Abstract
We assessed the relationship of fecal environmental contamination and environmental enteropathy. We compared markers of environmental enteropathy, parasite burden, and growth in 119 Bangladeshi children (≤ 48 months of age) across rural Bangladesh living in different levels of household environmental cleanliness defined by objective indicators of water quality and sanitary and hand-washing infrastructure. Adjusted for potential confounding characteristics, children from clean households had 0.54 SDs (95% confidence interval [CI] = 0.06, 1.01) higher height-for-age z scores (HAZs), 0.32 SDs (95% CI = −0.72, 0.08) lower lactulose:mannitol (L:M) ratios in urine, and 0.24 SDs (95% CI = −0.63, 0.16) lower immunoglobulin G endotoxin core antibody (IgG EndoCAb) titers than children from contaminated households. After adjusting for age and sex, a 1-unit increase in the ln L:M was associated with a 0.33 SDs decrease in HAZ (95% CI = −0.62, −0.05). These results are consistent with the hypothesis that environmental contamination causes growth faltering mediated through environmental enteropathy.
Introduction
Children living in low-income countries continue to suffer widely from undernutrition: a recent survey in Bangladesh reported that 41% of children under age 5 years were stunted, 36% of children under age 5 years were underweight, and 16% of children under age 5 years were wasted by World Health Organization (WHO) standards.1 Childhood undernutrition negatively affects cognitive development, increases infection risk, limits physical capacity and childbearing, reduces adult economic productivity, and increases mortality risk.2 A recent pooled analysis of nine studies concluded that diarrhea is a minority contributor to growth faltering: 25% of stunting in 24-month-old children was associated with a higher burden of diarrhea (five or more episodes) in the first 24 months of life.3 Also, most nutrition interventions, such as complementary feeding programs, fail to achieve expected growth improvements, and on average, increase length-for-age scores by only 0.2–0.5 SD.4,5 Environmental enteropathy (also referred to as tropical enteropathy) was estimated to explain 40–64% of growth faltering in a small cohort of children in The Gambia.6,7 Furthermore, children with severe forms of undernutrition, marasmus and kwashiorkor, manifest attributes typical of environmental enteropathy.8
Environmental enteropathy is a disorder featuring a small bowel with abnormal morphology and physiology. Biopsies of patients presenting with environmental enteropathy are characterized by crypt hyperplasia, villous atrophy, lymphocyte infiltration into the lamina propria and epithelium, reduced mucosal surface area, and increased intestinal permeability.9 Because biopsies are invasive and often infeasible, investigators use indirect measures of gut function to characterize environmental enteropathy. The non-invasive dual sugar permeability assay measures the ratio of lactulose to mannitol (L:M) excreted in urine.10 The increased absorption of L, a disaccharide normally not absorbed, is thought to reflect gut inflammation, resulting in impaired functioning of the tight junctions. The decreased permeability of M, a monosaccharide passively absorbed, is likely an indication of villous atrophy and decreased absorptive surface. A high L:M ratio is associated with environmental enteropathy.10 Elevated plasma immunoglobulin G (IgG) endotoxin core antibody (EndoCAb) titers reflect exposure to endotoxin, a cell wall component of many gram-negative bacteria that could potentially translocate across a leaky or damaged mucosa. Total IgG is also measured, because elevated IgG levels suggest infection or chronic immunostimulation.
Potentially modifiable environmental and socioeconomic exposures may contribute to environmental enteropathy.4,11,12 Poor sanitation conditions and environmental enteropathy are commonly observed in low-income households and countries.4 A previous multinational study revealed an association between lower gross domestic product (GDP) per capita and more severe environmental enteropathy.13 In Zimbabwe, household socioeconomic status positively correlated with intestinal absorptive capacity.14 A small-scale handwashing promotion intervention in Nepal failed to achieve improvements in environmental enteropathy markers.15 Other than these studies, there is scant evidence to support the association between environmental enteropathy and environmental conditions distinct from other exposures in a low-income area. If environmental enteropathy is potentially caused by widespread fecal contamination and chronic insults to the gastrointestinal tract, we do not know whether reasonably achievable changes in household environmental conditions would have a meaningful impact on child gut function and growth patterns.
We hypothesized that children living in clean households with good hygiene would have lower prevalence of parasites and environmental enteropathy and better growth (less stunting, wasting, and underweight conditions) compared with children living in contaminated households with poor hygiene. Our rationale for these hypotheses was that children from clean hygienic environments would ingest fewer parasites and fecal bacteria, which would decrease infections, systemic inflammation, and likelihood that the children would acquire environmental enteropathy. Children living in clean household environments would develop normal functioning intestines that would properly absorb nutrients, and they would experience better growth compared with children living in more contaminated household environments. We leveraged an existing cohort of children from 993 households in rural Bangladesh to measure the specific association between household environmental conditions and measures of parasitic infection, environmental enteropathy, and growth.16
Materials and Methods
Ethical approval.
Parents of study participants provided informed consent. The institutional review boards of the International Center for Diarrheal Disease Research, Bangladesh (icddr,b) and the University of California at Berkeley approved the study protocol.
Study population and selection criteria.
In 2007, the Government of Bangladesh Department of Public Health Engineering in collaboration with the United Nations Children's Fund and the Department for International Development of the British Government launched the nationwide Sanitation, Hygiene Education, and Water Supply—Bangladesh (SHEWA-B) program targeting 20 million low-income rural residents in Bangladesh. From July of 2007 to September of 2009, detailed household information, including sanitary infrastructure, defecation practices, handwashing practices, drinking water sources, and drinking water contamination, was collected on 993 households from 100 communities to evaluate the impact of the SHEWA-B interventions.16 We conducted this follow-up study in 2010.
We selected households at two extremes of the distribution of household environments to maximize the difference between the clean and contaminated environments. Among cohorts from both households that were provided the intervention and households from areas that did not receive the intervention (control areas), we identified children who were less than 4 years old in March of 2010 and lived in households that met our criteria for clean and contaminated hygienic environments. We chose a 4-year age cutoff after adjusting the age criteria upward from birth until we could enroll a sufficient number of children to test our hypotheses (determined by our sample size calculation). Household environment classifications were defined a priori. A clean environment was defined as a household with good water quality (median Escherichia coli < 10 CFU/100 mL in quarterly drinking water samples collected over 24 months), improved sanitation (flush/septic/piped sewerage or a pit latrine with a slab and a water seal), and hygienic handwashing conditions (a dedicated location to wash hands stocked with soap and water). In contrast, a contaminated environment was defined as a household with poor water quality (median E. coli ≥ 10 CFU/100 mL), inadequate sanitation (open defecation, open pit latrines, slabs with broken water seals, toilets that flush to somewhere else, or hanging toilets), and unhygienic handwashing conditions (a dedicated location that lacked either water or soap or the absence of a dedicated location to wash hands). We used these criteria to classify households as clean or contaminated household environments, because they are conditions that could feasibly be improved through interventions. We chose 10 E. coli CFU/100 mL as the water quality cutoff point based on WHO drinking water classification level of high risk17; we also considered a cutoff point of 100 but found it to be too restrictive: only 26 households had a median E. coli concentration > 100 CFU/100 mL. These criteria identified 136 children for the follow-up study (74 children in clean households and 62 children in contaminated households), which was 11% (136/1,187) of the SHEWA-B cohort.
Household water, sanitation, and hygiene conditions.
Using the protocols from the 2007 survey, fieldworkers conducted observational spot checks of water, sanitation, and hygiene (one spot check per household) to evaluate the household conditions again in selected households in 2010.16 Fieldworkers noted the water source that was offered by the respondent for drinking. They inspected the toilet facility used by members of each household. In addition to collecting data on the type and condition of each toilet facility, fieldworkers recorded the presence or absence of stool on the slab or floor. Fieldworkers asked caregivers to show them where children (≤ 48 months old) mainly defecated. They asked survey respondents to “show me where you usually wash your hands after you use the toilet” and recorded the presence or absence of water and soap.
Stool collection and parasite assays.
The methods for sample collection and analysis closely followed previously described methods.18 To measure intestinal parasites, fieldworkers collected one stool specimen from each target child and additional stool samples from a convenience sample of 69 siblings (< 1 month to 20 years) of the target children. Stool specimens were transported on dry ice to icddr,b within 24 hours after collection and stored at −80°C. Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used for the detection of Cryptosporidium parvum (Cryptosporidium Test; TechLab, Inc., Blacksburg, VA), Giardia lamblia (Giardia Test; TechLab, Inc., Blacksburg, VA), and Entamoeba histolytica (E. histolytica II Test; TechLab, Inc., Blacksburg, VA). Direct microscopy was used to examine aliquots of each stool sample (preserved in 10% formalin) for the presence of helminth ova. The endemic helminth species that were identified included Ascaris lumbricoides, Trichuris trichiura, and hookworm (Ancylostoma duodenale and Necator americanus). We used the concentrations of helminth ova to identify children with moderate or heavy infections using WHO levels.19
Anthropometrics.
Fieldworkers were trained for 10 weeks on sample collection and anthropometry protocols, and they were blinded to the household environmental classifications to reduce the potential for biased outcome assessment. At enrollment in SHEWA-B (2007), fieldworkers recorded anthropometric measurements for each child, and fieldworkers took new measurements when they revisited the households 3 years later (2010). Fieldworkers used standard techniques to measure the child's weight with an electronic scale (Tanita HD-318; Japan), head circumference with a pediatric head circumference measuring band (Seca 212; United Kingdom), and height (≥ 24 months old) or supine length (< 24 months old) with a portable stadiometer.20,21 Fieldworkers measured height (length) and head circumference to the nearest 0.1 cm and measured weight with 0.05-kg precision. Fieldworkers measured child height in duplicate, and if the measurements fell outside of the acceptable range of difference (0.5 cm), they recorded a third measurement. During training and at regular intervals during the study, we checked the accuracy and precision of fieldworkers' measurements by unannounced spot checks of household visits. We used height, weight, and head circumference measurements to calculate z scores standardized to the WHO 2006 growth standards using publicly available software.22
Urine collection and intestinal permeability assay.
Urine collection closely adhered to the protocol previously described by Goto and others.23 The oral solution administered in the dual sugar permeability assay consisted of L (250 mg/mL) and M (50 mg/mL). Children received an oral dose of 2 mL LM solution per 1 kg body weight. Fieldworkers requested mothers to fast their child for 1 hour before oral intake of the LM solution. From each child, fieldworkers collected urine specimens in a pediatric urine collection bag (Pediatric/Non-Sterile U-Bag; Hollister Limited, Aurora, ON, Canada). They collected urine over the course of 5 hours in a graduated cylinder containing 0.5 mL thimerosal, an antiseptic. After 5 hours, fieldworkers measured the total volume, transferred a 10-mL aliquot on dry ice to icddr,b, and stored it at −80°C. We excluded children with diarrhea, vomiting, or a perigenital skin infection on the day of the LM test (N = 3). We calculated the LM excretion ratio based on L and M concentrations determined by high-performance liquid chromatography (HPLC) combined with pulsed amperometric detection (PAD).24
Blood collection and immunological assays.
Fieldworkers collected a finger-prick blood sample (400 μL) from each child. We used a standard sandwich ELISA to quantitate the concentration of total IgG (IgG ELISA Kit; Bethyl Laboratories, Inc., TX) and EndoCAb (EndoCab ELISA Kit; Hycult Biotech Inc., Uden, The Netherlands) in the blood samples.
Statistical methods.
Primary outcomes included the urine and serum biomarkers L:M ratio, total IgG, and EndoCAb IgG. We estimated that a sample with 50 children per group could detect a difference of between 0.2 and 0.3 in the ln L:M ratio assuming 90% power, a type I error rate of 5%, and an outcome SD of 0.323 to 0.5.25 All three biomarker distributions were right-skewed, and therefore, we log-transformed them for the analysis. We repeated all analyses of continuous outcomes on their original scale and standardized versions (subtracting their mean and dividing by their SD).
The parameter of interest for all comparisons between children from clean and contaminated household environments was a difference in means. For binary outcomes, the parameter corresponds to a prevalence difference, and for standardized outcomes, the parameter corresponds to a difference in SDs. For each outcome, we estimated adjusted differences between groups using a generalized linear model; for binary outcomes, we fit linear binomial models. We selected covariates measured at enrollment in 2007 to include in adjusted analyses using the following pre-specified criteria: (1) imbalanced between groups in 2007, (2) prevalence in both groups between 10% and 90% (to ensure sufficient variation), and (3) associated with the outcome (defined in a univariate analysis as P < 0.2 or a difference of ±0.2 SDs [continuous outcomes] or a risk ratio between 0.8 and 1.25 [binary outcomes]). Supplemental Table 1 lists the full set of possible covariates that we considered, and the set that met criteria 1 and 2 was child age, age squared, sex, household head works in low-skilled labor, number of people in the household, number of rooms in the household, household owns land other than homestead land, house floor material, house wall material, and household has electricity, tables, chairs, watches or clocks, beds, radio, television, or bicycle. In all analyses, we calculated robust SEs clustered at the village level to allow for outcome correlation between children in the same village.26 As an additional robustness check, we repeated adjusted analyses using two matching estimators: Mahalanobis and Genetic Matching.27 We additionally measured the association between our urine and serum biomarkers and anthropometric measurements using linear regression with and without adjustment for child age and sex.
Results
Study population.
Of 136 children in the sample, 17 children were not enrolled in the follow-up survey because of migration (N = 13) or death (N = 4). The remaining 119 children were distributed across 83 villages in rural Bangladesh and included 66 children from clean environments and 53 children from contaminated environments.
In 2007, at the time of enrollment in the SHEWA-B study, children from the clean and contaminated household environment groups differed across several measures of socioeconomic status (Supplemental Table 1). Mean age at enrollment in 2007 was 9 months (range = 1, 17), and mean age at follow-up in 2010 was 35 months (range = 10, 48); 47 children included in this follow-up study were born in the 31 months that elapsed between the 2007 and 2010 surveys. Age and sex distributions were similar between the two groups. Objective, observed hygiene spot-check measures that were not used in the formal definitions of the groups differed between the two groups. For example, clean households were more likely than contaminated households to have respondents with clean fingernails (38% versus 11%; P = 0.002) and children with clean fingernails (20% versus 8%; P = 0.061). Furthermore, children in clean household environments had lower diarrhea prevalence in the 24 months after enrollment than children in contaminated household environments (11.7% versus 15.6%; difference = −3.9%, 95% confidence interval [95% CI] = −8.5%, −0.6%). Nonetheless, households that were classified as having clean hygiene conditions still had high levels of contamination by some spot-check measures. For example, among households classified as clean, 21% had stool visible on the latrine floor, and 79% disposed of child feces in the bushes or no designated place (Supplemental Table 1). When asked to guess household status (contaminated versus clean), blinded outcome assessors correctly guessed in 67% (71/106) of cases but were confident of their guess in only 22% (Supplemental Table 2).
Parasitic infection.
Children living in clean household environments had lower prevalence of all detectable parasites measured compared with children in contaminated household environments (Supplemental Table 3). Children (< 5 years old) from clean households had lower Ascaris prevalence than children from contaminated households (8% versus 21%), and much of this difference resulted from moderate or heavy infections (3% versus 14%) (Supplemental Table 3). After statistical adjustment for confounding variables, children in clean households had 12% lower Ascaris prevalence (95% CI = −30%, 6%) compared with children in contaminated households (Table 1). After statistical adjustment, we detected no differences in Trichuris or Giardia between groups that were significant at the 95% CI level. Cryptosporidium infection was rare (< 2%), and no child tested positive for hookworm or Entamoeba (Supplemental Table 3).
Table 1.
Unadjusted and adjusted differences in outcomes among children living in different household environments measured in 2010
| Outcome | Clean environment mean | Contaminated environment mean | Unadjusted difference (95% CI) | Age- and sex-adjusted difference (95% CI) | Fully adjusted* difference (95% CI) |
|---|---|---|---|---|---|
| Ascaris, proportion infected | 0.08 | 0.22 | −0.14 (−0.30, 0.02) | −0.12 (−0.28, 0.04) | −0.12 (−0.30, 0.06) |
| Trichuris, proportion infected | 0.11 | 0.16 | −0.05 (−0.18, 0.09) | −0.05 (−0.18, 0.08) | 0.02 (−0.13, 0.17) |
| Giardia, proportion infected | 0.34 | 0.37 | −0.02 (−0.20, 0.16) | −0.02 (−0.19, 0.16) | 0.01 (−0.21, 0.23) |
| HAZ | −1.66 | −2.57 | 0.91 (0.17, 1.65) | 0.96 (0.51, 1.41) | 0.54 (0.06, 1.01) |
| WAZ | −1.62 | −2.04 | 0.42 (0.02, 0.83) | 0.48 (0.08, 0.88) | 0.04 (−0.48, 0.55) |
| WHZ | −0.99 | −0.86 | −0.12 (−0.54, 0.30) | −0.10 (−0.52, 0.32) | −0.19 (−0.61, 0.24) |
| HCZ | −2.12 | −1.82 | 0.30 (−0.05, 0.65) | 0.36 (0.02, 0.71) | 0.08 (−0.36, 0.53) |
| Proportion HAZ < −2 | 0.33 | 0.74 | −0.40 (−0.57, −0.24) | −0.42 (−0.59, −0.26) | −0.22 (−0.42, −0.02) |
| Proportion WAZ < −2 | 0.33 | 0.49 | −0.16 (−0.34, 0.02) | −0.17 (−0.34, 0.01) | −0.02 (−0.26, 0.22) |
| Proportion WHZ < −2 | 0.11 | 0.09 | 0.01 (−0.10, 0.13) | 0.004 (−0.11, 0.12) | 0.10 (−0.03, 0.23) |
Differences are clean households minus contaminated households (95% CI). All estimates are restricted to the children from the original SHEWA-B sample < 4 years old (N = 119 for anthropometry, N = 118 for enteropathy biomarkers, N = 116 for parasitic infections). HCZ = head circumference-for-age z score.
Fully adjusted models adjust for age, age squared, sex, household head occupation, land ownership other than the homestead, number of people in the household, number of rooms in the house, house floor materials, house wall materials, house electricity, and asset ownership (tables, watches, beds, radio, television, and/or bicycle). The text has details on the model selection process.
Growth in 2007 and 2010.
Children from clean household environments grew better than children from contaminated environments at the time of enrollment in SHEWA-B in 2007 and the follow-up visit in 2010. Unadjusted differences in mean height-for-age z scores (HAZs) between the clean and contaminated households were 1.01 in 2007 and 0.91 in 2010 (Supplemental Figure 1 and Supplemental Tables 4 and 5). HAZ declined during the 3 years between measurements (in 2007: clean = −0.90 SDs, contaminated = −1.90 SDs; in 2010: clean = −1.66 SDs, contaminated = −2.57 SDs) (Supplemental Figure 1 and Supplemental Tables 4 and 5). Between 2007 and 2010, stunting prevalence increased from 27% to 33% in clean households and increased from 48% to 74% in contaminated households (Supplemental Figure 1 and Supplemental Tables 4 and 5). After statistical adjustment for potential confounders, children from clean households had 0.54 SDs (95% CI = 0.06, 1.01) higher HAZ and −22% (95% CI = −42%, −2%) lower stunting prevalence compared with children from contaminated households (Table 1). After statistical adjustment, we observed no detectable differences between the groups in weight-for-age z score (WAZ), weight-for-height z score (WHZ), and underweight or wasting prevalence.
Intestinal permeability.
Children living in clean household environments had lower L:M ratios (improved gut function) than children from contaminated households (Table 2). The lower L:M ratio in children from improved household environments resulted from both lower L recovery and higher M recovery (Table 2). After standardized log transformation and statistical adjustment for potential confounders, ln L:M ratio values were −0.32 SDs lower (95% CI = −0.72, 0.08) in children from clean household environments than children from contaminated household environments (Table 2 and Supplemental Figure 2).
Table 2.
Unadjusted and adjusted differences in environmental enteropathy biomarker measurements among children living in different household environments measured in 2010
| Biomarker | Clean environment | Contaminated environment | Unadjusted difference* (95% CI) | Age- and sex-adjusted difference (95% CI) | Fully adjusted† difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Total IgG concentrations | |||||||
| Total IgG (mg/mL) | 28.19 | 30.58 | 38.77 | 37.10 | |||
| Ln total IgG (mg/mL) | 2.79 | 1.08 | 3.11 | 1.12 | −0.33 (−0.76, −0.09) | −0.32 (−0.74, −0.10) | −0.60 (−1.05, −0.14) |
| Standardized ln total IgG | −0.14 | 0.97 | 0.16 | 1.02 | −0.30 (−0.69, −0.08) | −0.29 (−0.67, −0.09) | −0.54 (−0.95, −0.13) |
| IgG EndoCAb antibody titers | |||||||
| EndoCAb (MU/mL) | 50.02 | 37.01 | 62.42 | 44.06 | |||
| Ln EndoCAb (MU/mL) | 3.02 | 2.24 | 3.60 | 1.71 | −0.58 (−1.30, 0.14) | −0.66 (−1.40, 0.07) | −0.48 (−1.29, 0.33) |
| Standardized ln EndoCAb | −0.12 | 1.10 | 0.16 | 0.84 | −0.29 (−0.64, 0.07) | −0.33 (−0.69, 0.04) | −0.24 (−0.63, 0.16) |
| Dual sugar urine assay | |||||||
| L (mg per 100 ml) | 134.19 | 184.92 | 206.52 | 567.12 | |||
| M (mg per 100 mL) | 688.82 | 788.97 | 636.99 | 567.20 | |||
| L:M ratio | 0.21 | 0.21 | 0.31 | 0.32 | |||
| Ln L:M ratio | −1.92 | 0.88 | −1.55 | 0.88 | −0.37 (−0.68, −0.06) | −0.28 (−0.60, 0.04) | −0.29 (−0.64, 0.07) |
| Standardized ln L:M ratio | −0.19 | 0.98 | 0.23 | 0.98 | −0.42 (−0.77, −0.07) | −0.31 (−0.67, 0.05) | −0.32 (−0.72, 0.08) |
Differences are clean households minus contaminated households (95% CI). IgG EndoCAb antibody titers: N = 64 clean and N = 53 contaminated. Total IgG and dual sugar permeability assay: N = 65 clean and N = 52 contaminated. EndoCAb standard median units (MUs) IgG are arbitrary and based on medians of ranges for 1,000 healthy adults in a specific location.
We only presented unadjusted differences for log-transformed variables, because the distributions of the untransformed variables are highly right-skewed.
Fully adjusted models adjust for age, age squared, sex, household head occupation, land ownership other than the homestead, number of people in the household, number of rooms in the house, house floor materials, house wall materials, house electricity, and asset ownership (tables, watches, beds, radio, television, and/or bicycle). The text has details on the model selection process.
Immunostimulation.
After statistical adjustment for potential confounders, children from clean households had −0.24 SDs lower (95% CI = −0.63, 0.16) EndoCAb titers than children from contaminated households (Table 2 and Supplemental Figure 3). After statistical adjustment for potential confounders, ln total IgG titers were −0.54 SDs lower (95% CI = −0.95, −0.13) in children from clean households than children from contaminated households (Table 2 and Supplemental Figure 4).
Associations between gut function or immunostimulation and growth.
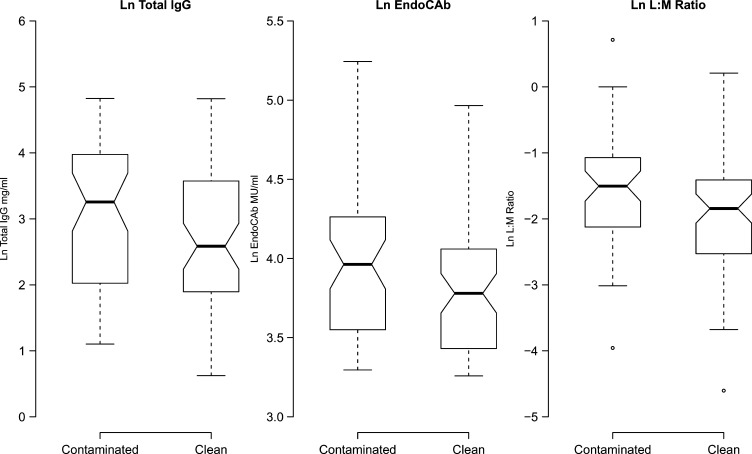
Consistent with the mean differences between groups, the distribution of the total IgG levels, EndoCAb titers, and L:M ratios were shifted lower for children in clean household environments (Figure 1). Average IgG EndoCAb and total IgG titers increased slightly with age, and the L:M ratio declined until age 30 months and then remained flat (Supplemental Figure 5). After adjusting for age and sex, the L:M ratio was strongly associated with HAZ and WAZ scores. A 1-unit increase in the ln L:M was associated with a −0.36 SDs reduction in HAZ (95% CI = −0.62, −0.05) and a −0.24 SDs reduction in WAZ (95% CI = −0.47, −0.01) (Supplemental Table 6). None of the anthropometric measurements were strongly associated with IgG EndoCAb or total IgG titers.
Figure 1.
Tukey box plots of total IgG, EndoCAb titer, and L:M ratio distributions by environmental group. Heavy horizontal lines mark median values, and box edges mark the interquartile range. If the box notches do not overlap between groups, there is strong evidence that the median values differ (there is slight overlap in both cases, consistent with the marginally significant differences reported in Table 2). EndoCAb standard median units (MUs) are arbitrary and are based on medians of ranges for 1,000 healthy adults in a specific location.
Robustness checks.
When we re-estimated differences between groups using two different matching estimators, which make fewer assumptions about the relationship between the adjustment covariates and household environmental group, we obtained results consistent with our primary analysis for all outcomes (Supplemental Table 7).
Discussion
To our knowledge, this study is the first to show a joint association between poor household environmental conditions, markers of environmental enteropathy, and growth faltering. After adjustment for potentially confounding covariates, children living in clean household environments had higher HAZs (+0.54 SDs), lower L:M ratios (−0.32 SDs), lower IgG EndoCAb titers (−0.23 SDs), and 12% lower prevalence of Ascaris than children living in contaminated households. Furthermore, the L:M ratio was strongly associated with HAZ in the cohort. The internal consistency of group differences across a broad set of outcomes lends support to the hypothesis that contaminated environmental conditions can adversely influence growth in young children and that the effect may be mediated, in part, through chronic gut dysfunction.
Even in the absence of drastic infrastructure improvements, children in clean households fared better in measures of parasitic infection, gut function, and growth compared with children in contaminated households. Fieldworkers blinded to household environmental classifications guessed the correct group in 67% of households and were confident of their guess in only 22% (Supplemental Table 6); this result underscores the general context of the study, in which clean households had improved environmental conditions compared with contaminated households but were not radically different (Supplemental Table 1).
Furthermore, clean household environments were not spatially clustered in the SHEWA-B cohort—the 119 children in this follow-up study were located in 83 villages that were spread across nearly all of Bangladesh. Our study showed that, even when children from clean households were surrounded by other households that did not meet our definition of a clean environment, these children still had dramatically lower stunting prevalence, lower levels of parasitic infection, and better gut function. This observation suggests that the household or compound may be a relevant unit for intervention.
In our adjusted analyses, stunting prevalence was 22% points lower and HAZ was 0.54 SDs higher among children living in clean households compared with those children living in contaminated households. The magnitudes of the growth differences observed (0.54 SDs) are commensurate with improvements achieved with nutrition interventions.4 Our findings are consistent with past observational studies that have showed that gains in height are largest with joint improvements in water and sanitation conditions.28–31 However, multiple intervention studies have documented no effect of household environmental improvements on HAZ, and the relationship between environmental improvements and growth remains ambiguous.15,32,33 Of the intervention studies to date, just one has measured the effects on markers of environmental enteropathy.15 Langford and others15 enrolled households in a Kathmandu slum and randomly assigned them to a handwashing promotion intervention. The study reported large reductions in diarrhea but no improvements in HAZ or environmental enteropathy markers. It is possible that these diverging study results derive from differences in study design: our study deliberately selected households representing two extremes of the distribution of household environments to maximize the observed differences, whereas Langford and others15 conducted a randomized trial that included a small number of children (N = 88) from a range of environments and only intervened on handwashing (not water quality and sanitation).
The mean L:M ratio was 0.21 and 0.31 for children living in clean and contaminated environments, respectively. The L:M ratios in both groups are higher than the mean ratio (0.12; a value considered to be the norm) previously found in a UK infant cohort, and they are within or above the range reported in Bangladeshi infants and children (0.15–0.24).23,34–36 It is possible that these previous studies in rural Bangladesh reported less sample variability compared with our study, because they were conducted over a narrow age and/or geographical range, whereas our sample was drawn from an age distribution that ranged from 10 to 48 months and was spread over 83 distant villages. High L:M ratios were strongly associated with growth faltering (lower HAZ and WAZ scores) in our cohort, which was consistent with results from rural Gambian and Bangladeshi infant studies.6,7,23 Although Campbell and others7 reported a strong association between plasma IgG and IgG EndoCAb concentrations and growth (predicting > 40% of growth faltering), we did not observe these associations. The discrepancy may be because Campbell and others7 measured environmental enteropathy immune markers during the relevant period of growth faltering (< 24 months),37 whereas most of our cohort had already passed this growth-faltering window by the time of our follow-up measurement. Because of the small sample size of this study and the timing of the measurements, we view these results as supportive but not conclusive evidence for these associations or lack thereof.
Beyond small sample size, this study had some additional limitations. This study lacked the temporal ordering necessary to establish causal relationships: differences in growth faltering between clean and contaminated environments were already in place by 2007, but environmental enteropathy markers were not measured until 2010. Because the growth differences between the two groups were already present when the average age in this cohort was 9 months old, we cannot exclude the possibility that the differences are caused by prenatal or early post-natal exposures, such as maternal and child nutrition, which we did not measure in this study. The environmental enteropathy measures collected in this study reflected the gut conditions when the cohort was, on average, 35 months old. The L:M ratio was associated with past growth, but this study does not show that it is associated with future growth. A prospective study would be required to show the association between environmental enteropathy markers and future growth.
Because we did not randomize household environmental conditions in this cohort, it remains possible that observed differences in growth, environmental enteropathy biomarkers, and parasite prevalence between the children result from unmeasured differences between groups that could not be controlled for in this observational follow-up study. Evidence for confounding by observed characteristics was greatest among anthropometric outcomes, but unadjusted and adjusted differences were highly consistent for parasitic infections and measures of gut function, suggesting less confounding (Tables 1 and 2). A large-scale, randomized controlled trial that delivers high-impact household environmental interventions to a birth cohort would provide more robust evidence of a causal relationship for the associations that we observed in this study.
Although we observed lower diarrhea prevalence among children from clean households compared with children from contaminated households during the original study (longitudinal prevalence: 11.7% versus 15.6%; P < 0.05) (Supplemental Table 4), this study was not designed to evaluate the relative contributions of symptomatic and asymptomatic infection to growth faltering in early childhood. A longitudinal follow-up study that prospectively collects concurrent diarrhea surveillance, environmental enteropathy biomarkers, and growth measures from birth would be better suited to estimate the relative importance of these hypothesized pathways between environmental contamination and growth.
Because our study participants were drawn from a nationally representative sample of rural Bangladesh, we expect our results to generalize to this population. To maximize the difference between the clean and contaminated environments, we selected households at two extremes of the distribution of household environments. The differences in outcomes estimated in this study represent the effect of taking a child from the most environmentally contaminated household conditions in rural Bangladesh and placing them in the most environmentally clean household conditions. From an intervention standpoint, this type of improvement would only be observed in a small subset of the population—those children from the most contaminated environments; however, most children in the population live in households that fall somewhere on the continuum between these two environmental extremes. Therefore, we would expect the effect of interventions deployed to the general population to have smaller average improvements on parasitic infection, gut function, and growth than the improvements that we observed in this study, because most intervention households would experience less extreme environmental changes.
Conclusions
In rural Bangladesh, young children living in environmentally clean households had lower levels of parasitic infection, improved measures of gut function, and improved growth compared with similar children living in contaminated environments. The results from this study support the hypothesis that environmental contamination, mediated through environmental enteropathy, could be a cause of growth faltering in contaminated settings. Randomized trials that modify environmental enteropathy risk factors by delivering high-impact environmental interventions to children in their first years of life would provide more robust evidence to test this hypothesis.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully thank all of our study participants; our extremely dedicated icddr,b field and laboratory staff; the Economic Empowerment of the Poorest/Stimulating Household Improvements Resulting in Economic Empowerment Programme of the UK Department for International Development for assistance with anthropometry training; Atiya Sharmeen and A. M. Shamsir Ahmed of the Malnutrition and Enteric Diseases (MAL-ED) Study for assistance with training and supplies; members of the icddr,b Parasitology Laboratory, Nutritional Biochemistry Laboratory, Hematology Laboratory, and Virology Laboratory for laboratory support; and Sania Ashraf of the icddr,b Health Systems and Infectious Diseases Division and Upi Singh and Katharine Ng of Stanford University for helpful comments and support.
Footnotes
Financial support: This research was financially supported by Global Development Grant OPPGD759 from the Bill & Melinda Gates Foundation (to the University of California at Berkeley; A.L., B.F.A., S.A., L.U., J.M.C., and S.P.L.); a National Science Foundation Graduate Research Fellowship (to A.L.); a Stanford Graduate Fellowship (to A.L.); the Stanford Center for Clinical and Translational Education and Research (Spectrum) and the Lucile Packard Foundation for Children's Health (A.L.); Centers for Disease Control and Prevention (S.L.); and United Nations Children's Fund (S.L.)
Authors' addresses: Audrie Lin, Benjamin F. Arnold, and John M. Colford Jr., Division of Epidemiology, School of Public Health, University of California, Berkeley, CA, E-mails: audrielin@berkeley.edu, benarnold@berkeley.edu, and jcolford@berkeley.edu. Sadia Afreen, Tarique Mohammad Nurul Huda, Rashidul Haque, Rubhana Raqib, Leanne Unicomb, and Stephen P. Luby, Health Systems and Infectious Diseases, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh, E-mails: afreen@icddrb.org, n_huda@icddrb.org, rhaque@icddrb.org, rubhana@icddrb.org, leanne@icddrb.org, and sluby@icddrb.org. Rie Goto, Department of Archaeology and Anthropology, Division of Biological Anthropology, University of Cambridge, Cambridge, United Kingdom, E-mail: rg277@cam.ac.uk. Tahmeed Ahmed, Public Health Sciences, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh, E-mail: tahmeed@icddrb.org.
References
- 1.National Institute of Population Research and Training (NIPORT), Mitra and Associates, and ICF International . Bangladesh Demographic and Health Survey 2011. Dhaka, Bangladesh and Calverton, MD: NIPORT, Mitra and Associates, and ICF International; 2013. pp. 1–430. [Google Scholar]
- 2.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 3.Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, Molbak K, Valentiner-Branth P, Lanata CF, Black RE. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37:816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374:1032–1035. doi: 10.1016/S0140-6736(09)60950-8. [DOI] [PubMed] [Google Scholar]
- 5.Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4((Suppl 1)):24–85. doi: 10.1111/j.1740-8709.2007.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991a;338:907–910. doi: 10.1016/0140-6736(91)91772-m. [DOI] [PubMed] [Google Scholar]
- 7.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133:1332–1338. doi: 10.1093/jn/133.5.1332. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DI, Murch SH, Elia M, Sullivan PB, Sanyang MS, Jobarteh B, Lunn PG. Chronic T cell-mediated enteropathy in rural West African children: relationship with nutritional status and small bowel function. Pediatr Res. 2003;54:306–311. doi: 10.1203/01.PDR.0000076666.16021.5E. [DOI] [PubMed] [Google Scholar]
- 9.Haghighi P, Wolf PL. Tropical sprue and subclinical enteropathy: a vision for the nineties. Crit Rev Clin Lab Sci. 1997;34:313–341. doi: 10.3109/10408369708998096. [DOI] [PubMed] [Google Scholar]
- 10.Lunn PG. The impact of infection and nutrition on gut function and growth in childhood. Proc Nutr Soc. 2000;59:147–154. doi: 10.1017/s0029665100000173. [DOI] [PubMed] [Google Scholar]
- 11.McKay S, Gaudier E, Campbell DI, Prentice AM, Albers R. Environmental enteropathy: new targets for nutritional interventions. In Health. 2010;2:172–180. doi: 10.1016/j.inhe.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg. 2012;86:756–763. doi: 10.4269/ajtmh.2012.11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menzies IS, Zuckerman MJ, Nukajam WS, Somasundaram SG, Murphy B, Jenkins AP, Crane RS, Gregory GG. Geography of intestinal permeability and absorption. Gut. 1999;44:483–489. doi: 10.1136/gut.44.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas G, Clain DJ, Wicks AC. Tropical enteropathy in Rhodesia. Gut. 1976;17:888–894. doi: 10.1136/gut.17.11.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langford R, Lunn P, Panter-Brick C. Hand-washing, subclinical infections, and growth: a longitudinal evaluation of an intervention in Nepali slums. Am J Hum Biol. 2011;23:621–629. doi: 10.1002/ajhb.21189. [DOI] [PubMed] [Google Scholar]
- 16.Huda TM, Unicomb L, Johnston RB, Halder AK, Yushuf Sharker MA, Luby SP. Interim evaluation of a large scale sanitation, hygiene and water improvement programme on childhood diarrhea and respiratory disease in rural Bangladesh. Soc Sci Med. 2012;75:604–611. doi: 10.1016/j.socscimed.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 17.WHO . Guidelines for Drinking-Water Quality, Fourth Edition. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 18.Haque R, Mondal D, Kirkpatrick BD, Akther S, Farr BM, Sack RB, Petri WA., Jr Epidemiologic and clinical characteristics of acute diarrhea with emphasis on Entamoeba histolytica infections in preschool children in an urban slum of Dhaka, Bangladesh. Am J Trop Med Hyg. 2003;69:398–405. [PubMed] [Google Scholar]
- 19.WHO . Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. WHO Technical Report Series. Geneva: World Health Organization; 2002. [PubMed] [Google Scholar]
- 20.Lohman T, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetic Books; 1988. [Google Scholar]
- 21.de Onis M, Onyango AW, Van den Broeck J, Chumlea WC, Martorell R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull. 2004;25:S27–S36. doi: 10.1177/15648265040251S104. [DOI] [PubMed] [Google Scholar]
- 22.WHO . WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva: World Health Organization; 2006. p. 312. [Google Scholar]
- 23.Goto R, Mascie-Taylor CG, Lunn PG. Impact of intestinal permeability, inflammation status and parasitic infections on infant growth faltering in rural Bangladesh. Br J Nutr. 2009b;101:1509–1516. doi: 10.1017/S0007114508083554. [DOI] [PubMed] [Google Scholar]
- 24.Barboza MS, Silva TMJ, Guerrant RL, Lima AAM. Measurement of intestinal permeability using mannitol and lactulose in children with diarrheal diseases. Braz J Med Biol Res. 1999;32:1499–1504. doi: 10.1590/s0100-879x1999001200008. [DOI] [PubMed] [Google Scholar]
- 25.Northrop-Clewes CA, Rousham EK, Mascie-Taylor CN, Lunn PG. Anthelmintic treatment of rural Bangladeshi children: effect on host physiology, growth, and biochemical status. Am J Clin Nutr. 2001;73:53–60. doi: 10.1093/ajcn/73.1.53. [DOI] [PubMed] [Google Scholar]
- 26.Freedman DA. On the so-called “Huber Sandwich Estimator” and “Robust Standard Errors.”. Am Stat. 2006;60:299–302. [Google Scholar]
- 27.Sekhon JS. Opiates for the matches: matching methods for causal inference. Annual Review of Political Science. 2009;12:487–508. [Google Scholar]
- 28.Cousens SN, Mertens TE, Fernando MA. The anthropometric status of children in Kurunegala district in Sri Lanka: its relation to water supply, sanitation and hygiene practice. Trop Med Parasitol. 1990;41:105–114. [PubMed] [Google Scholar]
- 29.Esrey SA, Habicht JP, Casella G. The complementary effect of latrines and increased water usage on the growth of infants in rural Lesotho. Am J Epidemiol. 1992;135:659–666. doi: 10.1093/oxfordjournals.aje.a116345. [DOI] [PubMed] [Google Scholar]
- 30.Esrey SA. Water, waste, and well-being: a multicountry study. Am J Epidemiol. 1996;143:608–623. doi: 10.1093/oxfordjournals.aje.a008791. [DOI] [PubMed] [Google Scholar]
- 31.Checkley W, Gilman RH, Black RE, Epstein LD, Cabrera L, Sterling CR, Moulton LH. Effect of water and sanitation on childhood health in a poor Peruvian peri-urban community. Lancet. 2004;363:112–118. doi: 10.1016/S0140-6736(03)15261-0. [DOI] [PubMed] [Google Scholar]
- 32.Stanton BF, Clemens JD, Khair T. Educational intervention for altering water-sanitation behavior to reduce childhood diarrhea in urban Bangladesh: impact on nutritional status. Am J Clin Nutr. 1988;48:1166–1172. doi: 10.1093/ajcn/48.5.1166. [DOI] [PubMed] [Google Scholar]
- 33.Arnold BF, Khush RS, Ramaswamy P, London AG, Rajkumar P, Ramaprabha P, Durairaj N, Hubbard AE, Balakrishnan K, Colford JM., Jr Causal inference methods to study nonrandomized, preexisting development interventions. Proc Natl Acad Sci USA. 2010;107:22605–22610. doi: 10.1073/pnas.1008944107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goto R, Mascie-Taylor CG, Lunn PG. Impact of anti-Giardia and anthelminthic treatment on infant growth and intestinal permeability in rural Bangladesh: a randomised double-blind controlled study. Trans R Soc Trop Med Hyg. 2009a;103:520–529. doi: 10.1016/j.trstmh.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Rousham EK, Northrop-Clewes CA, Lunn PG. Maternal reports of child illness and the biochemical status of the child: the use of morbidity interviews in rural Bangladesh. Br J Nutr. 1998;80:451–456. [PubMed] [Google Scholar]
- 36.Lunn PG, Northrop-Clewes CA, Downes RM. Recent developments in the nutritional management of diarrhoea. 2. Chronic diarrhoea and malnutrition in The Gambia: studies on intestinal permeability. Trans R Soc Trop Med Hyg. 1991b;85:8–11. doi: 10.1016/0035-9203(91)90137-n. [DOI] [PubMed] [Google Scholar]
- 37.Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–e480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.