Abstract
Schistosomiasis control efforts mainly target school–aged children. We studied the epidemiology of schistosomiasis in two high-risk communities in south Côte d'Ivoire, placing particular emphasis on pre-school–aged children. We used a suite of diagnostic techniques, including Kato–Katz, urine filtration, reagent strips, and urine circulating cathodic antigen cassettes. Risk factors for schistosomiasis were determined by focus group discussions and a structured questionnaire. The prevalence of Schistosoma mansoni in the two study villages among the pre-school–aged children (age < 6 years) was 20.9% and 25.0%, whereas several-fold higher prevalences were found in school–aged children (58.7–68.4%) and adolescents/adults (59.5–61.7%). The prevalence of S. haematobium in the three age groups was 5.9–17.3%, 10.9–18.4%, and 3.8–21.3%, respectively. Most participants had light-intensity infections. Mothers' occupations and older siblings play important roles in the epidemiology of schistosomiasis in pre-schoolers. In the current epidemiologic settings, more attention is warranted on pre-school–aged children and adolescents/adults for successful schistosomiasis control.
Introduction
Schistosomiasis remains of considerable public health importance in sub-Saharan Africa.1–4 It is widely acknowledged that schistosomiasis infection prevalence and intensity curves show peaks in children aged 6–15 years, and then, prevalence and intensities decline gradually with age.5,6 Hence, the current global strategy emphasizes preventive chemotherapy (i.e., regular administration of the anthelmintic drug praziquantel), which is primarily targeted to school–aged children. Conversely, pre-school–aged children (below the age of 5 or 6 years) and adolescents/adults (16 years and above) are often neglected from preventive chemotherapy. Justification for the exclusion of the latter age group is given by the lower frequency of water contact compared with school–aged children and the development of an acquired protective immunity against schistosomiasis.7–9 With regard to pre-school–aged children, they are thought to be at low risk of schistosomiasis because of infrequent contact with freshwater bodies. Moreover, there is a paucity of pharmacokinetic data, including safety, on praziquantel in the pre-school–aged child population.10,11 Finally, the current lack of an appropriate formulation (e.g., syrup) of praziquantel for young children12 is an important barrier for the inclusion of pre-school–aged children in preventive chemotherapy campaigns.
However, there is growing recognition that, in areas of high endemicity, pre-schoolers are at considerable risk of schistosomiasis.12–14 Hence, discussions are underway whether pre-school–aged children should be included in preventive chemotherapy campaigns, at least in areas that are highly endemic. Recent studies carried out in the Azaguié district, south Côte d'Ivoire, revealed that both Schistosoma mansoni and S. haematobium coexist, and particularly high levels of coinfections were found in the villages of Azaguié Makouguié and Azaguié M'Bromé.15,16 However, the epidemiology of schistosomiasis and risk factors for early childhood infection have not been investigated in this part of Côte d'Ivoire. To fill this gap, we designed a cross-sectional parasitologic and questionnaire survey to determine the prevalence and intensity of S. mansoni and S. haematobium infection in pre-school–aged children, including risk factors for early child infection. For comparison, we also screened older individuals (school–aged children and adolescents/adults) for schistosome infections. Our findings are discussed in the frame of integrated schistosomiasis control and indeed, new efforts that aim at elimination of schistosomiasis.17
Materials and Methods
Ethical consideration and treatment.
Ethical clearance was obtained from the Ministry of Health and Public Hygiene of Côte d'Ivoire (reference no. 4248/2010/MSHP/CNER). Village authorities were informed, and after they had agreed, the objectives, procedures, and potential risks and benefits of the study were explained to the heads of households by three of the authors (J.T.C., Y.K.N., and E.K.N.). Written informed consent or fingerprints (of illiterate people) were obtained from participants aged 16 years and above and parents or legal guardians on behalf of children (age < 16 years).
At the end of the study, free anthelmintic treatment was offered to the whole population in both villages (i.e., praziquantel, 40 mg/kg body weight using a dose pole against schistosomiasis; albendazole, 400 mg against soil-transmitted helminthiasis). For children aged < 6 years, praziquantel was given according to their weight using crushed 600-mg tablets; results on the efficacy and safety of crushed praziquantel tablets have been reported elsewhere.18
Study area.
The study was carried out between June and September of 2011 in two villages, Azaguié Makouguié (geographic coordinates 05°37′33″ N latitude, 04°09′04″ W longitude) and Azaguié M'Bromé (05°39′42″ N, 04°08′38″ W), both located in the district of Azaguié, south Côte d'Ivoire. Subsistence farming is the main economic activity in both villages. Many households lack access to permanent clean water, and open defection is frequently practiced. S. mansoni and S. haematobium are coendemic in this area.16,18
Population census and sample size calculation.
A detailed census was carried out in June of 2011 as follows. After discussions with village authorities, four community members in each of the two villages were trained to conduct the census. All households were visited, and sociodemographic characteristics were collected (e.g., name, age, sex, relationship with household head, and main activity of each individual). The primary source for obtaining the children's age was their birth certificate. In cases where birth certificates were not available, we checked the children's vaccination card, which includes the birth date. The age of children without any of these two official documents was declared by the mothers. Unique identifiers (IDs) were attached to all households and the individual inhabitants. As a household, we considered a structure where people regularly share their food (most commonly, the household was a male household head, a woman, and the parents' children). The village census revealed 931 and 783 individuals in Azaguié M'Bromé and Azaguié Makouguié, respectively. Among them, there were 209 children below the age of 6 years in Azaguié M'Bromé and 158 children below the age of 6 years in Azaguié Makouguié.
Sample size calculation for pre-school–aged children was done as follows. First, we assumed a prevalence of Schistosoma (either S. mansoni or S. haematobium) of 20% among pre-school–aged children (i.e., approximately one-quarter of the 80% S. mansoni infection prevalence observed in school–aged children in Azaguié in 2010).16 Second, we allowed for a relatively low compliance rate (70%) because of the difficulty of obtaining stool and urine samples from pre-school–aged children. Third, we considered an α-error of 5%. Thus, we needed approximately 300 pre-school–aged children. Because the total number of pre-school–aged children in the two villages was only slightly higher, we decided to invite all of them. For the sample size in the older age group (≥ 6 years), one-quarter of the total population was selected in each village (drawing every fourth household member using random number lists). Whenever the selection fell onto a pre-school–aged child, we selected the next individual until we reached the required sample size.
Field procedures.
The geographic coordinates of all households where at least one pre-school–aged child was registered and water contact points were recorded using a handheld global positioning system (GPS) device (Garmin GPS map 62 ST; Bucher+Walt SA, St-Blaise, Switzerland).
Pre-school–aged children were asked to provide two stool and two urine samples over consecutive days. The day before sample collection, children's mothers/guardians were given two empty containers, one for stool collection and one for urine collection. Filled containers (small portion of stool and at least 10 mL urine) were collected and labeled with unique IDs, and mothers/guardians were issued new empty containers for sample collection the next day. Given the difficulty of collecting biological samples in this age group, mothers/guardians were allowed to obtain samples from their young children at any time of the day (usually in the early morning hours). To have comparable data, stool and urine samples from older participants (school–aged children, adolescents, and adults) were also collected in the morning hours.
Laboratory procedures.
Stool and urine samples were transferred to a laboratory in the Azaguié health center. Diagnostic work-up was completed on the day of sample collection.16,18 Duplicate Kato–Katz thick smears, using 41.7-mg templates,19 were prepared on microscope slides from each stool sample. Hence, our diagnostic approach consisted of quadruplicate Kato–Katz thick smears (two stool samples each subjected to duplicate Kato–Katz). The slides were allowed to clear for at least 30 minutes before microscopic examination for eggs of S. mansoni and soil-transmitted helminths by an experienced laboratory technician.
Four hundred eighty-eight urine samples were examined as follows. First, the samples were visually inspected and classified into clear, cloudy, or bloody (macrohematuria). Second, microhematuria was determined using reagent strips (Combur-test; Roche Diagnostics, Basel, Switzerland) using a semiquantitative assessment scheme: negative, 1+ (approximately 5–10 erythrocytes/μL urine), 2+ (approximately 25 erythrocytes/μL urine), 3+ (approximately 50 erythrocytes/μL urine), and 4+ (approximately 250 erythrocytes/μL urine). Third, urine samples were subjected to a circulating cathodic antigen (CCA) cassette test (Rapid Medical, Pretoria, South Africa) for S. mansoni diagnosis.16 Fourth, samples were subjected to a filtration method. In brief, samples were vigorously shaken, 10 mL were filtered using small-sized filters (aperture = 20 μm; Sefar, Heiden, Switzerland), samples were placed on a slide, and S. haematobium eggs were enumerated under a microscope by an experienced laboratory technician.
Questionnaire survey and focus group discussions.
A structured questionnaire was administered to mothers/guardians of pre-school–aged children to determine risk factors for schistosomiasis. Before administration, our questionnaire was pre-tested with six women not otherwise involved in the study. The interviews were conducted in the local language (Abbey) by trained enumerators. Mothers/guardians' education and profession, knowledge about schistosomiasis, recent history of migration, personal hygiene, common playing and recreational activities (placing particular emphasis on water activities of their children), and access to healthcare were determined.
Subsequently, in each village, based on the parasitological data, one focus group discussion (FGD) was done with mothers/guardians of Schistosoma-infected pre-school–aged children, and a second FGD was done with mothers/guardians of non-infected children. The FGDs were built around occupational, bathing, washing, cooking, and recreational behaviors of the mothers/guardians and the children for whom they care.
Statistical analysis.
Parasitological and questionnaire data were entered two times into an Excel spreadsheet, transferred onto EpiInfo 3.2 (Centers for Disease Control and Prevention, Atlanta, GA), and cross-checked. All analyses were carried out in STATA version 10 (Stata Corp., College Station, TX). Participants with complete parasitologic data (i.e., quadruplicate Kato–Katz thick smears and duplicate urine filtration for all participants and an additional two urine CCA tests and two reagent strips [for microhematuria] for pre-school–aged children) were included in the final analysis. Means and proportions of interest were calculated, and comparisons were done using Kruskal–Wallis and Pearson's χ2 tests.
The intensity of S. mansoni was expressed as eggs per 1 g stool (EPG) and categorized into light (1–99 EPG), moderate (100–399 EPG), and heavy (≥ 400 EPG) infections. The intensity of S. haematobium infection was grouped into light (1–49 eggs/10 mL urine) and heavy (≥ 50 eggs/10 mL urine) infections.
We used a logistic regression model to assess significant associations between a Schistosoma infection and sex, age, village, and mothers/guardians behavioral factors. For both Schistosoma species, a baseline model was established, with infected pre-school–aged children defined as cases. Sex (as binary variable), age (as categorical variable), village (as binary variable), and mothers/guardians' behavioral factors (as binary or categorical variable) were incorporated into the model. A backward elimination approach was used, and non-significant associations (P > 0.2) were removed one at a time. Adjusted odds ratios (ORs), including 95% confidence intervals (CIs), were calculated.
To establish village-specific schistosomiasis risk maps for pre-schoolers, the geographic coordinates of each household inhabited by at least one child younger than 6 years and the water contact points indicated by the mothers/guardians were transformed into a universal transverse mercator (UTM) system and transferred into ArcView 3.2 (ESRI, Redlands, CA). Pre-school–aged children were stratified into schistosome-free, single species infection with either S. mansoni or S. haematobium, or coinfection with both schistosome species.
Results
Study adherence.
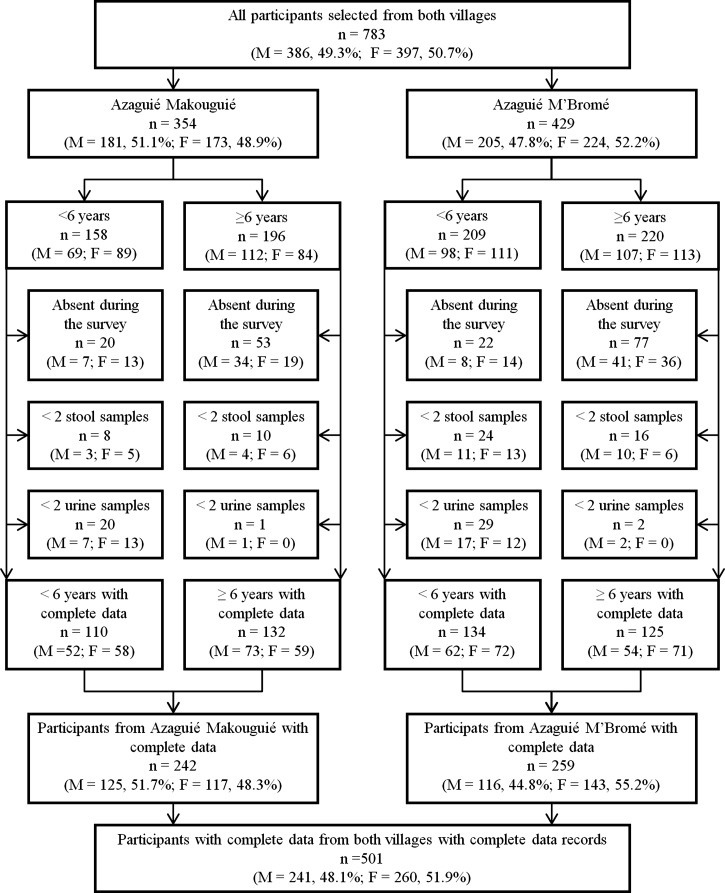
Figure 1 shows that 783 individuals were selected from both villages: 429 (54.8%) in Azaguié M'Bromé and 354 in Azaguié Makouguié. In Azaguié M'Bromé, there were 209 pre-school–aged children and 220 participants aged 6 years and above. In Azaguié Makouguié, there were 158 pre-schoolers and 196 older participants. Overall, 242 and 259 individuals had complete parasitologic data (i.e., quadruplicate Kato–Katz thick smears and two urine filtrations and for pre-school–aged children, two additional CCA cassette tests and two reagent strips) in Azaguié Makouguié and Azaguié M'Bromé, respectively.
Figure 1.
Flowchart detailing the study participation and adherence of pre-school–aged children and older participants (6 years and above) to submission of stool and urine samples for the diagnosis of S. mansoni and S. haematobium in Azaguié, south Côte d'Ivoire, in mid-2011 (M = males; F = females).
Demographic characteristics.
Table 1 summarizes the demographic characteristics of the study population stratified by pre-school–aged children (< 6 years), school–aged children (6–15 years), and adolescents/adults (> 15 years). Overall, the age of the pre-school–aged children ranged from 3 months to 5.5 years, with a mean of 3.2 years. There were slightly more young girls than boys, but the difference was not statistically significant (130 versus 114; P > 0.05). Study participants from both villages showed similar age and sex profiles. For instance, the mean age of the three study groups in Azaguié Makouguié was 2.9 years (pre-school–aged children), 9.1 years (school–aged children), and 38.1 years (adolescents/adults), whereas the respective mean ages in Azaguié M'Bromé were 3.3, 9.4, and 38.2 years. Regardless of the age group considered, no statistical difference was found between sexes in both villages (P > 0.05).
Table 1.
Characteristics of the study population in the two villages of Azaguié Makouguié and Azaguié M'Bromé, district of Azaguié, south Côte d'Ivoire
| Age group (years) | Azaguié Makouguié | Azaguié M'Bromé | P† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Male n (%) | Female n (%) | P* | Mean age (SD) | N | Male n (%) | Female n (%) | P* | Mean age (SD) | ||
| Total | 242 | 125 (51.7) | 117 (48.3) | 0.675 | 17.6 (18.7) | 259 | 116 (44.8) | 143 (55.2) | 0.170 | 15.1 (17.7) | 0.881 |
| < 6 | 110 | 52 (47.3) | 58 (52.7) | 0.640 | 2.9 (1.3) | 134 | 62 (46.3) | 72 (53.7) | 0.480 | 3.3 (1.5) | 0.975 |
| 6–15 | 38 | 27 (71.1) | 11 (28.9) | 0.032 | 9.1 (2.7) | 46 | 20 (43.5) | 26 (56.5) | 0.470 | 9.4 (2.7) | 0.968 |
| > 15 | 94 | 46 (48.9) | 48 (51.1) | 0.866 | 38.1 (13.9) | 79 | 34 (43.0) | 45 (57.0) | 0.312 | 38.2 (15.0) | 0.990 |
Pearson's χ2 test: P value comparing males with females.
Kruskal–Wallis test: P value of mean age between villages (< 6 years, pre-school–aged children; 6–15 years, school–aged children; > 15 years, adolescents and adults).
In Azaguié Makouguié, more than two-thirds of the mothers/guardians of pre-school–aged children were engaged in subsistence farming. Trading local goods was the second main occupation, which was reported by 27.5% of the interviewees. In Azaguié M'Bromé, among the 122 mothers/guardians interviewed, 59 (48.4%) and 38 (31.1%) were engaged in subsistence farming and local trading, respectively (Table 2).
Table 2.
Mothers' main activity, children care, and water contact practice
| Azaguié Makouguié, n (%) | Azaguié M'Bromé, n (%) | χ2 | P* | |
|---|---|---|---|---|
| Mother involved in subsistence farming | 56 (70.0) | 59 (48.4) | 2.47 | 0.116 |
| Mother involved in local trade | 22 (27.5) | 38 (31.1) | 0.17 | 0.682 |
| Mother with main activity strongly linked to water | 22 (27.5) | 24 (19.7) | 1.05 | 0.306 |
| Location of pre-school–aged children during main activity | ||||
| At the back of their mother | 8 (10.0) | 7 (5.7) | 1.09 | 0.296 |
| In the water | 0 (0.0) | 1 (0.8) | 0.65 | 0.419 |
| In close proximity to the water | 1 (1.3) | 4 (3.3) | 0.79 | 0.375 |
| At home with their older siblings | 13 (16.3) | 12 (9.8) | 1.41 | 0.234 |
| Knows about schistosomiasis | 19 (23.8) | 45 (36.9) | 2.05 | 0.153 |
| Knows about the place where one becomes infected | 17 (21.3) | 39 (32.0) | 0.60 | 0.206 |
| Main source of drinking water: traditional well | 78 (97.5) | 102 (83.6) | 0.55 | 0.460 |
Pearson's χ2 test.
Complete questionnaire data were available from 122 mothers in Azaguié M'Bromé and 80 mothers in Azaguié Makouguié.
Infection with S. mansoni.
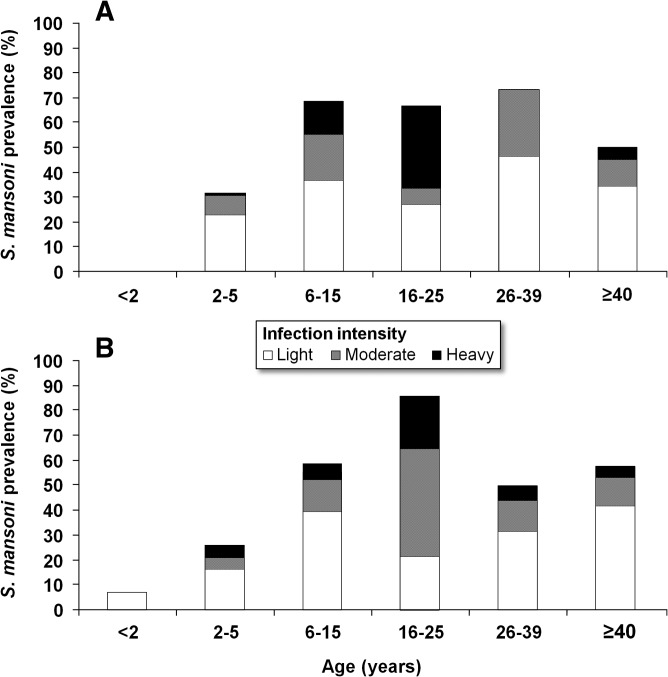
Figure 2 shows the prevalence and intensity of S. mansoni infection stratified by age group and study village. According to quadruplicate Kato–Katz thick smears, the overall prevalence of S. mansoni was 42.7%, with a prevalence of 46.3% in Azaguié Makouguié and 39.4% in Azaguié M'Bromé (no statistical difference between villages; P = 0.324).
Figure 2.
Prevalence and intensity categories of S. mansoni infection stratified by age categories for Azaguié Makouguié (A) and Azaguié M'Bromé (B) in Azaguié district, south Côte d'Ivoire, in mid-2011. S. mansoni infection intensities were categorized into light (1–99 EPG), moderate (100–399 EPG), and heavy (≥ 400 EPG).
In pre-school–aged children, based on a single Kato–Katz thick smear, the prevalence of S. mansoni was 14.3% (N = 35). Microscopic examination of quadruplicate Kato–Katz thick smears found 57 pre-school–aged children with S. mansoni eggs in their stool: 28 (25.5%) in Azaguié Makouguié and 29 (21.6%) in Azaguié M'Bromé. More than twofold higher prevalence was found in school–aged children (Azaguié Makouguié: 68.4%, Azaguié M'Bromé: 58.7%) and adolescents/adults (61.7% and 59.5%, respectively). Three-quarters of the infected pre-school–aged children had light infections (1–99 EPG), whereas only six pre-school–aged children had heavy S. mansoni infection (≥ 400 EPG): five in Azaguié M'Bromé and one in Azaguié Makouguié. None of the children below the age of 24 months were found to be S. mansoni-positive in Azaguié Makouguié, but two such individuals (6.9%) were found in Azaguié M'Bromé.
With regard to duplicate urine CCA cassette tests, including trace as positive results, 89 (81.7%) of the pre-school–aged children were found infected with S. mansoni in Azaguié Makouguié and 96 (72.2%) were found infected with S. mansoni in Azaguié M'Bromé. Considering trace results as negative, the respective prevalences were 44.0% and 45.9%.
Table 3 summarizes the groups' arithmetic means of S. mansoni (and S. haematobium) egg counts. No difference was found in the arithmetic mean fecal egg counts of S. mansoni between males and females and between villages, regardless of whether analysis was done for pre-school–aged children or all age classes combined (all P > 0.05). However, village-specific analysis revealed that female school-aged children and adolescents/adults in Azaguié Makouguié had statistically significantly higher S. mansoni fecal egg counts (P < 0.001), whereas in Azaguié M'Bromé, the fecal egg counts of males in both the school–aged (P < 0.001) and adolescents/adults groups were significantly higher compared with their female counterparts (P = 0.008).
Table 3.
Arithmetic mean of S. mansoni and S. haematobium egg counts in pre-school–aged children (< 6 years), school–aged children (6–15 years), and adolescents/adults (> 15 years) stratified by sex and village
| Azaguié Makouguié arithmetic mean | Azaguié M'Bromé arithmetic mean | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Male | Female | P* | n | Male | Female | P* | |
| S. mansoni (EPG) | ||||||||
| Total | 242 | 54.0 (24.7–83.2) | 85.3 (49.4–121.2) | 0.431 | 259 | 67.1 (38.7–95.5) | 53.2 (32.6–73.8) | 0.659 |
| < 6 years | 110 | 19.6 (0.5–38.6) | 27.9 (6.4–49.4) | 0.430 | 134 | 24.9 (1.8–48.0) | 24.1 (3.9–44.2) | 0.945 |
| 6–15 years | 38 | 118.0 (1.5–234.7) | 235.0 (0.0–482.4) | < 0.001 | 46 | 112.3 (22.6–201.9) | 64.7 (13.3–116.1) | < 0.001 |
| > 15 years | 94 | 55.3 (17.9–92.6) | 120.4 (57.2–183.5) | < 0.001 | 79 | 117.5 (48.3–186.6) | 93.1 (44.7–141.5) | 0.008 |
| S. haematobium (eggs/10 mL urine) | ||||||||
| Total | 242 | 3.0 (1.2–4.9) | 3.9 (1.5–6.3) | 0.149 | 259 | 1.8 (0.4–3.1) | 1.2 (1.0–1.3) | 0.629 |
| < 6 years | 110 | 2.4 (1.3–3.7) | 4.9 (0.3–9.5) | 0.812 | 134 | 1.1 (0.9–1.2) | 1.3 (0.9–1.6) | 0.986 |
| 6–15 years | 38 | 3.2 (0.6–5.8) | 5.6 (0.0–14.7) | 0.699 | 46 | 5.4 (0.0–13.6) | 1.0 (0.9–1.1) | 0.520 |
| > 15 years | 94 | 3.7 (0.0–8.4) | 2.2 (1.4–3.0) | 0.877 | 79 | 1.0 (0.9–1.04) | 1.1 (0.9–1.1) | 0.991 |
Kruskal–Wallis test.
Infection with S. haematobium.
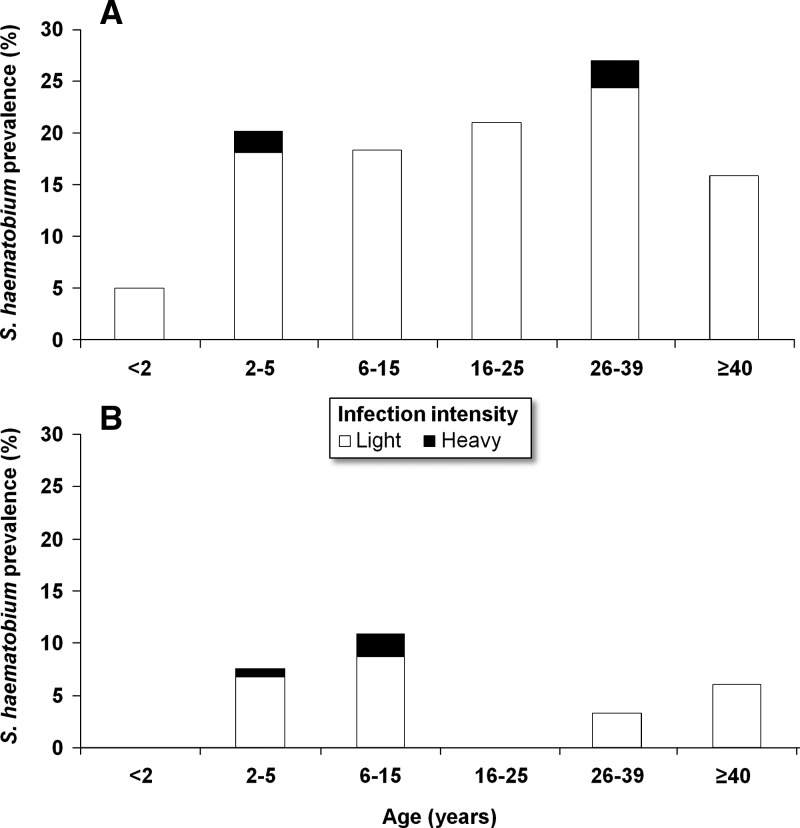
Figure 3 shows the prevalence and intensity of S. haematobium infection stratified by age group and study village. In total, 62 individuals were found with S. haematobium eggs in their urine; hence, there was an overall prevalence of 12.4%. The prevalence of S. haematobium was considerably higher in Azaguié Makouguié compared with Azaguié M'Bromé (19.0% versus 6.2%). Among 110 pre-school–aged children in Azaguié Makouguié and 134 pre-school–aged children in Azaguié M'Bromé, based on duplicate urine filtrations, 19 (17.3%) and 8 (5.9%) individuals were found with patent S. haematobium infections, respectively. The prevalence of S. haematobium was similar for males and females in both study villages (P > 0.05).
Figure 3.
Prevalence and intensity categories of S. haematobium infection stratified by age categories for Azaguié Makouguié (A) and Azaguié M'Bromé (B) in Azaguié district, south Côte d'Ivoire, in mid-2011. S. haematobium infection intensities were categorized into light (1–49 eggs/10 mL urine) and heavy (≥ 50 eggs/10 mL urine).
Visual inspection of urine among pre-schoolers revealed one case of macrohematuria in a 2-year-old child in Azaguié Makouguié; 19 (17.3%) and 14 (10.5%) pre-school–aged children had microhematuria in Azaguié Makouguié and Azaguié M'Bromé, respectively.
Among participants aged ≥ 6 years, 7 school–aged children (18.4%) and 20 adolescents/adults (21.3%) were found with patent S. haematobium infection in Azaguié Makouguié. In Azaguié M'Bromé, five school–aged children (10.9%) and three adolescents/adults (3.8%) were infected with S. haematobium. In both villages, most of the individuals had light S. haematobium infections (1–49 eggs/10 mL urine). No statistical difference was found between sex and between villages in arithmetic mean S. haematobium egg counts (P > 0.05).
Other helminths and coinfection.
The overall prevalence of hookworm, Trichuris trichiura, and Ascaris lumbricoides in the two study villages was 14.2%, 5.4%, and 2.4%, respectively. For each soil-transmitted helminth species, lower prevalences were found in Azaguié Makouguié compared with Azaguié M'Bromé (T. trichiura: 0.4% versus 10.0%; A. lumbricoides: 1.6% versus 3.1%; hookworm: 11.6% versus 16.6%). Multiple species parasitic infections were common in both villages; 38.7% of the participants were infected with at least two helminth species concurrently. We found one 5-year-old boy in Azaguié M'Bromé who harbored a quadruplicate helminth species infection (i.e., S. mansoni, S. haematobium, T. trichiura, and hookworm).
Parasite association in pre-school–aged children.
Table 4 shows pairwise associations between Schistosoma infection, age, village, and mothers/guardians' behavioral factors. Infection with S. mansoni showed significant positive associations with S. haematobium (OR = 9.4, P < 0.001). Pre-schoolers with an age above 24 months were at an 8.8-fold higher odds to be infected with S. mansoni than their younger counterparts (< 24 months). Pre-school–aged children staying at home when their mothers/guardians were involved in livelihood activities were at a 2.3-fold higher odds of S. mansoni infection compared with those children who accompanied their mothers/guardians. Infection with S. haematobium showed a significant positive association with S. mansoni (OR = 11.7, P < 0.001) and village (OR = 3.6, P = 0.008).
Table 4.
Association between schistosome infections in pre-schoolers adjusted by age, village, and mothers' behavioral factors (N = 244)
| Association | Adjusted OR (95% CI) | P |
|---|---|---|
| S. mansoni | ||
| S. haematobium | 9.4 (5.1–17.2) | < 0.001 |
| Children ages < 24 months (reference) | 1.0 | |
| Age group (2–5 years) | 8.8 (3.3–23.5) | < 0.001 |
| Children accompanying their mothers to livelihood activities (reference) | 1.0 | |
| Children stayed at home with their elders | 2.3 (1.5–3.5) | 0.017 |
| S. haematobium | ||
| S. mansoni | 11.7 (6.4–21.4) | < 0.001 |
| Azaguié M'Bromé (reference) | 1.0 | |
| Azaguié Makouguié | 3.6 (1.9–6.6) | 0.008 |
Logistic regression was used to assess the association between schistosome (S. mansoni and S. haematobium) infections adjusted by age and village and the association between S. mansoni or S. haematobium as outcome and S. mansoni or S. haematobium, age (< 24 months and 2–5 years), and village (Azaguié Makouguié and Azaguié M'Bromé) as explanatory variable. Age category < 24 months was used as baseline for comparison with age category 2–5 years. Azaguié M'Bromé was used as baseline for comparison with Azaguié Makouguié. S. mansoni and S. haematobium infections results were expressed as binary variables (positive/negative).
Schistosomiasis risk maps.
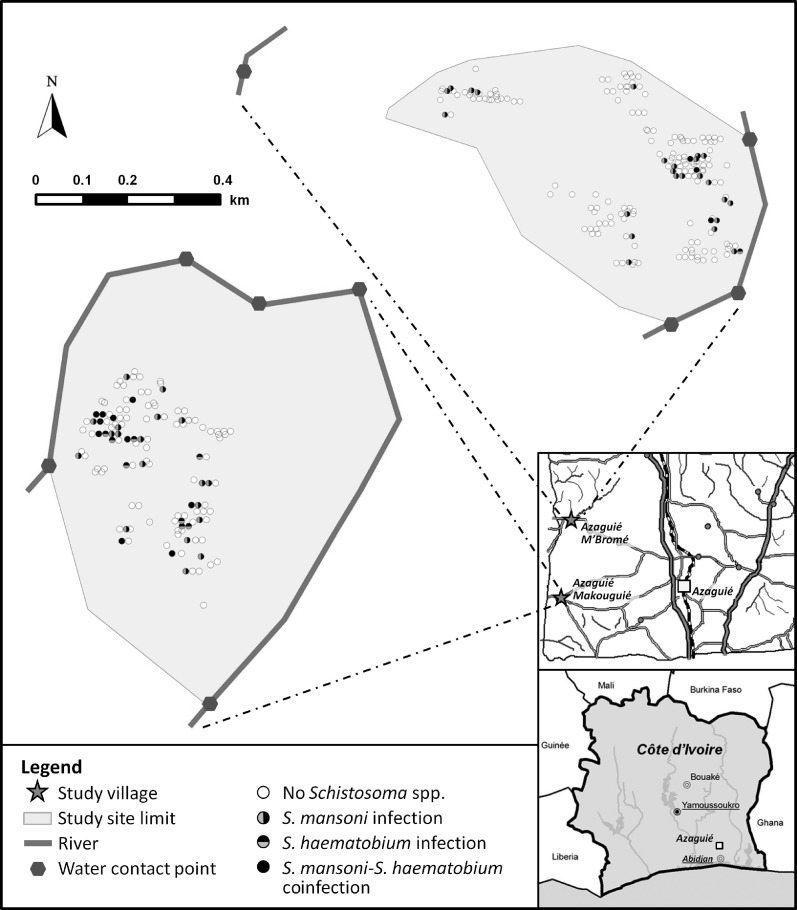
Figure 4 shows the spatial distribution of Schistosoma-infected pre-school–aged children as diagnosed by Kato–Katz (for S. mansoni) and urine filtration (for S. haematobium) and water contact points in Azaguié Makouguié and Azaguié M'Bromé. Schistosoma-infected pre-school–aged children were homogeneously distributed in Azaguié Makouguié. In Azaguié M'Bromé, Schistosoma cases were clustered among pre-schoolers living in households in close proximity to water contact sites.
Figure 4.
Map showing the town of Azaguié and its surrounding villages and settlements in south Côte d'Ivoire. The two villages where the current study was carried out are highlighted with asterisks. The accompanying risk maps show water contact points and the spatial distribution of households inhabited by at least one pre-school–aged child with no schistosome infection, monoinfection with either S. mansoni or S. haematobium, or coinfection with both schistosome species as determined by the Kato–Katz technique (for S. mansoni) and urine filtration (for S. haematobium).
Results from the questionnaire survey and FGDs.
Table 2 summarizes the results obtained from the questionnaire administered to 202 mothers/guardians of pre-school–aged children in the two study villages. In Azaguié Makouguié, 22 of 80 mothers interviewed (27.5%) responded that their daily activities are strongly linked to water contact patterns, somewhat higher than in Azaguié M'Bromé (24/122, 19.7%). In the latter village, one-half of these mothers reported that they take their pre-schoolers with them to the water contact sites. In Azaguié Makouguié, most pre-schoolers are left at home while mothers/guardians pursue their daily water chores. Knowledge about schistosomiasis was poor; only 19 (23.8%) mothers in Azaguié Makouguié and 45 (36.9%) mothers in Azaguié M'Bromé knew about the disease. In both villages, the main source of drinking water comes from a traditional well.
FGDs revealed no difference in the patterns of water use between the mothers/guardians of Schistosoma-infected pre-school–aged children and their non-infected counterparts. Results from the FGDs highlighted that mothers give special care to children below the age of 24 months. FGDs also revealed that some individuals use local remedies to manage schistosomiasis, especially the urogenital form of schistosomiasis. These local treatments are based on a mixture of plants and clay. The FGDs confirmed that most of the mothers lack detailed knowledge about schistosomiasis, but they know that contaminated water exposes their children to health risks.
Discussion
Studies going back as far as the 1960s documented that, in specific social–ecological settings, young children suffer from schistosomiasis before reaching school age.20–24 However, in view of peak infection prevalence and intensities usually observed in the school–aged population6 and ease of implementing preventive chemotherapy through the education system, schistosomiasis control is centered on school–aged children. The lack of pharmacological data and an appropriate formulation of praziquantel for pre-schoolers are important reasons why this age group is largely excluded from preventive chemotherapy.25,26 However, pre-school–aged children might be particularly vulnerable to the negative consequences of an early-life infection with Schistosoma, because they would not get treatment until entering school.27 Additionally, adolescents and adults are given far less attention than the school–aged population when it comes to schistosomiasis control. Indeed, World Health Assembly (WHA) resolution 54.19 sets clear treatment coverage targets for the school–aged population, but it remains comparatively silent on other age groups.
Recent studies confirmed that pre-school–aged children are at risk of schistosomiasis, but in-depth epidemiologic investigations are few.12–14,28,29 It has also been discussed whether preventive chemotherapy should be extended from school–aged to pre-school–aged children.27,30–32 However, there are a number of issues that must be addressed before policy recommendations can be made regarding the inclusion of pre-schoolers in preventive chemotherapy. First, what is the true extent of Schistosoma infection in pre-school–aged children in different epidemiologic settings? Second, what are the key risk factors that drive the epidemiology of schistosomiasis in pre-school–aged children? Third, can the prevalence and intensity of schistosomiasis in school–aged children serve as proxies for pre-school–aged children and adolescents/adults to better target control and enhance disease burden assessment?
The current study, pursuing a cross-sectional design, deepened our understanding of the epidemiology of schistosomiasis in two villages in south Côte d'Ivoire that are highly endemic for schistosomiasis but have not been subjected to large-scale administration of praziquantel before. We used a reasonably sensitive diagnostic approach with quadruplicate Kato–Katz thick smears for the diagnosis of S. mansoni and duplicate urine filtration for detection of S. haematobium eggs in all study participants. Additionally, in pre-school–aged children, a reagent strip was used for assessment of microhematuria, and a urine CCA cassette test was used for diagnosis of S. mansoni.
Our study confirmed that pre-school–aged children are at risk of schistosomiasis. One boy, before reaching his first birthday (8 months), had a patent S. mansoni infection, which was revealed by the Kato–Katz technique. Considering urine CCA test results, the earliest infection with S. mansoni was found in a 3-month-old boy.18 Previous studies have highlighted such early Schistosoma infection in areas of high endemicity.25,28,33,34 The negative health impact of such early infections has been emphasized.31,32,35,36 Our observation of CCA detected in the urine of a child as young as 3 months in the absence of S. mansoni eggs in fecal samples is in line with recent observations from a study in Uganda using different approaches for detecting Schistosoma infections in pre-school–aged children.36 It might be explained by CCA from the mothers' colostrums37 or CCA produced by the young developing stages of the worm before patency (hence, before egg production commences).16,37 New research is needed to further elucidate this issue, which will be important to clarify operational research and control issues and help interpret diagnostic results. First, assuming that infants receive CCA from their mothers' colostrums would jeopardize the use of urine CCA as a diagnostic assay in very young children. Indeed, based on positive urine CCA test results in the pre-school–aged population, one might suggest extending preventive chemotherapy to this age group. Second, if CCAs are accrued from juvenile worms, CCA might be an early indicator, allowing for efficient surveillance of schistosomiasis in endemic areas. However, because praziquantel is not effective against the young developing stages of S. mansoni,38 treatment must be scheduled accordingly.
Overall, between 5.9% and 21.6% of the pre-school–aged children investigated had patent S. haematobium (the prevalence could be underestimated because of our urine sample collection approach), and between 17.3% and 25.5% of the pre-school–aged children investigated had patent S. mansoni infections. Conversely, between 5.2% and 10.9% of the pre-school–aged children were coinfected, which is considerably higher than what would have been expected by chance (i.e., 1.0–5.5%). Our findings corroborate with recent results presented by Garba and others12 from highly endemic villages in Niger. The consequences of dual species infections in the same body of pre-school–aged children have yet to be investigated.
In the present study, most of the S. mansoni and S. haematobium infections in pre-school–aged children were of light intensity, which is in line with studies carried out elsewhere in sub-Saharan Africa.36,37 Nevertheless, we observed 12 (4.9%) pre-school–aged children heavily infected with either S. mansoni (≥ 400 EPG) or S. haematobium (≥ 50 eggs/10 mL urine). All of these children were above 3 years of age. To our knowledge, pre-school–aged children never received praziquantel treatment in the current study settings. Hence, the heavy infections observed in 12 pre-school–aged children above the age of 3 years are the likely result of very early infection followed by cumulative infections over time.36
For both Schistosoma species, we observed specific age–prevalence curves and intensities in both study villages. Although it is commonly believed that the peak of Schistosoma infection prevalence occurs in children between 6 and 15 years of age (school age), age patterns in the intensity of infection are less clear cut, with variations generally attributed to the level of contact with contaminated freshwater bodies.9,39 In our study, considering the burden of schistosomiasis as expressed by arithmetic mean egg counts, we concur that, in both communities, pre-school–aged children, regardless of their sex, were similarly exposed to contaminated freshwater bodies. However, in school–aged children, adolescents, and adults, S. mansoni infection intensities were sex-dependent (higher risk in males), whereas for S. haematobium, no sex difference was observed. Hence, sociocultural factors might govern sex-specific water use.39 Additionally, setting-specific abiotic and biotic features govern the development of intermediate host snails. Taken together, the interplay of these factors could explain the observed age prevalence and intensities profiles.40,41
In addition to our parasitologic investigations, we also determined risk factors for schistosomiasis in pre-school–aged children by conducting a series of FGDs with mothers and administering a structured questionnaire. Interestingly, we found no difference in common water use practices between mothers of Schistosoma-infected and non-infected children. Hence, no specific behavior of mothers could explain the difference in the infection status of pre-school–aged children. The questionnaire showed that, in both villages, most mothers left their young children at home with their elder siblings when pursuing daily livelihood activities in the fields, near water bodies, or at the market. Multivariable logistic regression revealed that pre-school–aged children staying at home were at a twofold higher odds of Schistosoma infection than their counterparts accompanying their mothers during daily livelihood activities. It is conceivable that most of the infections in pre-school–aged children occur at times during which pre-school–aged children are taken along to the river by their elder siblings for swimming, bathing, washing, or fishing activities. FGDs revealed that mothers are aware that the river is an important source of ill health, but the river remains essential for their many needs because of a lack of alternative water sources (for washing and drinking purposes). In the absence of a functioning health system, villagers use herbal remedies to treat schistosomiasis, particularly urogenital schistosomiasis.
In Azaguié Makouguié, we observed a rather homogenous distribution of Schistosoma-infected pre-school–aged children, whereas in Azaguié M'Bromé, there was clear clustering of schistosome infections in households located in close proximity to the main river. It is important to note that, in Azaguié Makouguié, only one traditional well exists. However, in Azaguié M'Bromé, several water sources are available (several traditional wells and one water pump). It follows that a minimum improvement in safe water supply could significantly influence the risk for pre-school–aged children and other community members to be exposed to Schistosoma infections. Tchuem Tchuenté and others42 in 2001 showed that the installation of a single water pump in Kinding Ndjabi village, Cameroon, was an important feature in reducing schistosomiasis transmission.
In conclusion, our study shows that schistosomiasis is governed by social–ecological contexts. Indeed, this parasitic disease is intimately linked with poverty, lack of essential social services and infrastructure (e.g., clean water, improved sanitation, hygiene, and health systems), and close proximity to small rivers and standing freshwater bodies on which people depend for their daily occupational and recreational activities. Community members are at high risk of schistosomiasis, and infections occur very early in childhood. However, in our study, prevalence and intensity of infection in pre-school–aged children were relatively low, and hence, young children might warrant treatment just one time before age 5 years. Improvement of water supply, sanitation, and hygiene and strengthening of the health system are necessary to reduce people's exposure to infested freshwater bodies.
ACKNOWLEDGMENTS
We are grateful to the district health and village authorities of Azaguié for their support and facilitating the implementation of this study. The authors thank the participants (children and their mothers/guardians) for their commitment throughout the study. We would like to express our sincere thanks to the laboratory technicians from the different institutions of Côte d'Ivoire for their support in this study. We are grateful to Rapid Medical Diagnostics in South Africa for providing 1,000 urine CCA cassette tests for our research purposes free of charge. The authors thank the team of Réseau International Schistosomoses, Environnement, Aménagement et Lutte (RISEAL-Niger) in Niger, led by Dr. Amadou Garba, which generously provided us with praziquantel to treat the surveyed communities. We are grateful to the team of the Laboratoire de Zoologie et de Biologie Animale at the Université Félix Houphouët Boigny for their support in the field and the laboratory.
Disclaimer: The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This study received financial support from the Carolito Foundation for a PhD fellowship granted to J.T.C. The research of J.U. and E.K.N. is financially supported by Fairmed the and Swiss National Science Foundation project no. IZ70Z0_123900.
Authors' addresses: Jean T. Coulibaly, Department of Epidemiology and Public Health, Swiss Tropical and Public Health Institute and University of Basel, Basel, Switzerland; Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny; and Centre Suisse de Recherches Scientifiques en Côte d'Ivoire, Abidjan, Côte d'Ivoire, E-mail: jean.coulibaly@unibas.ch. Yves K. N'Gbesso, Departement d'Agboville, Centre de Santé Urbain d'Azaguié, Azaguié, Côte d'Ivoire, E-mail: koutouann@yahoo.fr. Nicaise A. N'Guessan, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, Abidjan, Côte d'Ivoire, E-mail: nicaisayan@yahoo.fr. Mirko S. Winkler and Jürg Utzinger, Department of Epidemiology and Public Health, Swiss Tropical and Public Health Institute, Basel; and University of Basel, Basel, Switzerland, E-mails: mirko.winkler@unibas.ch and juerg.utzinger@unibas.ch. Eliézer K. N'Goran, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny; and Centre Suisse de Recherches Scientifiques en Côte d'Ivoire, Abidjan, Côte d'Ivoire, E-mail: eliezerngoran@yahoo.fr.
Reprint requests: Eliézer K. N'Goran, Unité de Formation et de Recherche Biosciences, Université Félix Houphouët-Boigny, 22 BP 770, Abidjan 22, Côte d'Ivoire, E-mail: eliezerngoran@yahoo.fr.
References
- 1.Southgate VR, Rollinson D, Tchuem Tchuenté LA, Hagan P. Towards control of schistosomiasis in sub-Saharan Africa. J Helminthol. 2005;79:181–185. doi: 10.1079/joh2005307. [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, Sachs JD. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med. 2006;3:e102. doi: 10.1371/journal.pmed.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 4.Utzinger J, Raso G, Brooker S, de Savigny D, Tanner M, Ørnbjerg N, Singer BH, N'Goran EK. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 2009;136:1859–1874. doi: 10.1017/S0031182009991600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sleigh AC, Mott KE, Hoff R, Barreto ML, Mota EA, Maguire JH, Sherlock I, Weller TH. Three-year prospective study of the evolution of Manson's schistosomiasis in north-east Brazil. Lancet. 1985;326:63–66. doi: 10.1016/s0140-6736(85)90177-1. [DOI] [PubMed] [Google Scholar]
- 6.Woolhouse MEJ. Patterns in parasite epidemiology: the peak shift. Parasitol Today. 1998;14:428–434. doi: 10.1016/s0169-4758(98)01318-0. [DOI] [PubMed] [Google Scholar]
- 7.Wilkins HA, Goll PH, Marshall TF, Moore PJ. Dynamics of Schistosoma haematobium infection in a Gambian community. III. Acquisition and loss of infection. Trans R Soc Trop Med Hyg. 1984;78:227–232. doi: 10.1016/0035-9203(84)90283-9. [DOI] [PubMed] [Google Scholar]
- 8.Butterworth AE, Capron M, Cordingley JS, Dalton PR, Dunne DW, Kariuki HC, Kimani G, Koech D, Mugambi M, Ouma JH, Prentice MA, Richardson BA, Arap Siongok TK, Sturrock RF, Taylor DW. Immunity after treatment of human schistosomiasis mansoni. II. Identification of resistant individuals, and analysis of their immune responses. Trans R Soc Trop Med Hyg. 1985;79:393–408. doi: 10.1016/0035-9203(85)90391-8. [DOI] [PubMed] [Google Scholar]
- 9.Verani JR, Abudho B, Montgomery SP, Mwinzi PN, Shane HL, Butler SE, Karanja DM, Secor WE. Schistosomiasis among young children in Usoma, Kenya. Am J Trop Med Hyg. 2011;84:787–791. doi: 10.4269/ajtmh.2011.10-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen HE, Crompton DWT, de Silva N, LoVerde PT, Olds GR. New policies for using anthelmintics in high risk groups. Trends Parasitol. 2002;18:381–382. doi: 10.1016/s1471-4922(02)02386-3. [DOI] [PubMed] [Google Scholar]
- 11.Geary TG, Woo K, McCarthy JS, Mackenzie CD, Horton J, Prichard RK, de Silva NR, Olliaro PL, Lazdins-Helds JK, Engels DA, Bundy DA. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int J Parasitol. 2010;40:1–13. doi: 10.1016/j.ijpara.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Garba A, Barkire N, Djibo A, Lamine MS, Sofo B, Gouvras AN, Bosque-Oliva E, Webster JP, Stothard JR, Utzinger J, Fenwick A. Schistosomiasis in infants and preschool-aged children: infection in a single Schistosoma haematobium and a mixed S. haematobium-S. mansoni foci of Niger. Acta Trop. 2010;115:212–219. doi: 10.1016/j.actatropica.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Bosompem KM, Bentum IA, Otchere J, Anyan WK, Brown CA, Osada Y, Takeo S, Kojima S, Ohta N. Infant schistosomiasis in Ghana: a survey in an irrigation community. Trop Med Int Health. 2004;9:917–922. doi: 10.1111/j.1365-3156.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- 14.Dabo A, Badawi HM, Bary B, Doumbo OK. Urinary schistosomiasis among preschool-aged children in Sahelian rural communities in Mali. Parasit Vectors. 2011;4:21. doi: 10.1186/1756-3305-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.N'Guessan NA, Acka CA, Utzinger J, N'Goran EK. Identification des régions à haut risque de schistosomoses en Côte d'Ivoire. Bull Soc Pathol Exot. 2006;100:119–123. [PubMed] [Google Scholar]
- 16.Coulibaly JT, Knopp S, N'Guessan NA, Silué KD, Fürst T, Lohourignon LK, Brou JK, N'Gbesso YK, Vounatsou P, N'Goran EK, Utzinger J. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Côte d'Ivoire. PLoS Negl Trop Dis. 2011;5:e1384. doi: 10.1371/journal.pntd.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté LA, Garba A, Mohammed KA, Schur N, Person B, Colley DG, Utzinger J. 2012. Time to set the agenda for schistosomiasis elimination. Acta Trop. doi: 10.1016/j.actatropica.2012.04.013. doi:10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Coulibaly JT, N'Gbesso YK, Knopp S, Keiser J, N'Goran EK, Utzinger J. Efficacy and safety of praziquantel in preschool-aged children in an area co-endemic for Schistosoma mansoni and S. haematobium. PLoS Negl Trop Dis. 2012;6:e1917. doi: 10.1371/journal.pntd.0001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop São Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 20.Farooq M, Mallah MB. The behavioural pattern of social and religious water-contact activities in the Egypt-49 bilharziasis project area. Bull World Health Organ. 1966;35:377–387. [PMC free article] [PubMed] [Google Scholar]
- 21.Siegal FM. Schistosomiasis hematobia in preschool children of Ibadan, Nigeria. Am J Trop Med Hyg. 1968;17:737–742. doi: 10.4269/ajtmh.1968.17.737. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Salam E, Abdel-Fattah M. Prevalence and morbidity of Schistosoma haematobium in Egyptian children. A controlled study. Am J Trop Med Hyg. 1977;26:463–469. doi: 10.4269/ajtmh.1977.26.463. [DOI] [PubMed] [Google Scholar]
- 23.Perel Y, Sellin B, Perel C, Arnold P, Mouchet F. Use of urine collectors for infants from 0 to 4 years of age in a mass survey of urinary schistosomiasis in Niger. Med Trop. 1985;45:429–433. [PubMed] [Google Scholar]
- 24.Smith FM. Bilharziasis in the African infant and child in the Mtoko, southern Rhodesia. Cent Afr J Med. 1985;4:287–288. [PubMed] [Google Scholar]
- 25.Stothard JR, Sousa-Figueiredo JC, Betson M, Green HK, Seto EYW, Garba A, Sacko M, Mutapi F, Vaz Nery S, Amin MA, Mutumba-Nakalembe M, Navaratnam A, Fenwick A, Kabatereine NB, Gabrielli AF, Montresor A. Closing the praziquantel treatment gap: new steps in epidemiological monitoring and control of schistosomiasis in African infants and preschool-aged children. Parasitology. 2011;138:1593–1606. doi: 10.1017/S0031182011001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keiser J, Ingram K, Utzinger J. Antiparasitic drugs for paediatrics: systematic review, formulations, pharmacokinetics, safety, efficacy and implications for control. Parasitology. 2011;138:1620–1632. doi: 10.1017/S0031182011000023. [DOI] [PubMed] [Google Scholar]
- 27.Stothard JR, Gabrielli AF. Schistosomiasis in African infants and preschool children: to treat or not to treat? Trends Parasitol. 2007;23:83–86. doi: 10.1016/j.pt.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Sousa-Figueiredo JC, Basáñez MG, Mgeni AF, Khamis IS, Rollinson D, Stothard JR. A parasitological survey, in rural Zanzibar, of pre-school children and their mothers for urinary schistosomiasis, soil-transmitted helminthiases and malaria, with observations on the prevalence of anaemia. Ann Trop Med Parasitol. 2008;102:679–692. doi: 10.1179/136485908X337607. [DOI] [PubMed] [Google Scholar]
- 29.Ekpo UF, Oluwole AS, Abe EM, Etta HE, Olamiju F, Mafiana CF. Schistosomiasis in infants and pre-school-aged children in sub-Saharan Africa: implication for control. Parasitology. 2012;139:835–841. doi: 10.1017/S0031182012000029. [DOI] [PubMed] [Google Scholar]
- 30.Johansen MV, Sacko M, Vennervald BJ, Kabatereine NB. Leave children untreated and sustain inequity! Trends Parasitol. 2007;23:568–569. doi: 10.1016/j.pt.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Sousa-Figueiredo JC, Pleasant J, Day M, Betson M, Rollinson D, Montresor A, Kazibwe F, Kabatereine NB, Stothard JR. Treatment of intestinal schistosomiasis in Ugandan preschool children: best diagnosis, treatment efficacy and side-effects, and an extended praziquantel dosing pole. Int Health. 2010;2:103–113. doi: 10.1016/j.inhe.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stothard JR, Sousa-Figueiredo JC, Betson M, Bustinduy A, Reinhard-Rupp J. Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol. 2013;29:197–205. doi: 10.1016/j.pt.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekpo UF, Laja-Deile A, Oluwole AS, Sam-Wobo SO, Mafiana CF. Urinary schistosomiasis among preschool children in a rural community near Abeokuta, Nigeria. Parasit Vectors. 2010;3:58. doi: 10.1186/1756-3305-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namwanje H, Kabatereine NB, Olsen A. The acceptability and safety of praziquantel alone and in combination with mebendazole in the treatment of Schistosoma mansoni and soil-transmitted helminthiasis in children aged 1–4 years in Uganda. Parasitology. 2011;138:1586–1592. doi: 10.1017/S0031182011000138. [DOI] [PubMed] [Google Scholar]
- 35.Hotez PJ, Engels D, Fenwick A, Savioli L. Africa is desperate for praziquantel. Lancet. 2010;376:496–498. doi: 10.1016/S0140-6736(10)60879-3. [DOI] [PubMed] [Google Scholar]
- 36.Stothard JR, Sousa-Figuereido JC, Betson M, Adriko M, Arinaitwe M, Rowell C, Besiyge F, Kabatereine NB. Schistosoma mansoni infections in young children: when are schistosome antigens in urine, eggs in stool and antibodies to eggs first detectable? PLoS Negl Trop Dis. 2011;5:e938. doi: 10.1371/journal.pntd.0000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odogwu SE, Ramamurthy NK, Kabatereine NB, Kazibwe F, Tukahebwa E, Webster JP, Fenwick A, Stothard JR. Schistosoma mansoni in infants (aged < 3 years) along the Ugandan shoreline of Lake Victoria. Ann Trop Med Parasitol. 2006;100:315–326. doi: 10.1179/136485906X105552. [DOI] [PubMed] [Google Scholar]
- 38.Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni: chemotherapy of infections of different ages. Exp Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 39.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kariuki HC, Clennon JA, Brady MS, Kitron U, Sturrock RF, Ouma JH, Ndzovu ST, Mungai P, Hoffman O, Hamburger J, Pellegrini C, Muchiri EM, King CH. Distribution patterns and cercarial shedding of Bulinus nasutus and other snails in the Msambweni area, Coast province, Kenya. Am J Trop Med Hyg. 2004;70:449–456. [PubMed] [Google Scholar]
- 41.Clennon JA, King CH, Muchiri EM, Kitron U. Hydrological modeling of snail dispersal patterns in Msambweni, Kenya and potential resurgence of Schistosoma haematobium transmission. Parasitology. 2007;134:683–693. doi: 10.1017/S0031182006001594. [DOI] [PubMed] [Google Scholar]
- 42.Tchuem Tchuenté LA, Southgate VR, Webster BL, De Bont J, Vercruysse J. Impact of installation of a water pump on schistosomiasis transmission in a focus in Cameroon. Trans R Soc Trop Med Hyg. 2001;95:255–256. doi: 10.1016/s0035-9203(01)90228-7. [DOI] [PubMed] [Google Scholar]