Abstract
Global Program to Eliminate Lymphatic Filariasis (GPELF) guidelines call for using filarial antigen testing to identify endemic areas that require mass drug administration (MDA) and for post-MDA surveillance. We compared a new filarial antigen test (the Alere Filariasis Test Strip) with the reference BinaxNOW Filariasis card test that has been used by the GPELF for more than 10 years. Laboratory testing of 227 archived serum or plasma samples showed that the two tests had similar high rates of sensitivity and specificity and > 99% agreement. However, the test strip detected 26.5% more people with filarial antigenemia (124/503 versus 98/503) and had better test result stability than the card test in a field study conducted in a filariasis-endemic area in Liberia. Based on its increased sensitivity and other practical advantages, we believe that the test strip represents a major step forward that will be welcomed by the GPELF and the filariasis research community.
Introduction
Lymphatic filariasis (LF) is a deforming and disabling neglected tropical disease (NTD) that has been targeted for elimination by the year 2020.1 The Global Program to Eliminate Lymphatic Filariasis (GPELF) aims to interrupt transmission of the nematode worms that cause LF using periodic, repeated mass drug administration (MDA) of antifilarial medications to entire at-risk populations. Four billion doses of these drugs were distributed in more than 50 disease-endemic countries between the years 2000 and 2011,1,2 which makes the GPELF the largest public health intervention program to date based on MDA. The World Health Organization (WHO) has provided guidelines and protocols for mapping, monitoring, and evaluating LF programs with diagnostic tests that include detection of microfilariae (Mf) by microscopic examination of stained blood smears and detection of circulating filarial antigen (CFA) in human blood.3 CFA tests detect a 200 kDa parasite antigen that is a sensitive and specific biomarker for the presence of adult Wuchereria bancrofti, the parasite species that is responsible for 90% of the LF disease burden in the world.4 CFA testing is much more sensitive than thick smear microscopy for detecting W. bancrofti infections, and it is also more convenient, because it can be performed with blood collected during the day or night in the field with no requirement for electricity, special equipment, or skilled microscopists.5
The first sensitive CFA tests used monoclonal antibodies in antigen-capture assays such as radioimmunoassay and microplate enzyme-linked immunosorbent assay (ELISA).6–8 However, the development of a commercial, point-of-care (POC) immunochromatographic (ICT) test in the late 1990s allowed CFA testing to escape the confines of the research laboratory and assume an important role as a tool for public health use. Initially developed as the ICT Filariasis card test in 1996 by ICT Diagnostics in Australia, the test has been produced as the BinaxNOW Filariasis test in the United States by Alere Scarborough (Scarborough, ME; formerly Binax, Inc.) since 2000. Although it took some time for this test to gain acceptance by the LF research and control communities, it is now integrated into the GPELF protocols for mapping LF endemicity, stopping MDA, and post-MDA surveillance.9,10 Although this test is a valuable tool, its short shelf life (3 months at ambient temperatures in the tropics) and cost have hampered its use by the GPELF. Another problem with the test is that it has a narrow time window for reading the test result. The manufacturer's instructions call for reading the test 10 minutes after one closes the card to start the test. False-positive results are common if the tests are read too late (after 20 minutes).11
Recognizing the importance of affordable and reliable diagnostic testing for the GPELF, the Bill and Melinda Gates Foundation canvassed filariasis experts to outline a target product profile for an improved CFA test and provided a grant to the manufacturer for test development. This paper reports results of an independent evaluation of the fruit of that effort, the Alere Filariasis Test Strip. POC technologies have improved in the past 15 years, and our results show that the new test has significant advantages over its predecessor; it will be marketed in 2013.
Material and Methods
Test materials and protocol.
Test materials were provided at no cost by Alere Scarborough, Inc. Test protocols were developed by the authors together with personnel at Alere Scarborough, Inc. to comply with rigorous industry standards required for Conformite Europeene (CE) marking and test registration. Test performance, interpretation of test results, data analysis, and manuscript preparation were conducted independently by the authors.
Ethical approval.
Laboratory evaluations with existing serum or plasma samples were conducted under human studies protocols approved by institutional review boards (IRBs) at the Centers for Disease Control (CDC) and Washington University. The field study in Liberia was approved by IRBs at Washington University and the University of Liberia in Monrovia. All adult participants in the field study provided informed consent; assent by the child and consent from at least one parent were required for children to participate in the study.
Laboratory evaluation of the two filarial antigen tests.
This evaluation was performed in two laboratories with well-characterized panels of serum or plasma. The Washington University laboratory tested a panel of previously frozen serum or plasma samples from human subjects with parasitologically proven helminthic infections and control samples collected in St. Louis, Missouri, which is non-endemic for human filariasis and other human helminthic infections. The CDC laboratory tested samples from Haiti that were collected in separate areas of the country that were highly endemic and non-endemic for LF. In both settings, venous blood samples were collected at night from consenting subjects, and Mf status was assessed by nuclepore filtration (1 mL). Mf counts for positive Haitian samples ranged from 1 to 3,144/mL. Additional non-endemic samples tested at the CDC were collected from US residents and residents of an Argentinean community with a high prevalence of soil-transmitted helminth infections and strongyloidiasis.12
Testing with the BinaxNOW Filariasis card test and the Alere Filariasis Test Strip was performed according to the manufacturer's instructions. For the card test, 100 μL serum or plasma were placed on the sample application pad, the card was closed, and the test result was assessed at 10 minutes. For the test strip, 75 μL serum or plasma were placed on the sample application pad, and the test result was read by two independent readers at 10 minutes. For the Washington University evaluation, if the two readers' results did not agree, a final decision regarding the test result was made by a third reader. Cards and test strips were also read by two readers 24 hours after adding samples to the tests. Serum and plasma samples were coded, and test readers read the samples blindly without knowing the type of sample being tested or the result obtained by the other reader. Analytical sensitivity of the two antigen tests was compared by parallel testing of serial dilutions of a filarial antigen (DATH; prepared as previously described).13
Field evaluation of the filarial antigen tests.
The field study was performed in Lofa County in northwestern Liberia. The study villages were endemic for lymphatic filariasis, onchocerciasis, schistosomiasis, and soil-transmitted helminth infections. A single round of community-directed ivermectin (MDA) had been distributed for onchocerciasis control in the study area in November of 2011, approximately 5 months before this study. Capillary blood was collected during the day by finger prick with a disposable contact-activated lancet (Becton Dickinson, Franklin Lakes, NJ). The blood was collected directly into a small 75-μL blood collection pipette for the Alere Filariasis Test Strip and a 100-μL capillary tube supplied with the BinaxNOW Filariasis card test. All testing was performed in the study villages immediately after blood was collected. For the card test, 100 μL blood were placed on the sample application pad, the card was closed, and the test result was assessed at 10 minutes. For the test strip, 75 μL blood were added to the sample application pad, and the test result was assessed at 10 minutes. Both types of test were independently read and scored by two readers. If the two readers' results did not agree, a final decision regarding the test result was made by a third reader. Cards and test strips were also read at 30 minutes and approximately 24 hours after adding blood to the tests. Test scores were recorded as follows: 0 = no test line visible (a negative test); 1+, the test line is present but weaker than the control line; 2+, the test line is equal to the control line; 3+, the test line is stronger than the control line. Tests with no control line were considered to have invalid results. A majority of subjects enrolled in the study was also tested for Mf by microscopic examination of stained thick blood films (60 μL) prepared with finger-prick blood collected between 9 pm and 12 am.
Results
Laboratory evaluation.
The analytical sensitivity of the two tests was compared by testing serial dilutions of DATH filarial antigen with a starting concentration of 4 ng/mL. The test strip produced stronger, sharper positive test lines than the card, and the test strip was more sensitive than the card test (Figure 1) . The card test was weakly positive with antigen diluted 1:4, and higher dilutions were cleanly negative. The test strip was clearly positive with antigen diluted at 1:8, and a weak shadow line was observed with antigen at 1:16. Thus, the minimum concentration of antigen detected with the test strip was two to four times lower than the minimum detected with the card test.
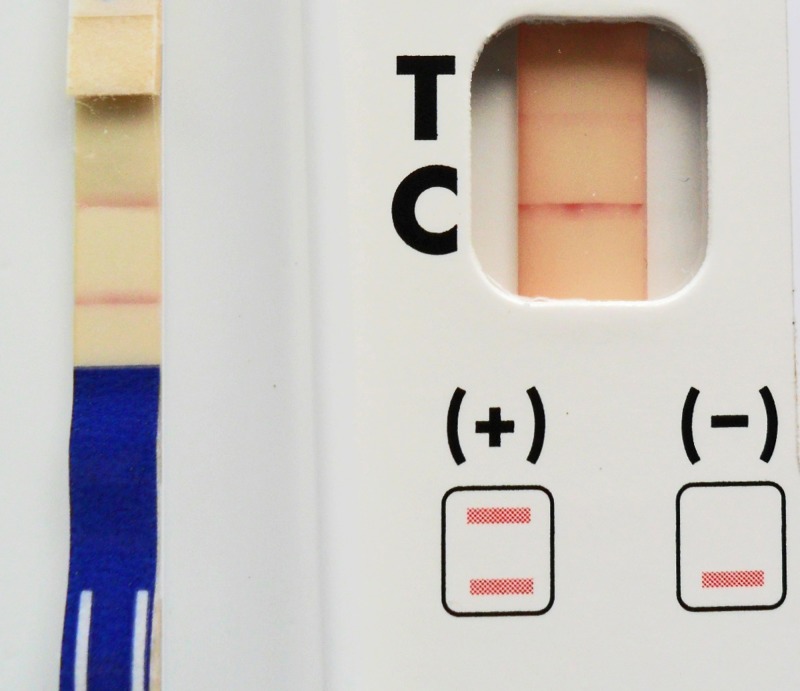
Figure 1.
Left shows a strongly positive Alere Filariasis Test Strip (the lower line is the T or test line; score of 2+). Right shows a weakly positive BinaxNOW Filariasis card test (score of 1+). The tests were performed with the same blood sample, and the photograph was taken 10 minutes after starting the tests.
Test results obtained with various types of infection and control sera are summarized in Table 1. The two tests produced the same results for 220 of 222 samples that had valid results for both tests (99.1% agreement). Apart from one invalid card test, both tests detected filarial antigenemia in all 88 samples from subjects with W. bancrofti microfilaremia. Turning to specificity results, 1 of 10 samples from Indonesian subjects with brugian filariasis was positive by both tests, 1 was positive by the test strip only, and 1 of 10 samples from Ugandan subjects with strongyloidiasis was positive by the test strip only. The positive brugian filariasis serum samples (from Alor Island) and the positive strongyloidiasis serum sample were from subjects who had lived in areas that were coendemic for bancroftian filariasis.14 No positive results were observed with either test for samples from subjects with no history of exposure to bancroftian filariasis, regardless of whether they had other parasitic infections or rheumatoid factor.
Table 1.
Laboratory comparison of the BinaxNOW Filariasis card test and the Alere Filariasis Test Strip using banked serum/plasma samples
| Serum group | Origin of samples | No. tested | No. of positive tests | Remarks | |
|---|---|---|---|---|---|
| Card | Strip | ||||
| W. bancrofti | Sri Lanka* | 29 | 28 | 29 | One card had an invalid result |
| W. bancrofti | Haiti† | 59 | 59 | 59 | One card had an invalid result |
| Brugia timori | Indonesia* | 10 | 1 | 2 | Sera were collected in an area that was coendemic for W. bancrofti |
| Onchocerca volvulus | Cameroon* | 14 | 0 | 0 | Five individuals were coinfected with L. loa |
| Mansonella perstans | Uganda* | 10 | 0 | 0 | Two card tests had invalid results |
| Strongyloides stercoralis or hookworm | Uganda* | 10 | 0 | 1 | Sera were collected in an area that was coendemic for W. bancrofti |
| Non-endemic | Haiti† | 20 | 0 | 0 | |
| Non-endemic | Argentina† | 20 | 0 | 0 | |
| Non-endemic | United States* | 20 | 0 | 0 | One test strip had an invalid result |
| Non-endemic | United States† | 25 | 0 | 0 | One test strip had an invalid result |
| Non-endemic, RF ≥ 1:64 | United States* | 10 | 0 | 0 | RF > 1:64 |
| Total | 227 | 88 | 91 | ||
RF = rheumatoid factor.
Samples tested at Washington University.
Samples tested at The Centers for Disease Control and Prevention.
Card tests and test strips were reevaluated at 24 hours to assess the stability of test results. In the Washington University laboratory, 7 of 71 card tests that were negative at 10 minutes were scored positive at 24 hours by both readers. Two of these samples were samples that were positive by the test strip at 10 minutes; one of these samples was from a subject with strongyloidiasis, and the other was from a subject with brugian filariasis. An additional 17 card tests that were negative at 10 minutes had very faint shadow T lines at 24 hours that were scored borderline positive by one of two readers; 1 of 71 test strips that were negative at 10 minutes was scored positive at 24 hours by both readers. This test strip was a non-endemic serum sample from the United States. Six other test strips that were negative at 10 minutes had weak shadow T lines that were scored borderline positive by one of two readers at 24 hours.
The CDC laboratory also reported that some tests that were negative at 10 minutes were positive at 24 hours, although some of these tests were only judged to be positive at the later time point by one reader; 5 of 65 test strips and 6 of 65 card tests that were negative at 10 minutes were judged to be positive at 24 hours by at least one reader. All of these late positive tests occurred with samples from subjects with no history of exposure to filariasis.
Field evaluation.
Sixteen test strips (3%) and one card test (0.2%) had invalid results with no control line. Most of the invalid test strip results occurred because the volume of blood tested was less than 75 μL or blood was partially clotted before testing. Most invalid test results occurred during the first few days of using the test, and this problem became rare as technicians gained experience using the plastic micropipettes supplied with the test. It is important to avoid bubbles and hold the micropipettes slightly above the horizontal plane during blood collection. The single invalid card test result occurred when too little blood was tested.
Whole-blood antigen test results are summarized in Table 2. Study participants ranged in age from 6 to 89 years, and approximately one-half of the subjects tested were female. The sensitivity of the tests was compared for 503 blood samples that had valid results for both tests. The test strip produced 26 (26.5%) more positive test results than the card test (124/503 versus 98/503). All but one of the samples with positive card tests were also positive by the test strip. Positive test lines were sharper and easier to read in the test strip than the card test. Night blood testing for Mf was performed for 109 of 124 subjects with positive filarial antigen tests. Only eight subjects had Mf by thick smear (range = 1–51 Mf per 60 μL). Seven samples from these subjects were positive for filarial antigenemia by both tests; the other sample was positive by the card test but had an invalid test strip result. No Mf were detected in any of 344 night blood smears examined from subjects with negative filarial antigen tests (Table 3).
Table 2.
Test results by village from the field study in northwestern Liberia that compared the performance of the BinaxNOW Filariasis card test and the Alere Filariasis Test Strip
| Village | No. tested | Mean age (years; range) | Female (%) | No. (%) of positive tests* | No. (%) of invalid tests | ||
|---|---|---|---|---|---|---|---|
| Card test | Test strip | Card test | Test strip | ||||
| Sasanin† | 102 | 29 (6–89) | 54.9 | 11 (10.9) | 16 (16.8) | 1 (1.0)‡ | 7 (6.7) |
| Felaloe | 71 | 28 (6–80) | 39.4 | 9 (12.7) | 14 (20.0) | 0 | 1 (1.4) |
| Sakawo | 143 | 25 (6–70) | 49.7 | 27 (18.9) | 34 (24.5) | 0 | 4 (2.8) |
| Kilima Bendu | 97 | 26 (6–75) | 47.4 | 26 (26.8) | 27 (28.4) | 0 | 2 (2.1) |
| Medikorma | 106 | 38 (6–80) | 55.7 | 27 (25.5) | 33 (31.7) | 0 | 2 (1.9) |
| Total | 519 | 29 (6–80) | 50.1 | 100 (19.3) | 124 (24.7) | 1 (0.2)‡ | 16 (3.1) |
Percent shown only considers tests with valid results.
The first village examined.
Both tests were invalid for this sample.
Table 3.
Comparison of results obtained with the BinaxNOW Filariasis card test and the Alere Filariasis Test Strip with blood samples collected in Liberia
| Card test result | Test strip result | ||
|---|---|---|---|
| Positive | Negative | Positive (%) | |
| Positive (N = 98) | 97 | 1 | 99.0 |
| Negative (N = 405) | 27 | 378 | 6.0 |
| Total (% positive) | 124 (78.2%) | 379 (0.3%) | – |
Results presented are for 503 blood samples, with valid results for both tests.
For 97 blood samples that were positive by both antigen tests, test strip scores were approximately one point higher than card test scores (2.52, SD = 0.70 versus 1.58, SD = 0.68; P < 0.001, Wilcoxon signed ranks test). Only 12 of 36 samples that produced weak (1+) positive test lines in the test strip had positive card test results. However, 85 of 88 samples that produced strong (2 + or 3+) test lines in the test strip had positive card test results. This difference in agreement rates between the two tests was statistically significant (P < 0.001 by χ2) (Table 4).
Table 4.
Comparison of test scores obtained during the field study with the BinaxNOW Filariasis card test and the Alere Filariasis Test Strip for samples that were positive by either test
| Test strip score | Card test score | Total | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| 0 | 0 | 1 | 0 | 0 | 1 |
| 1 | 25 | 11 | 0 | 0 | 36 |
| 2 | 2 | 20 | 4 | 0 | 26 |
| 3 | 1 | 19 | 32 | 10 | 62 |
| Total | 28 | 51 | 36 | 10 | 125 |
These results were obtained with human blood samples (10-minute time point).
Turning to the issue of test result stability, 4 of 379 (1.1%) test strips that were negative at 10 minutes were positive at 30 minutes, and 36 (9.5%) were positive at 24 hours; 3 of 417 (0.7%) card tests that were negative at 10 minutes were positive at 30 minutes, and 264 (63.3%) were positive at 24 hours. These results show that, although the test strip has better test result stability than the card test, late positive tests were observed with both tests at 24 hours, and both tests should be read at 10 minutes as instructed in the package inserts.
Discussion
The BinaxNOW Filariasis card test is widely used in the GPELF as an epidemiological tool for mapping filariasis endemicity and assessing the success of LF elimination programs after MDA. It is also useful for detecting active filarial infections in individual patients suspected of having LF. The Alere Filariasis Test Strip is a next generation filarial antigen test that was developed to improve on the card test. This study was performed to validate the new test and compare its performance with the performance of the card test.
Laboratory studies showed that the test strip has better analytical sensitivity than the card test for detecting filarial parasite antigen. However, the sensitivity and specificity of the two tests were comparable in studies performed in two laboratories with banked serum or plasma samples from individuals with W. bancrofti microfilaremia and various types of control samples. Both tests detected antigenemia in all samples from subjects with microfilaremic W. bancrofti infections, and neither test detected antigenemia in sera from people with no history of exposure to bancroftian filariasis. The high specificity of the new test was not surprising, because it is based on the same reagents that are used in the card test, which has high specificity. No single study can guarantee that a new test will perform well in all settings; additional testing with serum and whole-blood samples should be performed to verify the apparent high specificity of the test strip.
The superior sensitivity of the test strip was more evident in the field study than in the laboratory evaluation, because the positive laboratory samples were all from Mf carriers, who tend to have higher levels of antigenemia than people with amicrofilaremic infections. The test strip generated 26.5% more positive results in the field study than the card test. Differences in antigen detection results between the two tests may be even greater in areas where residual antigen levels are low after multiple rounds of MDA. Additional studies should be performed in such areas to test this hypothesis. The higher sensitivity of the new test may significantly raise the bar for LF elimination programs that use antigen test results to guide decisions on when to stop MDA and for post-MDA surveillance activities such as transmission assessment surveys (TAS).10
Both antigen tests detected CFA in all samples from W. bancrofti Mf carriers tested in the laboratory and field studies. However, relatively few of the antigen-positive subjects in the field study had Mf detected by night blood thick smears. This result probably reflects the impact of ivermectin MDA that study communities received a few months before the field study. Pilot surveys performed in the study area less than 1 year before our study (and before ivermectin) found that 46% of those individuals with positive card tests for filarial antigenemia had Mf detected in 60 μL night blood thick smears. This percentage is consistent with results reported from other areas that were studied before MDA.15,16 The small number of Mf carriers in the field study does not detract from the results; the card test and test strip detect an adult filarial worm antigen in human blood, and they are not designed to detect Mf.
Other than improved sensitivity, other advantages of the test strip relative to the card test are that it is expected to have a longer shelf life at ambient temperatures (15–37°C) and significantly lower purchase and shipping costs for the GPELF. These factors should further improve the field applicability of antigen testing for resource-challenged LF elimination programs. Although the test strip requires slightly less blood than the card test, some practice was required to properly use the micropipettes supplied with the test strips. Users should carefully follow the manufacturer's instructions for the new test.
Our results show that the new test has not completely solved the problem of test result stability. Preliminary observations suggest that this problem can be solved by removing the sample application pad from the test strip with a scissors at the 10-minute time point and placing a drop of isopropyl alcohol on the nitrocellulose membrane. Readers should understand that this off-label modification of the test protocol has not been evaluated or validated by the manufacturer. However, the authors believe that it may be useful when tests are performed in field situations with poor lighting conditions that prevent accurate reading of test results at 10 minutes.
In summary, our results show that the new Alere Filariasis Test Strip has significant technical and practical advantages over the BinaxNOW Filariasis card test. Although additional studies are needed to compare the performance of these two CFA tests in areas with low residual LF endemicity rates after multiple rounds of MDA, we believe that the new test represents a major step forward that will be welcomed by the GPELF and the filariasis research community.
ACKNOWLEDGMENTS
Filarial antigen tests for this study were provided at no cost by Alere Scarborough, Inc. The authors would like to thank the staff of the Liberian Institute for Biomedical Research for their assistance. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Financial support: This work was supported by grants from the Bill and Melinda Gates Foundation.
Disclosure: The filarial antigen tests evaluated in this study use reagents licensed from Barnes-Jewish Hospital, an affiliation of G.J.W. All royalties from sales of these tests are donated to the Barnes-Jewish Hospital Foundation, a registered not-for-profit organization (http://www.barnesjewish.org/groups/default).
Authors' addresses: Gary J. Weil, Kurt C. Curtis, Kerstin Fischer, Andrew C. Majewski, and Peter U. Fischer, Infectious Diseases Division, Department of Internal Medicine, Washington University School of Medicine, St. Louis, MO, E-mails: gweil@dom.wustl.edu, kcurtis@dom.wustl.edu, kefische@dom.wustl.edu, amajewsk@dom.wustl.edu, and pufische@dom.wustl.edu. Lawrence Fakoli, Lincoln Gankpala, and Fatorma K. Bolay, Liberian Institute for Biomedical Research (LIBR), Charlesville, Margibi County, Liberia, E-mails: lawfako2008@yahoo.com, lincolngankpala@yahoo.com, and director.libr@gmail.com. Patrick J. Lammie, Sonia Pelletreau, and Kimberly Y. Won, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: pjl1@cdc.gov, ikh8@cdc.gov, and kfw7@cdc.gov.
References
- 1.WHO . The Global Programme to Eliminate Lymphatic Filariasis: Progress Report 2000–2009 and Strategic Plan 2010–2020. Geneva: World Health Organization; 2011. pp. 1–78. [Google Scholar]
- 2.WHO Global programme to eliminate lymphatic filariasis: progress report, 2011. Wkly Epidemiol Rec. 2012;87:346–356. [PubMed] [Google Scholar]
- 3.Ottesen EA, Duke BO, Karam M, Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- 4.Michael E, Bundy DA, Grenfell BT. Re-assessing the global prevalence and distribution of lymphatic filariasis. Parasitology. 1996;112:409–428. doi: 10.1017/s0031182000066646. [DOI] [PubMed] [Google Scholar]
- 5.Weil GJ, Ramzy RM. Diagnostic tools for filariasis elimination programs. Trends Parasitol. 2007;23:78–82. doi: 10.1016/j.pt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 6.More SJ, Copeman DB. A highly specific and sensitive monoclonal antibody-based ELISA for the detection of circulating antigen in bancroftian filariasis. Trop Med Parasitol. 1990;41:403–406. [PubMed] [Google Scholar]
- 7.Weil GJ, Jain DC, Santhanam S, Malhotra A, Kumar H, Sethumadhavan KV, Liftis F, Ghosh TK. A monoclonal antibody-based enzyme immunoassay for detecting parasite antigenemia in bancroftian filariasis. J Infect Dis. 1987;156:350–355. doi: 10.1093/infdis/156.2.350. [DOI] [PubMed] [Google Scholar]
- 8.Forsyth KP, Spark R, Kazura J, Brown GV, Peters P, Heywood P, Dissanayake S, Mitchell GF. A monoclonal antibody-based immunoradiometric assay for detection of circulating antigen in bancroftian filariasis. J Immunol. 1985;134:1172–1177. [PubMed] [Google Scholar]
- 9.Gass K, Beau de Rochars MV, Boakye D, Bradley M, Fischer PU, Gyapong J, Itoh M, Ituaso-Conway N, Joseph H, Kyelem D, Laney SJ, Legrand AM, Liyanage TS, Melrose W, Mohammed K, Pilotte N, Ottesen EA, Plichart C, Ramaiah K, Rao RU, Talbot J, Weil GJ, Williams SA, Won KY, Lammie P. A multicenter evaluation of diagnostic tools to define endpoints for programs to eliminate bancroftian filariasis. PLoS Negl Trop Dis. 2012;6:e1479. doi: 10.1371/journal.pntd.0001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . Lymphatic Filariasis Transmission Assessment Surveys. Geneva: World Health Organization; 2011. pp. 1–100. [Google Scholar]
- 11.Simonsen PE, Magesa SM. Observations on false positive reactions in the rapid NOW filariasis card test. Trop Med Int Health. 2004;9:1200–1202. doi: 10.1111/j.1365-3156.2004.01326.x. [DOI] [PubMed] [Google Scholar]
- 12.Krolewiecki AJ, Ramanathan R, Fink V, McAuliffe I, Cajal SP, Won K, Juarez M, Di Paolo A, Tapia L, Acosta N, Lee R, Lammie P, Abraham D, Nutman TB. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin Vaccine Immunol. 2010;17:1624–1630. doi: 10.1128/CVI.00259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weil GJ, Malane MS, Powers KG, Blair LS. Monoclonal antibodies to parasite antigens found in the serum of Dirofilaria immitis-infected dogs. J Immunol. 1985;134:1185–1191. [PubMed] [Google Scholar]
- 14.Supali T, Wibowo H, Ruckert P, Fischer K, Ismid IS, Purnomo, Djuardi Y, Fischer P. High prevalence of Brugia timori infection in the highland of Alor Island, Indonesia. Am J Trop Med Hyg. 2002;66:560–565. doi: 10.4269/ajtmh.2002.66.560. [DOI] [PubMed] [Google Scholar]
- 15.Ramzy RM, El Setouhy M, Helmy H, Ahmed ES, Abd Elaziz KM, Farid HA, Shannon WD, Weil GJ. Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet. 2006;367:992–999. doi: 10.1016/S0140-6736(06)68426-2. [DOI] [PubMed] [Google Scholar]
- 16.Weil GJ, Kastens W, Susapu M, Laney SJ, Williams SA, King CL, Kazura JW, Bockarie MJ. The impact of repeated rounds of mass drug administration with diethylcarbamazine plus albendazole on bancroftian filariasis in Papua New Guinea. PLoS Negl Trop Dis. 2008;2:e344. doi: 10.1371/journal.pntd.0000344. [DOI] [PMC free article] [PubMed] [Google Scholar]