Abstract
Three patients diagnosed with scrub typhus through serology and polymerase chain reaction tests, experienced delayed administration of effective antibiotics after the appearance of symptoms, presented with subdural hemorrhage, intracerebral hemorrhage, or cerebral infarction in the late acute phase. Orientia tsutsugamushi should be considered as a causal or provoking factor for cerebrovascular accidents in regions where scrub typhus is endemic, especially in those who receive delayed treatment.
Introduction
Orientia tsutsugamushi, the causative agent of scrub typhus, infects principally endothelial cells in all organs.1 Acute infection, such as a systemic respiratory tract infection, may temporarily increase the risk of myocardial infarction or stroke.1 This study presents two cases of cerebral hemorrhage (one case of subdural hemorrhage and one case of intra-cerebral hemorrhage) and one case of cerebral infarction that occurred during antibiotic treatment of three scrub typhus patients, in whom the effective antibiotic therapy was relatively delayed after the initial onset of symptoms.
Case 1
An 81-year-old man was admitted to Chosun University Hospital, a tertiary hospital in Gwangju City, South Korea. He began to go on walks to pick ginkgo nuts at the beginning of autumn. He had a 2-week history of fever and chills, along with a 1-week history of skin rash. He was initially admitted to a local clinic, where he was diagnosed with scrub typhus and given ceftriaxone and doxycycline for 2 days. His mental status deteriorated, and he was transferred to our hospital. He had no significant medical, social, or family history. He presented with a blood pressure of 100/50 mm of Hg, a respiratory rate of 30/min, a pulse rate of 79/min, and a body temperature of 38°C. The peripheral blood tests performed at presentation indicated a white blood cell count of 5,090/mm3, a hemoglobin level of 10.9 g/dL, and platelets of 62,000/μL. The patient's coagulation test results were as follows: prothrombin time (PT), no coagulation; activated partial prothrombin time (aPTT), 35.5 sec; fibrinogen, no coagulation; fibrin degradation products (FDP), 11.6 μg/mL (normal range, 0–5.0 Ug/mL); and D-dimer, 2,466 ng/mL (normal range, 0–255 ng/mL).
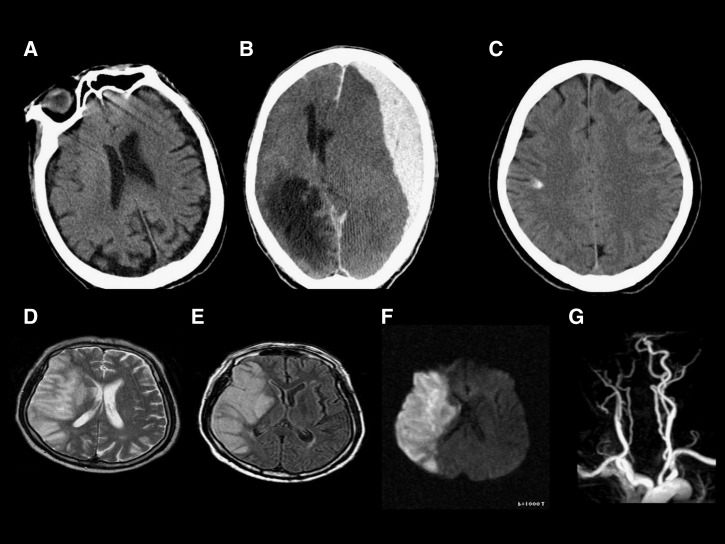
A brain computed tomography (CT) was obtained because of the coagulation defects (Figure 1A). An acute hemorrhage, an infarction or structural abnormalities were not found. Blood cultures showed no growth at presentation. An indirect immunofluorescence test for O. tsutsugamushi, performed at presentation, indicated immunoglobulin M (IgM) 1:256 and IgG 1:2,048, and a nested polymerase chain reaction (PCR) targeting the O. tsutsugamushi 56-kDa protein-encoding gene in the blood buffy coat was positive. The patient was diagnosed with scrub typhus. A comparative analysis of the O. tsutsugamushi DNA sequence from the patient, with those in the GenBank, confirmed that he was infected with the Boryong genotype2; a 500-mg azithromycin intravenously was initiated after hospital admission. However, the patient progressed to a semi-coma on the third day of hospitalization. An additional brain CT scan was performed (Figure 1B). Extensive subdural hemorrhage was detected in the left frontotemporoparital area, along with subfalcine herniation. The patient became comatose and died.
Figure 1.
An 81-year-old male patient with subdural hemorrhage associated with scrub typhus. Brain computed tomography (CT) scan indicated no hemorrhage or infarction on admission (A). Brain CT scan indicated subdural hemorrhage on the third day of hospitalization (B). A 53-year-old female patient with intracerebral hemorrhage associated with scrub typhus. Brain CT scan indicated focal hemorrhage in the right parietal lobe (C). A 74-year-old male patient with cerebral infarction associated with scrub typhus. T2W1 (D), T1W1 (E), and diffusion images (F) of brain magnetic resonance (MR) indicating recent-onset infarction of the territory supplied by the right middle cerebral artery. The MR angiogram (G) indicating occlusion of the right internal carotid artery and lack of display of the right middle cerebral artery.
Case 2
A 53-year-old woman presented at our hospital with a 20-day history of maculopapular skin rashes on the anterior chest and a 5-day history of fever, headache, and nausea. She had worked in an agricultural field twice a week before her admission. Her past medical history, social history, and family history were non-contributory. At presentation, the patient was conscious. Upon the physical examination, non-pruritic erythematous maculopapular rashes were observed on the anterior chest, and a 1 cm × 1 cm eschar was noted on the left axilla.
The blood coagulation test results were as follows: PT, 11.3 sec; international normalized ratio (INR), 0.9/; aPTT, 32.6 sec; fibrinogen, 257 mg/dL; FDP, 2.27 μg/mL; and D-dimer, 389 ng/mL. Immunofluorescence assays to detect antibodies against O. tsutsugamushi revealed IgM and IgG titers of 1:40 and 1:512, respectively, at presentation, and increases of at least 4-fold in the IgM titer (1:160) and IgG titer (1:4,096) were observed after 7 days. Nested PCR targeting the O. tsutsugamushi 56 kDa protein-encoding gene was negative, but nested PCR for an eschar was positive. The presence of O. tsutsugamushi Boryong was confirmed by a sequencing test.2
After a clinical diagnosis of scrub typhus, 600 mg of rifampin were given, but a severe headache persisted. The CT scans performed on the third hospital day revealed a focal hyperdensity and a small amount of blood in the right cerebral hemisphere (Figure 1C). At that time, the routine blood test results were normal and blood coagulation tests revealed the following: PT, 11.4 sec; INR, 0.92; aPTT, 28.6 sec; fibrinogen, 129 mg/dL; FDP, 5.18 μg/mL; and D-dimer, > 1,050 ng/mL. The serum levels of aspartate aminotransferase (227.6 IU/L) and alanine aminotransferase (417.6 IU/L) had increased, compared with the levels at the patient's initial presentation. The patient was discharged from the hospital without any specific sequelae on the ninth day of hospitalization.
Case 3
A 74-year-old man presented at our hospital with high fever and skin rashes. A generalized myalgia had occurred 2 weeks before presentation. He had a history of coronary interventions and took antiplatelet agents and oral hypoglycemic agents. There was no remarkable family history. At presentation, the patient was alert. He had a blood pressure reading of 130/80 mm of Hg, a pulse rate of 85/min, respiratory rate of 24/min, and body temperature of 38°C. The physical examination indicated conjunctional injection and nuchal rigidity. Non-pruritic maculopapular rashes were observed on the chest and abdomen, and a 1 cm × 1.5 cm eschar was noted on the anterior chest. Hematochemical tests revealed the following: white blood count, 11,950/μL; hemoglobin, 12.9 g/dL; platelets, 119,000/μL. Blood coagulation tests indicated the following: PT, 11.9 sec; INR, 0.98; aPTT, 27.9 sec; fibrinogen, 437 mg/dL; FDP 11.8 μg/mL; and D-dimer, > 1,050 ng/mL. Immunofluorescence assays to detect antibodies targeting O. tsutsugamushi revealed IgM and IgG titers of 1:80 and 1:128, respectively, at presentation, which increased to 1:320 and 1:11,024, respectively, after 13 days. Nested PCR targeting the O. tsutsugamushi 56-kDa protein-encoding gene was positive2; a comparative analysis of the O. tsutsugamushi DNA sequence of the patient and those in the GenBank confirmed that he had the Boryong genotype. Based on the clinical features at presentation, the patient was presumptively diagnosed with scrub typhus and given 600 mg of rifampin, after which his fever subsided. At 3:00 am on the fourth day, the patient fell because of muscle weakness during an attempt to get out of bed, and motor weakness was noted on his left side. The brain MRI indicated recent onset infarction in the right middle cerebral artery territory (Figure 1D–G). This study was approved by the Institutional Ethics Board (2012-12-008) for the Clinical Research of Chosun University Hospital.
Discussion
The main pathologic findings of scrub typhus are vasculitis and perivasculitis. Corresponding to this pathologic finding, hemorrhages in various organs, such as atraumatic hemoperitoneum or massive GI bleeding, and acute myocardial infarction or splenic infarctions have been reported as possible complications in scrub typhus patients.3,4 Endothelial cell dysfunction and disseminated intravascular coagulation may occur in response to endothelial cell injury by O. tsutsugamushi in scrub typhus patients,5,6 even though actual mechanism is not fully understood. According to the definition of disseminated intravascular coagulation (DIC) based on the criteria presented by the Scientific Subcommittee on DIC of the International Society of Thrombosis and Hemostasis (ISTH), overt DIC and thrombocytopenia were observed in two of the three patients with a cerebral vascular accident (CVA) in this study.7
Microorganisms that cause the spotted fever group rickettsial diseases induce coagulopathies and thrombotic events8,9; it has been shown that hemostatic and fibrinolytic changes occur as late manifestations of illness, rather than as initial clinical features. An interesting point is that the patients in the CVA cases in this study received relatively delayed administrations of effective antibiotics after the onset of symptoms. Although the time of the vascular event in case 2 (53/F) was unclear, in case 1 and case 3 (81/M and 74/M), a stroke occurred during effective treatment of scrub typhus, in the relatively late acute phase. The hypothesis that delayed treatment of scrub typhus can lead to CVAs is never explicitly stated. In this study, the average time from symptom onset to the administration of effective antibiotics was 16.3 days in scrub typhus patients with a CVA (Table 1). To clarify whether the scrub typhus patients with a CVA received delayed effective antibiotics, we assessed the average time of effective antibiotics after symptom onset for the confirmed scrub typhus patients who presented to our hospital in 2007. Considering that the average time for the administration of effective antibiotics after symptom onset was 6.15 days for the 47 patients who were diagnosed with scrub typhus by a positive PCR test in our hospital in 2007 (P < 0.001, data not shown), we were able to conclude that the scrub typhus patients with a CVA experienced a statistically significant delay in receiving effective antibiotics. Early effective antibiotic treatment may prevent the disease from progressing to DIC in scrub typhus patients. However, the homeostatic dysfunction of endothelial cells may continue if appropriate treatment is delayed. This endothelial dysfunction and vascular events, such as a CVA, may be related, and additional studies on this subject are necessary. In a comparative study of doxycycline and rifampin, conducted in Northern Thailand, in which some O. tsutsugamushi strains showed poor response to doxycycline, rifampin was reported to be more effective than doxycycline.10 Because rifampin is known to penetrate brain tissue effectively,11 rifampin was administered to the patients in case 2 (54/F) and 3 (75/M) in this study. However, Collazos and others12 reported that a patient infected with human immunodeficiency virus developed cerebral infarction and shock after rifampin re-exposure. Rifampin itself is a hepatotoxic drug, with hematological side effects. The possibility of a CVA provocation arising from the administration of rifampin could not be ruled out completely in patients suspected of having coagulation disorders in our study (one of the patients was treated with antiplatelet agents, and all of the patients had extremely high levels of D-dimer). In case 3, the 75-year-old male patient with a cerebral infarction had a history of coronary intervention and had been taking oral hypoglycemic agents for diabetes. In case 1, even though he had no significant medical, social, or family history, he was old enough to have CVA risks. It was difficult to conclude that O. tsutsugamushi was the direct cause of their CVA. However, it is very possible that O. tsutsugamushi may have acted as a provoking factor for CVA.
Table 1.
Clinical manifestations of scrub typhus patients with cerebrovascular accidents*
| Patient no. | Age/sex | Antibiotics | Eschar | Cerebrovascular accidents | Serum antibody titer | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Time of antibiotic administration after symptom onset (day) | Time of vascular event after symptom onset (day) | Acute phase | Convalescent phase | PCR | ||||||
| IgM | IgG | IgM | IgG | Genotype | |||||||
| 1 | 81/M | Doxycycline followed by azithromycin | No | Subdural hemorrhage | 12 | 14 | 256 | 2,048 | Boryong | ||
| 2 | 53/F | Rifampin | Yes | Intracerebral hemorrhage | 20 | 23 | 40 | 512 | 160 | 4,096 | Boryong |
| 3 | 74/M | Rifampin | Yes | Cerebral infarction | 17 | 20 | 80 | 128 | 320 | 11,024 | Boryong |
PCR = polymerase chain reaction.
In conclusion, scrub typhus is known to cause widespread endothelial injury, and this injury is also likely to occur in the cerebrovascular endothelium. Patients already predisposed to develop a CVA and who receive delayed treatment of scrub typhus are at an increased risk for developing a CVA.
Disclaimer: The authors do not have any commercial interests or other associations that might pose a conflict of interest.
Footnotes
Financial support: This study was supported by research funds from Chosun University, 2009.
Authors' addresses: Jong-Hoon Chung, Na-Ra Yun, Dong-Min Kim, Ji-Woon Lee, and Sung Ho Yoon, Department of Internal Medicine, Chosun University School of Medicine, Gwangju, Republic of Korea, E-mails: jschung@chosun.ac.kr, shine@chosun.ac.kr, drongkim@chosun.ac.kr, nolnol3618@hanmail.net, and drdbs@chosun.ac.kr. Seok-Won Kim, Department of Neurosurgery, School of Medicine, Chosun University, Gwangju City, Korea, E-mail: chosunns@chosun.ac.kr.
Reprint requests: Dong-Min Kim, Division of Infectious Diseases, Department of Internal Medicine, Chosun University School of Medicine, 588 Seosuk-dong, Dong-gu, Gwangju, 501-717, Republic of Korea, E-mail: drongkim@chosun.ac.kr.
References
- 1.Moron CG, Popov VL, Feng HM, Wear D, Walker DH. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol. 2001;14:752–759. doi: 10.1038/modpathol.3880385. [DOI] [PubMed] [Google Scholar]
- 2.Kim DM, Kim HL, Park CY, Yang TY, Lee JH, Yang JT, Shim SK, Lee SH. Clinical usefulness of eschar polymerase chain reaction for the diagnosis of scrub typhus: a prospective study. Clin Infect Dis. 2006;43:1296–1300. doi: 10.1086/508464. [DOI] [PubMed] [Google Scholar]
- 3.Lin WY, Lin GM, Chang FY. An unusual presentation of scrub typhus with atraumatic hemoperitoneum. Am J Gastroenterol. 2009;104:1067. doi: 10.1038/ajg.2009.8. [DOI] [PubMed] [Google Scholar]
- 4.Kim DG, Kim JW, Choi YS, Kim SH, Kim SM, Park CG, Seo HS, Oh DJ. Acute myocardial infarction following scrub typhus infection. Int J Cardiol. 2007;114:e18–e20. doi: 10.1016/j.ijcard.2006.07.131. [DOI] [PubMed] [Google Scholar]
- 5.Levine HD. Pathologic study of thirty-one cases of scrub typhus fever with especial reference to the cardiovascular system. Am Heart J. 1946;31:314–328. doi: 10.1016/0002-8703(46)90313-4. [DOI] [PubMed] [Google Scholar]
- 6.Ono Y, Ikegami Y, Tasaki K, Abe M, Tase C. Case of scrub typhus complicated by severe disseminated intravascular coagulation and death. Emerg Med Australas. 2012;24:577–580. doi: 10.1111/j.1742-6723.2012.01600.x. [DOI] [PubMed] [Google Scholar]
- 7.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Hemostasis (ISTH) Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. [PubMed] [Google Scholar]
- 8.Schmaier AH, Srikanth S, Elghetany MT, Normolle D, Gokhale S, Feng HM, Walker DH. Hemostatic/fibrinolytic protein changes in C3H/HeN mice infected with Rickettsia conorii–a model for Rocky Mountain spotted fever. Thromb Haemost. 2001;86:871–879. [PubMed] [Google Scholar]
- 9.Ben-Zvi IE, Meltzer O, Feld I. Bank. A case of murine typhus associated with large vessel infarct of the spleen. Am J Med Sci. 2008;6:502–503. doi: 10.1097/MAJ.0b013e3181586633. [DOI] [PubMed] [Google Scholar]
- 10.Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, Strickman D. Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomized trial. Lancet. 2000;356:1057–1061. doi: 10.1016/S0140-6736(00)02728-8. [DOI] [PubMed] [Google Scholar]
- 11.Mindermann T, Zimmerli W, Gratzl O. Rifampin concentrations in various compartments of the human brain: a novel method for determining drug levels in the cerebral extracellular space. Antimicrob Agents Chemother. 1998;42:2626–2629. doi: 10.1128/aac.42.10.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez E, Collazos J, Mayo J. Shock and cerebral infarct after rifampin re-exposure in a patient infected with human immunodeficiency virus. Clin Infect Dis. 1998;27:1329–1330. [PubMed] [Google Scholar]