Abstract
Post-treatment soil-transmitted helminth re-infection patterns were studied as part of a randomized controlled trial among school-aged children from an ethnic minority group in Yunnan province, People's Republic of China. Children with a soil-transmitted helminth infection (N = 194) were randomly assigned to triple-dose albendazole or placebo and their infection status monitored over a 6-month period using the Kato-Katz and Baermann techniques. Baseline prevalence of Trichuris trichiura, Ascaris lumbricoides, hookworm, and Strongyloides stercoralis were 94.5%, 93.3%, 61.3%, and 3.1%, respectively, with more than half of the participants harboring triple-species infections. For the intervention group (N = 99), the 1-month post-treatment cure rates were 96.7%, 91.5%, and 19.6% for hookworm, A. lumbricoides, and T. trichiura, respectively. Egg reduction rates were above 88% for all three species. Rapid re-infection with A. lumbricoides was observed: the prevalence 4 and 6 months post-treatment was 75.8% and 83.8%, respectively. Re-infection with hookworm and T. trichiura was considerably slower.
Introduction
More than 1 billion people are infected with soil-transmitted helminths, i.e., Ascaris lumbricoides, Trichuris trichiura, and the two hookworm species, Ancylostoma duodenale and Necator americanus.1 The collective global burden caused by soil-transmitted helminthiasis is estimated at 5.2 million disability-adjusted life years.2 Yet, soil-transmitted helminthiasis belongs to the group of infectious diseases commonly referred to as neglected tropical diseases.3,4 Although soil-transmitted helminth infections have largely disappeared from developed countries after sustained improvements in living and hygiene conditions,5 they continue to thrive where broad socio-economic development is elusive. Specifically, they are still common among the world's poorest populations in the Americas, Asia, and sub-Saharan Africa that lack access to clean water and improved sanitation, and where hygiene is poor.6,7 Children and pregnant women are most vulnerable to the detrimental effects of soil-transmitted helminth infections, among them anemia, nutritional deficiencies, and impairments in cognitive and physical development.8–11
Preventive chemotherapy, i.e., the periodic administration of anthelmintic drugs to entire populations or high-risk groups (e.g., school-aged children), is the hallmark of the control strategy currently advocated by the World Health Organization (WHO). Single-dose albendazole (400 mg) or mebendazole (500 mg) are used in virtually all preventive chemotherapy schemes against soil-transmitted helminthiasis.12–14 Although single-dose albendazole is highly efficacious against A. lumbricoides and reasonably so against hookworm, single-dose mebendazole reliably cures only A. lumbricoides infections but is considerably less efficacious against hookworm. The treatment of T. trichiura with a single dose of either drug results in low cure rates.15 As both drugs are rapidly cleared from the human body and thus do not confer protection against re-infection, other control strategies, such as health education, provision of clean water and improved sanitation, and shoe wearing are required to lower infection levels permanently. Indeed, a recent systematic review and meta-analysis has shown that post-treatment re-infection is common, with prevalences of A. lumbricoides and T. trichiura returning to almost pre-treatment levels within 12 months of drug administration.16 Proponents of preventive chemotherapy argue that this is acceptable because the primary aim of this strategy is to reduce infection intensity and thus, lower or eliminate morbidity from chronic helminth infections.17 Indeed, infection intensities usually recover much slower than prevalences after treatment. However, the long-term health impact and sustainability of preventive chemotherapy still needs further investigation18; the potential development and spread of drug resistance is another concern.19
We conducted a randomized controlled trial in Yunnan province, People's Republic of China (P.R. China), investigating the effects of triple-dose albendazole on physical fitness of school-aged children. We observed unexpectedly rapid re-infection with soil-transmitted helminths, and hence decided to report these findings (secondary outcomes of the trial) ahead of the primary outcomes, in consideration of their public health importance.
Materials and Methods
Study location and participants.
In a first step, a rapid appraisal was carried out to identify village schools where the prevalence of any soil-transmitted helminth was 70% or higher. The study finally included participants from five village schools located in the mountainous Bulangshan township, a sub-division of Menghai county in Xishuangbanna Dai Autonomous prefecture, situated in Yunnan province, P.R. China. The field work was carried out between October 2011 and May 2012. The five village schools are from 1) Sandui (geographical coordinates: 21°33′07″ N latitude and 100°19′34″ E longitude; altitude: 1,566 m above sea level [asl]); 2) Kongkan (21°32′34″ N and 100°20′25″ E; 1,195 m asl); 3) Laozhai (21°31′37″ N and 100°18′01″ E; 1,399 m asl); 4) Laonandong (21°33′28″ N and 100°21′45″ E; 1,188 m asl); and 5) Mannuo (21°33′27″ N and 100°23′53″ E; 1,352 m asl).
The county borders Myanmar and the study area is inhabited by the Bulang ethnic minority group. The sub-tropical climate is characterized by arid and cool winters, whereas summers are hot and rainy. Adult literacy rates are relatively low. Today, most children attend at least primary school, albeit often only for some years. Boys commonly receive part of their education in temple schools. Modern health care is lacking at village level, where only grassroots “doctors” with none or minimal formal medical training are present. Government-led health care facilities are located about 20 km away in the Bulangshan township. Water supply in the villages is piped, but water sources are unprotected and the water untreated. Power outages are common. Sanitation infrastructure is generally unavailable and open defecation takes place around the house or at the fringe of the village. Before our study, no survey or control activities targeting soil-transmitted helminthiasis have been implemented in the study villages. The epidemiology and control of soil-transmitted helminthiasis in other broadly similar Bulang communities have been studied by our group, with key findings reported elsewhere.11,20–22
Children 9–12 years of age were recruited from the village primary schools for a baseline screening for soil-transmitted helminth infections. All children attending school and falling into this age bracket, who had written informed consent from their parents/guardians, were invited to participate. The following inclusion criteria were applied in the randomized controlled trial: 1) submission of two stool samples at baseline; 2) detection of at least one soil-transmitted helminth egg in the samples; 3) no major systemic illnesses as determined by a medical doctor from the Bulangshan township hospital; 4) no known allergy to albendazole; 5) no deworming treatment over the previous 6 months; 6) no participation in other clinical trials; and 7) residency in the study area for at least 1 year before enrollment, as assessed by a parental questionnaire.
Study design.
The study was designed as a randomized, double-blind, placebo-controlled trial with multiple follow-ups. Triple-dose albendazole was administered in an attempt to achieve high cure rates, so that participating children had a fair chance of developing their physical fitness unaffected by ongoing intestinal helminth infections. The parasitological status of the children was assessed at baseline and at 1, 4, and 6 months after treatment.
Based on an estimated prevalence of 70% with any soil-transmitted helminth species and 50% loss to follow-up, we aimed at enrolling 250 children to submit stool samples at baseline to achieve a power of 80% at an alpha error of 5% for the detection of a 2.5 mL kg−1 min−1 difference in the maximum volume of oxygen that can be used during 1 min of exhaustive exercise (VO2 max estimate) between the treatment and placebo groups.23 Treatment allocation was determined by a statistician using block randomization with randomly varying block sizes of 2, 4, and 6 to ensure that both treatment arms had similar sample sizes.24 Triple-dose non-flavored albendazole (400 mg) and placebo treatments (tablets matched in color, size, taste, and shape) were prepared by staff not involved in the field work, in sealed envelopes marked with unique identifiers. Class lists were obtained from the schools and following the order of the list, each child who met the inclusion criteria was systematically assigned a random number from a list of random numbers generated from R.25 The assigned random number for each child corresponded to the treatment number on the sealed envelope and thus, determined the type of treatment to be allocated to the child.
Field and laboratory procedures.
After written informed consent was obtained from parents/guardians of the children, stool containers, labeled with the name and IDs, were distributed. Containers, filled with fresh morning stool samples, were collected on the following day. From each child, two stool samples were collected over consecutive days.
Stool samples were transferred to a nearby laboratory and processed on the day of collection. Both the Kato-Katz and Baermann techniques were used.26 In brief, for the Kato-Katz technique, a 41.7 mg template was used to prepare fecal thick smears. Thirty to 60 min after preparation, slides were read under a microscope at 100× magnification. The number of eggs was counted separately for A. lumbricoides, hookworm, and T. trichiura. For the Baermann technique, about 20 g of stool was placed on medical gauze in a glass funnel fitted with a rubber tube sealed by a clip and filled with tap water. The whole setup was illuminated with artificial light directed at the bottom of the funnel for 2 hours. The lowest 50 mL of the liquid was then collected and centrifuged. The sediment was subjected to microscopic examination for the larvae of Strongyloides stercoralis. In addition, each stool sample was visually inspected for Taenia spp. proglottids. Taking the presence of S. stercoralis and Taenia spp. into account was necessary to control for potential confounders with regard to the primary outcome (i.e., physical fitness, to be reported elsewhere), in the form of additional intestinal helminth infections.
The assigned triple dose treatment (i.e., 3 × 400 mg albendazole [GlaxoSmithKline; London, UK] or 3 × placebo [Fagron; Barsbüttel, Germany]), was started on treatment Day 1 with a single dose, with another dose administered every day until treatment Day 3. Drug ingestion was observed by trained medical staff. Parasitological follow-up involved the same sample collection and analysis procedures. In addition, the socioeconomic status of the participants was assessed through a short questionnaire asking for the education level of the children's parents and the main source of household income.
Statistical analysis.
Data were entered into Excel version 2008 (Microsoft Corp.; Redmond, WA), double-checked, and merged into a single database for statistical analysis with STATA version 10.0 (STATA Corp.; College Station, TX). The randomization code was broken after data entry and a series of internal consistency checks had been completed. A per-protocol analysis was carried out in an unblinded manner.
The parasitological status of the study participants was described in terms of prevalence, infection intensity (geometric mean [GM]27,28 eggs per gram of stool [EPG]), and multiparasitism (concurrent infections with more than one helminth species). Re-infection patterns were established from changes in prevalence and infection intensity after treatment. Comparisons were between the albendazole and placebo treatment groups. Efficacy of triple-dose albendazole against placebo was measured in terms of cure and egg reduction rates. Cure rate is defined as the proportion of egg-positive individuals who became egg-negative after treatment. Egg reduction rate is calculated using the following equation: (GM EPG at baseline among infected − GM EPG at follow-up among those infected at baseline)/GM EPG at baseline among infected × 100.27 All analyses were done on a per-species basis, except for multiparasitism. Test statistics included chi-square (χ2), Wilcoxon rank-sum, two-sample tests of proportions, and logistic regression, as appropriate.
Ethical considerations.
The results reported here were obtained as part of a randomized, double-blind, placebo-controlled trial that has been registered with Current Controlled Trials (identifier: ISRCTN 25371788). The study protocol was approved by the institutional research commission of the Swiss Tropical and Public Health Institute (Basel, Switzerland). Ethical clearance was obtained from the ethics committee of Basel (EKBB, reference no. 144/11) and the Academic Board of the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (Shanghai, P.R. China).
The village doctor, chief, and teachers of each village were briefed on the aims of the study and the planned procedures. With help from the teachers, the investigators further explained the study to the children and their parents/guardians. Written informed consent was obtained from parents/guardians and children assented orally. Parents/guardians were free to recall their children from the study anytime without further obligations, and children were informed that they could withdraw without negative impact for them or their family. Data were kept anonymous.
At study completion (i.e., after the 6-month follow-up), all children attending the five schools were given triple-dose albendazole (3 × 400 mg) treatment irrespective of their infection status, study participation, and treatment during the study (albendazole or placebo). In addition, children diagnosed with S. stercoralis during the study period were given a single dose of ivermectin (200 μg/kg). Brief education sessions on soil-transmitted helminth infections and prevention methods were conducted in all schools.
Results
Compliance and demographics of study participants.
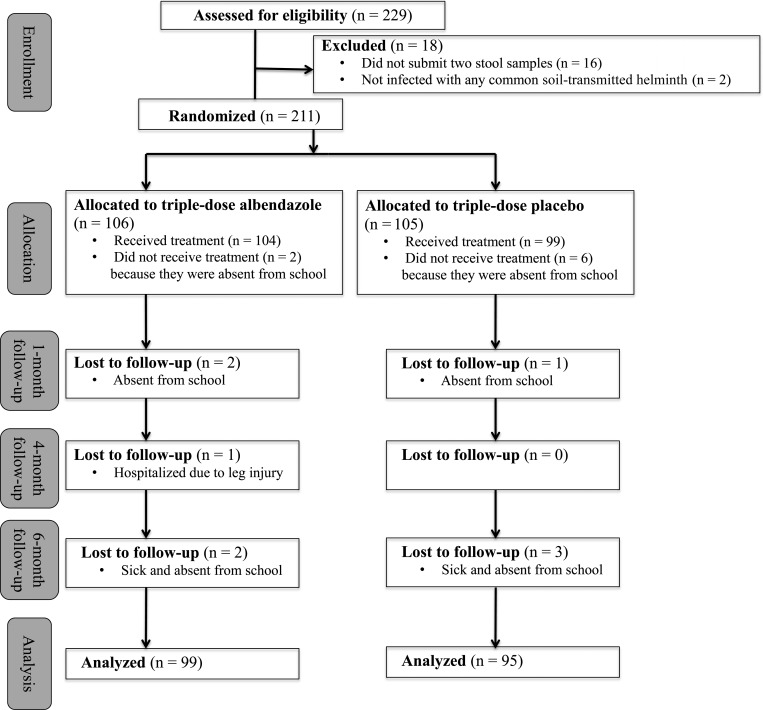
As shown in Figure 1, 229 children were interested in participating, and hence, they were assessed for eligibility. Eighteen children were excluded because they either did not submit two stool samples at baseline (n = 16) or were not infected with any soil-transmitted helminth species (n = 2). Randomization of treatments was done for 211 children and resulted in 106 subjects allocated to triple-dose albendazole and 105 children assigned to the placebo group. However, 2 and 6 children did not receive the full albendazole and placebo treatments, respectively, as they were sick or otherwise unavailable. Loss of children during the three follow-ups was small. Complete datasets were available for 194 children, 99 in the albendazole group, and 95 in the placebo group.
Figure 1.
Trial flow diagram of a randomized controlled trial conducted in south-west Yunnan province, P.R. China, from October 2011 to May 2012, according to CONSORT guidelines.29
The age of the cohort at the start of the study ranged between 9 and 12 years. At baseline, the mean age of the albendazole group was 10.4 years (standard deviation [SD]: 1.2 years), similar to the placebo group (mean 10.3 years, SD: 1.0 years; P = 0.494). The proportion of males in the albendazole and placebo groups was 46.5% and 50.5%, respectively (P = 0.571). Socioeconomic conditions across the five villages were comparable, and no significant difference was detected between the albendazole and placebo groups. All children originated from families where the main source of income was agriculture, and the majority of their parents (56.2%) had not received formal education (P = 0.108).
Baseline helminth infection status and efficacy of triple-dose albendazole.
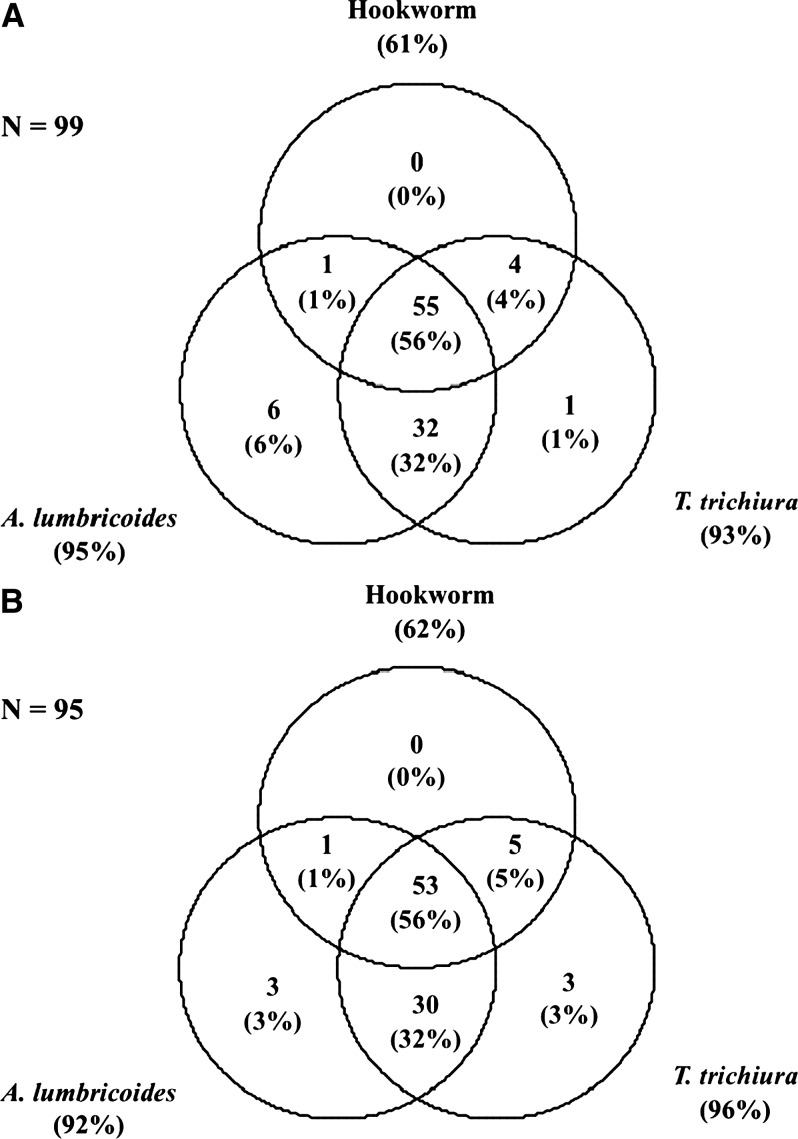
The baseline prevalence of T. trichiura, A. lumbricoides, and hookworm infection was 94.5%, 93.3%, and 61.3%, respectively. Six cases of S. stercoralis (3.1%) and one case of Taenia spp. (0.5%) were detected. Most of the infected children harbored light T. trichiura infections (1–999 EPG; 86.3%), whereas 13.1% had infections of moderate intensity (1,000–9,999 EPG). Only 1 heavy infection (≥ 10,000 EPG) was detected. In contrast, more than half of the A. lumbricoides-infected children had moderate intensity infections (5,000–49,999 EPG) and 27.6% had heavy infections (≥ 50,000 EPG). All but two hookworm infections were of light intensity (1–1,999 EPG). No significant differences in prevalence and intensity of infection were observed between the albendazole and placebo groups (Table 1). As illustrated in Figure 2 , multiparasitism was common: 55.7% of the children were infected with all three common soil-transmitted helminth species concurrently, whereas a single-species infection was detected in only 6.7% of the children.
Table 1.
Prevalence, cure rates, infection intensities, and egg reduction rates of Ascaris lumbricoides, Trichuris trichiura, and hookworm at baseline and 1-month follow-up among 194 children from Yunnan, P.R. China, from October 2011 to May 2012, stratified by treatment groups
| Baseline (Oct 2011) | 1-month follow-up (Dec 2011) | ||||
|---|---|---|---|---|---|
| Triple-dose albendazole (N* = 99) | Placebo (N* = 95) | Triple-dose albendazole (N* = 99) | Placebo (N* = 95) | ||
| n† (%) | n† (%) | n† (%) | n† (%) | P-value‡ | |
| Ascaris lumbricoides | |||||
| Prevalence | 94 (95.0) | 87 (91.6) | 8 (8.1) | 89 (93.7) | < 0.001 |
| New positives at 1-month follow-up | n.a. | n.a. | 0 | 5 | |
| Cure rate§ (% [95% CI]) | n.a. | n.a. | 91.5 (83.9–96.3) | 3.4 (0.7–9.7) | |
| Difference between albendazole and placebo cure rates (% [95% CI]) | n.a. | n.a. | 88.1 (81.3–94.9) | Reference | < 0.001 |
| Infection intensity¶ | |||||
| Mean EPG∥ (95% CI) | 15,850 (10,834–23,189) | 19,101 (13,198–27,644) | 1.3 (1.0–1.7) | 21,001 (12,835–34,362) | < 0.001 |
| Light (1–4,999) | 13 (13.1) | 15 (15.8) | 7 (7.1) | 13 (13.7) | < 0.001 |
| Moderate (5,000–49,999) | 54 (54.6) | 49 (51.6) | 1 (1.0) | 45 (47.4) | |
| Heavy (≥ 50,000) | 27 (27.3) | 23 (24.2) | n.r. | 31 (32.6) | |
| Egg reduction rate (% [95% CI]**) | n.a. | n.a. | > 99.9 (96.2–100) | n.a. | |
| Trichuris trichiura | |||||
| Prevalence | 92 (92.9) | 91 (95.8) | 74 (74.8) | 92 (96.8) | < 0.001 |
| New positives at 1-month follow-up | n.a. | n.a. | 0 | 1 | |
| Cure rate§ (% [95% CI]) | n.a. | n.a. | 19.6 (12.0–29.1) | 0 | |
| Difference between albendazole and placebo cure rates (% [95% CI]) | n.a. | n.a. | 19.6 (11.5–27.7) | Reference | < 0.001 |
| Infection intensity¶ | |||||
| Mean EPG∥ (95% CI) | 216.3 (160.2–292.0) | 284.4 (207.2–390.4) | 24.3 (16.2–36.3) | 304.7 (227.4–408.3) | < 0.001 |
| Light (1–999) | 82 (82.8) | 76 (80.0) | 73 (73.7) | 73 (76.8) | < 0.001 |
| Moderate (1,000–9,999) | 9 (9.1) | 15 (15.8) | 1 (1.0) | 18 (19.0) | |
| Heavy (≥ 10,000) | 1 (1.0) | n.r. | n.r. | 1 (1.1) | |
| Egg reduction rate (% [95% CI]**) | n.a. | n.a. | 88.8 (80.9–94.7) | n.a. | |
| Hookworm | |||||
| Prevalence | 60 (60.6) | 59 (62.1) | 2 (2.0) | 57 (60.0) | < 0.001 |
| New positives at 1-month follow-up | n.a. | n.a. | 0 | 6 | |
| Cure rate§ (% [95% CI]) | n.a. | n.a. | 96.7 (88.5–99.6) | 13.6 (6.0–25.0) | |
| Difference between albendazole and placebo cure rates (% [95% CI]) | n.a. | n.a. | 83.1 (73.3–92.9) | Reference | < 0.001 |
| Infection intensity¶ | |||||
| Mean EPG∥ (95% CI) | 130.4 (93.3–182.2) | 121.5 (86.7–170.3) | 1.2 (0.9–1.5) | 62.2 (37.7–102.6) | < 0.001 |
| Light (1–1,999) | 59 (59.6) | 58 (61.1) | 2 (2.0) | 57 (60.0) | < 0.001 |
| Moderate (2,000–3,999) | 1 (1.0) | 1 (1.0) | n.r. | n.r. | |
| Heavy (≥ 4,000) | n.r. | n.r. | n.r. | n.r. | |
| Egg reduction rate (% [95% CI]**) | n.a. | n.a. | 99.1 (91.1–100) | 48.8 (35.9–62.5) | < 0.001 |
N = total sample size.
n = number of infected individuals.
P-values are calculated using χ2, two-sample test of proportions or Wilcoxon rank-sum test, as appropriate.
Cure rate excludes newly infected at first follow-up.
Stratified according to WHO guidelines.
Geometric mean among those infected at baseline.
Calculated by bootstrap resampling among those infected at baseline.
CI = confidence interval; EPG = eggs per gram of stool; n.a. = not applicable; n.r. = not represented.
Figure 2.
Extent of multiparasitism (common soil-transmitted helminths only) at baseline among 194 children from Yunnan, P.R. China, in October 2011, stratified by triple-dose albendazole (A) and placebo (B) treatment groups. No significant difference was detected between the groups (P = 0.831).
Cure rates of 96.7%, 91.5%, and 19.6% were achieved for hookworm, A. lumbricoides, and T. trichiura infections, respectively, with triple-dose albendazole. The treatment resulted in egg reduction rates of > 99.9%, 99.1%, and 88.8% for A. lumbricoides, hookworm, and T. trichiura infections, respectively. Cure and egg reduction rates achieved with triple-dose albendazole were all significantly different from those observed after placebo administration (Table 1).
Re-infection patterns and dynamics.
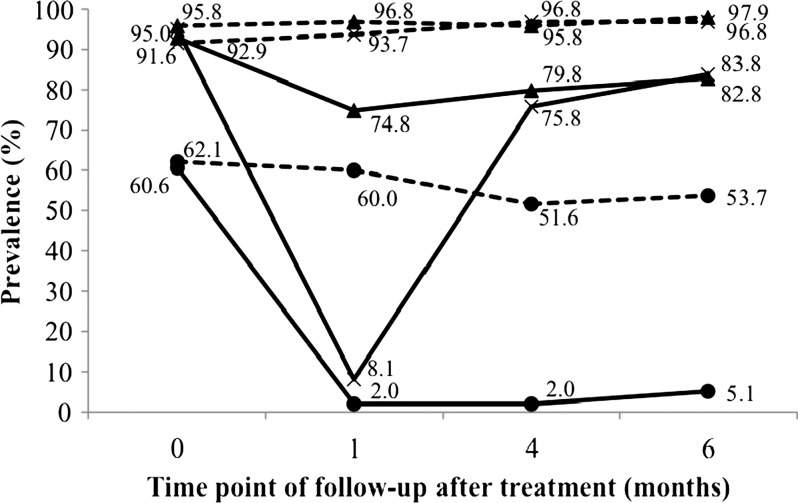
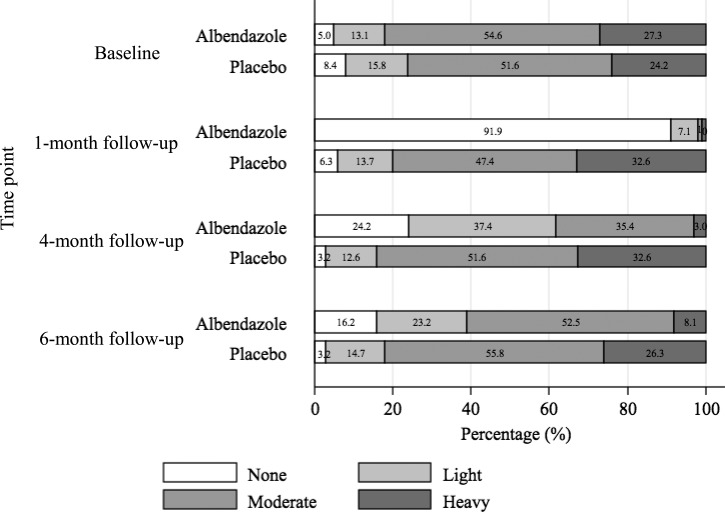
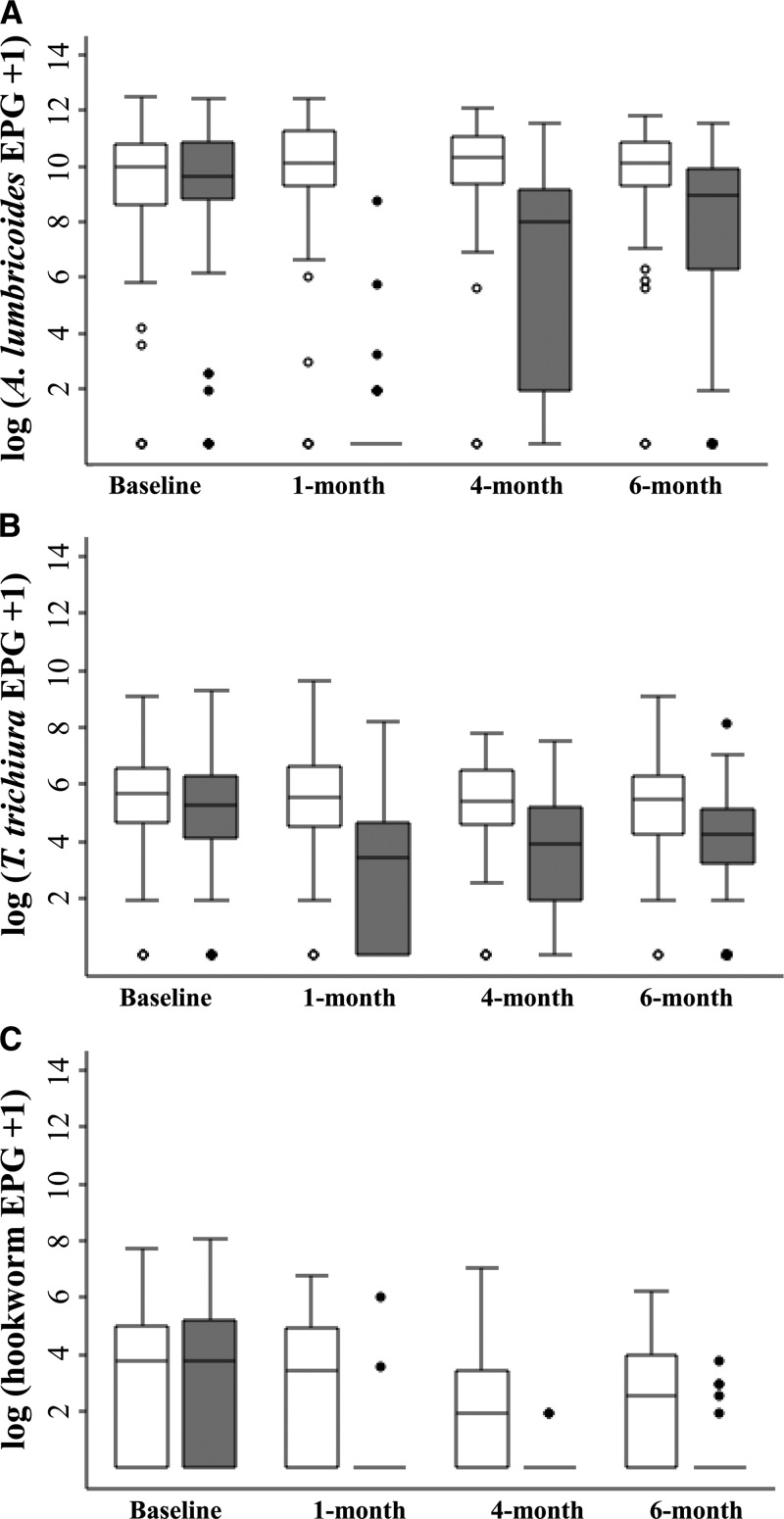
As shown in Figure 3 , re-infection with A. lumbricoides was rapid. About three-quarters of the treated children were infected with this parasite 4 months after drug administration. Six months post-treatment, 83.8% of the children were infected with A. lumbricoides. Stratification of the A. lumbricoides infection intensity at the various follow-ups according to WHO guidelines showed a gradual shift in infection intensity in most of the children, from no infection (91.9%) at the 1-month follow-up to moderate infection (52.5%) at the 6-month follow-up (Figure 4 ).
Figure 3.
Prevalence of Ascaris lumbricoides (×), Trichuris trichiura (▴), and hookworm (•) among 194 children from Yunnan, P.R. China, in October 2011–May 2012, stratified by triple-dose albendazole (solid lines) and placebo (dotted lines) treatment groups.
Figure 4.
Ascaris lumbricoides infection intensity levels, classified according to WHO guidelines, among 194 children from Yunnan, P.R. China, from October 2011 to May 2012.
In the case of T. trichiura infections, prevalence remained high even at the 1-month post-treatment follow-up (74.8%); as a consequence, subsequent re-infection was slow, with 82.8% of the treated children harboring T. trichiura 6 months after treatment. For the treatment group, an 87% drop in the intensity of T. trichiura infection was detected at the 1-month follow-up, but infection intensity increased over time and was back to 30% of the baseline level at the 6-month follow-up (Figure 5).
Figure 5.
Infection intensities of Ascaris lumbricoides (A), Trichuris trichiura (B), and hookworm (C) at different time points among 194 children from Yunnan, P.R. China, from October 2011 to May 2012, stratified by triple-dose albendazole (grey bars) and placebo (white bars) treatment group. Middle line of box: median; upper end of box: 75th percentile; lower end of box: 25th percentile; upper whisker: 95th percentile; lower whisker: 5th percentile; dots: outliers.
Hookworm re-infection was minimal throughout the 6-month follow-up period as only 5.1% of the treated children were harboring hookworm at the end of the study. The hookworm infection intensity was reduced by more than 90% and remained at this level across the various follow-ups.
When further stratified by baseline co-infection status, no significant difference in the prevalence of common soil-transmitted helminth infections at the follow-ups was detected among the treated children (results not shown). Among placebo recipients, no significant change in the prevalence and infection intensity levels of the three common soil-transmitted helminths at the various follow-ups was observed. Still, between the first and the second follow-up, an 8.4% decrease in the hookworm prevalence was noted.
Predictors of re-infection with A. lumbricoides.
As re-infection was minimal for hookworm and the cure rate for T. trichiura was very low, odds of re-infections were not calculated for these two species. According to the results of the univariate logistic regression analyses (Supplemental Table S1), children from Sandui and Laozhai had significantly higher odds to be re-infected with A. lumbricoides at the 4-month follow-up as compared with children from Kongkan (Sandui: odds ratio [OR] = 4.92, 95% confidence interval [CI]: 1.24–19.57; Laozhai: OR = 3.74, 95% CI: 0.93–15.14). Likewise, the odds of A. lumbricoides re-infection of children living in Laozhai were higher (OR = 4.58, 95% CI: 0.91–23.14) at the 6-month follow-up as compared with children from Kongkan. Baseline infections with A. lumbricoides and T. trichiura increased the odds of re-infection with A. lumbricoides by 2.18 and 2.54 times, respectively, at the 4-month follow-up and by 1.32 and 2.23 times, respectively, at the 6-month follow-up. However, these risk increases lacked statistical significance. Heavy baseline A. lumbricoides infection intensity was a positive but, for the current sample size, insignificant predictor of A. lumbricoides re-infection (OR = 8.33 at the 4-month follow-up, and OR = 6.50 at the 6-month follow-up). Age and sex showed no significant associations with A. lumbricoides re-infection.
In the multivariate logistic regression analyses, village location remained a significant predictor of A. lumbricoides re-infection. At the 4-month follow-up, the odds of re-infection of children living in Sandui were increased by a factor of 9.71 (95% CI: 1.72–54.61) as compared with that of children living in Kongkan.
Discussion
Few studies have assessed post-treatment re-infection patterns with soil-transmitted helminths as rigorously as reported here, with the study integrated into a randomized controlled trial. According to the national parasitological survey conducted in P.R. China from 2001 to 2004,30 soil-transmitted helminth infections were most prevalent in the provinces of Guizhou, Hainan, and Hunan, and Guangxi Zhuang Autonomous region. National prevalence rates were 12.7%, 6.1%, and 4.6% for A. lumbricoides, hookworm, and T. trichiura, respectively. Recent cross-sectional studies confirmed important variations in the prevalence and intensity of soil-transmitted helminth infections in different geographical regions across P.R. China.20,31 Among school-aged children, rural areas in Hainan province and Guangxi Zhuang Autonomous region reported prevalences of 18.5%, 14.7%, and 11.2% for A. lumbricoides, hookworm, and T. trichiura, respectively, with 16.7% of the infections being of moderate or heavy intensity.32 In another rural part of Yunnan province, prevalences of 35.7%, 5.4%, and 2.7% were reported for hookworm, T. trichiura, and A. lumbricoides, respectively.33 The baseline prevalence and intensity of soil-transmitted helminth infections documented here are among the highest ever reported from P.R. China. However, they are comparable to reports by Steinmann and co-workers34 from other Bulang communities characterized by similar socio-ecological contexts where multiple species helminth infection was also common.11,20–22 Taken together, these data emphasize that, although P.R. China has witnessed huge economic and social improvements over the past 30 years, hotspots of soil-transmitted helminthiasis and other neglected tropical diseases persist,35 with ethnic minority groups particularly vulnerable and thus, less benefiting from the country's development.36,37
Post-treatment re-infection patterns of soil-transmitted helminths are largely dependent on the degree of endemicity within the community.38,39 In the current high endemicity area of P.R. China, follow-ups at 1, 4, and 6 months after administration of triple-dose albendazole or placebo revealed that re-infection with A. lumbricoides was rapid, both in terms of prevalence and intensity. Indeed, the prevalence of A. lumbricoides reached 80% of the pretreatment prevalence 4 months after treatment. Our findings corroborate recent results from a systematic review and meta-analysis by Jia and colleagues.16 The high egg production and long survival of the infective stage (the ova) of A. lumbricoides in the environment could explain the quick re-infection wherever permissive hygiene and behavioral conditions coincide and no measures to prevent re-infection have been implemented.6
Recent publications have highlighted the low efficacy of albendazole against T. trichiura infections.40–43 A single oral dose of 400 mg of albendazole was reported to give a cure rate of 28% in a meta-analysis.15 Generally, the reported cure rates, particularly following triple-dose treatment, were considerably higher than the one reported in this trial. Indeed, the cure rate of only 19.6% for T. trichiura as observed in our trial is much lower than that reported from a previous trial with triple-dose albendazole treatment in the same area (56%).34 It is difficult to explain these observations because sampling and diagnostic efforts were comparable and the same field team was involved in both trials. It should be noted, however that from a public health point of view, the egg reduction rate rather than the cure rate should be considered when determining anthelmintic drug efficacy.44 Indeed, the egg reduction rate more accurately reflects the reduction in morbidity, because it is associated with decreases in soil-transmitted helminths infection intensity. We observed an egg reduction rate of 88.8% for T. trichiura, which is reasonably high. However, the very low cure rate reported in our trial is of concern and underscores the pressing need for the development of novel anthelmintics and the study of drug resistance. We were interested in re-infection patterns after treatment, but this proved to be difficult to determine for T. trichiura as only a few of the infections completely cleared even after triple-dose albendazole administration.45,46
Interestingly, hookworm infections were greatly reduced by triple-dose albendazole and the resulting low prevalence was maintained over the 6-month follow-up period, whereas prevalences of A. lumbricoides and T. trichiura were steadily increasing. Re-infection with hookworm is known to be slower compared with other common soil-transmitted helminth species as the reproductive number (R0) of hookworm is the lowest among them,6 and the time between the 1- and 4-month follow-ups (December–March) was cold and dry, conditions known to be unsuitable for the development and survival of hookworm larvae.6,47 The latter could also explain the slight decrease in the hookworm prevalence and infection intensity noted in the placebo group during the second follow-up. Consequently, deworming should be deployed at the start of the dry season (co-incidentally coinciding with the return of the students to school after the summer break) to maximize the time they can expect to remain free from re-infection.
In terms of S. stercoralis infection, a prevalence of 3.1% detected in this school-based trial is considerably lower than what was observed in a previous community-based study in the same region (11.7%),48 which might again point to the fact that S. stercoralis prevalence increases with age.49 However, the few cases present were observed to be of higher intensity, as inferred from the larger number of first-stage (L1) larvae present, than noted in former work. Unfortunately, quantification of S. stercoralis infections to further substantiate such a claim is currently not possible.
We conclude that the observed re-infection patterns with soil-transmitted helminths after triple-dose albendazole re-emphasize the need for control programs that go beyond preventive chemotherapy, particularly for ascariasis and trichuriasis. In areas of such intense transmission, even the stated goal of preventive chemotherapy, namely the prevention of morbidity through the depression of infection intensity levels, may be unattainable, particularly in the case of A. lumbricoides. Of note, the studied population is hitherto not covered by regular deworming activities. The need for a more effective drug or combination therapy against T. trichiura is further highlighted.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the children from the five study villages for their enthusiastic participation in this trial. The support from the teachers, parents, and the community leaders was also invaluable. Appreciation is also given to the local team of field workers, from the Menghai Center for Disease Control and Prevention and Xishuangbanna Center for Disease Control and Prevention, for their hard work and dedication.
Disclaimer: None of the authors have any conflict of interest.
Footnotes
Financial support: This study received financial support from the Swiss Tropical and Public Health Institute in Basel, and the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention in Shanghai.
Authors' addresses: Peiling Yap, Jan Hattendorf, Jürg Utzinger, and Peter Steinmann, Swiss Tropical and Public Health Institute, and University of Basel, Basel, Switzerland, E-mails: p.yap@unibas.ch, jan.hattendorf@unibas.ch, juerg.utzinger@unibas.ch, and peter.steinmann@unibas.ch. Zun-Wei Du, Fang-Wei Wu, and Jin-Yong Jiang, Yunnan Institute of Parasitic Diseases, Pu'er, People's Republic of China, E-mails: dzw5509@163.com, wufangwei-03@163.com, and yipdjiang@126.com. Ran Chen, Menghai Center for Disease Control and Prevention, Menghai, People's Republic of China, E-mail: gf_mh859@163.com. Xiao-Nong Zhou, National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention, Shanghai, People's Republic of China, E-mail: ipdzhouxn@sh163.net.
References
- 1.Brooker S. Estimating the global distribution and disease burden of intestinal nematode infections: adding up the numbers: a review. Int J Parasitol. 2010;40:1137–1144. doi: 10.1016/j.ijpara.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabè E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT-A, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Lèon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Ehrlich Sachs E, Sachs JD, Savioli L. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 4.Utzinger J, Becker SL, Knopp S, Blum J, Neumayr AL, Keiser J, Hatz CF. Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly. 2012;142:w13727. doi: 10.4414/smw.2012.13727. [DOI] [PubMed] [Google Scholar]
- 5.Cox FEG. History of human parasitology. Clin Microbiol Rev. 2002;15:595–612. doi: 10.1128/CMR.15.4.595-612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooker S, Clements ACA, Bundy DAP. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–261. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotez PJ, Bundy DAP, Beegle K, Brooker S, Drake L, de Silva N, Montresor A, Engels D, Jukes M, Chitsulo L, Chow J, Laxminarayan R, Michaud C, Bethony J, Correa-Oliveira R, Xiao SH, Fenwick A, Savioli L. Helminth infections: soil-transmitted helminth infections and schistosomiasis. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease Control Priorities in Developing Countries. 2nd ed. Washington, DC: World Bank; 2006. [Google Scholar]
- 8.Crompton DWT, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 9.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nga TT, Winichagoon P, Dijkhuizen MA, Khan NC, Wasantwisut E, Wieringa FT. Decreased parasite load and improved cognitive outcomes caused by deworming and consumption of multi-micronutrient fortified biscuits in rural Vietnamese schoolchildren. Am J Trop Med Hyg. 2011;85:333–340. doi: 10.4269/ajtmh.2011.10-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yap P, Du ZW, Chen R, Zhang LP, Wu FW, Wang J, Wang XZ, Zhou H, Zhou XN, Utzinger J, Steinmann P. Soil-transmitted helminth infections and physical fitness in school-aged Bulang children in southwest China: results from a cross-sectional survey. Parasit Vectors. 2012;5:50. doi: 10.1186/1756-3305-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 13.WHO . Eliminating Soil-Transmitted Helminthiases as a Public Health Problem in Children. Geneva: World Health Organization; 2012. [Google Scholar]
- 14.Prichard RK, Basáñez MG, Boatin BA, McCarthy JS, Garcia HH, Yang GJ, Sripa B, Lustigman S. A research agenda for helminth diseases of humans: intervention for control and elimination. PLoS Negl Trop Dis. 2012;6:e1549. doi: 10.1371/journal.pntd.0001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 16.Jia TW, Melville S, Utzinger J, King CH, Zhou XN. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1621. doi: 10.1371/journal.pntd.0001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabrielli AF, Montresor A, Chitsulo L, Engels D, Savioli L. Preventive chemotherapy in human helminthiasis: theoretical and operational aspects. Trans R Soc Trop Med Hyg. 2011;105:683–693. doi: 10.1016/j.trstmh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P. Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, hemoglobin and school performance. Cochrane Database Syst Rev. 2012;11:CD000371. doi: 10.1002/14651858.CD000371.pub5. [DOI] [PubMed] [Google Scholar]
- 19.Humphries D, Nguyen S, Boakye D, Wilson M, Cappello M. The promise and pitfalls of mass drug administration to control intestinal helminth infections. Curr Opin Infect Dis. 2012;25:584–589. doi: 10.1097/QCO.0b013e328357e4cf. [DOI] [PubMed] [Google Scholar]
- 20.Steinmann P, Du ZW, Wang LB, Wang XZ, Jiang JY, Li LH, Marti H, Zhou XN, Utzinger J. Extensive multiparasitism in a village of Yunnan province, People's Republic of China, revealed by a suite of diagnostic methods. Am J Trop Med Hyg. 2008;78:760–769. [PubMed] [Google Scholar]
- 21.Steinmann P, Zhou XN, Du ZW, Jiang JY, Xiao SH, Wu ZX, Zhou H, Utzinger J. Tribendimidine and albendazole for treating soil-transmitted helminths, Strongyloides stercoralis and Taenia spp.: open-label randomized trial. PLoS Negl Trop Dis. 2008;2:e322. doi: 10.1371/journal.pntd.0000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziegelbauer K, Steinmann P, Zhou H, Du ZW, Jiang JY, Fürst T, Jia TW, Zhou XN, Utzinger J. Self-rated quality of life and school performance in relation to helminth infections: case study from Yunnan, People's Republic of China. Parasit Vectors. 2010;3:61. doi: 10.1186/1756-3305-3-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eng J. Sample size estimation: how many individuals should be studied? Radiology. 2003;227:309–313. doi: 10.1148/radiol.2272012051. [DOI] [PubMed] [Google Scholar]
- 24.Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008;43:215–221. doi: 10.4085/1062-6050-43.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Development Core Team . R: A Language and Environment for Statistical Analysis. Vienna: R Development Core Team; 2010. [Google Scholar]
- 26.Yap P, Fürst T, Müller I, Kriemler S, Utzinger J, Steinmann P. Determining soil-transmitted helminth infection status and physical fitness of school-aged children. J Vis Exp. 2012;66:3966. doi: 10.3791/3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montresor A, Crompton DWT, Hall A, Bundy DAP. Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level. Geneva: World Health Organization; 1998. [Google Scholar]
- 28.Danso-Appiah A, Garner P, Olliaro PL, Utzinger J. Treatment of urinary schistosomiasis: methodological issues and research needs identified through a Cochrane systematic review. Parasitology. 2009;136:1837–1849. doi: 10.1017/S0031182009005939. [DOI] [PubMed] [Google Scholar]
- 29.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340:c332. doi: 10.1097/AOG.0b013e3181d9d421. [DOI] [PubMed] [Google Scholar]
- 30.Coordinating Office of the National Survey on the Important Human Parasitic Diseases A national survey on current status of the important parasitic diseases in human population. Chin J Parasitol Parasit Dis. 2005;23:332–340. (in Chinese) [PubMed] [Google Scholar]
- 31.Balen J, Raso G, Li YS, Zhao ZY, Yuan LP, Williams GM, Luo XS, Shi MZ, Yu XL, Utzinger J, McManus DP. Risk factors for helminth infections in a rural and a peri-urban setting of the Dongting Lake area, People's Republic of China. Int J Parasitol. 2011;41:1165–1173. doi: 10.1016/j.ijpara.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Shang Y, Tang LH, Zhou SS, Chen YD, Yang YC, Lin SX. Stunting and soil-transmitted-helminth infections among school-age pupils in rural areas of southern China. Parasit Vectors. 2010;3:97. doi: 10.1186/1756-3305-3-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mofid LS, Bickle Q, Jiang JY, Du ZW, Patrick E. Soil-transmitted helminthiasis in rural south-west China: prevalence, intensity and risk factor analysis. Southeast Asian J Trop Med Public Health. 2011;42:513–526. [PubMed] [Google Scholar]
- 34.Steinmann P, Utzinger J, Du ZW, Jiang JY, Chen JX, Hattendorf J, Zhou H, Zhou XN. Efficacy of single-dose and triple-dose albendazole and mebendazole against soil-transmitted helminths and Taenia spp.: a randomized controlled trial. PLoS One. 2011;6:e25003. doi: 10.1371/journal.pone.0025003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hotez PJ. Engaging a rising China through neglected tropical diseases. PLoS Negl Trop Dis. 2012;6:e1599. doi: 10.1371/journal.pntd.0001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Utzinger J, Bergquist R, Olveda R, Zhou XN. Important helminth infections in Southeast Asia: diversity, potential for control and prospects for elimination. Adv Parasitol. 2010;72:1–30. doi: 10.1016/S0065-308X(10)72001-7. [DOI] [PubMed] [Google Scholar]
- 37.Hotez PJ, Ehrenberg JP. Escalating the global fight against neglected tropical diseases through interventions in the Asia Pacific region. Adv Parasitol. 2010;72:31–53. doi: 10.1016/S0065-308X(10)72002-9. [DOI] [PubMed] [Google Scholar]
- 38.Albonico M, Smith PG, Ercole E, Hall A, Chwaya HM, Alawi KS, Savioli L. Rate of reinfection with intestinal nematodes after treatment of children with mebendazole or albendazole in a highly endemic area. Trans R Soc Trop Med Hyg. 1995;89:538–541. doi: 10.1016/0035-9203(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 39.Saathoff E, Olsen A, Kvalsvig JD, Appleton CC. Patterns of geohelminth infection, impact of albendazole treatment and re-infection after treatment in schoolchildren from rural KwaZulu-Natal/South-Africa. BMC Infect Dis. 2004;4:27. doi: 10.1186/1471-2334-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vercruysse J, Behnke JM, Albonico M, Ame SM, Angebault C, Bethony JM, Engels D, Guillard B, Nguyen TV, Kang G, Kattula D, Kotze AC, McCarthy JS, Mekonnen Z, Montresor A, Periago MV, Sumo L, Tchuem Tchuenté LA, Dang TC, Zeynudin A, Levecke B. Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis. 2011;5:e948. doi: 10.1371/journal.pntd.0000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Namwanje H, Kabatereine NB, Olsen A. Efficacy of single and double doses of albendazole and mebendazole alone and in combination in the treatment of Trichuris trichiura in school-age children in Uganda. Trans R Soc Trop Med Hyg. 2011;105:586–590. doi: 10.1016/j.trstmh.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Speich B, Ame SM, Ali SM, Alles R, Hattendorf J, Utzinger J, Albonico M, Keiser J. Efficacy and safety of nitazoxanide, albendazole, and nitazoxanide-albendazole against Trichuris trichiura infection: a randomized controlled trial. PLoS Negl Trop Dis. 2012;6:e1685. doi: 10.1371/journal.pntd.0001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geary TG. Are new anthelmintics needed to eliminate human helminthiases? Curr Opin Infect Dis. 2012;25:709–717. doi: 10.1097/QCO.0b013e328359f04a. [DOI] [PubMed] [Google Scholar]
- 44.Montresor A. Cure rate is not a valid indicator for assessing drug efficacy and impact of preventive chemotherapy interventions against schistosomiasis and soil-transmitted helminthiasis. Trans R Soc Trop Med Hyg. 2011;105:361–363. doi: 10.1016/j.trstmh.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keiser J, Utzinger J. The drugs we have and the drugs we need against major helminth infections. Adv Parasitol. 2010;73:197–230. doi: 10.1016/S0065-308X(10)73008-6. [DOI] [PubMed] [Google Scholar]
- 46.Geary TG, Woo K, McCarthy JS, Mackenzie CD, Horton J, Prichard RK, de Silva NR, Olliaro PL, Lazdins-Helds JK, Engels DA, Bundy DA. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int J Parasitol. 2010;40:1–13. doi: 10.1016/j.ijpara.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 47.CDC Hookworm: Biology. 2013. http://www.cdc.gov/parasites/hookworm/biology.html Available at. Accessed February 21, 2013.
- 48.Steinmann P, Zhou XN, Du ZW, Jiang JY, Wang LB, Wang XZ, Li LH, Marti H, Utzinger J. Occurrence of Strongyloides stercoralis in Yunnan province, China, and comparison of diagnostic methods. PLoS Negl Trop Dis. 2007;1:e75. doi: 10.1371/journal.pntd.0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becker SL, Sieto B, Silué KD, Adjossan L, Koné S, Hatz C, Kern WV, N'Goran EK, Utzinger J. Diagnosis, clinical features, and self-reported morbidity of Strongyloides stercoralis and hookworm infection in a co-endemic setting. PLoS Negl Trop Dis. 2011;5:e1292. doi: 10.1371/journal.pntd.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.