Abstract
Matrix metalloproteinase (MMPs) is the extracellular zinc-dependent endopeptidase and is secreted for degrading extracellular matrix molecules of host tissues. A cDNA encoding MMP-like protein of Gnathostoma spinigerum larvae was amplified by reverse transcription-polymerase chain reaction, and was cloned into a prokaryotic expression vector, and expressed in Escherichia coli. Total immunoglobulin G class (total IgG) antibody responses to the recombinant MMP-like protein were analyzed by immunoblot diagnosis of human gnathostomiasis. Serum samples from proven and clinically suspected cases of gnathostomiasis, other parasitic diseases patients, and from healthy volunteers were tested. The immunoblotting gave high sensitivity (100%) and specificity (94.7%). Positive and negative predictive values were 85.4% and 100%, respectively. Recombinant MMP-like protein can be used as a diagnostic antigen and potentially replace native parasite antigens to develop a gnathostomiasis diagnostic kit.
Introduction
Human gnathostomiasis is an important food-borne parasitic zoonosis caused by the spirurid nematode Gnathostoma spp. and the disease is endemic in Asia and the Americas,1–4 and in returned travelers who had visited the endemic areas of this harmful parasite.5,6 Humans acquire infection by consuming raw or undercooked meat, i.e., freshwater fish, frogs, chicken, etc., which harbor Gnathostoma advanced third-stage larvae (AL3). Gnathostoma spinigerum is a causative agent mainly in Asian countries, i.e., Thailand, Japan, Vietnam, etc.2,7–9 The Gnathostoma AL3 migrates into the subcutaneous tissue and causes intermittent migratory swelling. Sometimes the worm migrates to vital organs, i.e., brain, eye, etc., producing severe pathologic signs and symptoms that can lead to harmful problems and death.6,10–12 Definitive diagnosis for human gnathostomiasis can be made by detecting the migrating larvae from the human body. Because direct detection of the parasite is difficult and often unsuccessful, diagnosis of gnathostomiasis is practically made by relying upon clinical features, history of eating parasite-contaminated foods, blood eosinophilia, and serological outcomes, i.e., enzyme-linked immunosorbent assays13–16 or immunoblotting using Gnathostoma AL3 extract, including an antigenic peptide with an approximate molecular mass of 24 kDa17–19 and below 27–29 kDa.20 Two-dimensional gel electrophoresis (2-DE) and immunoblotting revealed that G. spinigerum AL3 antigenic spots with an approximate molecular mass of 23–25 kDa and pI of 8.3–8.5 revealed a high potential for the serodiagnosis of human gnathostomiasis spinigerum.21 The amino acid sequence of these antigenic spots was determined by liquid chromatography tandem mass spectrometry (LC/MS-MS) and the LC/MS-MS spectra22 and one of the peptide sequences showed high similarity with a matrix metalloproteinase (MMP)-like protein of G. spinigerum database (GenBank accession no. AAF82802).23
Cloning and expression of G. spinigerum genes such as MMP-like protein,23 cathepsin L-like cysteine protease,24 and cyclophilin protein25 have been reported. However, the diagnostic values of those recombinant proteins for human gnathostomiasis have not been validated. In this study, we produced a recombinant MMP-like protein of G. spinigerum and evaluated its sensitivity and specificity in immunodiagnosis for human gnathostomiasis. We selected MMP-like protein because its molecular mass and pI corresponded well with the 2-DE immunoreactive spots detected by the confirmed human gnathostomiasis sera.22 The goal of this study is to setup a stable mass-production system for the standardized immunodiagnostic kit with recombinant G. spinigerum MMP-like protein antigen.
Materials and Methods
Human sera.
All serum samples were supplied by the serum bank of the Faculty of Medicine, Khon Kaen University. Serum samples consisted of three groups: 1) Negative control group, which included samples from healthy adult volunteers who were free from any intestinal parasitic infection and checked by stool examination by the formalin ethyl acetate concentration technique26 at the time of blood collection. A pooled sera from all those healthy individuals was also used as negative control for each assay. 2) Gnathostomiasis group, which included samples from parasitologically confirmed gnathostomiasis patients and from patients showing clinical symptoms of suspected cutaneous and visceral gnathostomiasis,2,27,28 with a history of eating food possibly contaminated with Gnathostoma larvae and were positive 24 kDa G. spinigerum antigen by immunoblotting.29 3) The third group (N = 83) consisted of serum samples from patients with other parasitic infections than gnathostomiasis. Their infections were confirmed by parasitological methods except that cysticercosis cases were diagnosed by a computerized tomography scan and found positive by the immunological method Table 1. Informed consent was obtained from all human adult participants and from parents or legal guardians of minors. The study protocol was approved by the Khon Kaen University Ethics Committee for Human Research (HE541293).
Table 1.
Type of human sera and immunoblotting results against the purified fusion-tagged recombinant MMP-like protein*
| Type of serum | Group | No. positive/no. total |
|---|---|---|
| Healthy control | 1 | 0/30 |
| Confirmed gnathostomiasis | 2 | 13/13 |
| Suspected gnathostomiasis | 2 | 22/22 |
| Cysticercosis | 3 | 0/8 |
| Taeniasis | 3 | 0/5 |
| Opisthorchiasis viverrini | 3 | 2/8 |
| Fascioliasis | 3 | 3/10 |
| Paragonimiasis | 3 | 0/10 |
| Angiostrongyliasis | 3 | 0/9 |
| Strongyloidiasis | 3 | 0/5 |
| Hookworm infection | 3 | 0/5 |
| Capillariasis | 3 | 1/9 |
| Ascariasis | 3 | 0/5 |
| Trichinosis | 3 | 0/9 |
MMP = matrix metalloproteinase.
Parasites, total RNA isolation, and synthesis of cDNA encoding MMP-like protein.
The G. spinigerum AL3 were collected from mice inoculated orally with early third-stage larvae recovered from copepod.30 The worms (N = 40) were then placed into RNAlater (Promega, Madison, WI). The total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) and was finally dissolved in diethylpyrocarbonate-treated deionized water and stored at −70°C unit use. Based on the DNA sequence of a G. spinigerum MMP-like protein from the published data23 (GenBank accession no. AF277294), we designed a primer pair to obtain the complete open reading frame of the MMP-like sequence. The primers used were as follows: GS-F1 5′-CAGTAAAGATGAAACTACAGAGTGTG-3′ and GS-R1 5′-GACGTTTACGGCATTGGAG-3′ (The start and stop codons are indicated in bold). A reverse transcription-polymerase chain reaction (RT-PCR) was performed using the RobusT II RT-PCR kit (Finnzymes, Espoo, Finland) according to the manufacturer's protocol. The PCR parameters were as follows: cDNA synthesis at 40°C for 60 minutes and at 94°C for 2 minutes; and then 35 cycles of 30 seconds at 94°C, 30 seconds at 55°C, and 1 minute at 72°C; and a final step at 72°C for 10 minutes. The PCR product obtained was checked by electrophoresis using 1% agarose gel, purified, and subcloned into pCR4-TOPO Vector using a TOPO TA Cloning kit (Invitrogen), and transformed into TOP10 competent cells (Invitrogen) for sequence confirmation.
DNA sequencing and analysis.
The DNA sequencing was performed using the MegaBACE 1000 DNA analysis system (GE Healthcare, Piscataway, NJ), and the sequence obtained was analyzed using software programs including BLAST (www.ncbi.nlm.nih.gov), Multalin (http://bioinfo.genotoul.fr/multalin/multalin.html), BioEdit version 7.0.9 (www.mbio.ncsu.edu/BioEdit/BioEdit.html), and Compute pI/Mw tool (http://web.expasy.org/compute_pi/).
Expression, purification, and cleavage of the recombinant MMP-like protein.
The primers carried restriction sites of EcoRI (GS-F2 5′- TGGCGTGGAATTCTATGAAAC TACAGAGTGTG -3′) and Hind III (GS-R2 5′CGGAGGAAAGCTTTTACGGC ATTGGAG -3′) (Restriction sites are indicated in bold) were designed. The PCR parameters were as follows: initial heating at 94°C for 3 minutes; and then 35 cycles of 30 seconds at 94°C, 30 seconds at 69°C, and 1 minute at 72°C; and a final step at 72°C for 10 minutes. The PCR product was subcloned into a pET-43.1(+) expression vector (Novagen, Darmstadt, Germany). The recombinant plasmids were then transformed into Escherichia coli JM 109 and the accuracy of the nucleotide sequence harbored in the bacterial clones was verified by sequencing. The plasmid DNA presenting the correct codons was used to transform in E. coli Rosetta-gami 2(DE3) expression host (Novagen). Protein expression was induced by 1 mM isopropyl-β-D-thiogalactopyranoside. Suspension of bacterial cell in a lysis buffer (50 mM Tris–HCl, pH 8.0, 5% glycerol, 50 mM NaCl, 0.5 mg/mL lysozyme) was sonicated on ice, and the recombinant protein fused with N utilization substance A (NusA)-tagged and 6-Histidine (6-His)-tagged residues, and was purified using Ni–NTA His Bind Resin (Novagen). The recombinant MMP-like protein was separated from fusion-tagged proteins by cleaving with recombinant enterokinase (rEK) (Novagen) according to the manufacturer's instructions.
Antigenicity of the purified recombinant MMP-like protein by immunoblotting.
The purified and rEK-cleaved MMP-like fusion-tagged proteins were electrophoresed on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli,31 and then transferred to a nitrocellulose membrane32 that was cut into strips for immunoblotting. The purified fusion-tagged protein was detected by a reaction with an anti-NusA mouse monoclonal antibody (Novagen), according to the manufacturer's protocol. Briefly, after blocking the membrane strips with (3%) bovine serum albumin (BSA) in phosphate buffered saline (PBS, pH 7.5) incubating with an anti- NusA antibody, and then probing with horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), the reaction was visualized with 3,3′-diaminobenzidine tetrahydrochloride (DAB) substrate. For demonstration of antigenicity of the cleaved recombinant MMP-like protein, the reaction was probed with a 1:100 diluted pooled sera of gnathostomiasis patients or pooled negative control sera (in 1% skimmed milk in PBS, pH 7.5) absorbed with E. coli lysate for 2 hours at room temperature. The membranes were washed with 1% skimmed milk in PBS, pH 7.5, containing 0.1% Tween-20 (PBST) (5 times), and incubated with goat anti-human IgG (H+L) HRP conjugate (Invitrogen) at a dilution of 1:4,000 (in 1% skimmed milk in PBST) for 2 hours at room temperature. After 5 washes with 1% skimmed milk in PBST, the strips were then developed with DAB substrate, and the reaction stopped with distilled water.
Evaluation of the purified fusion-tagged recombinant MMP-like protein as a diagnostic antigen for human gnathostomiasis.
The purified fusion-tagged recombinant MMP-like protein was electrophoresed on 10% SDS-PAGE and then electrotransferred to a nitrocellulose membrane. After blocking nonspecific binding sites with 1% skimmed milk in PBST, pH 7.5 for 30 minutes, the membrane was cut into ∼3 mm wide strips (9.8 μg protein/strip). Each strip was incubated with individual human serum samples absorbed with E. coli lysate and processed as described previously. The diagnostic parameters of sensitivity, specificity, and positive and negative predictive values were calculated as previously described.33
Results
Synthesis of the complete cDNA encoding a MMP-like protein.
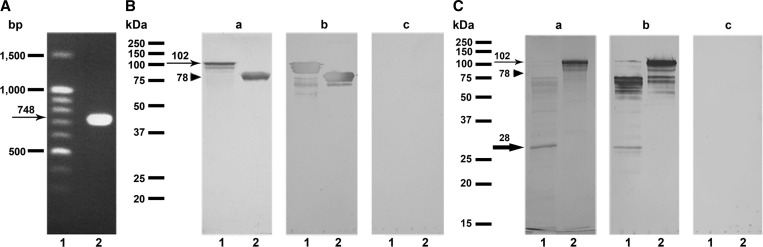
We successfully amplified cDNA encoding a MMP-like protein of G. spinigerum (Figure 1A). The gene consisted of a single open reading frame of 735 basepairs encoding 244 amino acids with a predicted molecular mass of 28 kDa and a theoretical pI of 7.8. The sequence was identical to the nucleotide sequence of a G. spinigerum MMP-like gene (GenBank accession no. AF277294).
Figure 1.
(A) Ethidium bromide stain patterns of the Gnathostoma spinigerum complete cDNA encoding a matrix metalloproteinase (MMP)-like protein on a 1.0% agarose gel. An arrow indicates band at approximate of 748 base pairs. Lane 1, DNA size markers (ZipEnzyme, Novosibirsk, Russia) and lane 2, the complete cDNA encoding a MMP-like protein amplified by reverse-transcriptase polymerase chain reaction (PCR) with gene-specific primers. (B) Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the fusion-tagged protein with and without recombinant MMP-like protein expressed in Escherichia coli. Coomassie Brilliant Blue stained (a) and immunoblotting probed with (b) and without (c) an anti- NusA antibody. Lane 1, the fusion-tagged protein containing the recombinant MMP-like protein (102 kDa, arrow) and lane 2, the fusion-tagged protein alone (78 kDa, arrow head). (C) SDS-PAGE analysis of the recombinant MMP-like protein cleaved with recombinant enterokinase (rEK) and the recombinant fused-tagged MMP-like proteins. Coomassie Brilliant Blue stained (a) and immunoblotting probed with sera from pooled gnathostomiasis patients (b) and pooled healthy negative controls (c). Lane 1, the rEK-cleaved MMP-like protein and lane 2, the fusion-tagged MMP-like recombinant protein. A thin arrow indicates expression of the fusion-tagged MMP-like protein (102 kDa). An arrow head indicates 78 kDa band of the fusion-tagged protein alone. A thick arrow indicates band of the rEK-cleaved MMP-like protein (28 kDa).
Expression, purification, cleavage, and immunocharacterization of the recombinant MMP-like protein.
A complete cDNA encoding the MMP-like protein was cloned into an expression vector and the recombinant MMP-like fusion-tagged protein was expressed in an E. coli expression system. The purified recombinant protein gave a single band in SDS-PAGE (Figure 1B, panel a). By immunoblot analysis, the fusion-tagged MMP-like protein and fusion-tagged protein alone were visualized by anti-NusA antibody at approximate molecular masses of 102 and 78 kDa, respectively (Figure 1B, panel b). The rEK-cleaved MMP-like protein has a molecular mass of ∼28 kDa (Figure 1C, panel a). The cleaved MMP-like protein showed strong positive reactivity with pooled gnathostomiasis patient serum (Figure 1C, panel b) but did not react with the pooled negative control serum (Figure 1C, panel c) by immunoblotting.
Evaluation of the diagnostic values of the purified fusion-tagged recombinant MMP-like protein.
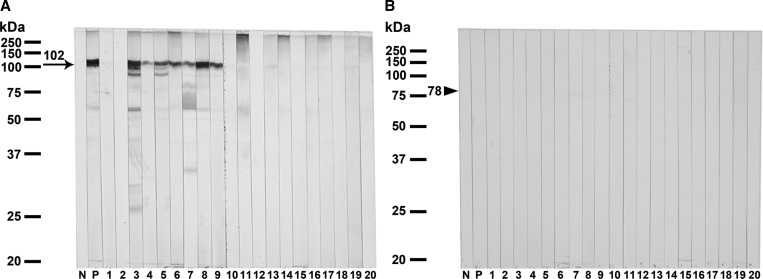
Immunoblot analysis employing the diagnostic values of the purified fusion-tagged recombinant MMP-like protein for human gnathostomiasis was evaluated using individual serum from healthy control, gnathostomiasis patients, and the patients with other parasitic diseases (Table 1; Figure 2A). All serum samples from the confirmed (N = 13) and suspected (N = 22) gnathostomiasis patients strongly reacted with the purified recombinant MMP-like fusion protein. In contrast, none of the 30 healthy control sera showed positive seroreactivity to this recombinant antigen. Some cross-reactivity was observed in serum samples of capillariasis (1 of 9), opisthorchiasis viverrini (2 of 8), and fascioliasis (3 of 10). The calculated diagnostic sensitivity, specificity, and positive and negative predictive values were 100%, 94.7%, 85.4%, and 100%, respectively. Non-reactive band was shown when all sera reacted with the fusion-tagged protein alone (78 kDa) (Figure 2B).
Figure 2.
Representative immunoblotting patterns reacted with the purified fusion-tagged MMP-like protein (A) compared with the fusion-tagged protein alone (B) expressed in Escherichia coli. Antigens were probed with 1:100 diluted sera from pooled negative controls (N), pooled human gnathostomiasis patients (P), healthy control (1, 2), parasitologically confirmed gnathostomiasis (3–7), clinically suspected visceral gnathostomiasis (8) and cutaneous gnathostomiasis (9), cysticercosis (10), angiostrongyliasis (11), paragonimiasis (12), fascioliasis (13), trichinosis (14), ascariasis (15), opisthorchiasis viverrini (16), strongyloidiasis (17), taeniasis (18), capillariasis (19), and hookworm infection (20). The arrow indicates the specific immunoreactive band at ∼102 kDa and arrow head indicates the fusion-tagged protein alone at ∼78 kDa.
Discussion
For the immunodiagnosis of gnathostomiasis, many attempts have been made34 to establish a specific diagnostic test system for the disease caused by Gnathostoma spp., such as Gnathostoma binucleatum,16,35 Gnathostoma doloresi,36 and G. spinigerum.17 However, the limitation is the small amount production of the specific antigen from native worm extracts. Maintenance of the lifecycle of Gnathostoma spp. in experimental animals in the laboratory is expensive and time consuming. The cDNA encoding MMP-like protein of G. spinigerum AL3 was cloned and the deduced amino acid sequence was correlated with that of the immunodominant spots22 and this protein was considered as the potential antigen for detecting specific antibodies in infected patients. Thus, in this study, we intended to produce the recombinant G. spinigerum MMP-like protein for massive antigen supply for the serodiagnosis of human gnathostomiasis in Asian countries. The MMP-like gene was expressed in E. coli using pET 43.1(+), which contained the Nus A and 6-His tagged residues as fusion proteins. This expression system resulted in a high yield (∼140 mg/one L of E. coli culture) and the fusion protein was obtained in the soluble fraction, as evaluated by Coomassie Brilliant Blue staining (shown in Figure 1B, panel a, lane 1). The fusion protein was seen as a molecular mass of ∼102 kDa, which was somewhat greater than the theoretical molecular mass of 89 kDa (fusion tag, 61 kDa; MMP-like protein, 28 kDa). This could possibly be caused by the alteration of the constant charge: mass ratio in binding between the SDS and the protein carrying a large size of the fusion tag.
The immunodominant antigenicity of the recombinant fusion protein against human sera was shown by immunoblotting. The recombinant fusion protein specifically reacted with the gnathostomiasis patient sera, but not with sera from healthy control or from patients infected with other parasites. Only faint cross-reactivity was observed with the sera of capillariasis (1 of 9), opisthorchiasis viverrini (2 of 8), and fascioliasis (3 of 10) patients. These cross-reactions are possibly explained because these patients might have a previous history of subclinical infection with G. spinigerum and mixed infections with these parasites. Even when cross-reactions with fascioliasis, capillariasis, and opisthorchiasis sera were observed, it does not cause a real problem in the clinical setting because these parasitic infections usually present with clinical features different from those of gnathostomiasis. However, subclinical infection with G. spinigerum and mixed infections sera with other parasites need to be evaluated with more samples. In addition, we have also tested the immunoblotting patterns using various human sera as revealed in Table 1 and reacted with the recombinant MMP-like protein cleaved with rEK (28 kDa) (see Supplemental Figure 1), the diagnostic sensitivity and specificity were quite similar and revealed the results as presented when testing with the purified fusion-tagged MMP-like protein (102 kDa). These results ensure both types of antigen can be used for supportive diagnostic purpose.
Previous immunoblotting reports have shown that the native 24 kDa G. spinigerum larval antigen reacting to total IgG antibody could contribute to the reliable diagnosis of human gnathostomiasis. The demonstrated sensitivity ranged from 83.3% to 100%, whereas the specificity ranged from 87.8% to 100%.17,19,21 The native 21 kDa antigenic band of G. spinigerum AL3 reacting faintly to IgG4 antibody gave the 100% sensitivity and specificity,18 whereas the 24 kDa G. spinigerum antigen revealed the sensitivity and specificity that ranged from 75% to 92.9% and 93.4% to 93.9%, respectively.18,19 In another study, the antigenic bands below 27–29 kDa of G. spinigerum AL3 showed the 100% sensitivity and specificity.20 This study showed high sensitivity and specificity of an immunoblot technique against the recombinant G. spinigerum MMP-like protein for detection of IgG antibody are quite similar with previous reports as described above.
In conclusion, we report the successful cloning of the G. spinigerum MMP-like gene and the fusion-tagged protein expressed as a soluble form in the E. coli cytoplasmic compartment. The recombinant G. spinigerum MMP-like protein has the potential for development of a serodiagnostic kit for human gnathostomiasis in endemic areas as a stable mass production system.
Supplementary Material
Footnotes
Financial support: This research was supported by grants from the Office of the Higher Education Commission, Thailand for supporting by grant fund under the program Strategic Scholarships for Frontier Research Network for the Ph.D. Program Thai Doctoral degree (CHE PhD scholarship); the Higher Education Research Promotion and National Research University Project of Thailand, and Faculty of Medicine, Khon Kaen University. PJ was supported by a CHE PhD scholarship. WM was supported by TRF Senior Research Scholar Grant, Thailand Research Fund grant no. RTA5580004. This research was also funded by a grant from the Association for Preventive Medicine of Japan in 2011 and 2012 to Wanchai Maleewong and Hiroshi Yamasaki.
Authors' addresses: Penchom Janwan, Pewpan M. Intapan, Porntip Laummaunwai, and Wanchai Maleewong, Department of Parasitology, Faculty of Medicine and Research, and Diagnostic Center for Emerging Infectious Diseases, Khon Kaen University, Khon Kaen, Thailand, E-mails: pair_wu@yahoo.com, pewpan@kku.ac.th, porlau@kku.ac.th, and wanch_ma@kku.ac.th. Hiroshi Yamasaki, Department of Parasitology, National Institute of Infectious Diseases, Tokyo, Japan, E-mail: hyamasak@nih.go.jp. Kittisak Sawanyawisuth, Department of Medicine, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, E-mail: kittisak@kku.ac.th. Chaisiri Wongkham, Department of Biochemistry, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, E-mail: chaisiri@kku.ac.th. Chatchai Tayapiwatana, Biomedical Technology Research Unit, National Center for Genetic Engineering and Biotechnology, National Science and Technology Development Agency at the Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand, E-mail: asimi002@chiangmai.ac.th. Amnat Kitkhuandee, Department of Surgery, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, E-mail: amnaki@kku.ac.th. Viraphong Lulitanond, Department of Microbiology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, E-mail: viraphng@kku.ac.th. Yukifumi Nawa, Research Division, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, E-mail: yukinawa@kku.ac.th.
References
- 1.Miyazaki I. On the genus Gnathostoma and human gnathostomiasis, with special reference to Japan. Exp Parasitol. 1960;9:338–370. doi: 10.1016/0014-4894(60)90040-0. [DOI] [PubMed] [Google Scholar]
- 2.Daengsvang S. Gnathostomiasis in southeast Asia. Southeast Asian J Trop Med Public Health. 1981;12:319–332. [PubMed] [Google Scholar]
- 3.León-Règagnon V, Osorio-Sarabia D, García-Prieto L, Akahane H, Lamothe-Argumedo R, Koga M, Messina-Robles M, Alvarez-Guerrero C. Study of the ethiological agent of gnathostomosis in Nayarit, Mexico. Parasitol Int. 2002;51:201–204. doi: 10.1016/s1383-5769(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 4.Waikagul J, Diaz Camacho SP. Gnathostomiasis. Murrell KD, Fried B, eds. World Class Parasites: Volume 11, Food-Borne Parasitic Zoonoses. New York, NY: Springer; 2007. pp. 235–261. [Google Scholar]
- 5.Moore DA, McCroddan J, Dekumyoy P, Chiodini PL. Gnathostomiasis: an emerging imported disease. Emerg Infect Dis. 2003;9:647–650. doi: 10.3201/eid0906.020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katchanov J, Sawanyawisuth K, Chotmongkoi V, Nawa Y. Neurognathostomiasis, a neglected parasitosis of the central nervous system. Emerg Infect Dis. 2011;17:1174–1180. doi: 10.3201/eid1707.101433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nawa Y. Historical review and current status of gnathostomiasis in Asia. Southeast Asian J Trop Med Public Health. 1991;22:217–219. [PubMed] [Google Scholar]
- 8.Xuan le T, Rojekittikhun W, Punpoowong B, Trang le N, Hien TV. Case report: intraocular gnathostomiasis in Vietnam. Southeast Asian J Trop Med Public Health. 2002;33:485–489. [PubMed] [Google Scholar]
- 9.Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484–492. doi: 10.1128/CMR.00003-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boongird P, Phuapradit P, Siridej N, Chirachariyavej T, Chuahirun S, Vejjajiva A. Neurological manifestations of gnathostomiasis. J Neurol Sci. 1977;31:279–291. doi: 10.1016/0022-510x(77)90113-7. [DOI] [PubMed] [Google Scholar]
- 11.Ratanarapee S, Jesadapatarakul S. A case of gnathostomiasis simulating acute appendicitis. J Med Assoc Thai. 1982;65:443–447. [PubMed] [Google Scholar]
- 12.Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33–50. doi: 10.1093/clinids/16.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Suntharasamai P, Desakorn V, Migasena S, Bunnag D, Harinasuta T. ELISA for immunodiagnosis of human gnathostomiasis. Southeast Asian J Trop Med Public Health. 1985;16:274–279. [PubMed] [Google Scholar]
- 14.Dharmkrong-at A, Migasena S, Suntharasamai P, Bunnag D, Priwan R, Sirisinha S Enzyme-linked immunosorbent assay for detection of antibody to Gnathostoma antigen in patients with intermittent cutaneous migratory swelling. J Clin Microbiol. 1986;23:847–851. doi: 10.1128/jcm.23.5.847-851.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maleewong W, Morakote N, Thamasonthi W, Charuchinda K, Tesana S, Khamboonruang C. Serodiagnosis of human gnathostomiasis. Southeast Asian J Trop Med Public Health. 1988;19:201–205. [PubMed] [Google Scholar]
- 16.Diaz Camacho SP, Zazueta Ramos M, Ponce Torrecillas E, Osuna Ramirez I, Castro Velazquez R, Flores Gaxiola A, Baquera Heredia J, Willms K, Akahane H, Ogata K, Nawa Y. Clinical manifestations and immunodiagnosis of gnathostomiasis in Culiacan, Mexico. Am J Trop Med Hyg. 1998;59:908–915. doi: 10.4269/ajtmh.1998.59.908. [DOI] [PubMed] [Google Scholar]
- 17.Tapchaisri P, Nopparatana C, Chaicumpa W, Setasuban P. Specific antigen of Gnathostoma spinigerum for immunodiagnosis of human gnathostomiasis. Int J Parasitol. 1991;21:315–319. doi: 10.1016/0020-7519(91)90033-4. [DOI] [PubMed] [Google Scholar]
- 18.Anantaphruti MT, Nuamtanong S, Dekumyoy P. Diagnostic values of IgG4 in human gnathostomiasis. Trop Med Int Health. 2005;10:1013–1021. doi: 10.1111/j.1365-3156.2005.01478.x. [DOI] [PubMed] [Google Scholar]
- 19.Laummaunwai P, Sawanyawisuth K, Intapan PM, Chotmongkol V, Wongkham C, Maleewong W. Evaluation of human IgG class and subclass antibodies to a 24 kDa antigenic component of Gnathostoma spinigerum for the serodiagnosis of gnathostomiasis. Parasitol Res. 2007;101:703–708. doi: 10.1007/s00436-007-0538-3. [DOI] [PubMed] [Google Scholar]
- 20.Tuntipopipat S, Chawengkirttikul R, Sirisinha S. A simplified method for the fractionation of Gnathostoma-specific antigens for serodiagnosis of human gnathostomosis. J Helminthol. 1993;67:297–304. doi: 10.1017/s0022149x00013304. [DOI] [PubMed] [Google Scholar]
- 21.Wongkham C, Maleewong W, Ieamviteevanich K, Intapan PM, Morakote N. Antigenic components of Gnathostoma spinigerum recognized by infected human sera by two-dimensional polyacrylamide gel electrophoresis and immunoblotting. Asian Pac J Allergy Immunol. 2000;18:47–52. [PubMed] [Google Scholar]
- 22.Laummaunwai P, Intapan PM, Wongkham C, Lulitanond V, Maleewong W. Identification of antigenic components of Gnathostoma spinigerum advanced-third stage larvae by two-dimensional gel electrophoresis and mass spectrometry. Southeast Asian J Trop Med Public Health. 2008;39:19–25. [Google Scholar]
- 23.Uparanukraw P, Morakote N, Harnnoi T, Dantrakool A. Molecular cloning of a gene encoding matrix metalloproteinase-like protein from Gnathostoma spinigerum. Parasitol Res. 2001;87:751–757. doi: 10.1007/s004360100440. [DOI] [PubMed] [Google Scholar]
- 24.Kongkerd N, Uparanukraw P, Morakote N, Sajid M, McKerrow JH. Identification and characterization of a cathepsin L-like cysteine protease from Gnathostoma spinigerum. Mol Biochem Parasitol. 2008;160:129–137. doi: 10.1016/j.molbiopara.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Laummaunwai P, Intapan PM, Wongkham C, Lulitanond V, Tayapiwatana C, Maleewong W. Gnathostoma spinigerum: molecular cloning, expression and characterization of the cyclophilin protein. Exp Parasitol. 2010;126:611–616. doi: 10.1016/j.exppara.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Elkins DB, Haswell-Elkins M, Anderson RM. The epidemiology and control of intestinal helminths in the Pulicat Lake region of Southern India. I. Study design and pre- and post-treatment observations on Ascaris lumbricoides infection. Trans R Soc Trop Med Hyg. 1986;80:774–792. doi: 10.1016/0035-9203(86)90384-6. [DOI] [PubMed] [Google Scholar]
- 27.Punyagupta S, Limtrakul C, Vichipanthu P, Karnchanachetanee C, Nye SW. Radiculomyeloencephalitis associated with eosinophilic pleocytosis. Report of nine cases. Am J Trop Med Hyg. 1968;17:551–560. doi: 10.4269/ajtmh.1968.17.551. [DOI] [PubMed] [Google Scholar]
- 28.Boongird P, Phuapradit P, Siridej N, Chirachariyavej T, Chuahirun S, Vejjajiva A. Neurological manifestations of gnathostomiasis. J Neurol Sci. 1977;31:279–291. doi: 10.1016/0022-510x(77)90113-7. [DOI] [PubMed] [Google Scholar]
- 29.Intapan PM, Khotsri P, Kanpittaya J, Chotmongkol V, Sawanyawisuth K, Maleewong W. Immunoblot diagnostic test for neurognathostomiasis. Am J Trop Med Hyg. 2010;83:927–929. doi: 10.4269/ajtmh.2010.10-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maleewong W, Sithithaworn P, Tesana S, Morakote N. Scanning electron microscopy of the early third-stage larvae of Gnathostoma spinigerum. Southeast Asian J Trop Med Public Health. 1988;19:643–647. [PubMed] [Google Scholar]
- 31.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galen RS. Predictive value and efficiency of laboratory testing. Pediatr Clin North Am. 1980;27:861–869. doi: 10.1016/s0031-3955(16)33930-x. [DOI] [PubMed] [Google Scholar]
- 34.Chaicumpa W. Immunodiagnosis of gnathostomiasis. Siriraj Med J. 2010;62:79–83. [Google Scholar]
- 35.Zambrano-Zaragoza JF, Durán-Avelar Mde J, Messina-Robles M, Vibanco-Pérez N. Characterization of the humoral immune response against Gnathostoma binucleatum in patients clinically diagnosed with gnathostomiasis. Am J Trop Med Hyg. 2012;86:988–992. doi: 10.4269/ajtmh.2012.11-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishiwata K, Diaz-Camacho SP, Ogata K, Nakamura-Uchiyama F, Hiromatsu K, Nawa Y. Evaluation of the antigenic similarities of adult-worm extracts from three Gnathostoma species, using sera from Mexican and Japanese patients with Gnathostoma infections. Ann Trop Med Parasitol. 2003;97:629–637. doi: 10.1179/000349803225001490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.