Abstract
Canine African trypanosomosis (CAT) is rarely reported in the literature. In this preliminary study, we evaluated the performance of loop-mediated isothermal amplification (LAMP) against microscopy to detect CAT in six exotic dog breeds naturally infected with trypanosomes from Zambia's South Luangwa National Park and Chiawa Game Management Area. To our knowledge, this is the first report of CAT in Zambia. The patients exhibited a variety of aspecific clinical signs. The LAMP did not only confirm all six parasitologically positive CAT cases detected passively between April 2010 and January 2012, but was also critical in trypanosome speciation. According to LAMP, the majority of the dogs had monolytic infections with either Trypanosoma congolense or Trypanosoma brucei rhodesiense. The LAMP is thus a potential simple and cost-effective tool for trypanosome diagnosis in endemic regions. The rare report of zoonotic trypanosomes in dogs in Zambia has public health implications and justifies further investigations of CAT.
Throughout their long history of domestication, dogs have served as a link for parasite exchange among livestock, wildlife, and humans and hence remain an important source of emerging and reemerging diseases in man.1 African trypanosomes that affect dogs include Trypanosoma brucei subspecies, Trypanosoma congolense, and Trypanosoma evansi.2,3 Although trypanosomosis is commonly reported in farm animals,4 canine African trypanosomosis (CAT) is rarely reported in the literature.3,5 This communication evaluates the performance of loop-mediated isothermal amplification (LAMP) in detecting CAT from six exotic dogs naturally infected with trypanosomes from Zambia's South Luangwa National Park (SLNP) and Chiawa Game Management Area (CGMA), against routine microscopy.
During the period between April 2010 and January 2012, the Show Grounds Veterinary Clinic in Lusaka, Zambia, recorded six cases of CAT. All of the patients reported were adult exotic dog breeds between 2 and 5 years of age that were exposed to tsetse bites in the known tsetse-infested Luangwa or Zambezi valleys in the vicinity of national parks. Table 1 summarizes the patient data.
Table 1.
Summary of patient data for canine African trypanosomosis (CAT) cases from South Luangwa National Park and Chiawa Game Management Area during the period 2010–2012
| Patient ID | Gender | Age (yrs) | Breed | Contracted CAT from | Month/year |
|---|---|---|---|---|---|
| 1 | Female | 3 | Boerboel | Chiawa Game Management Area | April 2010 |
| 2 | Male | 3 | Boerboel | Chiawa Game Management Area | November 2010 |
| 3 | Male | 5 | Labrador | Siavonga, near Chiawa Game Management Area | November 2010 |
| 4 | Male | 3 | Labrador | Chiawa Game Management Area | May 2010 |
| 5 | Male | 4 | Boerboel | Mfuwe, South Luangwa National Park | September 2011 |
| 6 | Female | 2 | Jack Russel | Mfuwe, South Luangwa National Park | January 2012 |
The reported symptoms included anorexia, lethargy, loss of vision, and loss of weight. At that stage, parasitic infections were suspected. Clinical examination revealed enlarged lymph nodes, oedematous forelimbs, distended abdomen, bilateral corneal opacity in some cases, conjunctivitis with mucopurulent discharge, average pyrexia of 40.5°C with labored breathing. In addition, most of the patients were found to have severe anemia, evidenced by pale mucous membranes coupled with a low average packed cell volume value of 14%.
After clinical examination, patients were bled and microscopic examination of a wet mount revealed the presence of motile trypanosomes, suggesting that they were suffering from CAT. Patient 6 had a highly acute infection characterized by fulminate parasitemia and died before treatment could be effected (Table 2). The other five patients were admitted to the Clinic for further investigation and received intramuscularly treatment with samorin (Table 2) for a total of 10 days. The patients were also given supportive therapy, became more active within a week, and were discharged after about 2 weeks at which point trypanosomes were no longer detectable in their blood.
Table 2.
Summary of clinical data for canine African trypanosomosis (CAT) patients from South Luangwa National Park and Chiawa Game Management Area during the period 2010–2012
| Patient ID | Breed | Contracted CAT from | Type of parasites detected | Treatment | Outcome |
|---|---|---|---|---|---|
| 1 | Boerboel | CGMA* | Trypanosoma congolense | Samorin | Recovered |
| 2 | Boerboel | CGMA* | Trypanosoma brucei rhodesiense | Samorin | Recovered |
| 3 | Labrador | Siavonga, near CGMA* | Trypanosoma brucei brucei + Trypanosoma congolense | Samorin | Recovered |
| 4 | Labrador | CGMA* | Trypanosoma congolense | Samorin | Recovered |
| 5 | Boerboel | Mfuwe, SLNP† | Trypanosoma congolense | Samorin | Recovered |
| 6 | Jack Russel | Mfuwe, SLNP† | Trypanosoma brucei rhodesiense | Nil | Died |
CGMA = Chiawa Game Management Area.
SLNP = South Luangwa National Park.
The rest of the collected blood samples were sent to the School of Veterinary Medicine, University of Zambia, for LAMP-based trypanosome-species identification. About 200 mL of each sample was placed on a labeled FTA Elute card (Whatman FTA Elute Cards, Whatman, UK) for DNA extraction according to the manufacturer's suggested protocol. The resultant DNA was stored at −30°C until use. A LAMP reaction of 25 mL was performed using a Loopamp DNA Amplification Kit (Eiken Chemical, Tochigi, Japan) and the extracted DNA as template.6,7 This study made use of the recently designed primers specifically targeting the 18S rRNA gene of T. congolense (CON2-LAMP),7 the repetitive insertion mobile element (RIME) gene of the Trypanozoon subgenus group (RIME-LAMP)8 and the human serum resistance-associated (SRA) gene uniquely expressed by T.b. rhodesiense (SRA-LAMP),9 respectively. All RIME-LAMP positive samples were screened for T.b. rhodesiense using SRA-LAMP. RIME-LAMP-positive and SRA-LAMP-negative samples were considered to be T.b. brucei.
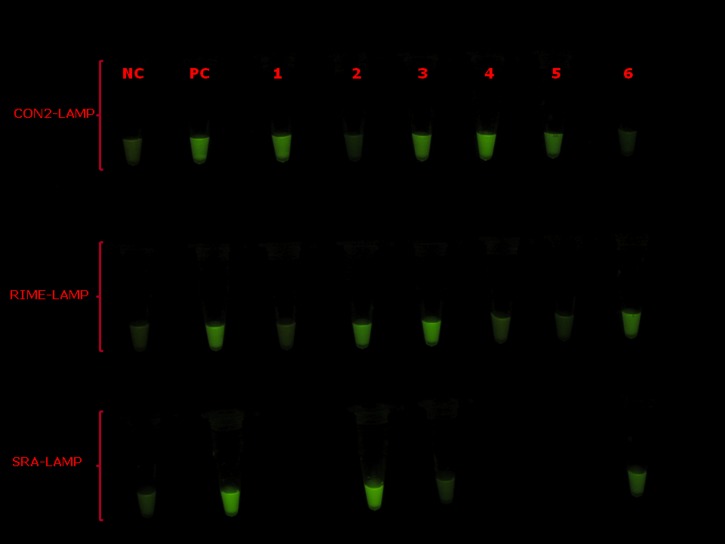
According to CON2-LAMP, three patients were monolytically infected with T. congolense (Figure 1). Figure 1 also shows that one patient was co-infected with T. congolense and T.b. brucei, whereas the other two patients had monolytic infections with T.b. rhodesiense.
Figure 1.
Visual appearance of representative results for the CON2 18S rRNA (CON2)-LAMP (upper row), repetitive insertion mobile element (RIME)-LAMP (middle row), and human serum resistance-associated gene (SRA)-LAMP used for confirmation of canine African trypanosomiasis (CAT). Loopamp Fluorescent detection reagent was added to the reaction mixture at the beginning of the assay. The reactions were incubated at 64°C for 30 minutes. In contrast to the light green background fluorescence in the negative samples, positive samples exhibit a bright fluorescent green color when visualized under the transilluminator. NC = negative control (distilled water); PC = positive control (Trypanosoma congolense for CON2-LAMP; Trypanosoma brucei rhodesiense for RIME-LAMP and SRA-LAMP); 1–6: dog blood sample identity.
The few published reports of CAT mainly involve exotic dog breeds.3,5 Similarly, we report herein six cases of CAT exclusively involving exotic dog breeds that naturally contracted the disease from Zambia's SLNP and CGMA. According to previous reports,10 indigenous dog breeds in tsetse-infested regions of sub-Saharan Africa seem to be trypanotolerant. The CAT in such dogs is either subclinical or asymptomatic.
In the absence of a vaccine, control of trypanosomosis partially depends on early and accurate diagnosis coupled with effective case management.6 However, microscopy, the current gold standard diagnostic test for trypanosomosis, suffers from low sensitivity. Here, we evaluated the performance of LAMP against microscopy to detect CAT in clinical specimens obtained from six patients from SLNP and CGMA. Trypanosome infections in all the cases were initially diagnosed by microscopy and later confirmed by LAMP, showing good correlation between the two methods. Trypanosome species-specific LAMP assays were able to identify specific species of trypanosomes affecting the dogs and tested negative for other species. Of importance, two clinically sound male dogs (a Jack Russel and a Labrador from SLNP and CGMA, respectively) that were brought to the clinic with the patients for examination, tested negative for CAT by microscopy and LAMP (data not shown). According to CON2-LAMP, the majority of dogs were infected with T. congolense. In agreement with previous reports,3 T. congolense-infected dogs tended to exhibit chronic infections unlike those infected with T. brucei subspecies that showed more acute symptoms. Such chronically infected dogs may act as a source of infection to other domestic animals4 in tsetse-infested regions.
In agreement with previous reports,11 all SRA-LAMP-positive cases were also RIME-LAMP positive, indicating their similar sensitivities and that they complimented each other. According to RIME-LAMP, three dogs had infections caused by Trypanozoon subgenus. Interestingly, SRA-LAMP revealed that two of those infections were caused by the human-infective T.b. rhodesiense. In view of the sporadic cases of re-emerging human African trypanosomiasis (HAT) being reported in the old foci,6 the finding of SRA gene in trypanosomes isolated from some dogs in this communication deserves further investigations. Considering the close relationship between dogs and humans, dogs may be a potential source of HAT, which may result when tsetse flies bite humans after taking a blood meal from a trypanosome-infected dog.
In this study, LAMP was not only sensitive, but also very specific and confirmed the microscopic observation of trypanosomes in patient blood. Despite its simplicity, LAMP is highly sensitive, specific, rapid, and user-friendly. Such a cost-effective technique has the potential for being the future principal molecular tool in the diagnosis of trypanosomes and related infections in resource-limited endemic regions. Visual detection of LAMP amplicons is convenient and easy, mainly when parasitemia is sufficiently high, yielding a strong fluorescence. Although LAMP has very high sensitivity,7–9 moderate to weakly positive samples (e.g., sample 5 for CON2-LAMP and sample 6 for SRA-LAMP) as a result of scanty parasitemia sometimes pose challenges caused by a weak fluorescence signal. To reduce subjectivity of result interpretation, plans are underway in our laboratory to start quantifying the fluorescence intensity and consider samples to be positive after subtracting the background fluorescence of the negative control. To our knowledge, this is the first report of CAT in Zambia. Our preliminary data indicate the potential of 1) LAMP in the diagnosis of CAT, and 2) dogs as sources of human trypanosome infections. Future studies should investigate the performance of LAMP in CAT diagnosis, with emphasis on zoonotic trypanosomes, among locally bred dogs in tsetse-infested GMAs and/or national parks.
Footnotes
Financial support: This study was part of the main project entitled “Establishment of rapid diagnostic tools for Tuberculosis and Trypanosomiasis and screening of candidate compounds for Trypanosomiasis in Zambia,” supported by the Japanese International Cooperation Agency (JICA). It also received financial support from the Center for Zoonosis Research, Hokkaido University, Japan, through the fund under the project entitled “Surveillance studies of emerging and re-emerging zoonoses”. This study was also financially supported by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) programmed by the MEXT, Japan.
Authors' addresses: Boniface Namangala and Ladslav Moonga, Department of Paraclinical studies, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia, E-mails: boniface_1020@yahoo.com and ladslavm@yahoo.com. Elizabeth Oparaocha, Show Grounds Veterinary Clinic, Lusaka, Zambia, E-mail: showgroundsvet@gmail.com. Kiichi Kajino, Kyoko Hayashida, Yasuhiko Suzuki, and Chihiro Sugimoto, Research Centre for Zoonosis Control, Hokkaido University, Sapporo, Japan, E-mails: kiichi@czc.hokudai.ac.jp, kyouko-h@czc.hokudai.ac.jp, suzuki@czc.hokudai.ac.jp, and sugimoto@czc.hokudai.ac.jp. Noboru Inoue, National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Japan, E-mail: ircpmi@obihiro.ac.jp.
References
- 1.Dantas-Torres F. Canine vector-borne diseases in Brazil. Parasit Vectors. 2008;1:25. doi: 10.1186/1756-3305-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matete GO. Occurrence, clinical manifestation and the epidemiological implications of naturally occurring canine trypanosomosis in western Kenya. Onderstepoort J Vet Res. 2003;70:317–323. doi: 10.4102/ojvr.v70i4.296. [DOI] [PubMed] [Google Scholar]
- 3.Gow AG, Simpson JW, Picozzi K. The first report of canine African trypanosomosis in the UK. J Small Anim Pract. 2007;48:658–661. doi: 10.1111/j.1748-5827.2007.00423.x. [DOI] [PubMed] [Google Scholar]
- 4.Laohasinnarong D, Thekisoe OM, Malele I, Namangala B, Ishii A, Goto Y, Kawazu SI, Sugimoto C, Inoue N. Prevalence of Trypanosoma sp. in cattle from Tanzania estimated by conventional PCR and loop-mediated isothermal amplification (LAMP) Parasitol Res. 2011;109:1735–1739. doi: 10.1007/s00436-011-2513-2. [DOI] [PubMed] [Google Scholar]
- 5.Keck N, Herder S, Kaba D, Solano P, Gomez J, Cuny G, Davoust B. Epidemiological study of canine trypanosomosis in an urban area of Ivory Coast. Parasite. 2009;16:305–308. doi: 10.1051/parasite/2009164305. [DOI] [PubMed] [Google Scholar]
- 6.Namangala B, Hachaambwa L, Kajino K, Mweene AS, Hayashida K, Simuunza M, Simukoko H, Choongo K, Chansa P, Lakhi S, Moonga L, Chota A, Ndebe J, Nsakashalo-Senkwe M, Chizema E, Kasonka L, Sugimoto C. The use of loop-mediated isothermal amplification (LAMP) to detect the re-emerging Human African Trypanosomiasis (HAT) in the Luangwa and Zambezi Valleys. Parasit Vectors. 2012;5:282. doi: 10.1186/1756-3305-5-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thekisoe OM, Kuboki N, Nambota A, Fujisaki K, Sugimoto C, Igarashi I, Yasuda J, Inoue N. Species-specific loop-mediated isothermal amplification (LAMP) for diagnosis of trypanosomosis. Acta Trop. 2007;102:182–189. doi: 10.1016/j.actatropica.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Njiru ZK, Mikosza AS, Armstrong T, Enyaru JC, Ndung'u JM, Thompson AR. Loop-mediated isothermal amplification (LAMP) method for rapid detection of Trypanosoma brucei rhodesiense. PLoS Negl Trop Dis. 2008b;2:e147. doi: 10.1371/journal.pntd.0000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Njiru ZK, Mikosza AS, Matovu E, Enyaru JC, Ouma JO, Kibona SN, Thompson RC, Ndung'u JM. African trypanosomiasis: sensitive and rapid detection of the sub-genus Trypanozoon by loop-mediated isothermal amplification (LAMP) of parasite DNA. Int J Parasitol. 2008a;38:589–599. doi: 10.1016/j.ijpara.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horchner F, Zillmann U, Metzner M, Schonefeld A, Hehlitz D. West African dogs as a model for research on trypanotolerance. Trop Med Parasitol. 1985;36:257–258. [PubMed] [Google Scholar]
- 11.Matovu E, Kuepfer I, Boobo A, Kibona S, Burri C. Comparative detection of trypanosomal DNA by loop-mediated isothermal amplification and PCR from flinders technology associates cards spotted with patient blood. J Clin Microbiol. 2010;48:2087–2090. doi: 10.1128/JCM.00101-10. [DOI] [PMC free article] [PubMed] [Google Scholar]