Foschi A, Corvasce S, Colomba C, Titone L, Parravicini C, Cascio A, Corbellino M. Is real-time polymerase chain reaction (PCR) more useful than a conventional PCR for the clinical management of leishmaniasis? Am J Trop Med Hyg. 2009;81:46–51. [PubMed] [Google Scholar]
- 21.Mary C, Faraut F, Lascombe L, Dumon H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J Clin Microbiol. 2004;42:5249–5255. doi: 10.1128/JCM.42.11.5249-5255.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riera C, Fisa R, Ribera E, Carrió J, Falcó V, Gállego M, Moner L, Molina I, Portús M. Value of culture and nested PCR of blood in the prediction of relapses in patients co-infected with Leishmania and HIV. Am J Trop Med Hyg. 2005;73:1012–1015. [PubMed] [Google Scholar]
- 23.Mary C, Faraut F, Drogoul MP, Xeridat B, Schleinitz N, Cuisenier B, Dumon H. Reference values for Leishmania infantum parasitemia in different clinical presentations: quantitative polymerase chain reaction for therapeutic monitoring and patient follow-up. Am J Trop Med Hyg. 2006;75:858–863. [PubMed] [Google Scholar]
- 24.Antinori S, Calattini S, Longhi E, Bestetti G, Piolini R, Magni C, Orlando G, Gramiccia M, Acquaviva V, Foschi A, Corvasce S, Colomba C, Titone L, Parravicini C, Cascio A, Corbellino M. Clinical use of polymerase chain reaction performed on peripheral blood and bone marrow samples for the diagnosis and monitoring of visceral leishmaniasis in HIV-infected and HIV-uninfected patients: a single-center, 8-year experience in Italy and review of the literature. Clin Infect Dis. 2007;44:1602–1610. doi: 10.1086/518167. [DOI] [PubMed] [Google Scholar]
- 25.Bourgeois N, Lachaud L, Reynes J, Rouanet I, Mahamat A, Bastien P. Long-term monitoring of visceral leishmaniasis in patients with AIDS: relapse risk factors, value of polymerase chain reaction, and potential impact on secondary prophylaxis. J Acquir Immune Defic Syndr. 2008;48:13–19. doi: 10.1097/QAI.0b013e318166af5d. [DOI] [PubMed] [Google Scholar]
- 26.Gutiérrez J, Maroto MC, Piédrola G, Higuera A. Prevalence of anti-Leishmania antibodies in parenteral drug addicts. Yield value of 2 study techniques. Med Clin (Barc) 1993;100:168–170. [PubMed] [Google Scholar]
- 27.Riera C, Fisa R, López-Chejade P, Serra T, Girona E, Jiménez M, Muncunill J, Sedeño M, Mascaró M, Udina M, Gállego M, Carrió J, Forteza A, Portús M. Asymptomatic infection by Leishmania infantum in blood donors from the Balearic Islands (Spain) Transfusion. 2008;48:1383–1389. doi: 10.1111/j.1537-2995.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 28.Riera C, Fisa R, Udina M, Gállego M, Portus M. Detection of Leishmania infantum cryptic infection in asymptomatic blood donors living in an endemic area (Eivissa, Balearic Islands, Spain) by different diagnostic methods. Trans R Soc Trop Med Hyg. 2004;98:102–110. doi: 10.1016/s0035-9203(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 29.García-García JA, Martín-Sánchez J, Gállego M, Rivero-Román A, Camacho A, Riera C, Morillas-Márquez F, Vergara S, Macías J, Pineda JA. Use of noninvasive markers to detect Leishmania infection in asymptomatic human immunodeficiency virus-infected patients. J Clin Microbiol. 2006;44:4455–4458. doi: 10.1128/JCM.00921-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riera C, Fisa R, Lopez P, Ribera E, Carrió J, Falcó V, Molina I, Gállego M, Portús M. Evaluation of a latex agglutination test (KAtex) for detection of Leishmania antigen in urine of patients with HIV-Leishmania coinfection: value in diagnosis and post-treatment follow-up. Eur J Clin Microbiol Infect Dis. 2004;23:899–904. doi: 10.1007/s10096-004-1249-7. [DOI] [PubMed] [Google Scholar]
- 31.Alvar J, Aparicio P, Aseffa A, Den Boer M, Cañavate C, Dedet JP, Gradoni L, Ter Horst R, López-Vélez R, Moreno J. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourgeois N, Bastien P, Reynes J, Makinson A, Rouanet I, Lachaud L. Active chronic visceral leishmaniasis’ in HIV-1-infected patients demonstrated by biological and clinical long-term follow-up of 10 patients. HIV Med. 2010;11:670–673. doi: 10.1111/j.1468-1293.2010.00846.x. [DOI] [PubMed] [Google Scholar]
- 33.Colomba C, Saporito L, Vitale F, Reale S, Vitale G, Casuccio A, Tolomeo M, Maranto D, Rubino R, Di Carlo P, Titone L. Cryptic Leishmania infantum infections in Italian HIV infected patients. BMC Infect Dis. 2009;9:199. doi: 10.1186/1471-2334-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]