Abstract
Plasmodium infection in pregnancy causes substantial maternal and infant morbidity and mortality. In Colombia, both P. falciparum and P. vivax are endemic, but the impact of either species on pregnancy is largely unknown in this country. A cross-sectional study was carried out with 96 pregnant women who delivered at their local hospital. Maternal, placental, and cord blood were tested for malaria infection by microscopy and real-time quantitative polymerase chain reaction (qPCR). A high frequency of infection was detected by qPCR (45%). These infections had low concentrations of parasite DNA, and 79% were submicroscopic. Submicroscopic infections were associated with placental villitis and intervillitis. In conclusion, the overall frequency of Plasmodium infection at delivery in Colombia is much higher than previously reported. These data prompt a re-examination of the local epidemiology of malaria using molecular diagnostics to establish the clinical relevance of submicroscopic infections during pregnancy as well as their consequences for mothers and newborns.
Introduction
Malaria in pregnancy causes substantial maternal and infant morbidity and mortality in tropical areas because of increased risk of maternal anemia and low birth weight (LBW) infants.1 In 2007, an estimated 125.2 million pregnancies occurred in areas with malaria transmission.2 In highly endemic areas, up to 50% of LBW deliveries can be attributed to malaria in pregnancy, leading to approximately 100,000 infant deaths.2,3 All species of Plasmodium cause malaria infection in pregnant women, but only P. falciparum has been extensively studied in pregnancy, with few studies of P. vivax. However, both infections can cause adverse pregnancy outcomes.4–7
Women living in different geographical areas experience variable levels of exposure to Plasmodium, which influences the course of infection in pregnancy.8 In areas of high, stable transmission, including sub-Saharan Africa, where P. falciparum predominates, pregnancy malaria is often asymptomatic but associated with severe maternal anemia and fetal growth retardation. In these areas, placental infection is most frequent in first-time mothers, because women develop immunity against placental parasites that provides protection over successive pregnancies. In areas of low, unstable transmission, malaria infection is typically symptomatic in women of all parities and associated with high rates of fetal loss and maternal death.9
In Latin America, both P. falciparum and P. vivax are endemic in areas of low, unstable transmission. According to the World Malaria Report (2011), malaria transmission occurs in 21 countries in the Americas, with Colombia and Brazil accounting for more than 60% of cases.10 In Colombia, 115,884 cases of malaria were reported in 2010, and the Urabá-Altos Sinú-San Jorge-Bajo Cauca region accounted for 60% of all cases. There are no official reports on the prevalence of infection within the pregnant population in Colombia. In a recent study of 2,117 pregnant women in the Urabá region, 220 cases of malaria were diagnosed by microscopy.11 In a subset of 79 pregnant women at delivery, the frequency of plasmodial infection in peripheral and placental blood samples detected by microscopy was 13% and 9%, respectively, whereas the frequency of infection detected by nested polymerase chain reaction (PCR) was 32% and 26%, respectively.12 Importantly, the application of more sensitive molecular tests identified 2.5 times more infections than microscopy, classifying these infections as submicroscopic. Similar discrepancies between PCR and microscopy are reported from other endemic population surveys.13,14 However, the epidemiology and clinical significance of submicroscopic malaria infections during pregnancy are not well-understood.13,15–20 The present pilot study evaluated the frequency of submicroscopic plasmodial infections in parturient women in the Urabá-Altos Sinú-San Jorge-Bajo Cauca region of Colombia and their associations with clinical outcomes of pregnancy.
Materials and Methods
Study site and population.
Women were recruited at one hospital obstetric facility in each of the municipalities of Turbo (08°05′ N, 76°44′ W), Necoclí (08°25′ N, 76°47′ W; Antioquia), and Puerto Libertador (07°54′ N, 75°40′ W; Córdoba). The three hospitals are public institutions that provide equivalent primary-level healthcare, servicing populations with comparable demographics and socioeconomic status in the region. Both Antioquia and Córdoba comprise the malaria transmission area termed Urabá-Altos Sinú-San Jorge-Bajo Cauca, which has an estimated area of 43,506 km2 and a malaria at-risk population of 2.5 million. Epidemiologic characteristics of this region are described elsewhere.21–23 This region is homogeneous in terms of ecoepidemiology and malaria transmission. Transmission intensity is low and stable, with no marked fluctuations in the number of malaria cases during the year. The mean annual parasitic index (malaria cases/1,000 inhabitants) during 2000–2009 was 46.6 in Turbo, 74.4 in Necoclí, and 23.4 in Puerto Libertador. P. vivax is reported in approximately 70% of cases.24,25 No national malaria control strategies specific for pregnant women have been implemented, and the use of bed nets is uncommon. Treatment guidelines for malaria in pregnancy include chloroquine for vivax malaria and quinine-clindamycin (first trimester) or artemether-lumefantrine (second and third trimesters) for falciparum malaria.26
Study and sample design.
A cross-sectional pilot study was carried out to measure the frequency of submicroscopic infection at delivery in Urabá-Altos Sinú-San Jorge-Bajo Cauca. A sample size of 96 randomly selected women was calculated from a larger epidemiological study of 2,550 parturient women recruited between 2005 and 2011 using the formula n = NZ2p(1 − p)/[(Ne2) + (Z2p(1 − p))],27 where N is the reference population (N = 2,550), Z is the confidence level (95%), p is the reported frequency of submicroscopic infection at delivery (18%),12 and e is the sampling error (7.5%). From the entire list of 2,550 women, 96 women were selected using a simple random sampling without replacement method in accordance with the inclusion criteria (see below).
Inclusion and exclusion criteria.
The inclusion criteria were permanent residence in the malaria-endemic community of Turbo, Necoclí, or Puerto Libertador, absence of serious general disease or complicated pregnancy, availability of filter papers with peripheral, placental, and cord blood, and signature on the informed consent form. The exclusion criterion was consent withdrawal.
Data and specimen collection.
A questionnaire was completed with data on age, number of pregnancies, and number of malaria episodes during the pregnancy reported by the mother and based on her health card that documents the diagnosis (by microscopy) and treatment. Labor/delivery and infant outcomes data as well as maternal hemoglobin level at delivery were collected from the hospital chart.
Within 8 hours of delivery, a blood sample from each compartment was collected in EDTA tubes (BD Vacutainer®, USA) and used to prepare thick smears and dried blood spots on filter paper (Whatman™ Grade 3). Blood spots were prepared with approximately 100 μL blood (two drops). After drying at room temperature, spots were sealed in plastic bags (one bag per sample per woman) and stored at 4°C. Maternal peripheral blood was collected by venipuncture. Cord blood was collected by sectioning a fragment to expose a fresh segment and then draining the blood into the tube. After cleaning with saline, small (∼1 cm3) sections of placenta were removed from the maternal side, and the blood pooled was collected by pipet aspiration. Three tissue fragments were obtained by sectioning the placenta (2 cm2 surface area through the entire thickness). Fragments were fixed in 10% neutral buffered formalin and paraffin-embedded within 48 hours. Sections of 5-μm thickness were stained with hematoxylin & eosin and read under 100× and 400× magnifications according to standard procedures.
Malaria diagnosis.
Field-stained thick films were read by an experienced microscopist. Thick smears were defined as negative if 200 fields (1,000× magnification) were free of parasites.
A hole punch circle (∼6 mm) of each filter paper was used for DNA extraction, corresponding to approximately 25 μL blood. DNA was extracted with the saponin-Chelex method.28 The extracted DNA was resuspended in 50 μL water. Real-time quantitative PCR (qPCR) was performed as described elsewhere.29 Samples were first tested for Plasmodium using genus-specific primers and a hydrolysis probe (Plasprobe). PCR was run on the ABI 7500 FAST platform. Samples with a Cycle Threshold (Ct) < 45 were tested in duplex species-specific reactions for P. falciparum and P. vivax.29 Additional confirmation of mixed and weak infections (Ct = 42–45) was performed using newly designed reverse primers specific for P. falciparum (5′-AGCAATCTAAAAGTCACCTCGAA-3′) and P. vivax (5′-AGCAATCTAAGAATAAACTCCGAAGA-3′). These primers were combined with the forward Plasmo1 primer and the Plasprobe in singleplex reactions. Discordant samples in the three reactions (genus-specific, duplex species-specific, and singleplex species-specific reactions) were called negative. DNA copy number was quantified from the genus-specific reaction against a standard curve using a plasmid containing a fragment of the 18S gene from P. falciparum. The lower limit for quantitation with this plasmid is 10 copies per PCR (or 2 copies/μL template). The sensitivity of the qPCR assay for the detection of parasite DNA in clinical samples is limited by the input volume in the qPCR reaction, which corresponds to ∼2.5 μL whole blood. Positive samples were further confirmed by nested and seminested PCR to amplify other sequences within the 18S gene30 and other microsatellite and gene regions.31–33
Histopathology.
A reader with experience in placental histology read an entire slide from each placenta. The reader established protocols and definitions based on her experience and data reported from other works.8,34–39 Placental histological analyses included deciduitis, > 8 high-power fields (HPF) with immune cells in the decidua; villitis, > 10 immune cells/HPF in stromal villi; villi infarct, at least 1 HPF with an ischemic area with degenerative lesions; fibrin deposits, at least 1 HPF with accumulation of fibrin in the intervillous space; intervillitis, > 10 immune cells/HPF in the intervillous space; increased syncytial knots, > 3 HPF with aggregates of syncytial nuclei at the surface of villi; and malaria pigment deposits, at least 1 HPF with malaria pigment in fibrinoid or immune cells.
Definitions and statistical analysis.
Malaria infection was defined by a positive diagnosis by microscopy and/or qPCR for P. falciparum and/or P. vivax. Submicroscopic infections were negative by microscopy and positive by qPCR. Definitions used were anemia, hemoglobin < 11 g/dL; LBW, birth weight < 2,500 g; and pre-term delivery, birth before 37 weeks of gestation.
According to the data distribution, the variable number of DNA copies per microliter was categorized in terciles as < 2 (below the linear range of the standard curve), 2–100, and > 100 DNA copies/μL. Data were analyzed using Excel and Epi Info 3.5.3. Kruskal–Wallis and χ2 tests were used for comparison of continuous and categorical variables, respectively. Significance was set at P < 0.05.
Ethical considerations.
Pregnant women or guardians signed a voluntary consent form. The study involved minor risk, and approval was granted by the Comité de Ética of Instituto de Investigaciones Médicas (ethical approval numbers: IIM2484, IIM2509, IIM2530, and IIM2557), Universidad de Antioquia, and the Health Research Ethics Board of the University of Alberta (ethical approval number: Pro00017660).
Results
General characteristics of study women are shown in Table 1; 59% of women had at least one previous delivery. Frequencies of pre-term delivery and LBW were low (6% and 4%, respectively). However, the overall frequency of anemia was high; of 88 women with known hemoglobin levels at delivery, 52% were anemic.
Table 1.
General characteristics of the study women
| Variable | Value |
|---|---|
| Age (years) mean ± SD (range) | 22.8 ± 6.4 (14–44) |
| Parity n (%) | 1.7 ± 2.1 (0–11) |
| Primiparous | 31 (33.4) |
| Secundiparous | 24 (26.7) |
| Multiparous | 35 (38.8) |
| Gestational age (weeks) | 38.6 ± 2.1 (27–42) |
| Preterm delivery percent (n) | 6 (5) |
| Birth weight (g) mean ± SD (range) | 3,230 ± 473 (1,850–4,800) |
| LBW percent (n) | 4 (4) |
| Hemoglobin (g/dL) mean ± SD (range) | 10.8 ± 1.5 (7.6–18.3) |
| Anemia percent (n) | 52 (46) |
Malaria infection at delivery.
The frequency of malaria infection in the three compartments varied significantly between microscopy and qPCR (Table 2). Based on qPCR, 65% of women had at least one positive compartment, 25% of women had two positive compartments, and 23% of women were positive in all three compartments. Twelve women (13%) had infections only in the placenta, whereas five women (5%) were positive only in peripheral blood; no women had infection in the cord exclusively. The highest frequency of infection was in the placenta (57%) followed by peripheral blood (49%) and cord (29%). To confirm the specificity of these results, the positive samples were further genotyped by nested or seminested PCR and capillary electrophoresis of five molecular markers per species40; 74% of samples were also positive for at least one species-specific marker (Supplemental Table 1).
Table 2.
Number of microscopy- and qPCR-positive samples in each compartment
| Peripheral blood | Placental blood | Cord blood | All compartments | |||||
|---|---|---|---|---|---|---|---|---|
| Microscopy (N = 95) | qPCR (N = 96) | Microscopy (N = 95) | qPCR (N = 96) | Microscopy (N = 95) | qPCR (N = 96) | Microscopy (N = 285) | qPCR (N = 288) | |
| P. falciparum | 1 | 23 | 2 | 28 | 0 | 16 | 3 | 67 |
| P. vivax | 11 | 22 | 6 | 20 | 0 | 10 | 17 | 52 |
| Mixed | 0 | 2 | 0 | 7 | 0 | 2 | 0 | 11 |
| Total positive | 12 | 47 | 8 | 55 | 0 | 28 | 20 | 130 |
| Total negative | 83 | 49 | 87 | 41 | 95 | 68 | 265 | 158 |
The distribution of P. vivax and P. falciparum in the infected samples differed according to the diagnostic method. By microscopy, the majority of infections (75%) were identified as P. vivax, whereas over 50% of the infections detected by qPCR were caused by P. falciparum (Table 2). Sixteen different species combinations were detected across the compartments. Of 46 women with infections in two or more compartments, 40 women (87%) had the same species, whereas the other 6 women had discordant species, mainly because of mixed infections in the placenta and monoinfections in peripheral or cord blood (Supplemental Table 2).
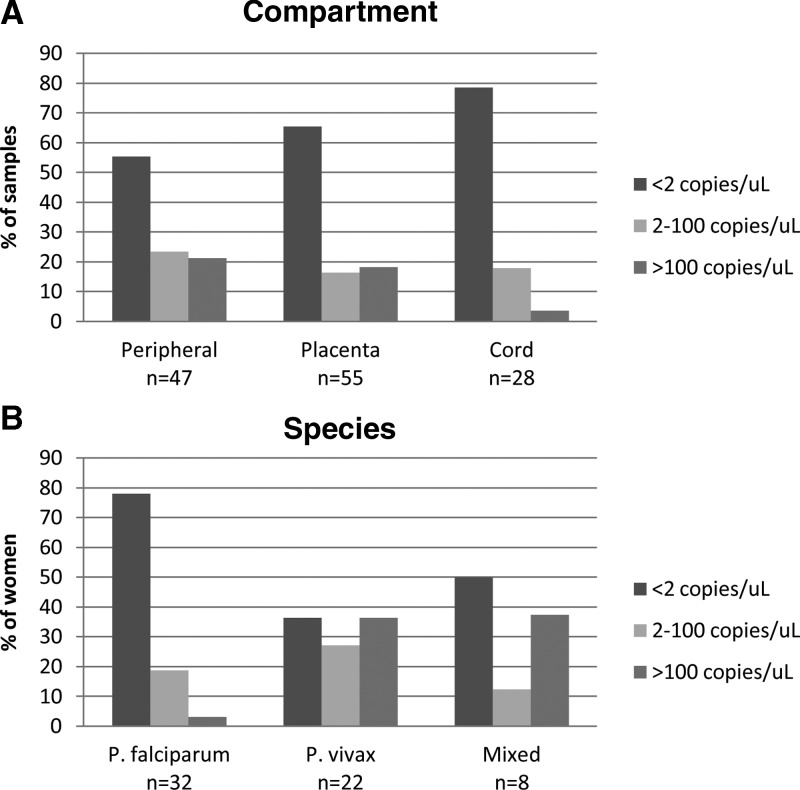
In general, the level of infection in these women was very low (Figure 1). Based on qPCR, 84 of 130 infections (65%) had < 2 DNA copies/μL template. Cord was the compartment with the lowest level of infection (Figure 1A). A comparison of the DNA concentrations by species revealed significantly higher DNA concentrations in P. vivax and mixed infections compared with P. falciparum (P = 0.0082). Across all three compartments, most of the P. falciparum infections (78%) had < 2 DNA copies/μL (Figure 1B).
Figure 1.
Quantitation of parasite DNA by qPCR. (A) Samples from each compartment were categorized into terciles based on quantitation of DNA copies by qPCR. (B) Quantitation of DNA copies according to the species of infection.
Clinical and histological outcomes of infected women.
Malaria infection was detected by qPCR but not by microscopy in 48 women. These submicroscopic infections account for 79% of the infections. The clinical characteristics were compared between women who were negative for malaria and women with microscopic and submicroscopic infections (Table 3). Age, hemoglobin level, gestational age, parity, and birth weight were similar among the three groups. Infant birth weight was significantly lower in primiparae and adolescents (≤ 18 years of age), but this finding was not associated with malaria infection. The mean infant birth weight in primiparae was 3,061 ± 467 g, and the mean infant birth weight in multiparous women was 3,333 ± 463 g (P = 0.086). Adolescent women had infants with a mean weight of 2,992 ± 284 g, whereas the infant birth weight in older women was 3,325 ± 486 g (P = 0.0009).
Table 3.
Clinical characteristics and histological changes of women with or without plasmodial infection at delivery
| Variable | Negative (N = 34)* | Submicroscopic (N = 48)* | Microscopic (N = 13) | P value |
|---|---|---|---|---|
| Age (years) mean ± SD (range) | 22.5 ± 7.0 (14–44) | 22.6 ± 5.8 (15–36) | 24.9 ± 7.4 (14–38) | 0.4647 |
| Gestational age (weeks) mean ± SD (range) | 38.7 ± 1.8 (32–41) | 38.5 ± 2.4 (27–42.3) | 38.6 ± 2.3 (32–40.8) | 0.9201 |
| Hemoglobin (g/dL) mean ± SD (range) | 10.9 ± 1.8 (8.4–18.3) | 10.7 ± 1.0 (9.0–13.3) | 10.8 ± 1.6 (7.6–13.3) | 0.8758 |
| Birth weight (g) mean ± SD (range) | 3,271 ± 469 (2,350–4,500) | 3,186 ± 508 (1,850–4,800) | 3,283 ± 348 (2,800–4,000) | 0.5861 |
| Parity percent (n) | ||||
| Primiparous | 40 (13) | 32 (14) | 33 (4) | 0.7254 |
| Secundiparous | 30 (10) | 27 (12) | 17 (2) | 0.7254 |
| Multiparous | 30 (10) | 41 (18) | 50 (6) | 0.7254 |
| Deciduitis percent (n) | 11 (3) | 26 (12) | 25 (3) | 0.2691 |
| Villitis percent (n) | 7 (2) | 28 (13) | 39 (5) | 0.0397 |
| Increased villi infarct percent (n) | 11 (3) | 20 (9) | 15 (2) | 0.6018 |
| Intervillitis percent (n) | 7 (2) | 33 (15) | 31 (4) | 0.0382 |
| Fibrin deposits percent (n) | 46 (13) | 63 (29) | 69 (9) | 0.2605 |
| Increased syncytial knots percent (n) | 15 (4) | 11 (5) | 39 (4) | 0.2214 |
| Malaria pigment deposits percent (n) | 14 (4) | 15 (7) | 62 (8) | 0.0009 |
The histological study was available for 26 women with negative results of placental infection and 46 women with submicroscopic placental infection.
Placental histological analysis was available for 87 women (91%): 28 women were negative for plasmodial infection, 46 women had submicroscopic infections, and 13 women had microscopic infections. Placentas from women with both microscopic and submicroscopic infections had a higher frequency of villitis and intervillitis than uninfected women (P = 0.04). This inflammation was observed in women infected with either P. falciparum or P. vivax. In addition, malaria pigment deposits were more frequent in women with microscopic infection (62%) than women with submicroscopic infection (15%) or uninfected women (14%) (Table 3).
History of malaria in pregnancy.
The history of malaria during the current pregnancy was known for 66 of 96 (69%) women. A total of 48 women (73%) had at least one recorded episode of malaria; 34 women had a history of P. vivax, and 6 women had a history of P. falciparum. In 8 women, the species was not recorded. All women received treatment at the time of diagnosis. Of these women with a history of malaria in the pregnancy, 73% had at least one positive compartment at delivery by qPCR; P. vivax was detected in 24 of 34 women with a history of P. vivax, whereas P. falciparum was detected at delivery in 4 of 6 women with a history of P. falciparum.
A comparison of the clinical characteristics of women with and without a history of malaria in the pregnancy revealed a significant association between malaria in pregnancy and lower birth weight (LBW) infants (Table 4). In addition, the species detected during the pregnancy influenced the birth weight; infants born from women with a history of P. vivax had a mean birth weight of 3,053 ± 427 g, whereas the infant birth weight from women with a history of P. falciparum was 3,300 ± 379 g. Infant birth weight from women without a history of malaria was 3,578 ± 559 g (P = 0.010). In contrast, the species detected at delivery by qPCR was not statistically associated with birth weight (P = 0.254); however, the mean infant birth weight in women with P. vivax infection at delivery was lower (3,059 ± 459 g) than the mean infant birth weight in women with P. falciparum infection (3,320 ± 512 g) and the mean infant birth weight in women without infection (3,271 ± 469 g). Furthermore, deposits of malaria pigment on placental tissue were only detected in women with a history of malaria in pregnancy, consistent with a past infection. The other placental histological variables were similar among women with and without history of malaria during the pregnancy (Table 4).
Table 4.
Clinical characteristics of women with or without history of malaria in the pregnancy
| Variable | History of malaria in pregnancy | P Value | |
|---|---|---|---|
| No (N = 18) | Yes (N = 48) | ||
| Age (years) mean (range) | 24.3 (16–32) | 22.2 (14–38) | 0.1014 |
| Gestational age (weeks) mean (range) | 39.1 (37–41.3) | 38.5 (27–42.3) | 0.4211 |
| Hemoglobin (g/dL) mean (range) | 11.4 (9.4–18.3) | 11.0 (7.6–13.3) | 0.8003 |
| Birth weight (g) mean (range) | 3,578 (2,800–4,800) | 3,132 (1,850–4,000) | 0.0081 |
| Parity percent (n) | |||
| Primiparous | 22 (4) | 38 (18) | 0.2256 |
| Secundiparous | 17 (3) | 25 (12) | 0.2256 |
| Multiparous | 61 (11) | 38 (18) | 0.2256 |
| Deciduitis percent (n) | 11 (2) | 31 (12) | 0.0987 |
| Villitis percent (n) | 22 (4) | 30 (12) | 0.3908 |
| Increased villi infarct percent (n) | 11 (2) | 23 (9) | 0.2609 |
| Intervillitis percent (n) | 22 (4) | 35 (14) | 0.2559 |
| Fibrin deposits percent (n) | 83 (15) | 76 (31) | 0.4485 |
| Increased syncytial knots percent (n) | 17 (3) | 20 (8) | 0.5365 |
| Malaria pigment deposits percent (n) | 0 | 48 (19) | < 0.001 |
Discussion
This pilot study is the first in Latin America to evaluate malaria in pregnancy using qPCR diagnostics. The frequency of infection detected by qPCR was far greater than previously reported; 65% of pregnant women in our study had at least one positive compartment. The few previous reports in Colombia on the frequency of malaria infection during pregnancy or at delivery were carried out in municipalities of Urabá; according to microscopy, the frequency of malaria infection in maternal peripheral blood was 11–14%, the frequency of malaria infection in placental blood was 9–12%, and the frequency of malaria infection in cord blood was 2–4%.11,12,41,42 One study used nested PCR in addition to microscopy to diagnose malaria infection in the three compartments, and the frequency of infection increased markedly from 13% to 32% in peripheral blood, from 9% to 26% in placenta, and from 2% to 13% in cord blood.12
Given the unexpectedly high frequency of malaria in our study, consideration of false-positive results is critical. In the data reported here, false positives are unlikely based on the inclusion of controls and rigorous criteria to call positives. (1) Each run included at least two negative controls. (2) Only samples that were positive in two independent reactions with different sets of primers and probes (genus and species) were considered positive. (3) Mixed and weak infections were confirmed using a third set of primers. (4) Samples with discordant results among the three different reactions were considered negative. Positive results were further confirmed by nested PCR for genotyping in 74% of samples. Given that the majority of infections had < 2 DNA copies/μL, detection by nested PCR was limited by the sensitivity of the assay and Poisson distribution of template in solution.
In addition to the high overall frequency of infection, more cases of P. falciparum (56%) than P. vivax (44%) were detected by qPCR in this study. These data contradict findings based on microscopy and nested PCR, where the majority of infections in pregnant women from Urabá were caused by P. vivax.11,12,41,42 However, there are reports from the Brazilian and Peruvian Amazon that pregnant women are at higher risk of P. falciparum infection compared with the general population.43–45 The Brazilian study showed that the ratio of P. falciparum to P. vivax changed in pregnant women from 1:5.6 to 1:2.3,43 and in Peru, pregnant women were 2.3 times more likely to be infected with P. falciparum than non-pregnant women.44
Consistent with these findings, we detected the highest frequency of infection in the placenta, and the majority of infection was caused by P. falciparum. Furthermore, 24% of infected women had placental infection without peripheral infection, suggesting that placental sequestration of P. falciparum may be more common than previously reported.12 This hypothesis is further supported by the high rates of villitis and intervillitis detected in infected women. Although we did not observe an association between placental infection and LBW, we found that even submicroscopic infections cause significant placental inflammation. It is, therefore, important to design expanded studies to investigate the clinical outcomes that may result from these infections in this transmission setting. Based on a systematic review, the frequency of maternal anemia and LBW was significantly lower in uninfected women compared with women with a submicroscopic infection.16 Other reports also show increased risk of maternal anemia in submicroscopic infections compared with uninfected women46,47; however, those studies were carried out in Africa, where P. falciparum is dominant and the intensity of transmission is very high, unlike in Latin America.
We also observed that the levels of infection quantified by qPCR are significantly lower with P. falciparum than P. vivax. One hypothesis is that the strains of P. falciparum in Colombia are less virulent, and parasitemia is controlled by immune mechanisms, consistent with the fact that the women in our study are all asymptomatic. Alternatively, qPCR may be detecting gametocytes or free parasite DNA circulating in the blood rather than intact, pathogenic asexual parasites. This distinction is critical when considering sensitive methods such as qPCR as diagnostic tools to predict clinical malaria in pregnancy.
One of the limitations of this study is the small sample size. Nevertheless, the study was sufficiently powered to detect significant findings in the study population, notably a high frequency of submicroscopic malaria that is associated with placental injury at delivery. Although subsequent, larger studies are required to broaden the scope of these findings, the results of our pilot study warn that the magnitude of pregnancy-associated malaria in Colombia may be far greater than anticipated based on insensitive diagnostic tests. Importantly, our data prompt a re-examination of the local epidemiology of malaria in this lower transmission setting using molecular diagnostics to establish the clinical relevance of submicroscopic infections during pregnancy as well as their consequences for mothers and newborns.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the participating women, field assistants, employees, and managers of the local hospitals for their collaboration. The authors thank Sandra Shokoples for assistance in the laboratory and Dr. Sédami Gnidehou for comments on the manuscript.
Footnotes
Financial support: This work was supported by the Departamento Administrativo de Ciencia, Tecnología e Inovación Colciencias (Project Codes 111540820495 and 111549326134), the Universidad de Antioquia (Project Codes IIM 8764-2530 and IIM 8764-2557; Estrategia de Sostenibilidad 2013–2014), and the Canadian Institutes of Health Research (Catalyst Grant in Maternal Health: FRN 115440).
Authors' addresses: Eliana M. Arango, Olga M. Agudelo, Jaime Carmona-Fonseca, and Amanda Maestre, Grupo Salud y Comunidad, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia, E-mails: emarango@gmail.com, momag204@gmail.com, jaimecarmonaf@hotmail.com, and aemaestre@gmail.com. Roshini Samuel, Provincial Laboratory for Public Health, Edmonton, Canada, E-mail: roshini@ualberta.ca. Stephanie K. Yanow, School of Public Health, University of Alberta, Alberta, Canada and Provincial Laboratory for Public Health, Edmonton, Canada, E-mail: stephanie.yanow@albertahealthservices.ca.
References
- 1.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 2.Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 2010;7:e1000221.3. doi: 10.1371/journal.pmed.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 4.Rijken MJ, McGready R, Boel ME, Poespoprodjo R, Singh N, Syafruddin D, Rogerson S, Nosten F. Malaria in pregnancy in the Asia-Pacific region. Lancet Infect Dis. 2012;12:75–88. doi: 10.1016/S1473-3099(11)70315-2. [DOI] [PubMed] [Google Scholar]
- 5.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 6.Hartman TK, Rogerson SJ, Fischer PR. The impact of maternal malaria on newborns. Ann Trop Paediatr. 2010;30:271–282. doi: 10.1179/146532810X12858955921032. [DOI] [PubMed] [Google Scholar]
- 7.Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Warikar N, Seal A, McGready R, Sugiarto P, Tjitra E, Anstey NM, Price RN. Adverse pregnancy outcomes in an area where multidrug-resistant Plasmodium vivax and Plasmodium falciparum infections are endemic. Clin Infect Dis. 2008;46:1374–1381. doi: 10.1086/586743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muehlenbachs A, Fried M, McGready R, Harrington WE, Mutabingwa TK, Nosten F, Duffy PE. A novel histological grading scheme for placental malaria applied in areas of high and low malaria transmission. J Infect Dis. 2010;202:1608–1616. doi: 10.1086/656723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nosten F, Rogerson SJ, Beeson JG, McGready R, Mutabingwa TK, Brabin B. Malaria in pregnancy and the endemicity spectrum: what can we learn? Trends Parasitol. 2004;20:425–432. doi: 10.1016/j.pt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 10.WHO . World Malaria Report: 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- 11.Carmona-Fonseca J, Maestre A. Incidencia de las malarias gestacional, congénita y placentaria en Urabá (Antioquia, Colombia), 2005–2007. Rev Colomb Obstet Ginecol. 2009;60:19–33. [Google Scholar]
- 12.Campos IM, Uribe ML, Cuesta C, Franco-Gallego A, Carmona-Fonseca J, Maestre A. Diagnosis of gestational, congenital, and placental malaria in Colombia: comparison of the efficacy of microscopy, nested polymerase chain reaction, and histopathology. Am J Trop Med Hyg. 2011;84:929–935. doi: 10.4269/ajtmh.2011.10-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis. 2009;200:1509–1517. doi: 10.1086/644781. [DOI] [PubMed] [Google Scholar]
- 14.Uneke CJ. Diagnosis of Plasmodium falciparum malaria in pregnancy in sub-Saharan Africa: the challenges and public health implications. Parasitol Res. 2008;102:333–342. doi: 10.1007/s00436-007-0782-6. [DOI] [PubMed] [Google Scholar]
- 15.Rantala AM, Taylor SM, Trottman PA, Luntamo M, Mbewe B, Maleta K, Kulmala T, Ashorn P, Meshnick SR. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J. 2010;9:269. doi: 10.1186/1475-2875-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arango E, Maestre A, Carmona-Fonseca J. Effect of submicroscopic or polyclonal Plasmodium falciparum infection on mother and gestation product: systematic review. Rev Bras Epidemiol. 2010;13:373–386. doi: 10.1590/s1415-790x2010000300002. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra I, Dent A, Mungai P, Muchiri E, King CL. Real-time quantitative PCR for determining the burden of Plasmodium falciparum parasites during pregnancy and infancy. J Clin Microbiol. 2005;43:3630–3635. doi: 10.1128/JCM.43.8.3630-3635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adegnika AA, Verweij JJ, Agnandji ST, Chai SK, Breitling LP, Ramharter M, Frolich M, Issifou S, Kremsner PG, Yazdanbakhsh M. Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am J Trop Med Hyg. 2006;75:798–803. [PubMed] [Google Scholar]
- 19.Mankhambo L, Kanjala M, Rudman S, Lema VM, Rogerson SJ. Evaluation of the OptiMAL rapid antigen test and species-specific PCR to detect placental Plasmodium falciparum infection at delivery. J Clin Microbiol. 2002;40:155–158. doi: 10.1128/JCM.40.1.155-158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker-Abbey A, Djokam RR, Eno A, Leke RF, Titanji VP, Fogako J, Sama G, Thuita LH, Beardslee E, Snounou G, Zhou A, Taylor DW. Malaria in pregnant Cameroonian women: the effect of age and gravidity on submicroscopic and mixed-species infections and multiple parasite genotypes. Am J Trop Med Hyg. 2005;72:229–235. [PubMed] [Google Scholar]
- 21.Padilla-Rodríguez JC, Álvarez-Uribe G, Montoya-Araújo R, Caparro-Narváez P, Herrera-Valencia S. Epidemiology and control of malaria in Colombia. Mem Inst Oswaldo Cruz. 2011;106((Suppl 1)):114–122. doi: 10.1590/s0074-02762011000900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmona-Fonseca J. La malaria en Colombia, Antioquia y las zonas de Urabá y Bajo Cauca: panorama para interpretar la falla terapéutica antimalárica. Parte 1. Iatreia. 2003;16:299–318. [Google Scholar]
- 23.Carmona-Fonseca J. La malaria en Colombia, Antioquia y las zonas de Urabá y Bajo Cauca: panorama para interpretar la falla terapéutica antimalárica. Parte 2. Iatreia. 2004;17:34–53. [Google Scholar]
- 24.Antioquia Dirección Seccional de Salud de Antioquia-DSSA. Enfermedades Transmitidas por Vectores 2000–2010. 2010. http://www.dssa.gov.co/index.php/documentos/doc_download/669-enfermedades-transmitidas-por-vectores Available at. Accessed January 20, 2012.
- 25.Córdoba Secretaría de Desarrollo de la Salud de Córdoba-SDSC. Programa Prevención y Control de Enfermedades Transmitidas por Vectores 2009. 2009. http://www.saludcordoba.gov.co/presentaciones/presentacion_etv.pptx Available at. Accessed January 20, 2012.
- 26.Colombia Guía para la atención clínica integral del paciente con malaria. 2010. http://dssa.media.vcb.com.co/dssa.gov.co/documentos/Protocolos-Vectores-INS/Clinica-Malaria.pdf Available at. Accessed June 12, 2012.
- 27.Martínez-Bencardino C. Muestreo: algunos métodos y sus aplicaciones prácticas. Bogotá, Colombia: Ecoe; 1984. [Google Scholar]
- 28.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 29.Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol. 2009;47:975–980. doi: 10.1128/JCM.01858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 31.Anderson TJ, Su XZ, Bockarie M, Lagog M, Day KP. Twelve microsatellite markers for characterization of Plasmodium falciparum from finger-prick blood samples. Parasitology. 1999;119:113–125. doi: 10.1017/s0031182099004552. [DOI] [PubMed] [Google Scholar]
- 32.Felger I, Beck H. msp2 genotyping of Plasmodium falciparum by capillary electrophoresis and GeneMapper® Program. In: Moll K, Ljungström I, Perlmann H, Scherf A, Wahlgren M, editors. Methods in Malaria Research. Manassas, VA: Malaria Research and Reference Reagent Resource Center (MR4); 2008. pp. 243–247. [Google Scholar]
- 33.Koepfli C, Mueller I, Marfurt J, Goroti M, Sie A, Oa O, Genton B, Beck HP, Felger I. Evaluation of Plasmodium vivax genotyping markers for molecular monitoring in clinical trials. J Infect Dis. 2009;199:1074–1080. doi: 10.1086/597303. [DOI] [PubMed] [Google Scholar]
- 34.Galbraith RM, Fox H, Hsi B, Galbraith GM, Bray RS, Faulk WP. The human materno-foetal relationship in malaria. II. Histological, ultrastructural and immunopathological studies of the placenta. Trans R Soc Trop Med Hyg. 1980;74:61–72. doi: 10.1016/0035-9203(80)90012-7. [DOI] [PubMed] [Google Scholar]
- 35.Bulmer JN, Rasheed FN, Francis N, Morrison L, Greenwood BM. Placental malaria. I. Pathological classification. Histopathology. 1993;22:211–218. doi: 10.1111/j.1365-2559.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 36.Bulmer JN, Rasheed FN, Morrison L, Francis N, Greenwood BM. Placental malaria. II. A semi-quantitative investigation of the pathological features. Histopathology. 1993;22:219–225. doi: 10.1111/j.1365-2559.1993.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 37.Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31:85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 38.Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodium falciparum malaria at delivery: comparison of blood film preparation methods and of blood films with histology. J Clin Microbiol. 2003;41:1370–1374. doi: 10.1128/JCM.41.4.1370-1374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGready R, Davison BB, Stepniewska K, Cho T, Shee H, Brockman A, Udomsangpetch R, Looareesuwan S, White NJ, Meshnick SR, Nosten F. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. Am J Trop Med Hyg. 2004;70:398–407. [PubMed] [Google Scholar]
- 40.Arango EM, Samuel R, Agudelo OM, Carmona-Fonseca J, Maestre A, Yanow SK. Genotype comparison of Plasmodium vivax and Plasmodium falciparum clones from pregnant and non-pregnant populations in north-west Colombia. Malar J. 2012;11:392. doi: 10.1186/1475-2875-11-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carmona-Fonseca J, Franco A, Arango E, Agudelo O, Maestre A. Now ICT malaria Pf/Pv® frente a microscopía (gota gruesa-extendido) para diagnóstico de malaria en Urabá (Colombia) Iatreia. 2010;23:137–145. [Google Scholar]
- 42.Piñeros-Jiménez JG, Álvarez G, Tobón A, Arboleda M, Carrero S, Blair S. Congenital malaria in Urabá, Colombia. Malar J. 2011;10:239. doi: 10.1186/1475-2875-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martínez-Espinosa FE, Daniel-Ribeiro CT, Alecrim WD. Malaria during pregnancy in a reference centre from the Brazilian Amazon: unexpected increase in the frequency of Plasmodium falciparum infections. Mem Inst Oswaldo Cruz. 2004;99:19–21. doi: 10.1590/s0074-02762004000100003. [DOI] [PubMed] [Google Scholar]
- 44.Padilla A, Tiburcio H, Apolinario M. Gestación y Malaria. Ginecol Obstet (Lima) 1997;43:239–243. [Google Scholar]
- 45.Ramal Ayasag C, Pinedo Iglesias P. Malaria en gestantes entre marzo del 2002 y julio del 2003: experiencia en el Hospital Regional de Loreto, Peru. Acta Med Peruana. 2008;25:220–223. [Google Scholar]
- 46.Mayor A, Serra-Casas E, Bardají A, Sanz S, Puyol L, Cisteró P, Sigauque B, Mandomando I, Aponte JJ, Alonso PL, Menéndez C. Sub-microscopic infections and long-term recrudescence of Plasmodium falciparum in Mozambican pregnant women. Malar J. 2009;8:9. doi: 10.1186/1475-2875-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayor A, Moro L, Aguilar R, Bardají A, Cisteró P, Serra-Casas E, Sigaúque B, Alonso PL, Ordi J, Menéndez C. How hidden can malaria be in pregnant women? Diagnosis by microscopy, placental histology, polymerase chain reaction and detection of histidine-rich protein 2 in plasma. Clin Infect Dis. 2012;54:1561–1568. doi: 10.1093/cid/cis236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.