Abstract
Previously, we have shown that persons with human immunodeficiency virus 1 (HIV-1) infection and reduced CD4+ T-lymphocyte counts excrete significantly fewer Schistosoma mansoni eggs than HIV-1–negative persons with similar intensities of schistosome infections. To determine how antiretroviral therapy (ART) might affect egg excretion, we conducted a study of HIV+ adults living in an area highly endemic for S. mansoni as they began an ART program. Fecal egg excretion and CD4+ T-lymphocyte counts were evaluated at enrollment as well as 2 and 4 weeks after initiation of ART. Fourteen individuals who were Kato–Katz-negative at enrollment subsequently started excreting S. mansoni eggs accompanied by a significant increase in CD4+ T lymphocytes (P = 0.004). Study participants who were S. mansoni egg-positive at enrollment and received both praziquantel and ART also showed significantly increased CD4+ T-lymphocyte counts compared with baseline (P < 0.0001). Our data support a role for CD4+ T lymphocytes in S. mansoni egg excretion.
Coinfections with helminths and human immunodeficiency virus (HIV) are widespread in sub-Saharan Africa and result in unique immunologic and pathologic outcomes compared with single-agent infections.1 Our previous studies in western Kenya indicated that Schistosoma mansoni and HIV-1 coinfected car washers excrete fewer S. mansoni eggs than persons infected with similar levels of mature adult worms measured by circulating antigen concentrations.2 Other studies have similarly reported that egg excretion by S. mansoni or S. haematobium patients may be decreased if they have HIV-1 coinfections.3–5 However, these studies did not measure CD4+ T lymphocytes, and circulating antigen concentrations were not determined for either of the S. haematobium reports. The only investigation that ascertained both CD4+ T-lymphocyte counts and circulating antigen concentration for both S. mansoni and S. haematobium patients did not find an effect of HIV-1–related immunodeficiency on the ability to excrete eggs in low-intensity schistosome infections.6 To date, no study has explored the possible effects of antiretroviral therapy (ART) on schistosome egg excretion in individuals coinfected with schistosomiasis and HIV. The current study was performed to examine the relationship between S. mansoni egg excretion and CD4+ T-lymphocyte counts in HIV+ individuals who are receiving ART and examine whether an increase in CD4+ T lymphocytes during ART is associated with increased egg excretion from the human host.
The study was approved by the Institutional Review Board of the Kenya Medical Research Institute. Participants were drawn from three government HIV treatment centers (Naya, Madiany, and Ragengni) that were located within 5 km of Lake Victoria. This area is highly endemic for S. mansoni; thus, participants had a high likelihood of infection.7 The study participants were antiretroviral treatment-naive individuals ages 18 years and older at the time of enrollment. Informed written consent, study activities, and counseling were conducted confidentially in the native language of the participants (Luo) by qualified counselors. HIV treatment was given according to Kenya national guidelines for ART, which includes HIV+ persons with < 350 CD4+ cells/mm3, World Health Organization (WHO) clinical stage 3 or 4, or coinfection with tuberculosis or hepatitis B virus. Study participants provided stool for detection of S. mansoni or other helminth ova and blood for determination of CD4+ T-lymphocyte counts and HIV-1 viral load. They were also asked to answer a demographic and water contact questionnaire. Parasitological evaluations were carried out at baseline and 2 and 4 weeks after initiation of ART. Standard Kato–Katz thick smears were prepared for stool examinations for schistosome and other helminth eggs using two slides on the one stool sample collected at each time point.8 Slides were quantitatively evaluated for eggs of S. mansoni, Ascaris lumbricoides, hookworm, and Trichuris trichuria by well-trained laboratory technicians. Quality control was assured by randomized selection and examination of 25% of the samples by another technician who was blinded to the first technician's results. The study participants were treated with praziquantel and/or albendazole for schistosomiasis and other helminth infections, respectively, under the observation by a Kenyan ministry of health nurse.
Blood was collected in a vacutainer tube containing ethylenediaminetetraacetic acid (EDTA) anticoagulant at each visit. CD4+ T-lymphocyte counts were determined using flow cytometry (FacsCalibur; Becton Dickinson, San Jose, CA). Viral loads were determined by the Amplicor version 1.5 monitor test (Roche Diagnostics Systems, Branchburg, NJ). Comparisons between time points were performed using paired t tests analyzed by GraphPad Prism 5.03 (GraphPad Software, Inc., La Jolla, CA). P values < 0.05 were considered significant. An enzyme-linked immunosorbent assay (ELISA) to detect immunoglobulin G (IgG) to schistosome adult worm antigens (SWAP) was performed on baseline sera according to previously described methods.9
Study participants were categorized into three groups: patients negative for schistosome ova at baseline (at the start of ART) but found to excrete eggs later (N = 14); patients excreting schistosome ova at baseline (N = 53); and patients who were schistosome egg-negative throughout the study period (N = 23). The three groups were similar in terms of age, approximate number of years spent in proximity to the lake, and baseline CD4+ T-lymphocyte counts (Table 1).
Table 1.
Characteristics of the study population categorized by S. mansoni egg excretion
| S. mansoni egg-positive at baseline (N = 53) | S. mansoni egg-negative at baseline and became egg-positive (N = 14) | S. mansoni egg-negative throughout (N = 23) | |
|---|---|---|---|
| Age (in years; mean ± SD) | 36.8 ± 10.1 | 34.3 ± 13.3 | 35 ± 9.1 |
| Years spent in proximity to the lake (mean ± SD) | 21 ± 2.2 | 21 ± 2.7 | 23 ± 3.1 |
| CD4+ at baseline (median, range) | 201, 22–546 | 234, 69–507 | 189, 15–393 |
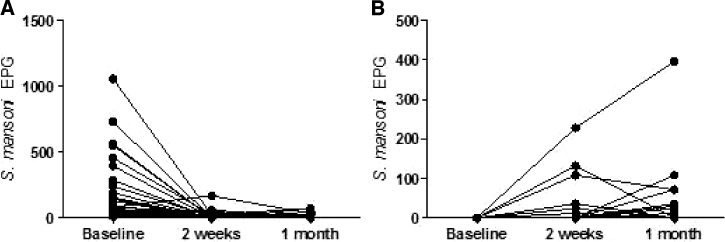
S. mansoni eggs per gram (EPG) of feces in study participants who were shedding eggs at enrollment are shown in Figure 1A. Although a few persons were still excreting eggs 2 weeks after initial drug treatment, these individuals had an average 96% decrease in their S. mansoni EPG (P < 0.0001) compared with baseline. Fourteen individuals who were S. mansoni egg-negative at baseline began excreting eggs after 2–4 weeks of ART (Figure 1B). For individuals excreting eggs at enrollment, 95.7% (45/47) were positive for anti-SWAP antibodies, whereas 69.2% (9/13) of the individuals who began excreting eggs after the start of ART were positive. Of the study participants who remained egg-negative for the entire follow-up period, 64.3% (9/14) were anti-SWAP antibody-positive.
Figure 1.
Comparison of EPG of feces at baseline and 2 and 4 weeks after treatment and the start of ART in patients who enrolled when (A) positive (N = 53) or (B) negative at baseline (N = 14) for S. mansoni eggs. Participants were offered praziquantel treatment (A) at baseline or (B) when they became egg-positive. Each line represents an individual study participant.
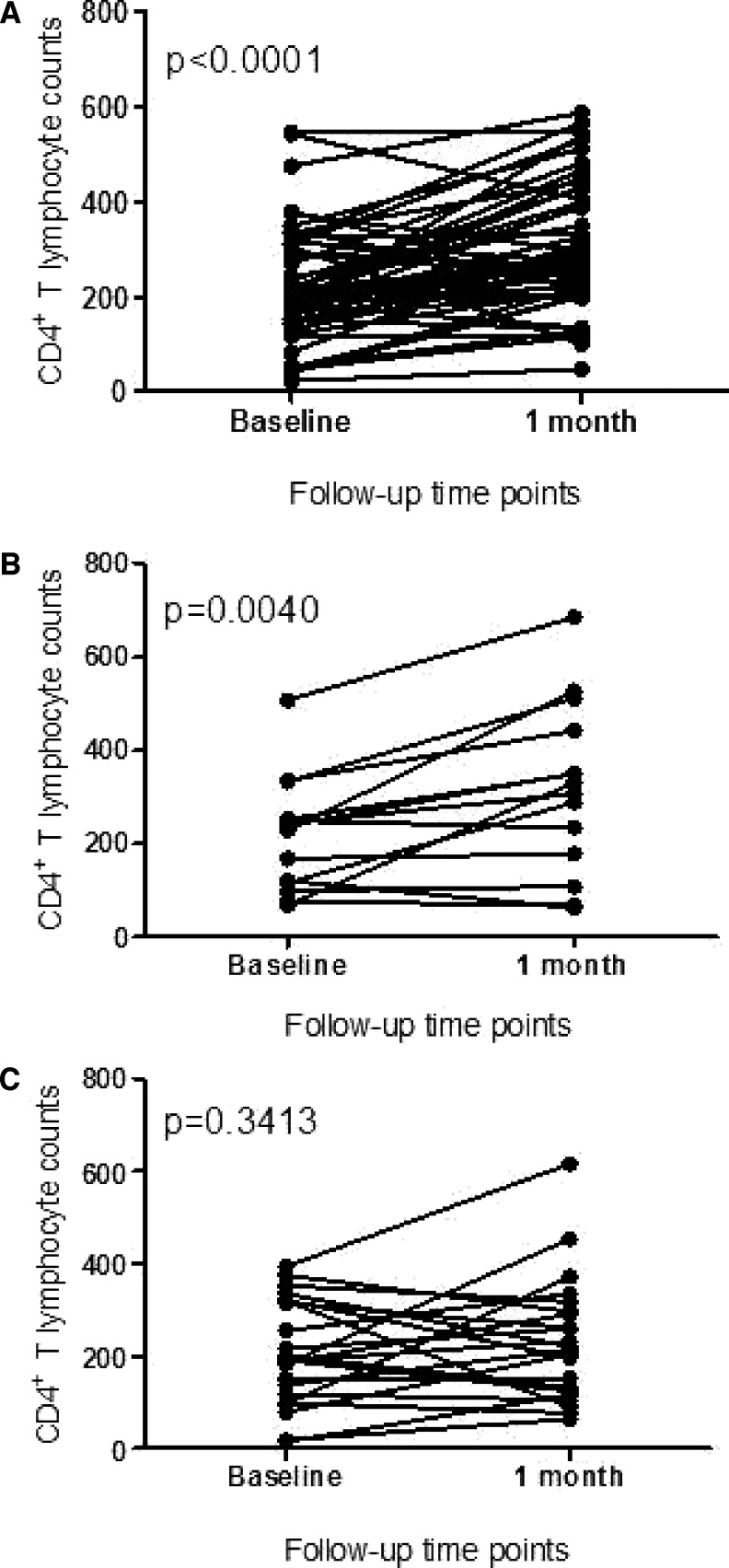
We compared the CD4+ T-lymphocyte counts at baseline and 1 month after the start of ART in the three groups (Figure 2). There was a significant increase in CD4+ T-lymphocyte counts after 1 month of ART in patients who excreted schistosome eggs at baseline (P < 0.0001). CD4+ T-lymphocyte counts also significantly increased in the group of patients who were negative at baseline but started egg excretion later (P = 0.004). The group that was negative for schistosome eggs throughout the study period showed no change in CD4+ T-lymphocyte counts after 1 month of ART (P = 0.3). The proportion of participants that increased CD4+ T-lymphocyte counts on ART was significantly higher (χ2 P = 0.0025) for those individuals who were egg-positive at baseline (39/53, 73.5%) or became egg-positive (11/14, 78.6%) than for individuals who remained egg-negative throughout the study (8/23, 37.8%). We do not know why ART did not increase CD4+ T-lymphocyte counts in most patients who remained egg-negative.
Figure 2.
Comparison of CD4+ T-cell counts on initial study enrollment and 1 month after the start of ART in individuals who were (A) egg-positive for S. mansoni (N = 53) at baseline, (B) egg-negative at baseline but became egg-positive after ART initiation (N = 14), or (C) remained egg-negative throughout the study (N = 23). Each line represents an individual participant.
Results from this study are consistent with our earlier observations and data from experimental models of schistosomiasis that suggest that CD4+ T lymphocytes are among the immune cells involved in the excretion of S. mansoni eggs from the infected host.2,10 Some schistosome and HIV coinfected study participants did not excrete eggs at baseline but started excreting eggs within 1 month after initiation of ART, corresponding with a significant increase in their CD4+ T-lymphocyte numbers. Thus, restoration of immune capacity was temporally associated with the increase of egg excretion. In contrast, the group that did not begin egg excretion after the initiation of ART did not show increased CD4+ T lymphocytes within the 1-month timeframe (Figure 2C). It may be possible that these individuals simply did not have active schistosome infections at the time of the study. However, the high rate of positivity in the SWAP ELISA and their proximity to Lake Victoria suggest that most of them would have been infected with schistosomes at some point in their lives. In the absence of previous mass praziquantel administration programs in this area, it is likely that at least some of these individuals were still actively infected.
Because study participants were treated with praziquantel whenever they were found to be egg-positive, we do not know whether ART would have increased egg excretion in the group of study participants who had positive stools at baseline and increased their CD4+ T-cell counts within 1 month. Nevertheless, egg counts in this group were reduced by 96% after praziquantel treatment, consistent with our previous results showing good drug efficacy in HIV-1 coinfected individuals.11
We have previously found that schistosome infection itself may be associated with reduced levels of circulating CD4+ T lymphocytes independent of HIV-1 infection.12 The stage of HIV infection may also have an influence on these results. Although it is not possible from the design of the current study to know whether praziquantel treatment of schistosomiasis in coinfected individuals promotes increases in CD4+ T cells greater than ART alone, attention to this question may provide advantages to HIV-1+ individuals living in schistosomiasis-endemic areas beyond the recognized benefits of clearing a person's schistosome infection. Furthermore, failure to attend to infections in persons starting on ART may put them at greater risk for immune reconstitution inflammatory syndrome (IRIS).13
The primary limitation of this study is that it relied on two slides from one stool sample for microscopy diagnosis at each time point. Because Kato–Katz stool examination can miss low-intensity infections and egg excretion can fluctuate daily, it is possible that we did not detect eggs at baseline of the participants who eventually became stool-positive or missed eggs at follow-up time points in the group that remained negative throughout the study. However, these initial results warrant additional study with more rigorous stool examinations in a larger sample size. The role that schistosomiasis may play in determining the pathology of HIV coinfected individuals at the initiation and during ART is an area of ongoing study.
ACKNOWLEDGMENTS
The authors thank Fridah Mualma, Geoffrey Muchiri, fieldworkers, and especially, all the study participants. This study is published with the permission of the Director, Kenya Medical Research Institute.
Disclaimer: The findings and conclusions in this report are the findings and conclusions of the authors, and they do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Financial support: This research was supported by an European and Developing Countries Clinical Trial Partnership Senior Fellowship awarded to P.N.M.M.
Authors' addresses: Erick M. O. Muok, Elizabeth A. Ochola, Diana M. S. Karanja, and Pauline N. M. Mwinzi, Center for Global Health Research, Kenya Medical Research Institute, Kisumu, Kenya, E-mails: EMuok@kemricdc.org, EAkinyi@kemricdc.org, DKaranja@kemricdc.org, and PMwinzi@kemricdc.org. Elses W. Simiyu, Zoology Department, Maseno University, Maseno, Kenya, E-mail: elsessim@yahoo.com. Zipporah W. Ng'ang'a, Department of Health Sciences, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya, E-mail: zipnganga@gmail.com. W. Evan Secor, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: was4@cdc.gov.
References
- 1.Bundy D, Sher A, Michael E. Good worms or bad worms: do worm infections affect the epidemiological patterns of other diseases? Parasitol Today. 2000;16:273–274. doi: 10.1016/s0169-4758(00)01689-6. [DOI] [PubMed] [Google Scholar]
- 2.Karanja DMS, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya. I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus co-infections. Am J Trop Med Hyg. 1997;56:515–521. doi: 10.4269/ajtmh.1997.56.515. [DOI] [PubMed] [Google Scholar]
- 3.Fontanet AL, Woldemichael T, Sahlu T. Epidemiology of HIV and Schistosoma mansoni infections among sugar-estate residents in Ethiopia. Ann Trop Med Parasitol. 2000;94:145–155. [PubMed] [Google Scholar]
- 4.N'Zoukoudi-N'Doundou MY, Dirat I, Akouala JJ, Penchenier L, Makuwa M, Rey JL. Bilharziasis and human immunodeficiency virus infection in Congo. Med Trop (Mars) 1995;55:249–251. [PubMed] [Google Scholar]
- 5.Mwanakasale V, Vounatsou P, Sukwa TY, Ziba M, Ernest A, Tanner M. Interactions between Schistosoma haematobium and human immunodeficiency virus type 1: the effects of coinfection on treatment outcomes in rural Zambia. Am J Trop Med Hyg. 2003;69:420–428. [PubMed] [Google Scholar]
- 6.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, van Dam GJ, Erikstrup C, Ullum H. Schistosomiasis and HIV-1 infection in rural Zimbabwe: implications of coinfection for excretion of eggs. J Infect Dis. 2005;191:1311–1320. doi: 10.1086/428907. [DOI] [PubMed] [Google Scholar]
- 7.Opisa S, Odiere MR, Jura WGZO, Karanja DMS, Mwinzi PNM. Malacological survey and geographical distribution of vector snails for schistosomiasis within informal settlements of Kisumu City, western Kenya. Parasit Vectors. 2011;4:226. doi: 10.1186/1756-3305-4-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 9.Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, Mwinzi PMN, Butler SE, Karanja DMS, Secor WE. Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in Western Kenya. PLoS Negl Trop Dis. 2011;5:e951. doi: 10.1371/journal.pntd.0000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner JD, Narang P, Coles MC, Mountford AP. Blood flukes exploit Peyer's Patch lymphoid tissure to facilitate transmission from the mammalian host. PLoS Pathog. 2012;8:e1003063. doi: 10.1371/journal.ppat.1003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karanja DMS, Boyer AE, Strand M, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya. II. Efficacy of praziquantel for schistosomiasis patients with human immunodeficiency virus co-infections. Am J Trop Med Hyg. 1998;59:307–311. doi: 10.4269/ajtmh.1998.59.307. [DOI] [PubMed] [Google Scholar]
- 12.Mwinzi PN, Karanja DM, Kareko I, Magak PW, Orago AS, Colley DG, Secor WE. Short report: evaluation of hepatic fibrosis in persons co-infected with Schistosoma mansoni and human immunodeficiency virus 1. Am J Trop Med Hyg. 2004;71:783–786. [PubMed] [Google Scholar]
- 13.de Silva S, Walsh J, Brown M. Symptomatic Schistosoma mansoni infection as an immune restoration phenomenon in a patient receiving antiretroviral therapy. Clin Infect Dis. 2006;42:303–304. doi: 10.1086/499109. [DOI] [PubMed] [Google Scholar]