Abstract
Abu Hamed, Sudan, the northernmost location of onchocerciasis in the world, began community-directed treatment with ivermectin (CDTI) in 1998, with annual treatments enhanced to semiannual in 2007. We assessed the status of the parasite transmission in 2011 entomologically, parasitologically, and serologically. O-150 pool screening showed no parasite DNA in 17,537 black flies collected in 2011 (95% confidence interval upper limit [95% CI UL] = 0.023). Skin microfilariae, nodules, and signs of skin disease were absent in 536 individuals in seven local communities. Similarly, no evidence of Onchocerca volvulus Ov16 antibodies was found in 6,756 school children ≤ 10 years (95% CI UL = 0.03%). Because this assessment of the focus meets the 2001 World Health Organization (WHO) criteria for interrupted transmission, treatment was halted in 2012, and a post-treatment surveillance period was initiated in anticipation of declaration of disease elimination in this area. We provide the first evidence in East Africa that long-term CDTI alone can interrupt transmission of onchocerciasis.
Introduction
Human onchocerciasis, also known as river blindness, is a parasitic disease caused by the nematode Onchocerca volvulus, which is transmitted by black flies of the genus Simulium. Current estimates suggest that over 123 million people are at risk of infection in 38 endemic countries; at least 25.7 million of these people are infected, and 1 million are blinded or have severe visual impairment.1 About 99% of the cases exist in a sub-Saharan belt extending from Senegal to Ethiopia. The disease is also endemic in Yemen and six countries in Latin America. Recent reports estimated that > 102 million individuals are at risk of the disease in the 20 countries of the African Program of Onchocerciasis Control (APOC), including Sudan.2
In Sudan, onchocerciasis is endemic in three main foci. The largest of the three foci exists in the southwest of the country, where infection prevalence was originally reported to be over 90% and blindness rates reached 10% in some villages.3 The disease in this focus is similar to the blinding form in the savanna regions of West Africa. The vast majority of this endemic area is now part of the new Republic of South Sudan, which was formed in 2011. The second focus of the disease exists in Galabat locality (Gedarif state, Eastern Sudan) along Atbara River close to borders with Ethiopia.4 Infection rates in Galabat reach 63%, but the disease is non-blinding and characterized primarily by severe localized skin manifestations, including a peculiar form of the skin discoloring called sowda.5 The third focus of onchocerciasis centers on the banks of the River Nile around the town of Abu Hamed in the Nubian desert.6 The disease here is also non-blinding and associated with severe skin manifestations. Skin microfilaria rates were originally described to reach 37%,7 ranking the Abu Hamed focus as mesoendemic based on World Health Organization (WHO) criteria.8 Molecular studies showed that the parasites from this focus differed from all of the O. volvulus isolates examined.9 In addition, cytological and molecular studies indicated that Abu Hamed S. damnosum s.l. black fly vectors were distinct from other S. damnosum subspecies.10 These studies suggested the amenability of the focus to disease elimination because of its biogeography and isolated nature.
Global efforts to control onchocerciasis were revolutionized by the introduction of ivermectin (Mectizan®) as a potent microfilaricidal agent for mass drug administration11 and its donation in 1987 by Merck for as long as needed to achieve disease control.12 APOC and the Onchocerciasis Elimination Program of the America (OEPA) subsequently adopted mass treatment with ivermectin as the sole measure for the control of morbidity and/or elimination of the disease transmission, foregoing vector control that had largely characterized earlier efforts.1 By mid-2012, semiannual mass treatments with ivermectin had eliminated or interrupted transmission of the disease in 10 of 13 foci of onchocerciasis in the Americas.13 In Africa, recent reports have shown that long-term annual or semiannual mass treatment with ivermectin may have eliminated the disease in foci in Mali and Senegal, West Africa.14,15
In this article, we present results from the 2011 assessment of the disease in the Abu Hamed focus, which has received annual and semiannual ivermectin mass treatment since 1998. The survey data presented here indicate that mass distribution with ivermectin has resulted in the interruption of onchocerciasis transmission in the Abu Hamed focus based on 2001 WHO guidelines.16
Materials and Methods
Study area.
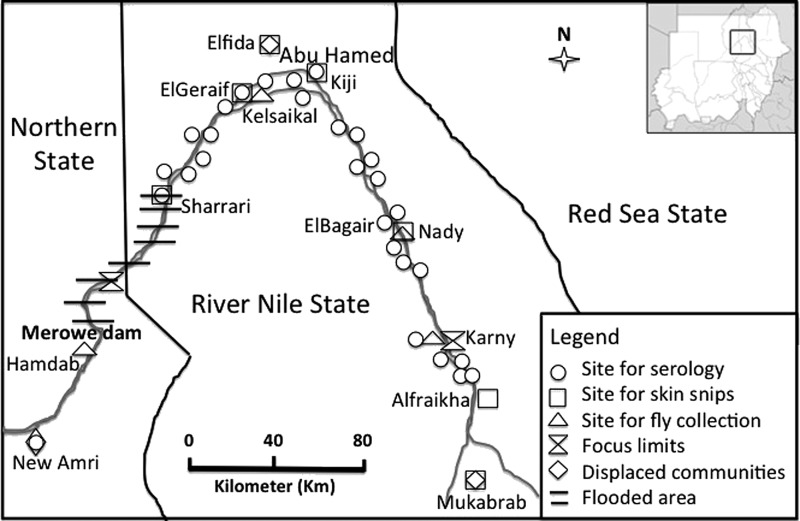
The Abu Hamed focus is the northernmost focus of onchocerciasis in the world. It is approximately 300 km long and 5 km wide. It is located between the fourth and fifth cataracts along the portion of the River Nile that runs from east to west, and it is surrounded by the Nubian subdivision of the Sahara desert in River Nile State of northern Sudan. The endemic area extends from near Sharrari in the west to Karny in the east, with the town of Abu Hamed (19°30′ N, 33°20′ E) being near the middle (Figure 1). Abu Hamad is about 500 km north of the capital Khartoum and 800 km away from the next nearest focus of onchocerciasis in Galabat.
Figure 1.
Map of the Abu Hamed focus in northern Sudan showing 2011 sites for fly collection in and around sentinel communities, sentinel sites for serology and skin snips, and the Merowe Dam west of the focus. Some of these sites were also used for assessment of the disease status in 2007.
Treatment activities.
Onchocerciasis control activities in Abu Hamed based on ivermectin mass treatments began in 1998 after Rapid Epidemiological Mapping of Onchocerciasis (REMO). Annual treatment was deployed using the APOC community-directed treatment with ivermectin (CDTI) strategy. In 2006, the policy of the government of Sudan was changed from one of morbidity control to one of complete elimination of the parasite from the Abu Hamed focus. With that change, treatments were expanded to the hypoendemic fringes of the Abu Hamed focus, and the frequency of treatment moved from annual to semiannual (e.g., every 6 months). The population eligible for treatment (targeted population) was determined through population census in every affected community using household registers. The goal was to treat at least 90% of the ultimate treatment goal (UTG) in the annual treatments and every round of treatment when semiannual treatments were provided. The UTG is the sum of all persons eligible for treatment among the at-risk population living in all at-risk communities in the onchocerciasis-endemic area.17
Historic parasitological data evaluating the status of the disease in the Abu Hamed focus were reported in 1985.7 In 2007, additional entomological, parasitological, and serological data were collected, concurrent with the onset of semiannual treatments. Entomological analysis revealed that low levels of disease transmission existed before the shift to semiannual treatment activities in the area.18 A more comprehensive follow-up assessment of entomological, serological, and parasitological data from the Abu Hamed focus was conducted in 2011.
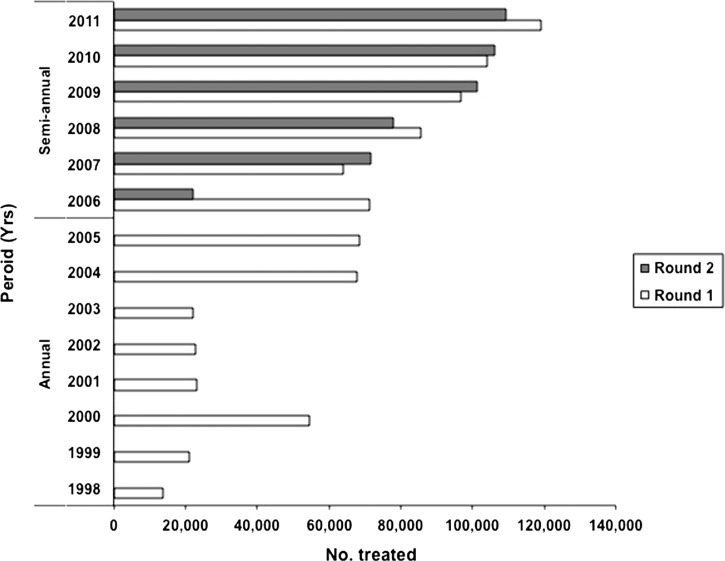
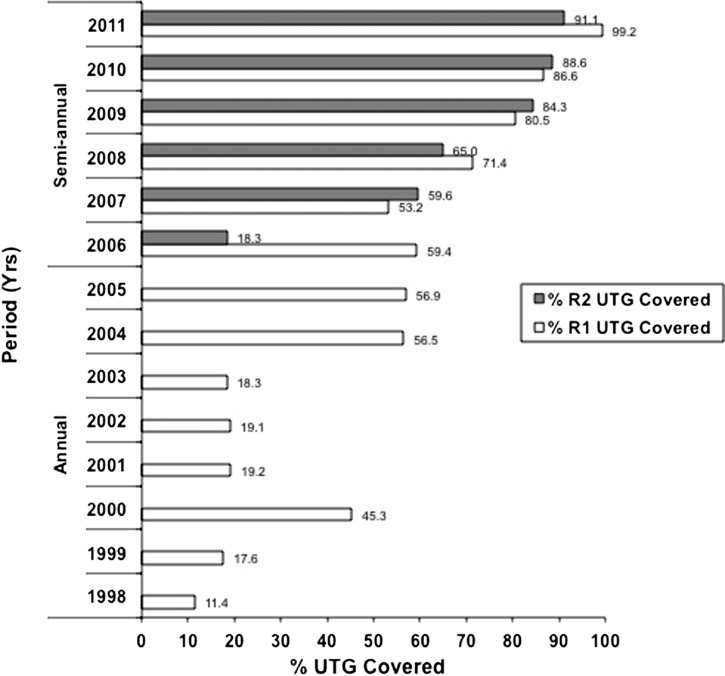
The Abu Hamed focus was expanded in 2007 to include suspected hypoendmic areas (Figure 1), resulting in an increase in the number of individuals eligible for treatment from about 61,000 in 1998 to about 120,000 by 2011 (Figure 2 ). The target population resides in 147 communities, including 18 islands. With the exception of 2000, treatment coverage in the first 6 years of the program was low, but this coverage improved in 2004 to 2006. The shift to semiannual treatment, along with enhanced health education and community mobilization, resulted in coverage exceeding 90% of the UTG by 2011 (Figure 3 ).
Figure 2.
History of mass treatments with ivermectin in the Abu Hamed focus from 1998 to 2011. In the semiannual treatment scheme that began in 2006, round 1 refers to ivermectin treatments provided in the first semester (January to June), and round 2 refers to treatments provided in the second semester (July to December).
Figure 3.
History of treatment coverage with ivermectin based on percentage of annual UTG from 1998 to 2005 and semiannual UTG (rounds 1 and 2) from 2006 to 2011 in the Abu Hamed focus from 1998 to 2011.
Population displacement.
A new hydroelectric dam was built at the fourth cataract of the River Nile near the town of Merowe about 30 km from the western end of the Abu Hamed focus (Figure 1). After it became operational in 2009, the Merowe Dam reservoir flooded some endemic villages, islands, and breeding sites nearby. Affected communities were moved to alternative locations within and nearby the focus. Examples of new settlements of the displaced populations are Elfida communities near the town of Abu Hamed, Mukabrab communities about 90 km south of the focus, and New Amri communities about 75 km downstream (southwest) of the Dam (Figure 1). These displaced communities were covered by regular semiannual CDTI activities in their new locations. Selected displaced communities were also included in the assessment of the disease status in 2011.
Entomologic studies.
S. damnosum s.l. black flies were collected from four sites in and around sentinel villages of the Abu Hamed focus from December of 2010 to November of 2011. Standard procedures19,20 were used to collect flies from (1) Kelsaikal village in Mograt island (19°29′ N, 33°14′ E, population = 932), (2) Nady (18°44′ N, 33°39′ E, population = 1,168),18 (3) Karni (18°25′ N, 33°45′ E, population = 978) at the southern end of the focus, and (4) Hamdab island (18°40′ N, 32°03′ E, population = 679) downstream of the Merowe Dam to the west of the focus (Figure 1). Collection sites in and around Kelsaikal village in Mograt island and Nady were originally used for baseline entomological studies in 2007.18 The Hamdab collection site was selected to investigate whether black fly breeding and transmission had been established in the spillways of the newly built dam. Flies were collected hourly 5 days per month from 6 am to 7 pm, and the number of flies collected per hour was recorded. Fly collectors were trained volunteers from the targeted communities who received regular semiannual ivermectin treatments. Flies from each sentinel village were preserved in isopropanol and brought to the Ministry of Health (MOH) molecular laboratory in Khartoum for processing.
The flies were divided into pools of 100 or less by site and date of collection and maintained in isopropanol until analysis. Heads of each black fly pool were first separated from bodies, and DNA was then extracted from pools of fly heads according to published procedures,18,21,22 including the use of a 96-well purification module (Qiagen Inc., Valencia, CA) to streamline the DNA extraction process. A sham extraction was included with every 11 extractions to serve as contamination control. Purified DNA was used as a template in the Onchocerca-specific O-150 polymerase chain reaction (PCR) followed by PCR-based enzyme-linked immunosorbent assay (ELISA) using probes specific for the parasite as previously reported.18,21–23 A sample was considered putatively positive if it had an ELISA score at or above the cutoff (i.e., the mean of 10 internal negative controls plus 3 SD). Only samples that were positive after a second independent PCR and ELISA were reported as confirmed positive.
Results were interpreted with respect to the 2001 WHO criteria,16 which assume that the threshold for interruption of transmission is below an infective rate in flies of 1/1,000 (0.1% in parous flies) or less than 1/2000 by PCR (0.05%). The latter assumes 50% nulliparous rate.14,24
Serologic studies.
The Ov16 ELISA assay uses a recombinant antigen of O. volvulus25 to measure prevalence of immunoglobulin G4 (IgG4) antibodies. Blood spots for the Ov16 assay were collected in July of 2007 and from December of 2010 to January of 2011. In both studies, sterile techniques were used to collect finger prick blood onto the 5 × 5-cm area of Whatman 2 filter paper. The saturated blood spots were dried, individually wrapped, and transported at 4°C to the laboratory, where they were stored at −20°C until further analysis. In the laboratory, sera were eluted from filter paper punches and used in standard ELISA assay as previously described.24 We used a standard curve on each ELISA plate to identify positive samples and permit comparisons between plates and over time. The cutoff was chosen as 40 arbitrary units as previously reported.24,26 A sample was reported positive only after a second independent positive repeat from the same stored specimen.
Ov16 serologic testing to determine transmission activity should be focused on a sufficient sample of children under 10 years of age to statistically exclude a prevalence of 0.1%.14 A 2007 survey in eight sentinel communities in Abu Hamed did not meet the minimum sample size needed to exclude the 0.1% threshold required by the WHO16,24 to indicate interruption of the disease transmission. In 2011, we were able to reach the required sample size (> 3,000) by sampling school children from 40 communities, including 29 communities within the focus, 7 communities close to but beyond the focus southern limits, and 4 displaced communities in and around the focus (Figure 1) consisting of people relocated because of flooding of their original land by the Merowe Dam reservoir.
Parasitologic studies.
Sentinel villages in this study included the three communities (Kiji, ElBagair, and ElGeraif) originally reported in the mid 1980s7,27 as having the highest endemicity in the focus. These were reinvestigated in 2007 and 2011 for the prevalence of O. volvulus skin microfilariae (mf), palpable nodules, and signs of the skin disease (onchodermatitis). Palpable nodules and onchodermatitis were used as additional indicators of infection, because the Abu Hamed focus is historically known for low infection intensity (average of < 3 mf/mg skin).7,27 In 2011, four additional communities in and around the Abu Hamed focus were assessed for parasitological indicators of the disease. These communities included three communities (Sharrari, Elfida, and Mukabrab) displaced by the Merowe Dam and relocated within and outside the focus together and another community (Alfiraikha) located about 33 km outside the southern limit of the known onchocerciasis focus that had not been covered by CDTI activities (Figure 1).
The disease infection rates in the villages were measured primarily in adults over 20 years old who lived in the area for at least 10 years. Parents consistently refused to assent to skin snip examinations of their children, although some children were included in the screening for skin disease; one sowda case in 2007 was found in a child of about 13 years old. For skin mf, two skin snips were taken from both hips of each participant using a corneoscleral biopsy punch. The snips were placed in sterile normal saline in a 96-well plate maintained at ambient temperature and examined microscopically for emerging O. volvulus mf after 2 hours and again after 24 hours. Palpable nodules and signs of O. volvulus skin disease (onchodermatitis) were determined as previously described.28–30
Data analysis.
Data were initially recorded on paper forms and then transferred into an Excel database. Black fly O-150 data were analyzed using Poolscreen algorithm Version 2.0,31 and the prevalence of infective larvae in the fly population, annual transmission potential (ATP), annual biting rate (ABR), and their associated 95% confidence intervals (95% CIs) were estimated using the OEPA version of the PoolScreen algorithm (http://www.soph.uab.edu/bst/directory?facname=3190). PoolScreen analysis of ABR and ATP required the hourly number of flies collected in the 5 catching days each month, the length of the transmission season (12 months), the latitudes of the fly collection sites, and the mean number of L3 per infective fly. This number was estimated to be one, which has been found to be the case in areas subject to effective control.24,32 Peak biting rates for fly collection sites were estimated by analyzing monthly data.
Ethical review.
Communities where the studies were performed were informed of the purpose and procedures of the screening process. Identification data (including name, age, sex, and length of residence in the village) were collected from all individuals enrolled in the study and kept confidential in locked files. Ethical approvals were obtained from the Federal MOH, the State MOH, and community leaders, and verbal informed consents were obtained from all eligible individuals and headmasters. The Emory University Institutional Review Board (eIRB–11 438) reviewed the study protocol and considered it as a non-research routine program evaluation.
Results
Treatment.
Figure 2 shows the increasing numbers of individuals treated with ivermectin in the Abu Hamed focus between 1998 and 2011. Annual treatment was given for 8 years followed by 6 years of semiannual CDTI administered treatment. Treatment coverage was low in the first six treatment rounds, ranging only between 11% and 19%, of the UTG, with the exception the year 2000, when 45% coverage was achieved (Figure 3). Treatment coverage improved to just under 60% between 2004 and 2006. Although in the first year of semiannual treatment, the second round coverage was under 20%, the shift to semiannual treatment overall later showed marked improved coverage, reaching more than 90% of the eligible population by 2011 (Figure 3).
Entomologic studies.
From December of 2010 to November of 2011, 17,537 black flies were collected over 2,340 hours from Kelsaikal in Mograt Island and Nady and Karny sites in and around sentinel communities near breeding sites within the Abu Hamed focus. The biting season was found to extend from November to May and peaked in January and February with rates of 22.4/hour in Nady and 77.6/hour in Mograt Island. No black flies were found in the Hamdab site in the spillway downstream of the newly built Merowe Dam (Table 1). The latter site is far away from sentinel communities (Figure 1).
Table 1.
Prevalence of O. volvulus-infective black flies in Abu Hamed focus from December of 2010 to November of 2011 based on O-150 pool screening
| Community | No. of flies | L3 infection per 2,000 flies | 95% CI |
|---|---|---|---|
| Kelsaikal | 9,008 | 0 | 0–0.43* |
| Nady | 5,173 | 0 | 0–0.74* |
| Karny | 3,178 | 0 | 0–1.2 |
| Hamdab | 0 | N/A | N/A |
| Abu Hamed total | 17,359 | 0 | 0–0.23* |
N/A = not applicable.
Below the 1/2,000 WHO/TDR indicator of transmission interruption.
The collected black flies, analyzed in 176 pools by O-150 PCR screening analysis, showed no evidence of O. volvulus L3 larvae (Table 1), thus indicating point estimate of zero prevalence of infective black flies with 95% CI upper limit (UL) of 0.23/2,000 flies. This rate is well below the WHO threshold of 1/2,000 for transmission interruption. Similarly, the point estimate of the ATP was calculated as zero, with a UL of the 95% CI of 1.99 L3/person per year.
Serologic studies.
Blood spots were collected from 6,756 school children ≤ 10 years in 2011 (Table 2) . They were obtained from 3,955 school children from 29 communities throughout the focus and 1,418 children from four communities displaced by the Merowe Dam reservoir in 2009, including the New Amri community that was relocated downstream of the dam (Figure 1). In addition, samples were obtained from 1,383 school children ≤ 10 years residing in seven communities just outside of the southern border of the focus. No children were found positive for Ov16 IgG4 antibodies in the assay (95% CI UL = 0.03%), well below the WHO threshold of 0.1% needed to declare transmission interruption.
Table 2.
Prevalence of O. volvulus Ov16 IgG4 antibodies in school children ≤ 10 years in Abu Hamed focus in 2011
| Location (no. of communities) | No. examined | No. positive (prevalence %) |
|---|---|---|
| Abu Hamed focus (29) | 3,955 | 0 (0)* |
| Beyond focus limits (7) | 1,383 | 0 (0) |
| Displaced (4) | 1,418 | 0 (0) |
| Total | 6,756 | 0 (0)* |
95% CI excludes 0.1%.
No O. volvulus antibodies were found in 349 school children ≤ 10 years old from eight communities screened in 2007. However, the 2007 serologic study did not meet the required sample size necessary to statistically exclude 0.1%.
Parasitologic studies.
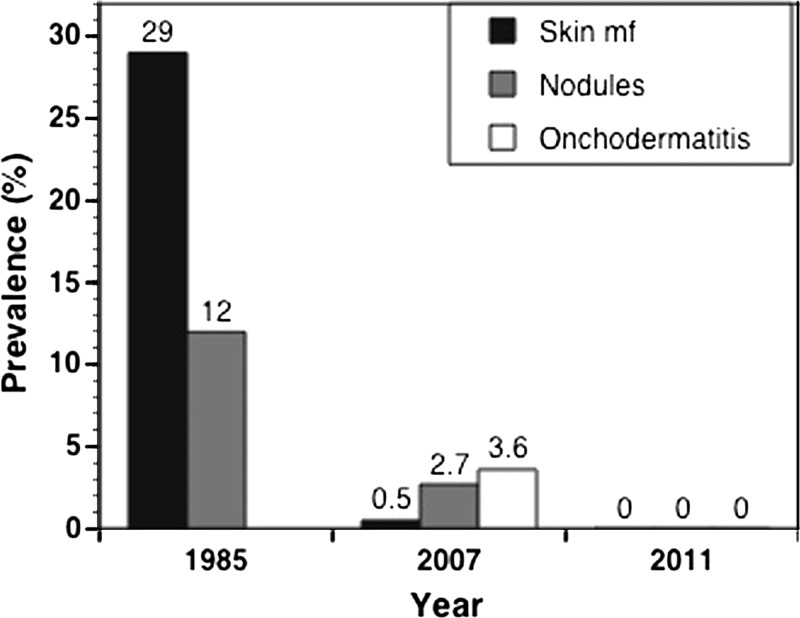
Figure 4 shows the history of the overall disease infection rates in the Abu Hamed focus. A skin mf prevalence of 29% and palpable nodules of 12% were reported among 208 outpatient clinic visitors from the Abu Hamed focus in 19857 (Figure 4). In comparison, the 2007 assessment showed O. volvulus skin mf in 2 adults of 442 adult and child residents (0.45%) of three sentinel communities in the Abu Hamed focus. The infected individuals were from the same community, indicating 1.6% infection rate compared with zero infection rate in the other two communities. In addition, 13 and 17 individuals of 474 adults from the same communities showed palpable nodules and signs of onchodermatitis, indicating a prevalence of 3.6% and 2.7%, respectively, in the same year (Figure 4).
Figure 4.
History of the prevalence of onchocerciasis in the Abu Hamed focus. Overall prevalence of the disease based on skin mf, presence of palpable nodules, and onchodermatitis; 1985 studies7 did not report prevalence for onchodermatitis.
The 2011 assessment of disease status showed no O. volvulus skin mf, palpable nodules, or onchodermatitis in 536 individuals from seven communities in and around the Abu Hamed focus (Figure 1). This result indicates a point estimate of zero infection (95% CI UL = 0.75%) in the Abu Hamed focus, which is below the WHO threshold for transmission interruption of < 1%.
Discussion
The 2011 evaluations in Abu Hamed indicated that onchocerciasis transmission has been interrupted in this northernmost onchocerciasis focus in the world. Although infective rates in vector black flies were below 1 in 2,000 when summed for the three sentinel collecting sites, Karny remained above the threshold (CI UL of 1.2), most likely because of the relatively small sample size. O. volvulus exposure among children born after the initiation of annual mass ivermectin treatment, as measured by the prevalence of Ov16 antibodies, was below 0.1%. There were no positive skin snips or evidence of palpable nodules or skin disease in adults.
Our 2007 data, although less comprehensive than the data from 2011, nevertheless indicated a low prevalence of skin mf, palpable nodules, and skin manifestations among adults and children after 9 years of annual ivermectin treatment in Abu Hamed. In addition, we reported low levels of transmission based on the presence of L3 larvae in black flies that same year.18 These reports indicated that the annual treatment regimen had achieved a high degree of onchocerciasis control in the area, with a considerable reduction in prevalence of dermal mf from the 34% to 37% reported in the mid-1980s,7,27 but had not yet interrupted transmission. It is important to note, however, that coverage during the years where annual treatment was offered was inadequate (less than 65%).
By the 2011 assessment, conducted in the fifth year after launching two times per year treatment, transmission in Abu Hamed had been interrupted. In this survey, sample sizes were expanded to meet or exceed the minimum requirements of the 2001 WHO guidelines. Statistical testing applied to the entomological and serological survey data excluded the UL transmission thresholds of 0.05% flies carrying infective larvae, for the ATP and 0.1% exposure in children. Mathematical modeling and field studies estimated threshold ATP for interruption of the disease transmission between 5 and 54,24,33,34 7.6 and 18,22,24,32 and 5 and 20 L3/person per year.24,35,36 The reported 95% CI UL ATP of 1.99 L3/person per year in Abu Hamed is well below all of these reported limits. Although the number of communities tested for mf was small, the data also satisfied the parasitological requirements of mf prevalence < 1% in 90% of sample villages and a prevalence < 5% in 100% of sample villages.14
In light of the documented effects of multiple semiannual ivermectin treatment on the parasite and its transmission,37 we believe that providing semiannual ivermectin treatment for 5 years was important in hastening the interruption of transmission in the Abu Hamed focus, particularly shown by the entomological changes in PCR positivity between 2007 and 2011.
The isolated nature of the Abu Hamed focus might have contributed to the interruption of transmission. The extreme geographic isolation of this focus in the Nubian Desert prevents vector migration and limits its breeding to between the fourth and fifth cataracts of the River Nile.10 Because of the riverine nature of the narrow endemic zone, it has always been relatively easy to identify areas of potential transmission (i.e., villages next to the Nile). Thus, we feel that the four sentinel sites selected for fly collections are representative of the whole endemic area. In addition, the local communities are rooted to their stretch of fertile land along the River Nile, and there is negligible human migration into or out of the focus. These factors also contribute to the parasites' reproductive isolation.9 Our data showed an absence of the disease and its transmission beyond the southern limit of the focus.
The establishment of the Merowe Dam close to the western limit of the focus in 2009 presented some challenges to the elimination program. The dam's artificial reservoir flooded a significant area of the River Nile at the western edge of the focus, rendering it unsuitable for vector breeding. However, it also flooded and displaced several island and shore communities to new locations within or around the focus, including a community that was displaced downstream of the Merowe Dam in New Armi. In addition, the dam created a new environment potentially suitable for black fly breeding sites, posing the threat of establishment of disease transmission downstream of the dam in Northern State. It was essential to extend semiannual treatments to all displaced communities and include them in the disease assessment and monitoring activities because of the potential colonization of the spillway downstream of the dam by vector black flies. Our data suggest that infections are not present in the displaced communities and that no black fly breeding sites were established. However, this major population and environmental change downstream of the dam needs to be followed during the post-treatment surveillance period.
In Africa, the first evidence that long-term ivermectin treatment alone might interrupt and eliminate onchocerciasis in some foci came from West Africa.14,15 An additional report from Nigeria showed absence of mf in skin after years of annual treatment but provided no entomological data as required by the WHO criteria.38 The Abu Hamed focus in Sudan and the Wadelai focus in Uganda26 are the first endemic regions reporting interruption of O. volvulus transmission using ivermectin alone in East Africa. However, interruption of the disease transmission in Wadelai might have been partially or completely because of the disappearance of the vector resulting from environmental change.26 In contrast, the S. damnosum s.l. vector in Abu Hamed still exhibits high biting rates, suggesting that the Abu Hamed focus is the first example in East Africa where expanded and intensified ivermectin mass treatment and health education alone, without vector control, could interrupt and possibly eliminate the disease.
The 2011 assessment data presented here were reviewed at a conference of partners in Khartoum in May of 2012. As a result of this review, the Sudan Federal MOH suspended ivermectin treatments in Abu Hamed and launched post-treatment surveillance (PTS) activities. The PTS phase will last for 3 years15,16,24 and is expected to provide the evidence needed to declare elimination of onchocerciasis from Abu Hamed. The success of the PTS surveys will provide the final demonstration of the operational feasibility of the elimination of this parasite, using ivermectin alone, from Africa.
ACKNOWLEDGMENTS
The authors thank the affected communities for their cooperation and support. The Lions Clubs SightFirst Program and The Carter Center funded treatment activities from 2004 to 2011 as well as the fieldwork and laboratory analysis associated with monitoring and evaluation activities. The African Program for Onchocerciasis Control (APOC) supported the ivermectin treatment program in Abu Hamed from 1998 to 2003. Ivermectin (Mectizan®) was donated by Merck.
Footnotes
Authors' addresses: Tarig B. Higazi, Department of Biological Sciences, Ohio University, Zanesville, OH, E-mail: higazi@ohio.edu. Isam M. A. Zarroug, Entomology, Ministry of Health, Khartoum, Sudan, E-mail: izarroug@yahoo.com. Hanan A. Mohamed, Wigdan A. ElMubark, and Tong Chor M. Deran, Ministry of Health, Khartoum, Sudan, E-mails: hanan.mohamed@gmail.com, wigdanelmubark@hotmail.com, and tong_schewitaak@yahoo.co.uk. Nabil Aziz, The Carter Center—Sudan, Khartoum, Sudan, E-mail: nabilazizm@hotmail.com. Moses Katabarwa, The Carter Center, Emory University, Atlanta, GA, E-mail: mkataba@emory.edu. Hassan K. Hassan and Thomas R. Unnasch, Global Health, University of South Florida, Tampa, FL, E-mails: hhassan@health.usf.edu and tunnasch@health.usf.edu. Charles D. Mackenzie, Pathobiology, Michigan State University, East Lansing, MI, E-mail: mackenz8@msu.edu. Frank Richards, Health Programs, The Carter Center, Atlanta, GA, E-mail: frich01@emory.edu. Kamal Hashim, Eye Hospital, Ministry of Health, Khartoum, Sudan, E-mail: kamalbinnawi@yahoo.com.
References
- 1.Crump A, Morel CM, Omura S. The onchocerciasis chronicle: from the beginning to the end? Trends Parasitol. 2012;28:208–288. doi: 10.1016/j.pt.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization African Programme for Onchocerciasis Control: meeting of national onchocerciasis task forces. Wkly Epidemiol Rec. 2011;86:541–549. [PubMed] [Google Scholar]
- 3.World Health Organization Drancunculiasis and onchocerciasis. Wkly Epidemiol Rec. 1997;72:297–301. [PubMed] [Google Scholar]
- 4.Abdalla R, Baker EA. A new focus of onchoceriasis in the Sudan. Trop Geogr Med. 1975;27:365–370. [PubMed] [Google Scholar]
- 5.Ghalib H, Mackenzie C, Williams J, Elsheikh H, Kron M. Severe onchocercal dermatitis in Ethiopian border region of Sudan. Ann Trop Med Parasitol. 1987;81:405–419. doi: 10.1080/00034983.1987.11812138. [DOI] [PubMed] [Google Scholar]
- 6.Morgan H. Onchocerciasis in northern Sudan. J Trop Med Hyg. 1958;61:145–147. [PubMed] [Google Scholar]
- 7.Williams JF, Abu Yousif AH, Ballard M, Awad R, el Tayeb M, Rasheed M. Onchocerciasis in Sudan: the Abu Hamed focus. Trans R Soc Trop Med Hyg. 1985a;79:464–468. doi: 10.1016/0035-9203(85)90066-5. [DOI] [PubMed] [Google Scholar]
- 8.Prost A, Hervouet JP, Thylefors B. Epidemiologic status of onchocerciasis. Bull World Health Organ. 1979;57:655–662. [PMC free article] [PubMed] [Google Scholar]
- 9.Higazi TB, Katholi CR, Mahmoud BM, Baraka OZ, Mukhtar MM, Qubati YA, Unnasch TR. Onchocerca volvulus: genetic diversity of parasite isolates from Sudan. Exp Parasitol. 2001;97:24–34. doi: 10.1006/expr.2000.4589. [DOI] [PubMed] [Google Scholar]
- 10.Higazi TB, Boakye DA, Wilson MD, Mahmoud BM, Baraka OZ, Mukhtar MM, Unnasch TR. Cytotaxonomic and molecular analysis of Simulium (Edwardsellum) damnosum sensu lato (Diptera: Simuliidae) from Abu Hamed, Sudan. J Med Entomol. 2000;37:547–553. doi: 10.1603/0022-2585-37.4.547. [DOI] [PubMed] [Google Scholar]
- 11.Greene BM, Taylor HR, Cupp EW, Murphy RP, White AT, Aziz MA, Schulz-Key H, D'Anna SA, Newland HS, Goldschmidt LP, Auer C, Hanson AP, Freeman SV, Reber EW, Williams PN. Controlled comparison of ivermectin and diethylcarbamazine in treatment of human onchocerciasis. N Engl J Med. 1985;313:133–138. doi: 10.1056/NEJM198507183130301. [DOI] [PubMed] [Google Scholar]
- 12.Sturchio JL. The case of ivermectin: lessons and implications for improving access to care and treatment in developing countries. Community Eye Health. 2001;14:22–23. [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Progress towards eliminating onchocerciasis in the WHO Region of the Americas in 2011: interruption of transmission in Guatemala and Mexico. Wkly Epidemiol Rec. 2012;87:309–316. [PubMed] [Google Scholar]
- 14.Diawara L, Traoré MO, Badji A, Bissan Y, Doumbia K, Goita SF, Konaté L, Mounkoro K, Sarr MD, Seck AF, Toé L, Tourée S, Remme JH. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLoS Negl Trop Dis. 2009;3:e497. doi: 10.1371/journal.pntd.0000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troare MO, Sarr MD, Badji A, Bissan Y, Diawara L, Doumbia K, Goita SF, Konate L, Mounkoro A, Sek AF, Toe L, Toure S, Remme JHF. Proof-of-principle of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: final results of a study in Mali and Senegal. PLoS Negl Trop Dis. 2012;6:1–14. doi: 10.1371/journal.pntd.0001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . Certification of Elimination of Human Onchocerciasis: Criteria and Procedures. Geneva: World Health Organization; 2001. pp. 1–36. [Google Scholar]
- 17.Richards FO, Miri ES, Katabarwa M, Eyamba A, Sauerbrey M, Zea-Flores G, Korve K, Mathai W, Homeida MA, Mueller I, Hilyer E, Hopkins DR. The Carter Center's assistance to river blindness control programs: establishing treatment objectives and goals for monitoring ivermectin delivery systems on two continents. Am J Trop Med Hyg. 2011;65:108–114. doi: 10.4269/ajtmh.2001.65.108. [DOI] [PubMed] [Google Scholar]
- 18.Higazi TB, Zarroug I, Mohamed HA, Mohamed WA, Deran TC, Aziz N, Katabarwa M, Hassan HK, Unnasch TR, Mackenzie CD, Richards F. Polymerase chain reaction pool screening used to compare prevalence of infective black flies in two onchocerciasis foci in northern Sudan. Am J Trop Med Hyg. 2011;84:753–756. doi: 10.4269/ajtmh.2011.11-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh J. Sampling Simuliid black flies. In: Service AY, editor. Pest and Vector Management in the Tropics. London, UK: Longman; 1983. pp. 93–99. [Google Scholar]
- 20.Rodríguez-Pérez MA, Lilley BG, Domínguez-Vázquez A, Segura-Arenas R, Lizarazo-Ortega C, Mendoza-Herrera A, Reyes-Villanueva F, Unnasch TR. Polymerase chain reaction monitoring of transmission of Onchocerca volvulus in two endemic states in Mexico. Am J Trop Med Hyg. 2004;70:38–45. [PubMed] [Google Scholar]
- 21.Rodríguez-Pérez MA, Katholi CR, Hassan HK, Unnasch TR. Large-scale entomologic assessment of Onchocerca volvulus transmission by poolscreen PCR in Mexico. Am J Trop Med Hyg. 2006;74:1026–1033. [PubMed] [Google Scholar]
- 22.Rodríguez-Pérez MA, Unnasch TR, Domínguez-Vázquez A, Morales-Castro AL, Richards F, Jr, Peña-Flores GP, Orozco-Algarra ME, Prado-Velasco G. Lack of active Onchocerca volvulus transmission in the northern Chiapas focus of Mexico. Am J Trop Med Hyg. 2010;83:15–20. doi: 10.4269/ajtmh.2010.09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meredith SE, Lando G, Gbakima AA, Zimmerman PA, Unnasch TR. Onchocerca volvulus: application of the polymerase chain reaction to identification and strain differentiation of the parasite. Exp Parasitol. 1991;3:335–344. doi: 10.1016/0014-4894(91)90105-6. [DOI] [PubMed] [Google Scholar]
- 24.Lindblade KA, Arana B, Zea-Flores G, Rizzo N, Porter CH, Dominguez A, Cruz-Ortiz N, Unnasch TR, Punkosdy GA, Richards J, Sauerbrey M, Castro J, Catú E, Oliva O, Richards FO., Jr Elimination of Onchocerca volvulus transmission in the Santa Rosa focus of Guatemala. Am J Trop Med Hyg. 2007;77:334–341. [PubMed] [Google Scholar]
- 25.Lobos E, Weiss N, Karam M, Taylor HR, Ottesen EA, Nutman TB. An immunogenic Onchocerca volvulus antigen: a specific and early marker of infection. Science. 1991;25:1603–1605. doi: 10.1126/science.2011741. [DOI] [PubMed] [Google Scholar]
- 26.Katabarwa MN, Walsh F, Habomugisha P, Lakwo TL, Agunyo S, Oguttu DW, Unnasch TR, Unoba D, Byamukama E, Tukesiga E, Ndyomugyenyi R, Richards FO. Transmission of onchocerciasis in Wadelai focus of northwestern Uganda has been interrupted and the disease eliminated. J Parasitol Res. 2012;2012:1–7. doi: 10.1155/2012/748540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams JF, Mackenzie CD, Dawood M. Current distribution of onchocerciasis in Sudan. Sudan Med J. 1985b;21:9–18. [Google Scholar]
- 28.Mackenzie CD, Williams JF, O'Day J, Flockhart HA, Ghalal I, Sisley BM, El Sheikh H. Onchocerciasis in southern Sudan: parasitological and clinical characteristics in the Bahr El Ghazal Province. Am J Trop Med Hyg. 1987;36:379–382. doi: 10.4269/ajtmh.1987.36.371. [DOI] [PubMed] [Google Scholar]
- 29.Murdoch ME, Hay RJ, Mackenzie CD, William JF, Ghalib HW, Cousens S, Abiose A, Jones B. A clinical classification and grading system of the cutaneous changes in onchocerciasis. Br J Dermatol. 1993;129:260–269. doi: 10.1111/j.1365-2133.1993.tb11844.x. [DOI] [PubMed] [Google Scholar]
- 30.Okoye IC, Onwuliri CO. Epidemiology and psycho-social aspects of onchocercal skin diseases in northeastern Nigeria. Filaria J. 2007;6:15. doi: 10.1186/1475-2883-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katholi CR, Unnasch TR. Important experimental parameters for determining infection rates in arthropod vectors using pool screening approaches. Am J Trop Med Hyg. 2006;74:779–785. [PubMed] [Google Scholar]
- 32.Porter CH, Collins RC, Brandling-Bennett AD. Vector density, parasite prevalence, and transmission of Onchocerca volvulus in Guatemala. Am J Trop Med Hyg. 1988;39:567–574. doi: 10.4269/ajtmh.1988.39.567. [DOI] [PubMed] [Google Scholar]
- 33.Wada Y. Theoretical approach to the epidemiology of onchocerciasis in Guatemala. Jpn J Med Sci Biol. 1982;35:183–196. doi: 10.7883/yoken1952.35.183. [DOI] [PubMed] [Google Scholar]
- 34.Basanez MG, Collins RC, Porter CH, Little MP, Brandling-Bennett D. Transmission intensity and the patterns of Onchocerca volvulus infection in human communities. Am J Trop Med Hyg. 2002;67:669–679. doi: 10.4269/ajtmh.2002.67.669. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Pérez MA, Lutzow-Steiner MA, Segura-Cabrera A, Lizarazo-Ortega C, Domínguez-Vázquez A, Sauerbrey M, Richards F, Jr, Unnasch TR, Hassan HK, Hernández-Hernández R. Rapid suppression of Onchocerca volvulus transmission in two communities of the Southern Chiapas focus, Mexico achieved by quarterly treatments with Mectizan. Am J Trop Med Hyg. 2008;79:239–244. [PMC free article] [PubMed] [Google Scholar]
- 36.Program Coordinating Committee and OEPA Staff Guide to detecting a potential recrudescence of onchocerciasis during the posttreatment surveillance period: the American paradigm. Res Rep Trop Med. 2012;3:21–33. doi: 10.2147/RRTM.S30482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cupp EW, Sauerbrey M, Richards F. Elimination of human onchocerciasis: history of progress and current feasibility using ivermectin (Mectizan®) monotherapy. Acta Trop. 2011;120S:100S–108S. doi: 10.1016/j.actatropica.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Tekle AH, Elhassan E, Isiyaku S, Amazigo UV, Bush S, Noma M, Cousens S, Abiose A, Remme JH. Impact of long-term treatment of onchocerciasis with ivermectin in Kaduna State, Nigeria: first evidence of the potential for elimination in the operational area of the African Programme for Onchocerciasis Control. Parasit Vectors. 2012;5:28. doi: 10.1186/1756-3305-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]