Abstract
This report summarizes the status of the global Dracunculiasis Eradication Program as of the end of 2012. Dracunculiasis (Guinea worm disease) has been eliminated from 17 of 21 countries where it was endemic in 1986, when an estimated 3.5 million cases occurred worldwide. Only 542 cases were reported from four countries in 2012, and 103 villages still had indigenous transmission. Most remaining cases were reported from the new Republic of South Sudan, whereas Chad, Ethiopia, and Mali each reported 10 cases or less. Political instability and insecurity in Mali may become the main obstacles to interrupting dracunculiasis transmission forever.
Introduction
The global Dracunculiasis Eradication Program has gotten much closer to its goal of stopping transmission of dracunculiasis (Guinea worm disease) since the status of the initiative was last reviewed here in 2008.1 This paper describes the status of the program as of the end of 2012.
We described in previous reports the parasite and the strategies and interventions being used to eradicate it.1,2 The infection (dracunculiasis or Guinea worm disease) is caused by the nematode parasite Dracunculus medinensis, and it is transmitted to humans in contaminated drinking water containing copepods (water fleas) that harbor infective larvae of the parasite. The larvae are expelled into water by adult female worms, and then, they emerge through the skin of infected persons about 1 year after infection. Emergence, the act of manually removing the Guinea worm, is slow, painful, and disabling (although usually not fatal), and therefore, it has a serious adverse socioeconomic impact on the health, agricultural productivity, and school attendance of affected populations. Persons are incapacitated for periods averaging 3 months. In the past, more than one-half of a village's population might have been affected simultaneously during the main harvest or planting season. Humans are the only reservoir of infection. Individual infections last only 1 year, but people do not develop immunity to the parasite. There is no effective treatment or vaccine; however, the infection may be prevented by (1) educating villagers about the origin of the disease, (2) preventing infected persons from entering sources of drinking water, (3) filtering all drinking water through a finely woven cloth that removes the copepods, (4) applying Abate larvicide (temephos; BASF Corp., Mount Olive, NJ) to kill the copepods in ponds and other stagnant sources of drinking water, and (5) providing clean drinking water from safe sources, such as protected hand-dug or borehole wells.
The global eradication campaign began at the Centers for Disease Control and Prevention (CDC) in 1980, it was adopted as a subgoal of the International Drinking Water Supply and Sanitation Decade (1981–1990), and it has been led since 1986 by The Carter Center, which is at the head of a coalition that includes the Ministries of Health of the endemic countries, the CDC, the World Health Organization (WHO), and the United Nations Children's Fund (UNICEF) as major partners and thousands of village volunteers and supervisory health staff. The coalition is supported by numerous donor agencies, governments, foundations, and other institutions. When The Carter Center began leading the global campaign after the CDC, there were an estimated 3.5 million cases of dracunculiasis worldwide.3 At the World Health Assembly in 2004, ministers of health of the remaining endemic countries set a target to stop transmission of dracunculiasis by the end of 2009.4 When that target date was not met, partly because of the ongoing civil war in Sudan as well as unexpected outbreaks in Chad, Ethiopia, and Mali, the global initiative resolved to interrupt transmission as soon as possible, expecting that interruption would occur before the end of 2012.
Current Status Of The Campaign
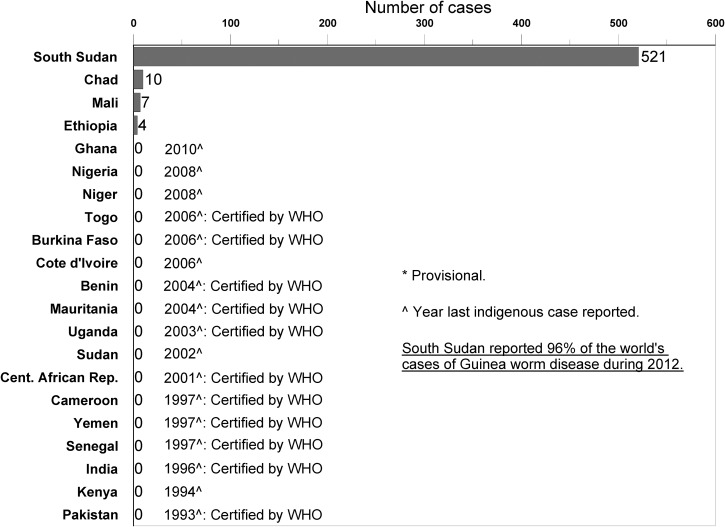
Worldwide in 2012, only 542 cases of dracunculiasis were reported (down from 4,619 cases in 2008), only 103 villages reported indigenous cases (down from 23,735 villages in 1993 and 1,019 villages in 2008), and only 4 countries remained with endemic disease (down from 21 countries in 1986 and 5 countries in 2008) (Figure 1 and Table 1). The number of cases exported from one country to another fell from a peak of 154 cases in 2002 to 6 cases in 2008 and 3 cases in 2012. Ghana, Niger, and Nigeria each stopped transmission of the disease in or since 2008, whereas Chad and Ethiopia became endemic again. When the Republic of South Sudan became officially independent from Sudan in July of 2011, South Sudan included all of the remaining endemic areas of the former Sudan, whereas the northern states of the old Sudan, which had reported their last indigenous cases in 2002, retained the name Sudan and became the latest Guinea worm-free country. South Sudan reported 521 cases, Chad reported 10 cases, Mali reported 7 cases, and Ethiopia reported 4 cases in 2012.
Figure 1.
Distribution of 542 indigenous cases of dracunculiasis reported during 2012.
Table 1.
Dracunculiasis eradication campaign: cases reported and contained, villages under active surveillance (VAS), and status of interventions in endemic villages: 2012 (provisional)
| Countries with endemic transmission in 2012 | Reported cases (indigenous) in 2012 | Reported cases (imported) in 2012* | All cases reported that were contained in 2012 (%) | Overall change in indigenous cases in VAS during the same period of 2011 (%) | Villages/localities | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAS: 2012 | Status of interventions in endemic villages in 2012 | |||||||||||||||
| No. | Reporting monthly (%) | Zero cases | One or more cases | Only imported cases† | Indigenous cases | Endemic villages 2011–2012 | Reporting monthly‡ (%) | Provided health education‡ (%) | Filters in all households‡ (%) | Using Abate‡ (%) | One or more sources of safe water‡ (%) | |||||
| South Sudan | 521 | 0 | 64 | −49 | 6,410 | 100 | 6,155 | 255 | 166 | 89 | 167 | 100 | 98 | 100 | 100 | 33 |
| Mali§ | 7 | 0 | 57 | −42 | 121 | 88 | 117 | 4 | 0 | 3 | 9 | 78 | 78 | 78 | 57 | 71 |
| Chad | 10 | 0 | 40 | 0 | 693 | 95 | 684 | 9 | 0 | 9 | 2 | 100 | 100 | 100 | 100 | 100 |
| Ethiopia | 4 | 0 | 50 | −50 | 77 | 100 | 75 | 3 | 2 | 2 | 3 | 100 | 100 | 100 | 100 | 67 |
| Total | 542 | 0 | 64 | −49 | 7,301 | 99 | 7,031 | 271 | 168 | 103 | 181 | 98 | 98 | 98 | 97 | 35 |
Imported from another country.
Imported from another country or another in-country disease-endemic village.
The denominator of the percentage is the number of villages/localities where the program applied interventions during 2011–2012.
Guinea worm eradication program operations (supervision, surveillance, and interventions) were interrupted in Kidal, Gao, and Timbuktu Regions as a result of the coup d'etat beginning in March of 2012. Three cases of dracunculiasis were exported to Niger in September and are included in Mali's total reported cases.
South Sudan.
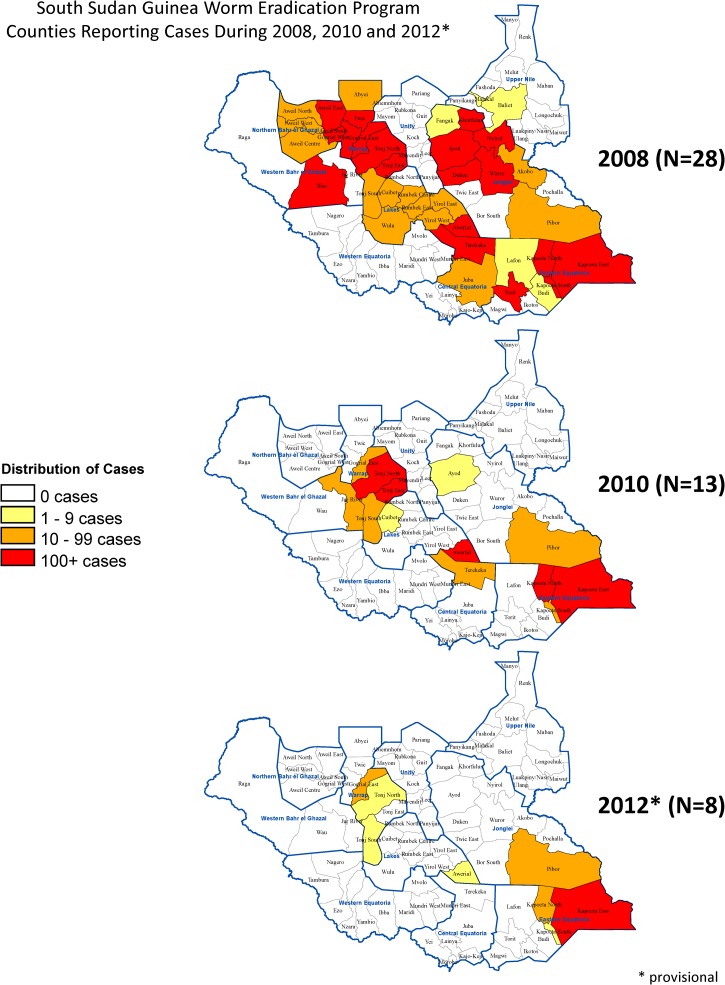
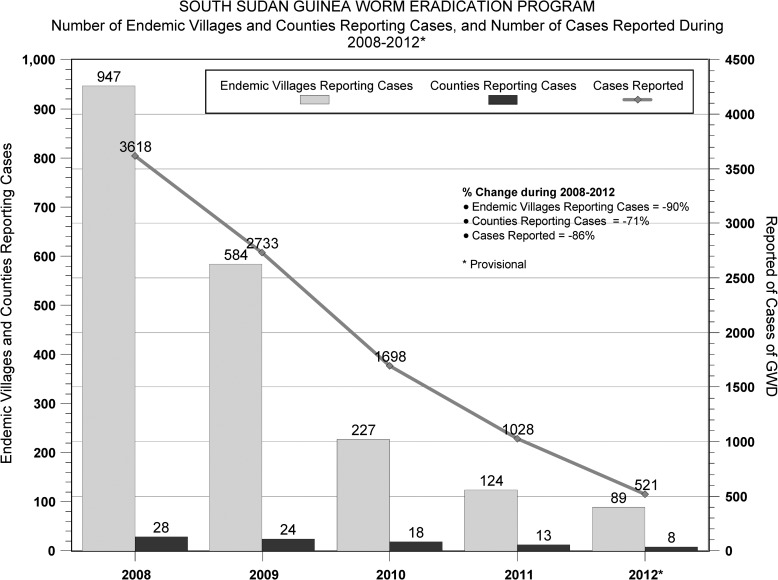
Despite considerable challenges of poor infrastructure, vast area, insecurity, long rainy season, and exceptionally complex epidemiology (e.g., seasonal migration, ethnic conflicts, and multiple potential transmission sites, including villages, gardens, more distant farms, cow cattle camps, bull cattle camps, pastures for smaller livestock, and temporary drinking water sources along pathways between areas), the area now known as the Republic of South Sudan has made steady progress. Between 2008 and 2012, South Sudan has reduced cases by 86%, reduced the number of counties reporting cases by 71%, and reduced the number of villages reporting indigenous cases by 91% (from 947 to 89) (Figures 2 and 3 ); 72 of South Sudan's 80 counties are now Guinea worm-free, with 81% of all remaining cases located in Kapoeta East County of Eastern Equatoria State. The intensity of interventions has increased steadily over the past 5 years. For example, cloth filters were in all households in 79% of endemic villages in 2008 and 100% of endemic villages in 2012. Additionally, the percentage of endemic villages covered by vector control with Abate increased from 34% in 2008 to 100% in 2012, and the proportion of reported cases that were contained increased from 49% to 64% during the same period. (A case is considered contained if all of the following conditions are met: [1] the disease is detected < 24 hours after worm emergence; [2] the patient has not entered any water source since the worm emerged; [3] a volunteer has managed the patient properly by cleaning and bandaging the lesion until the worm has been fully removed manually and providing health education to discourage the patient from contaminating any water source (if two or more emerging worms are present, transmission is not contained until the last worm is removed); and [4] the containment process, including verification of dracunculiasis, is validated by a supervisor within 7 days of emergence of the worm. All of these criteria must be achieved for each emerged worm for the case to be considered contained.5) Of 14 cattle camps with endemic disease in 2012, all submitted reports monthly and were provided with health education; none had a source of safe drinking water, and 33% applied Abate to one or more of their water sources. The number of supervisors has been increased from 181 during 2008 to 311 during 2012 to reduce the size of areas of supervisory responsibility. Incidents of political insecurity severe enough to disrupt program activities have decreased from 32 in 2009 to 3 in 2012. Former US President Jimmy Carter met with government leaders, representatives of the South Sudan Guinea Worm Eradication Program (SSGWEP) and its partners, and visited an endemic area in February of 2010. The SSGWEP has convened meetings in Juba, South Sudan, to review the state of its eradication campaign annually since 2006. Significant remaining challenges include inadequate surveillance for imported cases of dracunculiasis in Guinea worm-free areas (which now include most of the country), inadequate prioritization of villages for provision of safe sources of drinking water, and urgent need to repair the key Kauto Bridge that collapsed in Kapoeta East County in May of 2012, which has complicated SSGWEP access to key areas.
Figure 2.
SSGWEP: counties reporting cases during 2008, 2010, and 2012.
Figure 3.
SSGWEP: the number of endemic villages and counties reporting cases and the number of cases reported during 2008–2012.
Chad.
During a routine pre-certification visit in 2010, a WHO mission investigated rumors and discovered cases of dracunculiasis in Chad after the country had reported no cases in over a decade. The source of the new cases, from which several worms were confirmed as D. medinensis at the CDC, is still uncertain. Chad reported a total of 10 cases in eight villages in 2010, 10 cases in nine villages in 2011, and 10 cases in nine villages in 2012. Of the 26 villages, which are spread over 10 districts, only 2 villages (in Chari Baguirmi Region) reported cases in more than 1 year. Most cases occurred during the rainy season from July to October, with no marked preponderance among adults versus children or males versus females. Although almost one-half (49%) of villages under active surveillance have at least one source of safe drinking water, an investigation led by the CDC and assisted by The Carter Center in 2012 identified the use of secondary sources of drinking water as the main associated risk factor for infection (Sreenivasan N, unpublished data). Secondary sources were those sources used regularly in addition to the main source of water for the household and were typically lakes, ponds, or unprotected wells used in the fields while farming or fishing.
In 2012, the WHO Collaborating Center at the CDC examined worm specimens from several dogs from the same two regions but not from the same villages where human cases were reported in 2012. These specimens could not be distinguished from D. medinensis on preliminary microscopic- and molecular-level examination, but neither their speciation nor any relationship to the human cases of dracunculiasis is yet fully established. Additional studies are underway and will be reported separately. Guinea worms in dogs have been reported for almost a century in some areas of Asia, Africa, and North America, including in some recently endemic countries that have interrupted transmission,6 but disease caused by D. medinensis has never been reported again after human transmission was interrupted.
The Carter Center established a renewed presence in Chad in 2011 and has assisted the Ministry of Public Health in establishing active surveillance by 1,829 village volunteers in 693 villages in the affected areas. The village volunteers are supported by 103 supervisors and five expatriate technical assistants. In 2011, the Government of Chad began publicizing a cash reward equivalent to US$100 for reporting a case of dracunculiasis.7 Informal spot inquiries in October of 2012 suggested virtually complete awareness of the reward among people queried in endemic areas. A spot check in one small Guinea worm-free area of Chad in 2012 (such areas remain at risk of imported cases) found a 38% level of awareness of the reward. By the end of 2012, more than 95% of villages under active surveillance were receiving health education about dracunculiasis and reporting monthly. Cloth filters are distributed only in villages with cases or high-risk villages sharing a common water source with endemic villages. Abate larvicide is used sparingly, because most surface water sources are flooded during the rainy season. None of the cases reported in 2010 were contained; four cases each were contained in 2011 and 2012. After 3 successive years of indigenous cases, Chad was declared endemic again in 2012.
Mali.
Mali's Guinea Worm Eradication Program suffered a setback in 2007 as a result of two large unexpected outbreaks in Kidal and Gao Regions that were detected and reported after significant contamination of local water sources had already occurred.1 After reporting 417 cases in 2008, Mali's program reduced the number of cases by 97% (to 12 cases in 2011) before suffering another setback because of a coup d'etat in March of 2012. Since the coup, in which rebels, independent of the coup participants, seized control of the northern one-half of the country, regular surveillance and other program operations have been conducted only in the southern four regions of the country. Sporadic and unverifiable reports have been received from a few non-governmental and humanitarian organizations still operating in the northern regions. Mali had seven known cases detected in June, July, and September of 2012, including three cases that were exported to Niger from Gao Region in September. All three cases discovered in Niger and one of four other cases reported in Mali were contained. In 2011, Mali increased its cash reward for reporting a case of dracunculiasis to the equivalent of $40 from $10.8 Informal spot checks of reward awareness in 2011 found 71% awareness in an endemic district of Gao Region and 2% awareness in a Guinea worm-free district of Koulikoro Region.
Ethiopia.
Ethiopia's Dracunculiasis Eradication Program (EDEP) reported no indigenous cases of the disease between June of 2006 and March of 2008, and it was thought to have interrupted transmission at the time of the previous review.1 However, Ethiopia reported 41 cases in 11 villages of Gambella Region, including 38 indigenous cases and 3 cases imported from Sudan, in 2008. Ethiopia reported 24 cases in 2009, 21 cases in 2010, 8 cases in 2011 (including 2 cases imported from South Sudan), and 4 cases—1 case each in April, May, August, and December—in 2012. The cases in April and December of 2012 were not contained. All indigenous cases since 2008 occurred in Gambella Region. It now seems that a small number of cases continued to be transmitted in Gambella Region but were undetected before cases came to attention again in April of 2008. In 2011, Ethiopia increased the amount of its cash reward for reporting a case of dracunculiasis to the equivalent of $58 from $29. Informal spot checks of reward awareness in 2011 revealed 83% awareness among those individuals queried in an endemic district of Gambella Region and 2% awareness in a district of Guinea worm-free Amhara Region.
Global activities.
As before, the global campaign continues to convene an annual meeting of the remaining endemic countries each March or April to review progress, challenges, and resource requirements. Such meetings, which now also include representatives of recently endemic countries that have not yet been certified as Guinea worm-free by the International Commission for the Certification of Dracunculiasis Eradication (ICCDE), were held most recently in Bamako, Mali (2009), Atlanta, Georgia, United States (2010 and 2011), and Addis Ababa, Ethiopia (2012). The ICCDE held its seventh and eighth meetings at the WHO headquarters in Geneva, Switzerland, in October of 2009 and November/December of 2011, respectively. With the certification of formerly endemic Benin, Burkina Faso, Mauritania, Togo, Uganda, and seven other non-endemic countries in 2011, a total of 192 countries and territories has now been certified as free of the disease, whereas 14 countries remain to be certified.8 The Carter Center hosted a ceremony to honor the two latest countries to report zero indigenous cases for 12 consecutive months, Niger and Nigeria, in Atlanta in February of 2011. During 2009–2012, the WHO Collaborating Center for Research, Training, and Eradication of Dracunculiasis at the CDC examined 109 specimens thought to be Guinea worms submitted from 11 countries using microscopic and molecular differentiation techniques described previously1 and confirmed 44 to be D. medinensis. In association with The Carter Center, the Collaborating Center at the CDC continues to issue the newsletter of the global campaign, Guinea Worm Wrap-Up, almost monthly.
The World Health Assembly (WHA) adopted a new resolution (WHA64.16) on dracunculiasis eradication at its annual meeting in Geneva in May of 2011. In a departure from five previous resolutions, the new resolution requires the WHO to report to the WHA on the progress of dracunculiasis eradication “every year until eradication of dracunculiasis is certified.” The WHO has convened an “Informal Meeting” on the state of the campaign during the WHA each year since 2006 as an important advocacy event. These meetings feature brief reports on the status of the eradication campaign by The Carter Center and national program leaders and certification of eradication by WHO. They are increasingly well-attended by ministers and other senior health officials from endemic and formerly endemic countries, partner agencies, and donors. A new award-winning documentary of the campaign, “Foul Water Fiery Serpent,” sponsored by the former chairman of The Carter Center's Board of Trustees, Mr. John Moores, was released in 2010 and has been shown in special screenings around the United States and broadcast widely on US television.
Major funding for dracunculiasis eradication by the Bill & Melinda Gates Foundation, the United Kingdom's Department for International Development, the President of the United Arab Emirates His Highness Sheikh Khalifa bin Zayed Al Nahyan, the Children's Investment Fund Foundation, and other donors announced in 2008 and 2012 has provided the remaining estimated financial support needed to secure eradication and certification of eradication of dracunculiasis. Recent additional in-kind donation pledges to The Carter Center of Abate larvicide and nylon filter material by the international companies BASF and Vestergaard Frandsen, respectively, complete the necessary materiel for those two important interventions.
Discussion
The global campaign to eradicate dracunculiasis has made considerable progress since the previous review in this journal in 2008. Nigeria and Niger had their last cases late that year, and Ghana had its last cases in 2010. The endemic country with the largest number of remaining cases, South Sudan, obtained its independence, reduced disruptive insecurity incidents substantially, and continues to progress steadily to interruption of transmission. With full funding secured and only 542 cases reported worldwide in 2012, the end of Guinea worm disease is near but not yet achieved. Recent surprise outbreaks in Chad (after 10 years) and Ethiopia (after 22 months) have underscored the dangers of inadequate surveillance in areas believed to be free of the disease, with such surveillance insecurity replacing political insecurity as the major challenge in Chad, Ethiopia, and South Sudan. The scattered, hypoendemic epidemiology of dracunculiasis in Chad over the past 3 years is unusual. The use of secondary water sources, which are plentiful during the flooding that occurs in the rainy season, might explain the sporadic nature of cases, because few people necessarily use the same secondary water sources. Dilution of infected copepods in surface water sources during widespread seasonal flooding might explain the pattern, but there is no evidence to confirm this theory. There also is no evidence of human consumption of potential paratenic hosts of D. medinensis in Chad. Aggressive surveillance and containment of cases may eliminate transmission in Chad before the pattern of transmission is fully understood. In Mali, the March of 2012 coup d'etat removed an ardent advocate of dracunculiasis eradication from the presidency, and the unofficial partition of the country made much of the endemic area inaccessible to national authorities, including the national Guinea Worm Eradication Program. By its combined political and surveillance insecurity, Mali emerges as the most uncertain prospect for stopping transmission this year or next year. Improving surveillance in Guinea worm-free areas to detect any imported cases quickly requires high priority in all four remaining endemic countries.
ACKNOWLEDGMENTS
The authors thank Shandal Sullivan and Renn McClintic-Doyle for assistance in preparing this manuscript. We acknowledge the contributions of the national coordinators, village-based volunteers, other health workers in the disease-endemic countries, and other staff of The Carter Center, WHO, and UNICEF to these significant accomplishments. We dedicate this paper to the memory of Dr. Robert L. Kaiser.
Footnotes
Financial support: During 2008–2012, The Carter Center's work to eradicate Guinea worm disease has been made possible by financial and in-kind contributions from the Next Generation Fund of the Hugh J. Andersen Foundation; Apple Computer, Inc.; Arab Fund for Economic and Social Development; Atlanta Woman's Club; BASF Corporation; Canadian International Development Agency; Chevron Corporation; Children's Investment Fund Foundation UK; Crawford Family Foundation; Delta Medical Supplies; Edgar O. Dixon Charitable Trust; Elfenworks Foundation; First Congregational Church; Foundation Source; Bill & Melinda Gates Foundation; General Electric Company; Girl Scouts of America Brownie Troop 861; Global Aviation Holdings; Global Health Education Consortium, Inc.; Google, Inc.; Robert and Shirley Harris Family Foundation; Harris myCFO Foundation; Conrad N. Hilton Foundation; John C. and Karyl Kay Hughes Foundation; John P. Hussman Foundation, Inc.; Johns Hopkins University; Johnson & Johnson; Kendeda Fund; Leslie Family Foundation; John D. and Catherine T. MacArthur Foundation; McKenna Foundation; Mid-Continent University; Monsanto Company; Mount Pleasant Lutheran Church; National Democratic Institute for International Affairs; OPEC Fund for International Development; Roman Catholic Diocese of Joliet; Government of Saudi Arabia; Saudi Fund for Development; J. V. Schiro Zavela Foundation; Shod LLC; Stahl Family Foundation; St. Thomas Aquinas Parish; Sultanate of Oman; His Highness General Sheikh Mohamed bin Zayed Al Nahyan, Crown Prince of Abu Dhabi, in honor of His Highness Sheikh Khalifa bin Zayed, President of the United Arab Emirates; UNICEF; United Kingdom Department for International Development; US Agency for International Development; US Centers for Disease Control and Prevention; United Nations World Food Programme; Vanguard Charitable Endowment Program; Vestergaard Frandsen; Women's Leadership Foundation; YKK Corporation; and many generous individuals.
Authors' addresses: Donald R. Hopkins and P. Craig Withers Jr., Health Programs, The Carter Center, Atlanta, GA, E-mails: sdsulli@emory.edu and cwither@emory.edu. Ernesto Ruiz-Tiben and Adam Weiss, Guinea Worm Eradication Program, The Carter Center, Atlanta, GA, E-mails: eruizti@emory.edu and adam.j.weiss@emory.edu. Mark L. Eberhard, Division of Parasitic Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: mle1@cdc.gov. Sharon L. Roy, Waterborne Disease Prevention Branch, Division of Foodborne, Waterborne, and Environmental Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: str2@cdc.gov.
Reprint requests: Donald R. Hopkins, Health Programs, The Carter Center, 453 Freedom Parkway, Atlanta, GA 30307, E-mail: sdsulli@emory.edu.
References
- 1.Hopkins DR, Ruiz-Tiben E, Downs P, Withers PC, Jr, Roy S. Dracunculiasis eradication: neglected no longer. Am J Trop Med Hyg. 2008;79:474–479. [PubMed] [Google Scholar]
- 2.Hopkins DR, Ruiz-Tiben E. Strategies for eradication of dracunculiasis. Bull World Health Organ. 1991;69:533–540. [PMC free article] [PubMed] [Google Scholar]
- 3.Watts SJ. Dracunculiasis in Africa: its geographic extent, incidence, and at-risk population. Am J Trop Med Hyg. 1987;37:119–125. doi: 10.4269/ajtmh.1987.37.119. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Dracunculiasis eradication: Geneva declaration on guinea-worm eradication, Geneva, 2004. Wkly Epidemiol Rec. 2004;79:234–235. [Google Scholar]
- 5.World Health Organization Dracunculiasis eradication: case definition, surveillance and performance indicators. Wkly Epidemiol Rec. 2003;78:323–328. [PubMed] [Google Scholar]
- 6.Cairncross S, Muller R, Zagaria N. Dracunculiasis (Guinea worm disease) and the eradication initiative. Clin Microbiol Rev. 2002;15:223–246. doi: 10.1128/CMR.15.2.223-246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djidina MR, Guialoungou H, Dono BB, Ngarhor N, Padjaina M, Biswas G, Sankara A, Maiga N, Djimrassengar H, Roy SL, El Bcheraoui C, Walldorf JA. Renewed transmission of dracunculiasis-Chad, 2010. Morb Mortal Wkly Rep. 2011;60:744–748. [PubMed] [Google Scholar]
- 8.World Health Organization Dracunculiasis eradication-global surveillance summary, 2011. Wkly Epidemiol Rec. 2012;87:177–187. [PubMed] [Google Scholar]