Abstract
In central China, Plasmodium vivax accounts for all of the native reported cases of malaria. Chloroquine (CQ) plus primaquine (PQ) have been used for more than 60 years as the frontline drugs, but the risk of treatment failure remains unknown. To measure the effectiveness and safety of CQ-PQ among vivax malaria patients, a total of 39 subjects with monoinfection vivax malaria was enrolled in a study from 2008 to 2009. There were no recrudescence or danger signs observed within the 28-day follow-up period, showing that blood stage of P. vivax isolates from central China is still susceptible to CQ plus PQ combination therapy. However, the antirelapse efficacy of PQ is difficult to assess because of the high rate of loss to follow-up after 28 days; also, parasites persisted in a single case at 3 days post-antimalarial drug treatment, indicating that continuous annual monitoring is needed in central China.
Malaria is one of the most common parasitic diseases, with approximately one-half of the world's population at risk of infection. The disease is caused by multiple species of Plasmodium parasites with ranges that very often overlap. In central China, however, only one species, P. vivax, is endemic.1 Malaria was nearly eliminated from the central provinces in China during the World Health Organization's malaria eradication campaign in the mid-1950s. However, malaria outbreaks now occur frequently in many counties in the region. For example, in Suining County in Jiangsu Province, there were 488,029 (annual incidence of 70.1%) and 424,964 (49.6%) cases reported in 1960 and 1971, respectively.2 In central China, despite significant reductions in the overall malaria burden in the 20th century, vivax malaria has shown resilience to elimination and become increasingly prevalent in the past 10 years.3,4
The Ministry of Health in China launched an “action plan for malaria elimination” in 2010, which included measures to focus on improving capacity for early diagnosis and the radical treatment of P. vivax and also, strengthening surveillance systems.5 The frontline drugs used for vivax malaria treatment in central China are still chloroquine (CQ) plus primaquine (PQ), which they have been for the past 60 years. The risk of treatment failure with these drugs in this area remains unknown. CQ is well-tolerated, is affordable, has a long half-life, and provides protection from early relapses after treatment. However, CQ-resistant (CQR) P. vivax has been reported widely6–8 and raised great concerns for its continued use for treatment of vivax malaria. PQ is the only drug available for the prevention of relapse. However, P. vivax tolerance to PQ has been reported in Southeast Asia and Oceania, and the use of the drug needs to be carefully monitored because of the risk of hemolysis in patients with Glucose 6 Phosphate Dehydrogenase (G6PD) deficiency.9 Because the majority of people infected by P. vivax in central China are treated with CQ plus PQ, surveillance of therapeutic efficiency can support guidelines for antimalarial policy.
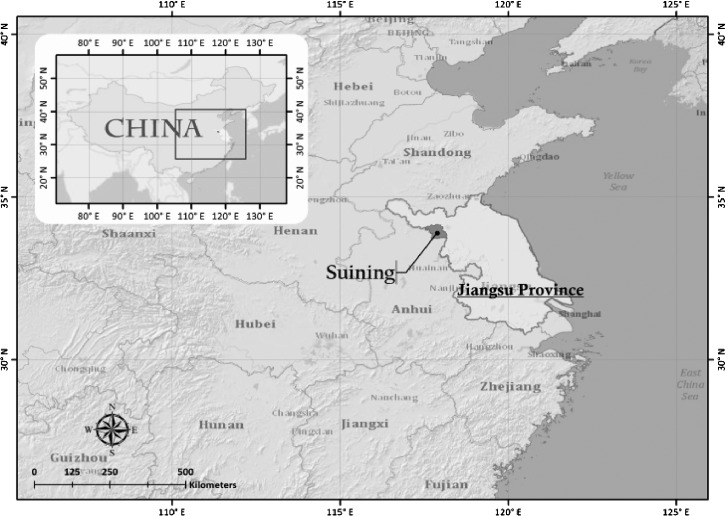
Surveillance of therapeutic efficiency was carried out in Suining County in Jiangsu Province from 2008 to 2009. Suining is located at 117.31–118.10° E longitude and 33.40–34.10° N latitude within the Jiangsu Province of China (Figure 1 ). The county is located in a warm temperate zone with a light maritime monsoon climate; the average temperature over the year is about 15°C, and the average humidity is 75.5% from May to October. The predominant vector mosquito is Anopheles sinensis.10 The pattern by which P. vivax relapses is subject to geographical variation: strains from tropical areas are characterized by early primary attack followed by a short latent period before the appearance of frequent relapses, whereas this occurrence was relatively late with the strains from temperate and subtropics areas, starting within months of the initial infection.11 However, P. vivax strains from the temperate areas of China are characterized by early primary attacks followed by a long latent period or late primary attacks followed by a long latent period; the latent period can range from 40 to 362 days.12 Patients with P. vivax monoinfections older than 5 years of age with parasitemia ≥ 250 asexual forms/μL, no signs of severe disease, no antimalarial treatment during the preceding 4 weeks, no history of hepatic or kidney diseases, and ability to swallow oral medication who were non-pregnant and non-lactating were enrolled. Written informed consent for study participation was obtained from all patients. The study was approved by the Institutional Ethics Committee of the National Institute for Parasitic Diseases (NIPD), Chinese Central for Diseases Control and Prevention. On enrolment, each patient underwent a physical examination and completed a symptom questionnaire. Study participants received treatment with CQ combined with PQ for eradication of hepatic stages of P. vivax parasites. CQ (250 mg CQ phosphate per tablet; Shanghai Zhongxi Pharmaceutical Company Limited, Shanghai, China) was given at a dose of 25 mg base/kg body weight and divided into single daily doses over 3 days (10 mg/kg on day 1 and 7.5 mg/kg on days 2 and 3). PQ (13.2 mg PQ phosphate per tablet; Shanghai Zhongxi Pharmaceutical Company Limited) was given daily for 8 days starting from day 3 and one time daily until day 10 (0.5 mg/kg daily for 8 days). Consumption of each treatment dose of drug was fully supervised and directly observed by medical staff. Subjects were closely observed for at least 30 minutes after each drug was ingested. Patients whose parasitemia was not cleared or patients who had a reappearance of P. vivax were treated with a rescue treatment (other artemisinin-based combination therapeutics officially recommended as second-line drugs for treatment according to the current national antimalarial drug policy recommendations). Any adverse effects were documented. All patients were routinely asked about previous and current symptoms. Blood smears and sampling of blood onto filter papers were performed on days 0, 1, 2, 3, 7, 14, 21, 28, and 180. Parasitemia and body temperature were evaluated immediately and on each occasion when an enrolled study participant returned to the clinic with fever or sign/symptoms of malaria.
Figure 1.
Sentinel site for antimalarial drug efficacy surveillance.
DNA was isolated from the blood filter papers using 5% Chelex-100 (Bio-Rad, Hercules, CA) as previously described, and all samples were confirmed by polymerase chain reaction (PCR).13
Treatment outcomes were assessed based on parasitological and clinical assessment after antimalarial treatment. The efficacy of treatment of P. vivax malaria was assessed by the clearance time of P. vivax parasites from the patient's blood after treatment and the continued absence of parasitemia throughout the follow-up period of 28 days. Parasite clearance time (PCT) was defined as the time taken for the parasite count to fall below the level of microscopic detection, and fever clearance time (FCT) was defined as the time taken for the temperature to return to normal (< 37.3°C) and remain so for at least 24 hours.
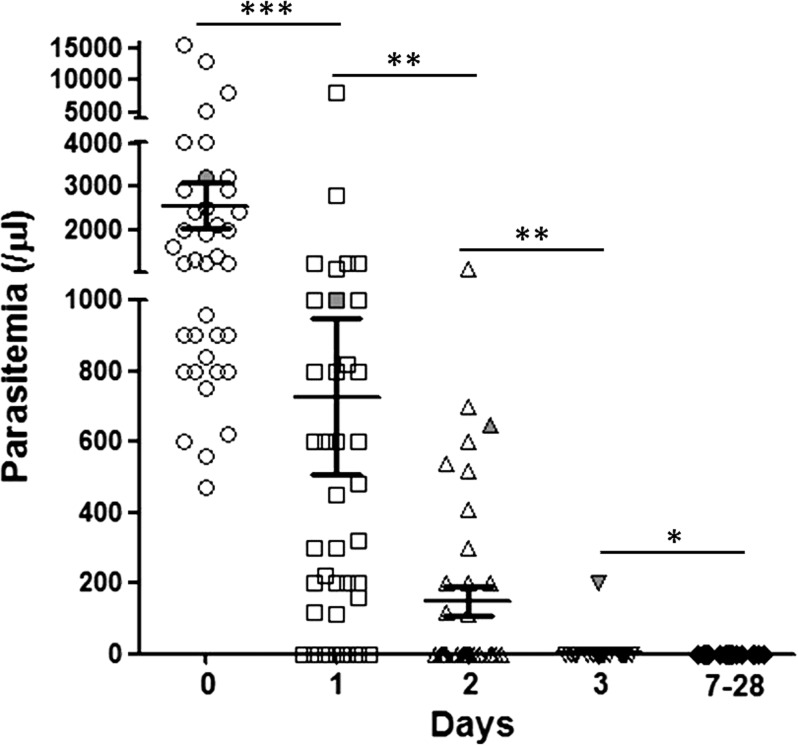
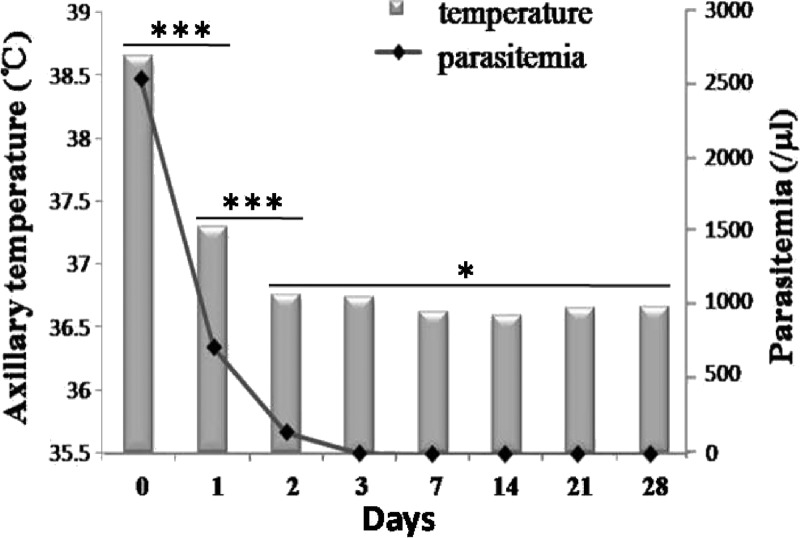
A total of 39 informed and consenting subjects (21 subjects in 2008 and 18 subjects in 2009) with uncomplicated vivax malaria was enrolled. However, two subjects were lost to follow-up: one subject at day 2 and one subject at day 7. In addition, only 15 subjects were followed-up successfully to day 180. The mean age and body weight were 43 (7–73) years and 55 (38–76) kg, respectively. Other information, including sex, age group, pre-treatment body temperature, and parasitemia, is given in Table 1. The PCTs of the majority of patients were less than 3 days, except for in one case, in which the parasite still remained on day 3 as assessed by microscopy (6.3% of the parasitemia on day 0) and confirmed by PCR. There were significant differences in the distribution of parasitemia among patients on days 0–3, but there was no statistical difference between parasitemia on day 3 and all subsequent days (Figure 2 ). The FCTs of all enrolled patients were also less than 3 days. Only one case had fever on day 3, and there was no fever found after day 3. There were no parasites or fever found after the date of PCTs and FCTs during the follow-up periods of 28 and 180 days. The average body temperature and parasitemia of patients during the 28-day follow-up period were consistent (Figure 3 ). The efficacy of CQ plus PQ in the treatment of P. vivax malaria in this study was 100%. There was no recrudescence within the follow-up period after drug treatment. No adverse treatment effects were observed.
Table 1.
Baseline characteristics of treatment group
| Variables | Value |
|---|---|
| Number of patients | 39 |
| Loss to follow-up at day 28 | 2* |
| Loss to follow-up at day 180 | 24 |
| Sex N (%) | |
| Female | 16 (42.1) |
| Male | 22 (57.9) |
| Age groups N (%) | |
| 5–15 years | 5 (13.2) |
| Adult | 33 (86.8) |
| Body temperature at day 0 (°C) mean ± SD | 38.6 ± 1.3 |
| Parasite count at day 0 (per microliter) mean (range) | 2,555 (470–15,500) |
One case was included in the analysis that had the information until day 7.
Figure 2.
The distribution of parasitemia during the follow-up period. ***P < 0.001; **P < 0.01; *No significant difference. (For one case, indicated in grey, parasitemia persisted until day 3.)
Figure 3.
Comparison of the average body temperature and parasitemia of patients during the 28-day follow-up period; t tests were performed for the temperature groups. *No significant difference, ***P < 0.001.
P. vivax has shown resilience to elimination and has become increasingly prevalent in central China since 2001.4 Although vivax malaria has traditionally been considered benign, recent evidence of severe disease manifestations associated with P. vivax infection has come to light.14,15 The ability of P. vivax to cause disease relapses adds significantly to the disease burden. Several methods can be used to monitor antimalarial drug efficiency, but clinical trials allow a direct assessment of treatment response in malaria patients. However, no clinical efficiency of CQ-PQ has been reported in central China until now.
Because parasitological cure is the goal of antimalarial therapy, to avoid the risk of inadequate treatment because of loss to follow-up, all study subjects were treated with PQ on day 3; one patient was treated with the rescue treatment, because parasites persisted in the blood on day 3. From the total of 37 patients enrolled in the study, 0 patients had a recurrence of parasitemia within the 28-day follow-up period, the PCTs and FCTs were less than 3 days, and no danger signs were observed, all of which suggest that CQ is still effective against P. vivax in central China. For subjects who were followed for up to 180 days, no relapses were observed. However, the antirelapse efficacy of PQ is difficult to assess because of the high rate of loss to follow-up to day 180, and it will be higher for follow-up for 1 year.
In a previous study, we analyzed the polymorphism of CQ resistance-associated genes,16 and no resistance-conferring mutations were discovered, which is consistent with the results of this study. In addition, our previous in vitro study also indicated that CQ remained effective against P. vivax malaria in central China.17 These consistent results offer additional confirmation that blood stage of P. vivax in central China is still susceptible to CQ plus PQ combination therapy, although continued surveillance is necessary because of the risk of emergence of CQ drug resistance.
ACKNOWLEDGMENTS
We acknowledge staff from Suining County Center for Diseases Control and Prevention for their assistance in samples collection. This work was supported by the World Health Organization Mekong Malaria Programme (WP/08/MVP/00051), Jiangsu Province's Medical High Tech Platform (ZX201108), and the National S & T Major Program (2012ZX10004220 and 2008ZX10004-011).
Footnotes
Authors' addresses: Guoding Zhu, Feng Lu, Jun Cao, Huayun Zhou, Yaobao Liu, and Qi Gao, Key Laboratory on Technology for Parasitic Disease Prevention and Control, Ministry of Health, Jiangsu Institute of Parasitic Diseases, Wuxi, Jiangsu, People's Republic of China, E-mails: jipdzhu@hotmail.com, lufeng981@hotmail.com, caojuncn@hotmail.com, zhou_huayun@hotmail.com, boao0721@hotmail.com, and gaoqi54@hotmail.com. Eun-Taek Han, Department of Medical Environmental Biology and Tropical Medicine, Kangwon National University School of Medicine, Chunchon, Gangwon-do, Republic of Korea, E-mail: etaekhan@yahoo.com.
References
- 1.WHO World Malaria Report 2011. 2011. http://www.who.int/malaria/world_malaria_report_2011/9789241564403_eng.pdf Available at. Accessed February 2012.
- 2.Tang YE, Zhong CY, Jiang CB, Wang WM. Results of surveillance of malaria in Suining County. China Trop Med. 2011;11:50–51. [Google Scholar]
- 3.Zhang W, Wang L, Fang L, Ma J, Xu Y, Jiang J, Hui F, Wang J, Liang S, Yang H, Cao W. Spatial analysis of malaria in Anhui province, China. Malar J. 2008;7:206. doi: 10.1186/1475-2875-7-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou SS, Wang Y, Tang LH. Malaria situation in the People's Republic of China in 2006. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2007;25:439–441. [PubMed] [Google Scholar]
- 5.The Ministry of Health of the People's Republic of China China Action Plan for Malaria Elimination. 2010. http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohjbyfkzj/s3593/201005/47529.htm Available at. Accessed May 2010.
- 6.Baird JK. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother. 2004;48:4075–4083. doi: 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnadas C, Ratsimbasoa A, Tichit M, Bouchier C, Jahevitra M, Picot S, Menard D. Plasmodium vivax resistance to chloroquine in Madagascar: clinical efficacy and polymorphisms in pvmdr1 and pvcrt-o genes. Antimicrob Agents Chemother. 2008;52:4233–4240. doi: 10.1128/AAC.00578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Santana Filho FS, Arcanjo AR, Chehuan YM, Costa MR, Martinez-Espinosa FE, Vieira JL, Barbosa MG, Alecrim WD, Alecrim MG. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg Infect Dis. 2007;13:1125–1126. doi: 10.3201/eid1307.061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 10.Zhou SS, Huang F, Wang JJ, Zhang SS, Su YP, Tang LH. Geographical, meteorological and vectorial factors related to malaria re-emergence in Huang-Huai River of central China. Malar J. 2010;9:337. doi: 10.1186/1475-2875-9-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Editorial Committee of Malaria Control and Research in China . Malaria Control and Research in China. Beijing, China: People's Medical Publishing; House; 1991. [Google Scholar]
- 13.Lu F, Gao Q, Zhou H, Cao J, Wang W, Lim CS, Na S, Tsuboi T, Han ET. Molecular test for vivax malaria with loop-mediated isothermal amplification method in central China. Parasitol Res. 2012;110:2439–2444. doi: 10.1007/s00436-011-2783-8. [DOI] [PubMed] [Google Scholar]
- 14.Anstey NM, Russell B, Yeo TW, Price RN. The pathophysiology of vivax malaria. Trends Parasitol. 2009;25:220–227. doi: 10.1016/j.pt.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 16.Lu F, Wang B, Cao J, Sattabongkot J, Zhou H, Zhu G, Kim K, Gao Q, Han E. Prevalence of drug resistance-associated gene mutations of Plasmodium vivax in Central China. Korean J Parasitol. 2012;50:379–384. doi: 10.3347/kjp.2012.50.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu F, Gao Q, Chotivanich K, Xia H, Cao J, Udomsangpetch R, Cui L, Sattabongkot J. In vitro anti-malarial drug susceptibility of temperate Plasmodium vivax from central China. Am J Trop Med Hyg. 2011;85:197–201. doi: 10.4269/ajtmh.2011.10-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]