Abstract
There is increasing interest in rearing modified mosquitoes for mass release to control vector-borne diseases, particularly Wolbachia-infected Aedes aegypti for suppression of dengue. Successful introductions require release of high quality mosquitoes into natural populations. Potential indicators of quality are body size and shape. We tested to determine if size, wing/thorax ratio, and wing shape are associated with field fitness of Wolbachia-infected Ae. aegypti. Compared with field-collected mosquitoes, released mosquitoes were larger in size, with lower size variance and different wing shape but similar in wing-thorax ratio and its associated variance. These differences were largely attributed to nutrition and to a minor extent to wMel Wolbachia infection. Survival potential of released female mosquitoes was similar to those from the field. Females at oviposition sites tended to be larger than those randomly collected from BG-Sentinel traps. Rearing conditions should thus aim for large size without affecting wing/thorax ratios.
Introduction
There is increasing interest in modifying mosquitoes for potential release into field populations to suppress disease, including modifications by the introduction of Wolbachia to interfere with disease transmission,1,2 and modifications to generate sterility and other changes.3–6 As in mass releases with other insects, the field success of these programs will ultimately depend on the release of high quality material, and on developing ways of measuring and maintaining quality. For example, biocontrol of agricultural moth pests using Trichogramma spp. has been ongoing for many years and a number of studies have assessed effects of mass rearing on quality of these wasps, based on measurements of life history traits under laboratory7,8 and field8–10 conditions. A variety of quality indicators have been proposed and developed for such insect systems including measures of body size.11,12
Aedes aegypti, a highly anthropophilic mosquito species,13,14 and the primary vector of dengue virus,15 has been successfully infected with Wolbachia2,16 and released in the field.1 The prospects of using this approach to reduce dengue transmission2,17–19 and/or reduce adult life span20 may lead to large-scale releases of these mosquitoes akin to releases being undertaken with other biocontrol agents.
To date, mass-rearing of high quality Wolbachia-infected mosquitoes for field release has relied on backcrossing schemes to introduce field nuclear backgrounds and thereby minimize laboratory adaptation, and mass rearing under semi-field conditions to control for environmental effects.1 Mosquitoes are reared under high nutrition conditions to ensure the production of a large number of mosquitoes in a synchronized manner. Based on past research, high nutrition produces large mosquitoes that are potentially fitter in terms of fecundity, sperm quantity, and survival (e.g., References 21–24) but might lack other beneficial attributes. However, few field studies on the effects of Ae. aegypti size on field fitness have been performed, even though these could be completed with a mark-release-recapture design,25 and through comparisons of resting and host-seeking females.26,27
Apart from size, field fitness may also be affected by the wing loading and shape of insects. Wing loading as measured by weight to wing span has been shown to affect dispersal in insects such as butterflies and damselflies.28–31 In Drosophila, there is evidence for an association between wing loading and resource finding ability that contributes to an antagonistic interaction between wing size and thorax length.32 An association between wing shape and fitness has also been suggested in insects including parasitoids (e.g., Reference 10), although some researchers have suggested that shape may be phenotypically invariable and unlikely to affect fitness.33
Here, we first analyze variation among populations of laboratory and field females for adult size, based on measurements of both wing size and thorax length. We also consider differences in wing loading and wing shape. Based on mosquito surveillance and Wolbachia releases, using different trapping methods and parity assessment, we assess whether wing size, thorax length, wing size/thorax length, and wing shape affect the ability of mosquitoes to locate breeding sites and blood feed under field conditions. The successful invasion of Ae. aegypti populations in Cairns, Queensland, Australia by Wolbachia in 20111 provided an opportunity to monitor changes in morphometric measures associated with Wolbachia invasion. We use these analyses to make recommendations about quality indicators and the generation of high fitness mosquitoes in mass release programs.
Materials and Methods
Three laboratory lines of Ae. aegypti were used in this study. Uninfected C67 and C89 were lines established from several hundred eggs collected from Cairns, Queensland, Australia, around January 2010 and August 2010, respectively. A wMel-infected line2 was generated through outcrossing to F1 C89 males and successive backcrossing female offspring with F1 C89 males for an additional three generations (Aug–Nov 2010); this line is identified as wC89.
Colony maintenance (University of Melbourne).
All life stages of the uninfected, and Wolbachia-infected lines were maintained in a controlled 12:12 L:D laboratory environment at 26°C and 75–85% humidity. Eggs were hatched in plastic trays (20 cm × 28.5 cm × 9 cm) containing 3 L of RO (reverse osmosis) water, yeast (∼0.09 mg), and one crushed tablet (300 mg) of TetraMin Tropical Fish Food tablets Rich Mix (Tetra Holdings Inc. [US], made in Germany). Density was controlled to 200 individuals at the second instar stage and larvae were transferred to shallow plastic trays (42.7 cm × 31.2 cm × 7.2 cm) (Modulab Systems, Gratnell Ltd., United Kingdom). The trays contained 4 L RO water (1 larva/20 mL) and were supplied with TetraMin in excess.
Pupae were sexed by size (females are larger than males) and sex was confirmed by checking eclosed adults. Adults were housed in plastic containers with mesh sides (20 cm × 20 cm × 30 cm, covered in a plastic bag to maintain high humidity) and allowed constant access to a wick attached to a 20 mL vial of 10% sucrose solution. Females were given access to a human arm for blood feeding within 1 week of emergence and provided with sandpaper strips (11.8 m × 3.83 cm) for oviposition inside a plastic cup containing 150 mL RO water. Oviposition strips were collected and replaced daily for 1 week following blood feeding. Eggs were allowed to embryonate by leaving strips wet for 2 days after collection, partially drying them on paper towel for 30 s and leaving them moist for one more day. Strips were then dried under rearing temperature conditions until only traces of moisture by touch were apparent (i.e., strips were not completely dried) on the smooth side of the strip and stored in sealed bags with moist cotton wool to maintain high humidity and prevent desiccation.
Colony maintenance (James Cook University [JCU], Cairns).
The wC89 line (see above) was established in two semi-field cages (8.0 m × 9.0 m × 4.1 m) described elsewhere34 at the end of 2010. The cage contains free flying adults (800–1,600 females) and males hatched from eggs of field collected eggs are regularly released into this cage to allow for further outcrossing that minimizes laboratory adaptation.1 Females were blood fed with human volunteers almost daily in this colony (JCU Human Ethics Approval H2250). Eggs were collected using red flannel cloth half submerged in ovibuckets and larvae were reared in a second field cage to simulate natural conditions. Eggs were hatched with yeast solution, fed excess TetraMin. Larvae were reared in white bucket 205 mm in diameter with ∼1,500 mL water. Density was controlled to 150 larvae per bucket. Adults were provided access to 50% honey water solution before release.
Field release.
There were two field releases between January and March 2011 over 10 weeks in Yorkeys Knob and Gordonvale, as described by Hoffmann and others1; during the release period, more than 10,000 mosquitoes were released each week.
Sampling.
Three types of traps were used in this study: BioGent-Sentinel (BGS) traps (Biogents AG, Regensburg, Germany), double sticky ovitraps, and felt-cloth ovitraps. The BGS traps are useful for surveillance of all physiological stages of adult Ae. aegypti, although teneral and blood-fed females were undersampled.35–37 BioGent-Sentinel trapping was conducted without olfactory cues. Trapped mosquitoes were identified as Ae. aegypti and stored in ethanol at −20°C. In some cases, ovarian parity was assessed before preservation in ethanol. Double sticky ovitraps (referred to here as sticky ovitraps) composed of an internal removable sticky panel placed above a container of water. Because females are attracted to the water source to lay their eggs, sticky traps are effective in catching egg-laying (gravid) female Ae. aegypti, with 99% of females being parous.38,39 Ovipositing females are likely to be inseminated because insemination triggers oviposition and increases fecundity.40–42 In contrast, virgin blood-fed females do not readily lay eggs (< 10% 1 week after blood feeding (Yeap HL, unpublished data). The sticky ovitraps were used to determine whether there were morphometric differences between egg-laying females and those in the overall population. Mosquitoes were retrieved 1–3× per week and Ae. aegypti were stored in a dry vial at 4°C. Ovarian parity status was determined before storing these mosquitoes in ethanol at −20°C. Finally, felt cloth ovitraps are effective in collecting Ae. aegypti eggs from the field,34,43 and can be hatched under laboratory conditions. For these traps, red felt cloth was used as the egg-laying substrate in the trap, 0.5 g of lucerne was added as an oviposition attractant, and the felt was collected 1 week after setting the trap. The cloth was dried to condition eggs and to prevent immediate hatching.
Ovarian parity.
Abdomens of cold-anaesthetized female mosquitoes were dissected in diluted detergent and assessed for parity status according to Detinova44 and Clements and Boocock.45 Three ovarian parity states were noted: nulliparous, gravid, and parous. We defined gravid females as those with eggs developing past Christopher's Stage II,45 ranging from ovarian tracheoles almost blocked from sight, to ovaries completely engorged with fully developed eggs. Because we used ovarian parity as a sign of blood feeding, we classified females that had traces of blood as gravid, particularly as blood feeding results in the development of mature ovules within 3 days.46 Parous females were defined here as completely emptied of eggs with some tracheolation, whereas nulliparous females still had uncoiled tracheoles. Unlike gravid females, parous females would not have fed within the last 3 days.
Wolbachia testing.
The BGS-trap samples from field releases in Yorkeys Knob, Gordonvale, and Machans Beach were tested for Wolbachia by real-time polymerase chain reaction (PCR) using a Roche Applied Science (Australia) LightCycler 480.47 Whole mosquitoes or their abdomens were homogenized in 250 μL or 150 μL of 5% Chelex 100 resin (Bio-Rad Laboratories, Hercules, CA), respectively. Homogenized tissue was subjected to 45–60 minutes at 65°C with Proteinase K (Roche Diagnostics Australia Pty. Ltd., Castle Hill NSW, Australia), and then 10–15 minutes at 90°C to lyse Proteinase K. An aliquot of 10 μL of each sample was then diluted 10× or 5× for whole mosquito or abdomen respectively in a 96-well plate.
Three PCR reactions were performed for each sample. Three sets of primers were used47: 1) universal primer pair (mRpS6_F: 5′-AGTTGAACGTATCGTTTCCCGCTAC; mRpS6_R: 5′-GAAGTGACGCAGCTTGTGGTCGTCC), which target the conserved region of the RpS6 gene, to detect presence of mosquito DNA; 2) Ae. aegypti specific primers (aRpS6_F: 5′-ATCAAGAAGCGCCGTGTCG; aRpS6_R: 5′- CAGGTGCAGGATCTTCATGTATTCG), which target the Ae. aegypti-specific polymorphisms within the highly variable region of RpS6, to distinguish between Ae. aegypti and non-Ae. aegypti specimens; or 3) a pair of Wolbachia-specific primers (w1_F: 5′- AAAATCTTTGTGAAGAGGTGATCTGC; w1_R: 5′-GCACTGGGATGACAGGAAAAGG) to detect the presence of Wolbachia DNA.
The PCR cycling conditions were as follows: 95°C for 10 minutes, 40 cycles of 95°C for 5 seconds, 58°C for 15 seconds, and 72°C for 15 seconds. Products were heated to 95°C for 1 minute, cooled to 40°C for 20 seconds, and then raised to 65°C. As temperature increases gradually from 65°C to 95°C, fluorescence data were acquired continuously. The amplification and melting profiles of each PCR were subsequently used to determine the crossing point (Cp) values and melting temperatures (Tm) using the Absolute Quantification and Tm calling modules of the LightCyler 480 software package (Roche Applied Science).
Morphometrics.
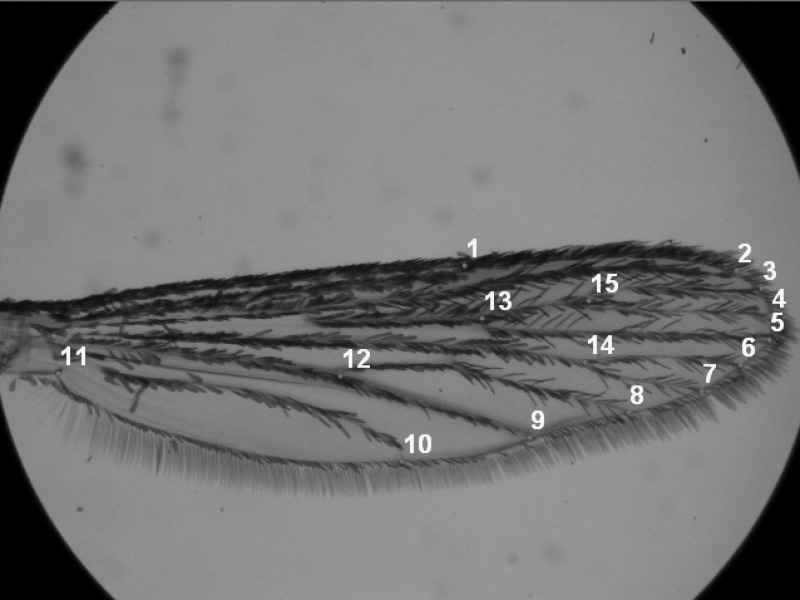
Left wings were detached and mounted on a slide with Hoyer's solution. A photograph was taken of each wing at 11.25× magnification using a camera (Nikon SMZ1500, Japan) mounted on a microscope. A 2 mm graticule was photographed to standardize size measurements. Photos were digitized with tPSUtil48 and tPSDig2 version 2.16.49 Fifteen landmarks were located on the wings to capture shape and size (Figure 1). This includes extra landmarks compared with an earlier study50 to allow wing shape to be accurately represented, but fewer points than used in Vargas and others51 because we found that some of their specified landmarks were difficult to locate accurately and reliably in our specimens. Wing centroid size is the square root of the sum of squares of the Euclidean distances between landmarks to the centroid.52
Figure 1.
Position of 15 landmarks on the wings of Aedes aegypti.
Wing length and centroid sizes are highly correlated to other measures of body size.53,54 Thorax length was also measured from the scutellum to the most anterior point of the thorax. The ratio of wing centroid size to thorax length was computed as the inverse of wing load, the body size to wing span ratio.
Repeatibility tests on measurements were initially performed by twice landmarking 10 samples from 25 groups of wing photos (250 images). One-way analysis of variance (ANOVA) was performed for each x- and y-coordinate, including centroid size, with individual as the fixed factor, thus the random error corresponds to within- individual or repeatibility errors.55 Repeatibility (R) was computed as the ratio of between individual variance to the sum of variance of between individual and within individual. All repeatibility values were > 0.99. The correlations between all repeated landmarks for all x-coordinates, y-coordinates, and overall centroid size were all high (> 0.99).
Comparisons and experiments.
Field and laboratory samples.
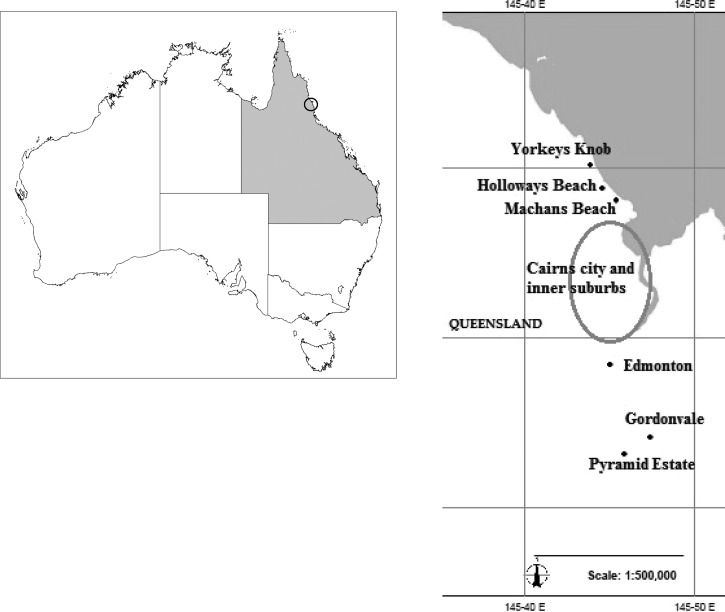
Samples were collected from BGS traps from within the release sites Yorkeys Knob (YK) and Gordonvale (GV) (Figure 2) at two time points from November to December 2009 and January to February 2010 before the release of laboratory-reared Wolbachia-infected Ae. aegypti. Approximately 80–100 female mosquitoes from each site and each time point were measured. Males were also obtained, but numbers were lower. These samples were considered representative of the uninfected field population during the wet season before the release. During the release period in January–March 2011, we collected more uninfected field samples from 20 BGS-traps placed in Holloways Beach (HB) and Machans Beach (MB) close to YK, and we also collected mosquitoes from 13 BGS traps in Pyramid Estate and Edmonton close to GV in January–February 2011 (Figure 2).
Figure 2.
Map of collection sites of Aedes aegypti and other sites mentioned in the work. This map was produced using MapConnect web Mapping http://www.ga.gov.au/mapconnect with GlobalMap 1 million data.
The C67 and C89 samples were obtained in May 2010 and May/June 2011, respectively (after both strains had been reared eight generations in the laboratory) to provide samples of uninfected mosquitoes reared under the laboratory environment. We also collected C67 and C89 at the same times from low nutrition conditions, by rearing them under identical conditions but providing larvae a quarter of the food given under the culture conditions described previously. The wMel-infected mosquitoes, wC89 were reared alongside uninfected C89 under both high and low nutrition conditions and used to examine the influence of infection status on measurements. The C67 laboratory strain was used in comparisons with field mosquitoes. Mosquitoes were stored in ethanol at −20°C after one gonotrophic cycle or directly after eclosion.
Field eggs from HB, MB, Edmonton, and Pyramid Estate were collected with ovitraps at the time of BGS trapping in those locations. Eggs were hatched under the laboratory conditions described previously and Ae. aegypti identified at the 3rd or 4th instar stage. These were then reared at one larva per 20 mL density with excess TetraMin. A day after eclosion, adults were stored in ethanol at −20°C. These F1 individuals from field females were compared with laboratory-reared mosquitoes.
Field comparisons.
The oviposition success of mosquitoes of different sizes was investigated by comparing mosquitoes retrieved from BGS traps and sticky ovitraps, as BGS-traps were expected to capture all mosquitoes, whereas sticky ovitraps captured ovipositing females. Eighty BGS traps were placed in the Cairns Central Business District (CBD), Cairns inner suburbs, and Machans Beach areas from March 8–18, 2011. Twenty of the traps with consistently high mosquito numbers were inspected daily. The others were checked once a week. Females were collected from sticky traps and BGS traps from Cairns CBD, Cairns inner suburbs, and Machans Beach in March 2011. Ovarian parity was assessed for all females from the BGS traps before preservation in ethanol. Size and shape were compared between females of different parity status. We compared gravid (and parous) females that would have survived long enough to feed, with nulliparous females that represented a population that had not yet undergone selection for feeding and survival.
All females caught in the double sticky ovitraps were parous or gravid. Parous females laid eggs onto the trap or inside the vials where they were stored. This “death stress oviposition” phenomenon leads to identification of captured gravid females as gravid rather than parous, and has been noted in previous double sticky ovitrap evaluations.38 From the BGS traps, we obtained 13 nulliparous, 17 parous, and 79 gravid females, along with 17 non-gravid females whose status was undefined because the ovarial tracheolation could not be visualized clearly.
Monitoring during release.
Infected mosquitoes were collected from YK and GV from 30 BGS traps in areas where weekly releases were undertaken.1 We assessed mosquitoes obtained in the third week of the release (week beginning 17 January), 2 weeks after the final release (week beginning 21 March) and in the last 2 weeks of May after sites were successfully invaded. Wolbachia-infected mosquitoes from the third week of the release are almost all likely to be field cage material, as there is insufficient time for a field generation to reach adulthood from the egg stage in two weeks. Infected females reared in the laboratory were tested at the same time as a representative sample of released material.
Statistical analyses.
Size measures from the samples were subjected to Shapiro-Wilk tests to check for normality. Variances were compared with check for the assumption of homoscedascity before performing ANOVAs or t-tests. The R2.11 program and SPSS version 20 (SPSS, Inc., Chicago, IL) were used for most of the analyses. Because of multiple comparisons in the tests, P values were corrected by the Dunn-Šidák procedure where appropriate. Size measures were almost always normally distributed and we used parametric tests, but in those rare cases where data were not normally distributed, we also confirmed any significant differences with non-parametric tests. Where variances differed, we used the unequal variance Welch t tests to compare groups. We also ran non-parametric tests to compare samples for wing thorax ratios. For all the analyses, we only considered females because sample sizes for males were small.
For analyses of wing shape, Procrustes superimposition was undertaken on landmark coordinates to remove orientation, location, and size effects.56 An initial exploratory analysis of shape variation observed within the datasets was undertaken with Principal Component Analyses (PCA) and/or Procrustes ANOVA. This was followed by Canonical Variate Analysis (CVA) with 10,000 permutations to test for pairwise distances and/or Discriminant Function Analysis (DFA) with 1,000 permutations on Procrustes distances to compare shape between groups. Canonical variates were graphed, although distances do not always reflect significance, because DFA and CVA maximize differences between groups. Shape variables were regressed with size to test for allometry. All shape analyses were performed in MorphoJ version 1.05a.57 We then generated shape changes for pairs of groups that were significantly different to visualize changes in shape.
Morphometric variation in laboratory and field populations.
To compare the field and laboratory samples of uninfected C67 mosquitoes, we first tested if the samples were homogeneous across the areas where field samples were obtained (YK and GV collected in 2010 before the release, or the four outer suburbs collected in 2011 during the release) using ANOVA and t-tests. We then compared the field samples to the laboratory-reared sample by unequal variance t-tests. For wing/thorax ratios, we used Mann-Whitney U tests to compare distributions among collections. We also used statistical tests to examine differences in coefficient of variation (CoV) between field samples, and between field and laboratory samples.58 For the shape analysis, in cases where Procrustes ANOVA indicated differences in shape between groups, we performed CVA on standardized shape variables to further elucidate the nature of these differences. Shape changes were visualized for pairs of groups that were significantly different after correction for multiple comparisons.
Field comparison.
Wing centroid size, thorax length, and wing/thorax ratio were compared between mosquitoes with a different parity states using t-tests, ANOVA, or non-parametric tests, whichever was appropriate. We also compared sticky ovitrap data and BGS-trap data to test for differences between ovipositing females collected in sticky ovitraps and a random sample of field mosquitoes (BGS traps) (Table 1). Gravid and parous females from BGS traps were compared with nulliparous to investigate size effects on blood feeding success. Gravid and parous females were also compared. Because BGS traps represent the general population, we acknowledge that the comparison between sticky ovitrap and BGS-trap samples may be prone to type II error, i.e., true difference will be underestimated and missed. This also applies to parity states because nulliparous mosquitoes will eventually give rise to gravid and then parous females; the parous state is therefore a subset of gravid females, whereas both are subsets of nulliparous mosquitoes.
Table 1.
Summary of samples included in main comparisons*
| Comparisons | 2010 | 2011 | During† | Post† | May† | BGS | Sticky | JCU | Laboratory | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| YK/GV | HB/MB | ED/PE | YK/GV | YK/GV | YK/GV | Cairns/MB | Cage | Uninfected | wMel | ||
| Laboratory vs. field | X | X | X | Field eggs/C67 | |||||||
| Infection | C89 | X | |||||||||
| Field | X | X | |||||||||
| Monitoring | X | X | |||||||||
| X | X | ||||||||||
| X | X | ||||||||||
All field collected mosquitoes (i.e., NOT JCU and Laboratory) are caught in BGS traps except for Sticky (sticky ovitrap).
YK = Yorkeys Knob; GV = Gordonvale; MB = Machans Beach; HB = Holloways Beach; ED = Edmonton; PE = Pyramid Estate.
During = Third week of wMel-infected release; Post = Two weeks post-final release; May = Samples from May.
We investigated shape variables for the BGS traps and sticky ovitraps with Procrustes to test for differences between groups. The CVA with pairwise comparisons was then performed to compare shape for the different parity status and sticky ovitrap-caught females. Principal component scores of standardized shape variables were obtained by PCA. The ANOVA was performed on principal components to identify if there was a significant effect of change in shape on ability to locate oviposition site.
We examined the relationship between ability to find an oviposition site and blood feeding success with each morphometric estimate and shape variables in the form of principal coordinates or canonical variates. For this, we used the cubic spline analysis using the “gam” or generalized additive models with integrated smoothness estimation function, from the “mgcv” or multiple smoothing parameter estimation by the general cross-validation or unbiased risk estimator (UBRE) package in R2.11.59 The cubic spline analyses involve selection of a smoothing parameter that minimizes the general cross-validation score to maximize the predictive ability of a model. The smoothed spline was plotted along with Bayesian prediction standard errors.
Monitoring the release.
With the samples collected at different time points at the release sites (Table 1), we examined how size changed over time. For all captured females, we separated wMel-infected and uninfected females and performed CoV to compare captured female mosquitoes with laboratory-reared and other field females. Cleveland plots were used to visualize the change in variance over different sampling time points with a modified command60 in R2.11. We also compared shape of these samples. Procrustes ANOVA was used to explore overall difference between groups. The CVA with pairwise comparison tests of groups were then run to explore how shape of captured female mosquitoes during the release over the three time points differed from laboratory-reared and field female mosquitoes before the release. We also compared captured infected females with other groups using DFA to obtain discriminant scores when comparing groups.
RESULTS
Comparison between field populations.
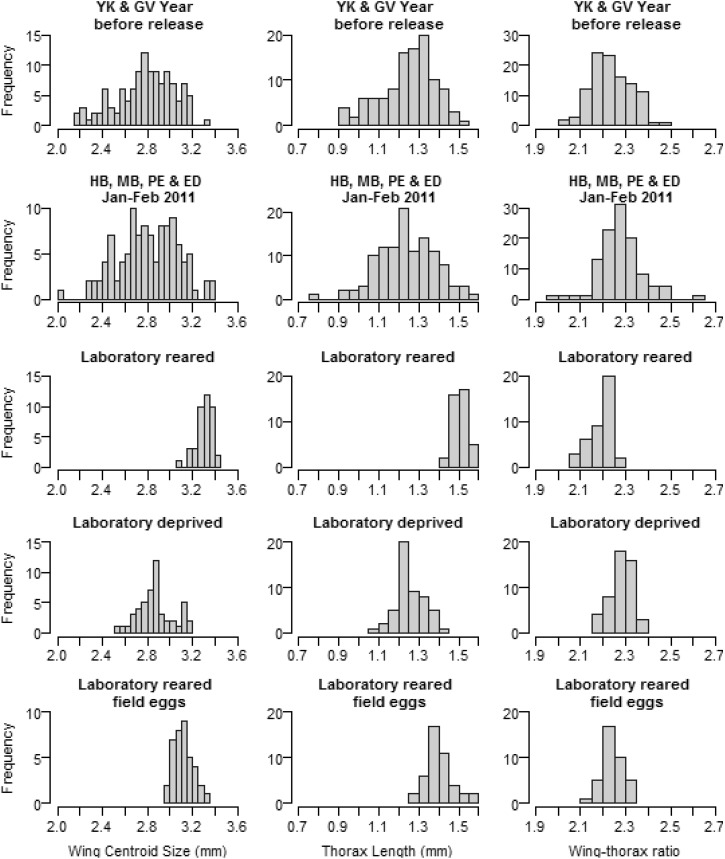
The 2011 BGS-trap collections of field mosquitoes from Holloways Beach and Machans Beach (within 5 km of YK) and Edmonton and Pyramid Estate (within 10 km of GV) did not differ significantly for wing centroid size (range of means 2.75–2.84 mm) or for thorax length (range of means 1.23–1.25 mm). Field mosquitoes from YK and GV collected in January–February 2010 (1 year before the wMel trial release) also did not differ from each other for wing centroid size (means of 2.75–2.85 mm) or thorax length (means of 1.21–1.27 mm). We therefore pooled into two datasets, field mosquitoes from 2011 and 2010, respectively (Figure 3 ). The 2010 and 2011 samples were not significantly different from each other by ANOVA with respect to size (Table 2). The CoVs for wing centroid size and thorax length did not differ significantly among the sites or years (Z < 1.3, P > 0.195), and neither did wing size/thorax ratios (range 2.23–2.26) (Figure 3).
Figure 3.
Wing centroid size, thorax length, and wing size/thorax length (left to right) of field and laboratory samples of Aedes aegypti. Samples were obtained from (top to bottom) Yorkeys Knob (YK) and Gordonvale (GV) collected the year before the release during the wet season; Holloways Beach (HB), Machans Beach (MB), Pyramid Estate (PE), and Edmonton (ED), all of which are either close to Yorkeys Knob or Gordonvale samples collected during the release; laboratory-reared C67 (eight generations in the laboratory) collected in May 2010; laboratory deprived (poor nutrition) C67; and mosquitoes reared in the laboratory, but derived from field eggs from the four outer sites.
Table 2.
Analysis of variance (ANOVA) and Procrustes ANOVA comparing wing size and shape between field samples and between 2011 field and laboratory samples
| Effect | MS | Df | F | P | |
|---|---|---|---|---|---|
| 2010 and 2011 field samples | |||||
| Size | Sample | 0.040 | 1 | 0.59 | 0.444 |
| Residual | 0.067 | 214 | |||
| Shape | Sample | 0.419* | 26 | 8.30 | < 0.001 |
| (Procrustes) | Residual | 0.051* | 5,564 | ||
| 2011 field and laboratory samples | |||||
| Size | Sample | 2.794 | 3 | 73.23 | < 0.001 |
| Residual | 0.038 | 224 | |||
| Shape | Sample | 0.386* | 78 | 9.48 | < 0.001 |
| (Procrustes) | Residual | 0.041* | 5,824 | ||
Actual value is the number stated multiplied by 10−3.
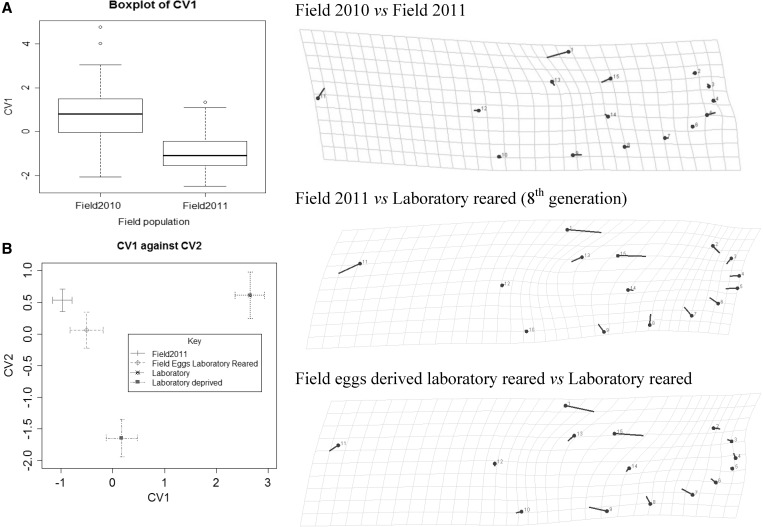
Procrustes ANOVA and CVA of Procrustes superimposed shape variables showed that GV and YK samples collected in 2010 could be classified as one group, as could the 2011 samples collected in the surrounding suburbs (all P > 0.05). However, in the Procrustes ANOVA of the pooled 2010 and 2011 samples, there were significant shape differences (Table 2) as evident from the boxplot of the canonical variate (Figure 4A). The difference in wing shape between years was associated with changes in landmarks 1, 15, and 11 (alular notch) (Figure 4), with landmark 1 in 2011 samples on average positioned toward the left of landmark 13. Further comparisons of shape were therefore only made between samples collected within the same year.
Figure 4.
(A) Differences in wing shape between the 2010 field and 2011 field samples of Aedes aegypti. The boxplot represents the significant canonical variate differentiating these samples, although the differences in shape (magnified 5×) are reflected in the landmark comparisons on the right. (B) Scatter plot of the two main canonical variates distinguishing the 2011 field, laboratory reared, and laboratory reared (but field egg derived) samples. The covariates explained 59.0% and 26.4% of the difference in wing shape. Landmark locations (magnified) reflecting differences between the field and laboratory-reared mosquitoes, and the differences between the laboratory-reared and field egg-derived samples are also given.
Comparison between laboratory and field.
Laboratory-reared mosquitoes (C67) were substantially larger on average than field mosquitoes (Figure 3, Table 2). Mean size was significantly different when compared with 2010 GV and YK field samples (wing centroid, Welch-t = 19.3, degrees of freedom [df] = 150, P < 0.001; thorax length, Welch-t = 18.3, df = 137, P < 0.001) and with 2011 field samples (wing centroid, Welch-t = 17.9, df = 140, P < 0.001; thorax length, Welch-t = 18.8, df = 145, P < 0.001). The mean wing size of laboratory females was 3.30 mm, in contrast to field females with means in the range 2.75–2.85 mm, whereas means for thorax length were 1.51 mm for laboratory-reared females, compared with 1.18–1.26 mm in field females.
Coefficients of variation of wing centroid size and thorax length were larger in field samples as is evident from the wider size distributions in the field mosquitoes (Figure 3). For comparisons of laboratory-reared females to both the 2010 and 2011 samples for wing centroid size and thorax length, Z values were > 7 and P values were < 0.001. Field CoVs were greater than 8%, compared with samples of laboratory-reared mosquitoes reared under excess nutrition that had CoVs < 4%.
In contrast to size measures, differences in wing/thorax ratios were much less pronounced between the samples (Figure 3). Field wing/thorax ratios were larger than those of laboratory-reared mosquitoes, suggesting a lower wing load (mean ratios of 2.22–2.26 in the field versus 2.20 in the laboratory, Mann-Whitney test, P = 0.005). The variation in wing load ratios was greater in the field population than in the laboratory-reared mosquitoes, although differences were smaller than for wing centroid size and thorax length (Figure 3) with a higher degree of overlap between the samples.
Morphometric traits were strongly affected by the laboratory nutrition treatments. Both wing centroid size and thorax length were reduced, whereas the wing size/thorax ratio was higher (all P < 0.001) in females reared under a restricted diet. Both the CoVs of wing centroid size and thorax length were higher in the low nutrition treatment than in the high nutrition treatment (Z > 5.39, P < 0.001), however the CoVs were still considerably smaller than in the field mosquitoes (compared with 2010 and 2011 field samples, Z = 3.73 and 3.94, respectively, P < 0.001).
When Ae. aegypti eggs were collected from the field and hatched under optimal laboratory conditions (i.e., first generation laboratory reared), the means and CoVs of wing centroid size, thorax size, and wing size/thorax length ratio were not significantly different from those of the laboratory-reared strain (eighth generation) (all P > 0.05) (Figure 3). In contrast to the field mosquitoes, the wing centroid size and thorax length were larger, whereas the wing size/thorax ratio was smaller (all tests, P < 0.001).
Wing shape exhibited significant differences between laboratory-reared (C67 8th generation) and field mosquitoes from 2011 (Table 2). The CVAs (Figure 4B) indicate differentiation in shape between laboratory-reared and field mosquitoes and with different nutritional levels, most apparent along the first and second canonical variates (CVs). Shape differences were not solely caused by allometry because they persisted when Procrustes distances were corrected for allometry (all pairwise comparisons between groups for field mosquitoes and groups for laboratory reared were significantly different, P < 0.0001). When visualizing the shape changes (Figure 4), it is apparent that laboratory rearing changed landmarks 11 (alular notch), 1 and 15, which were further away from landmark 12 and 10, whereas landmarks at the wing tip (2–9) were closer in. Landmark 1 was to the right of landmark 13 in laboratory-reared mosquitoes unlike in the field mosquitoes.
First generation laboratory-reared mosquitoes (field eggs, laboratory reared) differed significantly in shape (pairwise Procrustes distance) from laboratory-reared mosquitoes at the 8th generation, nutrition-deprived mosquitoes and field mosquitoes (all P < 0.001). This may reflect a subtle change in genetic variation for shape during laboratory rearing, although the sample size is small (N = 38). For the comparison with field mosquitoes from 2011, laboratory-reared mosquitoes at the 8th generation also had landmark 1 to the right of landmark 13, compared with mosquitoes at the first generation of laboratory rearing (Figure 4). In addition, landmarks 1, 15, and 11 were relatively further from landmark 12 and 10 in the well-established laboratory lines.
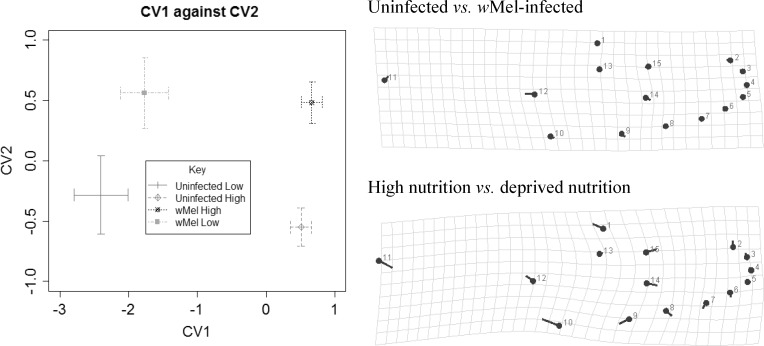
Under laboratory conditions, wMel-infected females were not significantly different in wing size compared with uninfected mosquitoes (C89) under low nutrition (2.29 mm versus 2.33 mm) and under high nutrition (uninfected versus infected, 2.95 mm versus 2.98 mm, P = 0.041) after adjusting for multiple comparison (Table 3). Procrustes ANOVA of shape variables (Table 3) indicated that the effect of infection status on shape was significant under high nutrition but not under low nutrition (Table 3). The shape change in infected compared with uninfected females under control conditions involved landmark 12 moving closer to landmark 11, away from the other inner landmarks (13–15). Unsurprisingly, there were also large effects of nutrition on size (P < 0.0001) consistent with effects observed in the other experiments (Figure 4). There was also an effect of nutrition on shape in both the infected and uninfected individuals (P < 0.001) (Table 3), as evident from differences in CV1 computed from the CVA (Figure 5). The shape differences between the high to low nutrition conditions involved the alular notch (landmark 11) moving closer to landmarks 12 and 10, whereas landmark 1 was located toward the left of landmark 13 (Figure 5).
Table 3.
Analysis of variance (ANOVA) and Procrustes ANOVA comparing wing size and shape between infected and uninfected mosquitoes scored under control (high) and low nutrition laboratory conditions; and comparing overall effect of nutrition levels
| Effect | MS | df | F | P | ||
|---|---|---|---|---|---|---|
| High nutrition | Size | Infection | 0.059 | 1 | 4.22 | 0.041 |
| Residual | 0.014 | 283 | ||||
| Shape | Infection | 0.133* | 26 | 3.38 | < 0.0001 | |
| (Procrustes) | Residual | 0.039* | 7,358 | |||
| Low nutrition | Size | Infection | 0.042 | 1 | 2.02 | 0.160 |
| Residual | 0.021 | 77 | ||||
| Shape | Infection | 0.049* | 26 | 1.21 | 0.213 | |
| (Procrustes) | Residual | 0.040* | 2002 | |||
| Overall laboratory | Size | Nutrition | 27.042 | 1 | 1,721.98 | < 0.001 |
| Residual | 0.016 | 362 | ||||
| Shape | Nutrition | 0.666* | 26 | 16.69 | < 0.001 | |
| (Procrustes) | Residual | 0.040* | 9,412 |
Actual value is the number stated multiplied by 10−3.
Figure 5.
(Left) The main canonical variates explaining differences between wMel and uninfected Aedes aegypti females at two different nutrition conditions (CV1, 77.3%; CV2, 15.3%). Non-overlapping error bars do not imply significant difference; (top right) shape change from uninfected to wMel infected; (bottom right) shape change from high nutrition to deprived nutrition. All shape changes were magnified 5×.
Field comparison.
Within the BGS traps, gravid and parous females not infected with Wolbachia together (mean wing size, 2.87 mm; thorax length, 1.27 mm) were not significantly different (P > 0.17) from nulliparous females (mean wing size, 2.76 mm; thorax length, 1.23 mm). If we separate gravid and parous females, parous females were smaller than gravid females (see Table 4) and this difference was significant (t = 2.34, df = 86, P < 0.05), but the number of parous females was low (Table 4).
Table 4.
Summary statistics (sample size, mean, SD, and coefficient of variation (CoV) of wing centroid size, thorax length and wing size/thorax ratio of mosquitoes obtained from BGS or sticky ovitraps in March 2011*
| Wing centroid size (mm) | Thorax length (mm) | Wing size/thorax ratio | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | CoV | n | Mean | SD | CoV | n | Median | SD | CoV | ||
| BGS traps | |||||||||||||
| Overall | 117 | 2.834 | 0.27 | 9.50% | 124 | 1.258 | 0.139 | 11.00% | 108 | 2.294 | 0.075 | 3.30% | |
| Gravid | 71 | 2.902 | 0.238 | 8.20% | 79 | 1.287 | 0.124 | 9.70% | 67 | 2.292 | 0.073 | 3.20% | |
| Parous (1) | 17 | 2.751 | 0.242 | 8.80% | 16 | 1.206 | 0.107 | 8.80% | 15 | 2.290 | 0.067 | 2.90% | |
| Nulliparous (2) | 12 | 2.765 | 0.331 | 12.00% | 13 | 1.240 | 0.184 | 14.80% | 12 | 2.294 | 0.086 | 3.80% | |
| Non-gravid (3) | 17 | 2.685 | 0.302 | 11.30% | 16 | 1.184 | 0.159 | 13.40% | 14 | 2.317 | 0.08 | 3.50% | |
| (1)+(2)+(3) | 46 | 2.73 | 0.285 | 10.50% | 45 | 1.208 | 0.149 | 12.30% | 41 | 2.296 | 0.077 | 3.40% | |
| Sticky ovitraps | 56 | 2.921 | 0.286 | 9.80% | 76 | 1.306 | 0.144 | 11.00% | 53 | 2.257 | 0.073 | 3.20% | |
Median of wing size/thorax ratio because we used Mann-Whitney U comparison of medians to test difference.
Females lacking Wolbachia and collected from sticky ovitraps tended to be larger than females collected in the BGS traps (Table 4), a difference that was just non-significant for wing centroid size (t = 1.93, df = 171, P = 0.06) and significant for thorax length (t = 2.32, df = 198, P = 0.02). The BGS-trap sample included gravid females that had similar means to those from the sticky ovitraps (see means in Table 4) and did not differ significantly from them (P > 0.38).
Turning to wing size/thorax ratio, sticky ovitrap females had a lower ratio (higher wing load) compared with BGS-trapped females (Table 4) and this difference was significant (Mann-Whitney U = 3739, P < 0.01). Unlike wing size and thorax length, gravid females from BGS traps were not significantly different from all other parity states for wing/size thorax ratio (Mann-Whitney U = 1377, P = 0.98), whereas gravid females in BGS-traps had a higher ratio compared with sticky ovitrap females (Table 4) (Mann-Whitney U = 2323. P < 0.01).
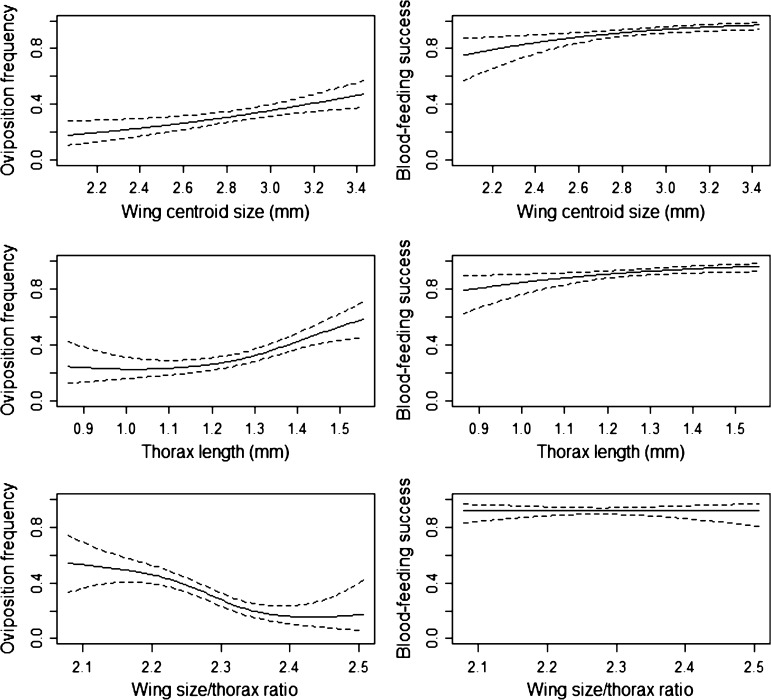
We investigated how relative fitness changed with morphometric measures fewer than two assumptions. First, we assumed sticky ovitrap females were relatively fitter than BGS-trap females, thereby testing for oviposition success. Second, we also compared gravid and parous females to nulliparous, to investigate possible differences between groups differing in blood feeding. As we have noted, these are likely to be conservative comparisons as we are comparing groups for which one is a subset of the other. We found that the likelihood of belonging to the ovipositing group increased with size (particularly for wing centroid size) (Figure 6). Likelihood of belonging to the ovipositing group tended to decrease with increasing wing size/thorax ratios, but there was no noticeable difference in likelihood between ratios of 2.1–2.3, which comprised 63–67% of all individuals in the analysis.
Figure 6.
Fitness function plots using cubic spline with Bayesian standard error based on wing centroid size, thorax length, and wing size/thorax ratio of Aedes aegypti. Fitness curves on the left assume only egg laying females from sticky ovitraps are fit. On the right, fitness curves consider successful blood-feeding as high fitness, which includes gravid, parous and sticky ovitrap females.
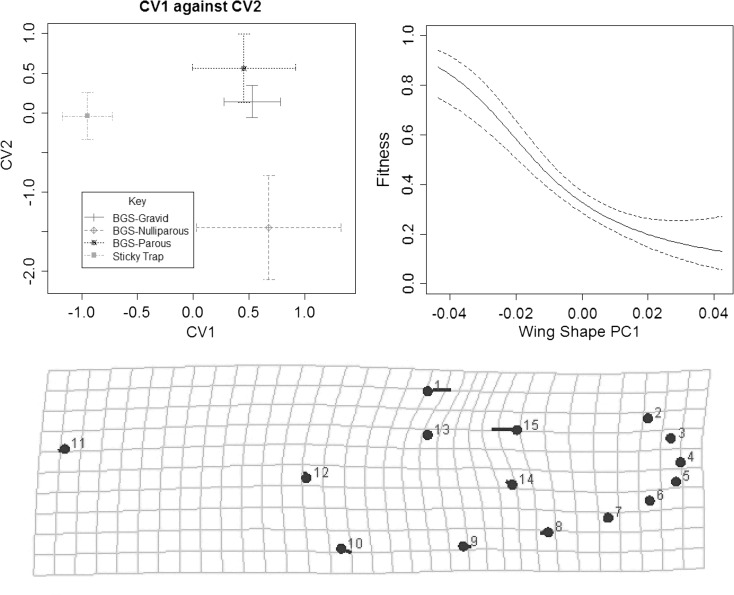
As in the case of wing/thorax ratio, we found a significant effect of trap type on wing shape. Based on Procrustes ANOVA, we found that sticky ovitrap females were significantly different from all categories of BGS-trapped females (P < 0.0001). When the BGS samples were separated and compared against the sticky ovitrap samples, the CVAs (Figure 7) indicated that the gravid, parous, and nulliparous females from BGS traps clustered as one group, and these were not different in pairwise comparisons of Procrustes distances (P > 0.05). We therefore only visualized how shape variables relate to ability to locate oviposition sites based on a comparison of sticky ovitrap versus BGS-trap females. The PCA was performed to obtain six orthogonal principal components (PCs) explaining > 5% of the variance: PC1 explained 24.6%, PC2 18.9%, PC3 12.5%, PC4 7.8%, PC5 6.5%, and PC6 5.3%. The ANOVAs on PCs indicated that after correction for multiple comparisons, only PC1 differed between gravid versus other parity states (F(1,145) = 5.61, P = 0.01) and its association was more pronounced when it was assumed that only egg laying females were fitter than BGS-trapped females (F(1,145) = 15.64, P < 0.0001), with trends plotted in Figure 7. Shape differences between the BGS-trapped and sticky ovitrap females involved central landmarks rather than those at the wing tip, with landmark 1 tending toward the right of landmark 13, whereas landmark 15 was closer to landmark 13 (Figure 7).
Figure 7.
(Top left) Main canonical variates differentiating mosquitoes from sticky ovitrap and BGS traps with parity states before size correction, CV1 and CV2 explained 56.8% and 23.0% of difference; (top right) main principal component versus fitness (ability to seek oviposition site) with Bayesian standard errors; (bottom) shape change between BGS-trapped female mosquitoes and sticky ovitrap female mosquitoes (magnified 5×).
Changes during the release.
The release of large field cage mosquitoes was expected to produce an influx of large infected mosquitoes in the field populations, with contributions from field-reared infected mosquitoes increasing over time as the Wolbachia frequency steadily increased throughout the release and immediate post-release periods. In Yorkeys Knob, Wolbachia frequency increased from 61% to 76.9% and then to 89.7% when comparing the three time points (Week 3, 2 weeks after final release and May), whereas in Gordonvale, frequencies were 53.3%, 65%, and finally 73.2%.1 Based on the comparison of laboratory and field reared mosquitoes, we expected some shape differences as well.
Third week of release.
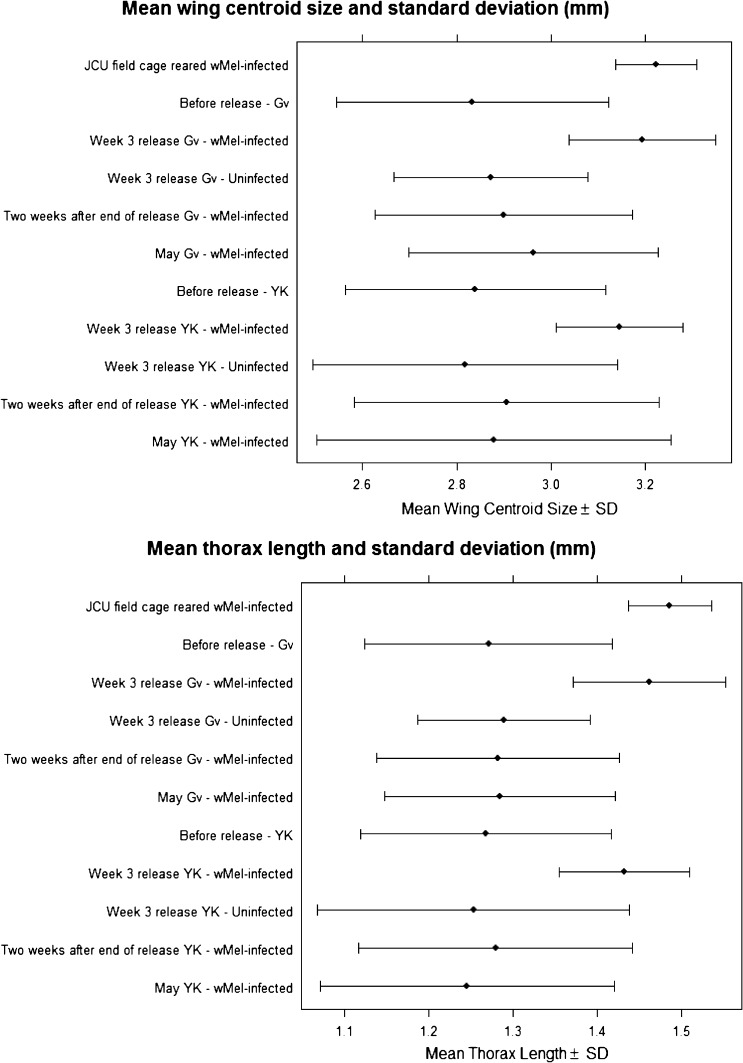
Captured wMel-infected females were not significantly different in size from the JCU cage-reared mosquitoes (mean wing centroid size: 3.17 versus 3.22 mm, Welch-t = 1.85, df = 55, P > 0.05, also see Table 5; thorax length: 1.44 versus 1.49 mm, Welch-t = 2.37, df = 62, P > 0.01). There was also no significant difference between wing size to thorax length ratio (median: 2.19 versus 2.17, P > 0.2). Because we expected almost all the mosquitoes from this period to be reared in the cage, the lack of significant differences in sizes suggest that there is no evidence of variation in survival between different sized mosquitoes from the field cage.
The CoVs of wing centroid size (overall: 4.6%) and thorax length (overall: 5.9%) of captured wMel-infected females were significantly larger than those of JCU field cage-reared mosquitoes (CoV: wing centroid size, 2.7%; thorax length, 3.3%) (Z > 1.96, P < 0.05) (Figure 8). When compared with captured uninfected females (overall CoV: wing centroid size, 9.8%; thorax length, 10.8%), the magnitude of the disparity was much greater and statistically significant (Z > 1.96, P < 0.05) (Figure 8). The CoVs of captured uninfected females from both sites were not significantly different from field mosquitoes captured the year before the release (for all comparisons: Z < 1.96, P > 0.05). As expected, these patterns reflect the fact that captured wMel-infected females represent release material, but they also point to mosquitoes with diverse sizes surviving in the field at least during the wet season.
Figure 8.
Cleveland plots of wing centroid size and thorax length of Aedes aegypti. YK = Yorkeys Knob; GV = Gordonvale; JCU = James Cook University cage-reared mosquitoes.
Procrustes ANOVAs (Table 5) revealed that captured infected females differ significantly from JCU field cage females and field mosquitoes from 2011. Pairwise permutation tests on Procrustes distances also confirmed these results with P < 0.05 for both comparisons. Canonical variate analysis suggests that captured infected females are still more similar to JCU field cage mosquitoes than to the field mosquitoes.
Table 5.
Analysis of variance (ANOVA) of wing size and Procrustes ANOVA of wing shape of captured third week of release, two weeks post release and two months post release (May) compared with field cage-reared and field mosquitoes from 2011
| Effect | MS | df | F | P | |||
|---|---|---|---|---|---|---|---|
| Third week of release | wMel captured vs. field cage | Size | Origin | 0.051 | 1 | 3.63 | 0.060 |
| Residual | 0.014 | 75 | |||||
| Shape | Individual | 0.127* | 26 | 3.50 | < 0.001 | ||
| Residual | 0.036* | 1,950 | |||||
| wMel captured vs. field 2011 | Size | Individual | 3.437 | 1 | 60.28 | < 0.001 | |
| Residual | 0.057 | 134 | |||||
| Shape | Individual | 0.093* | 26 | 2.43 | < 0.001 | ||
| Residual | 0.038* | 3,484 | |||||
| 2 weeks post-release | wMel captured vs. field cage | Size | Individual | 2.371 | 1 | 44.41 | < 0.001 |
| Residual | 0.053 | 92 | |||||
| Shape | Individual | 0.187* | 26 | 5.56 | < 0.001 | ||
| Residual | 0.034* | 2,392 | |||||
| wMel captured vs. field 2011 | Size | Individual | 0.291 | 1 | 3.82 | 0.053 | |
| Residual | 0.076 | 151 | |||||
| Shape | Individual | 0.099* | 26 | 3.73 | < 0.001 | ||
| Residual | 0.036* | 3926 | |||||
| May | wMel captured vs. field cage | Size | Individual | 1.905 | 1 | 33.71 | < 0.001 |
| Residual | 0.057 | 82 | |||||
| Shape | Individual | 0.534* | 26 | 14.42 | < 0.001 | ||
| Residual | 0.037* | 2,132 | |||||
| wMel captured vs. field 2011 | Size | Individual | 0.368 | 1 | 4.62 | 0.033 | |
| Residual | 0.080 | 141 | |||||
| Shape | Individual | 0.112* | 26 | 2.90 | < 0.001 | ||
| Residual | 0.038* | 3,666 |
Actual value is the number stated multiplied by 10−3.
Two weeks post-final release.
By this stage, the frequency of wMel infection in the two sites was high. Captured wMel-infected females had significantly reduced wing size and thorax length when compared with JCU field cage mosquitoes: wing centroid size (2.90 versus 3.22 mm; Welch-t = 7.44, df = 63, P < 0.0001), thorax length (1.28 versus 1.49 mm, Welch-t = 9.67, df = 76, P < 0.0001). Wing/thorax ratios of captured wMel-infected females were significantly higher than those of the JCU field cage females (2.27 versus 2.17, P < 0.0001).
As expected, field females had significantly larger CoVs compared with JCU field cage mosquitoes: wing centroid size (10.3% versus 2.7%) and thorax length (12.0% versus 3.3%) (all comparisons: Z > 1.96, P < 0.05) (Figure 8). The CoVs were similar to those from field female mosquitoes captured the year before the release (2010 sample) (all comparisons: Z < 1.96, P > 0.05) (Figure 8). Procrustes ANOVAs (Table 5) indicate that captured infected females were significantly different from JCU field cage mosquitoes in shape. Pairwise permutation tests on Procrustes distances and canonical variates also suggested a marginally significant (P < 0.05) difference in shape between captured infected females and field mosquitoes.
Because of the significant differences in size and shape between field cage and field mosquitoes, we estimated the survival of released females using individuals in the population with a large wing size and shape similar to those of the release sample. On the basis of wing centroid size only, we estimated that ∼22.5% of the field female mosquitoes (2010 and 2011 samples) were within the narrow size range observed in the release sample (JCU field cage). This compares with ∼34.2% (GV) and 31.2% (YK) of female mosquitoes' post-final release, which had wing centroid sizes within the range of the JCU field cage mosquitoes. We can estimate algebraically the frequency of individuals originating from the field cage to be 15.1% and 11.3% after 2 weeks from GV and YK, respectively. These translate into 87.4% and 85.6% survival per day. We also estimated survival in the same way by assigning mosquitoes based on shape instead of size using a discriminant analysis contrasting field cage and field mosquitoes, but daily survival estimates were 89.4% in YK and 90.3% in GV. Combining both size and shape constraints, the daily survival rate in YK was estimated to be 87% and in GV to be 79%. All of this was made possible by assuming no underlying Wolbachia effect on survival and size, constant population size of infected mosquitoes over the 2 weeks post release, and negligible survival of released mosquitoes before the last release.
Samples from May.
In May, ambient temperature in Cairns was on average 5°C lower than in January to March. This change could have an impact on morphological traits of mosquitoes.61,62 Wing centroid sizes of females from this sampling period were larger (2.92 versus 2.81 mm) than field females sampled in the warmer months (t = 2.25, df = 151, P = 0.03). There was, however, no evidence of a difference in thorax length (t = 0.98, df = 160, P = 0.33). The CoV for both measures were similar to field females from the year before the release. With regards to wing size/thorax ratios, these were significantly higher in May compared with field samples from the wet season (January–March) (median ratio = 2.33 versus 2.28, Mann-Whitney U = 3023, P values = 0.004), reflecting the fact that wing size changed without a concomitant change in thorax length.
Captured infected females in May were significantly different in shape from JCU field cage-reared females and when compared with field mosquitoes from 2011 (Table 5). However, based on canonical variate analysis, we found that captured infected females were more similar to field mosquitoes from 2011 than to JCU field cage mosquitoes. This is similar to the difference observed between infected females from 2 weeks post-final release and JCU field cage females.
Discussion
The findings in this study suggest that morphometric traits are not only strongly affected by environmental conditions but also linked to the likelihood of being collected from oviposition sites of Ae. aegypti females in the field. These traits can be used to assess the fitness of laboratory-reared colonies destined for release, and to assess survival of released individuals in the field. Large size seems to reflect oviposition site location in females. The effects may have been underestimated as a result of comparing non-exclusive groups, particularly as BGS-traps contained > 50% gravid females. This result may reflect the fact that large females have an increased fecundity.63–66 Larger females may also have a better flight range, higher survival, increased host finding, and blood feeding success, and improved ability to locate oviposition sites.67,68 Larger also means higher energy reserves,67 and by having a higher mass/surface area ratio, they might be less prone to desiccation than small mosquitoes.69 These results suggest that the strategy of rearing large mosquitoes in the mass-releases of wMel-infected mosquitoes1 may have contributed to the success of these releases. It is unclear if the large size of males also influences fitness in the field, although laboratory studies support the idea that larger males are fitter.22,70,71
We found no difference in measurements between gravid and parous versus nulliparous females. Field studies on blood feeding success suggests a negative association with size25,72 or no strong evidence of association.72 In contrast, we did find a significant difference in size between gravid and parous females and several factors might contribute to this difference. First, larger females may be more likely to have acquired a blood meal recently, particularly when the presence of mature ovules indicates successful blood feeding within at least the last 3 days.46 Second, blood-feeding rate may not depend on size but larger females may stay gravid for a longer time because of higher fecundity and a lengthy period of oviposition arising from skip oviposition behavior. Third, smaller females may live long enough to undergo complete oviposition, in contrast to the larger females. Finally, smaller females may be more likely to be inseminated. Because there is no strong evidence that smaller females have higher survival in smaller females,25,67 we suspect that the third reason is not likely to be important. We also suspect that there is unlikely to be an insemination bias given the results of laboratory studies22; the results may therefore reflect success in blood feeding or egg retention, but sorting this out requires further work.
Although the wing size to thorax length ratio also differed between the laboratory and field samples, this difference was much less than the difference in size. Given that previous studies have suggested that this trait may influence dispersal ability,32 and given the lower ratio in females from the sticky ovitraps, it would seem prudent to try to match ratios in released material with those from the field. Temperature manipulations may need to be monitored closely with changing seasons, as there is some evidence from our May sampling data and previous studies73,74 that temperature could affect wing load.
The shape analyses suggest that the available wing shape variation is associated with environmental effects and fitness based on ability to seek oviposition sites but not blood-feeding success. Effects of nutrition and other variables on wing shape have previously been documented in insects,53,75 and in the current study there was a distinct change in wing shape associated with low nutrition. Given the multivariate nature of shape, it is difficult to determine precisely the likely implications of this shape change on field fitness. Changes in landmarks 1 and 15 are affected by nutrition conditions and differ between BGS and sticky samples, so changes in these aspects of wing shape may influence field fitness.
Any effects of Wolbachia releases on morphometric variation are likely to be transient; because once releases were completed the morphometric traits and their variances converged rapidly on those of field populations before the release. This convergence also provides evidence that mass-rearing protocols and the backcrossing scheme did not markedly influence morphometric traits, with rearing in the laboratory and field cage environments likely to exert effects through phenotypic plasticity. Field environments presumably result in variability because of the wide array of environmental conditions available for juvenile stages.76,77
Finally, size and shape appeared useful in discrimination of released mosquitoes from field-reared mosquitoes. By combining the morphometrics with an assay of Wolbachia status, we were able to assess the survival potential of the released mosquitoes, which appeared similar to estimates of survival of Ae. aegypti mosquitoes in the field,78 corroborating the results of laboratory studies of wMel-infected mosquitoes,2 which indicated that survival of wMel-infected mosquitoes in the laboratory was not significantly impaired by the infection. Despite some previous studies suggesting that shape could be used to discriminate different environmental conditions,79,80 we suspect that it should be applied alongside size to discriminate populations from different conditions.
ACKNOWLEDGMENTS
We thank members of the Eliminate Dengue Project team who released mosquitoes and collected and identified BGS samples. We thank James Cook University staff, particularly Chris Paton, Clare Omodei and Gavin Omodei who organized mosquito rearing. We thank members of the Eliminate Dengue team for helping with BGS collections, Sharron Long and Karel von Herck from Queensland Health for assisting with sticky ovitrap collections. We also thank Jason K. Axford and Ashley G. Callahan for the assistance given in some of the laboratory based experiments and rearing. We are grateful to the residents of Cairns, Yorkeys Knob and Gordonvale for allowing us to set traps at their properties.
Footnotes
Financial support: This project was funded by a grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative of the Bill and Melinda Gates Foundation, the National Health and Medical Research Council, Australia, and the Urban Health Cluster of the CSIRO Climate Change Flagship program. AAH was funded by a Fellowship from the Australian Research Council.
Authors' addresses: Heng Lin Yeap, Nancy M. Endersby, and Ary A. Hoffmann, Department of Genetics, Parkville, VIC 3052, Australia, E-mails: hlyeap@unimelb.edu.au, nancye@unimelb.edu.au, and ary@unimelb.edu.au. Petrina H. Johnson, School of Biological Sciences, Faculty of Science, Monash University, Australia, E-mail: petrina.johnson@monash.edu. Scott A. Ritchie, Tropical Medicine and Rehabilitation Sciences, James Cook University, Cairns, Queensland, Australia, E-mail: scott.ritchie@jcu.edu.au.
References
- 1.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, O'Neill SL. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 2.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, Lloyd AL, Ritchie SA, O'Neill SL, Hoffmann AA. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 3.Bellini R, Calvitti M, Medici A, Carrieri M, Celli G, Maini S. Use of the sterile insect technique against Aedes albopictus in Italy: first results of a pilot trial. In: Vreysen MJ, Robinson AS, Hendrichs J, editors. Area-Wide Control of Insect Pests: From Research to Field Implementation. Springer, Dordrecht: The Netherlands; 2007. pp. 505–515. [Google Scholar]
- 4.de Valdez MR, Nimmo D, Betz J, Gong HF, James AA, Alphey L, Black WC. Genetic elimination of dengue vector mosquitoes. Proc Natl Acad Sci USA. 2011;108:4772–4775. doi: 10.1073/pnas.1019295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helinski ME, Hassan MM, El-Motasim WM, Malcolm CA, Knols BG, El-Sayed B. Towards a sterile insect technique field release of Anopheles arabiensis mosquitoes in Sudan: irradiation, transportation, and field cage experimentation. Malar J. 2008;7:65. doi: 10.1186/1475-2875-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, Dobson SL. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 2010;10:295–311. doi: 10.1089/vbz.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerutti F, Bigler F. Quality assessment of Trichogramma-brassicae in the laboratory. Entomol Exp Appl. 1995;75:19–26. [Google Scholar]
- 8.Dutton A, Bigler F. Flight activity assessment of the egg parasitoid Trichogramma brassicae (Hym: Trichogrammatidae) in laboratory and field conditions. Entomophaga. 1995;40:223–233. [Google Scholar]
- 9.Dutton A, Cerutti F, Bigler F. Quality and environmental factors affecting Trichogramma brassicae efficiency under field conditions. Entomol Exp Appl. 1996;81:71–79. [Google Scholar]
- 10.Kolliker-Ott UM, Blows MW, Hoffmann AA. Are wing size, wing shape and asymmetry related to field fitness of Trichogramma egg parasitoids? Oikos. 2003;100:563–573. [Google Scholar]
- 11.Kazmer DJ, Luck RF. Female body size, fitness and biological control quality: field experiments with Trichogramma pretiosum. Colloques de l'INRA. 1991;56:37–40. [Google Scholar]
- 12.Navarro-Campos C, Martinez-Ferrer MT, Campos JM, Fibla JM, Alcaide J, Bargues L, Marzal C, Garcia-Mari F. The influence of host fruit and temperature on the body size of adult Ceratitis capitata (Diptera: Tephritidae) under laboratory and field conditions. Environ Entomol. 2011;40:931–938. doi: 10.1603/EN10302. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery BL, Ritchie SA. Roof gutters: a key container for Aedes aegypti and Ochlerotatus notoscriptus (Diptera: Culicidae) in Australia. Am J Trop Med Hyg. 2002;67:244–246. doi: 10.4269/ajtmh.2002.67.244. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery BL, Ritchie SA, Hart AJ, Long SA, Walsh ID. Subsoil drain sumps are a key container for Aedes aegypti in Cairns, Australia. J Am Mosq Control Assoc. 2004;20:365–369. [PubMed] [Google Scholar]
- 15.Wilder-Smith A, Ooi E-E, Vasudevan S, Gubler D. Update on dengue: epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr Infect Dis Rep. 2010;12:157–164. doi: 10.1007/s11908-010-0102-7. [DOI] [PubMed] [Google Scholar]
- 16.McMeniman CJ, Lane AM, Fong AW, Voronin DA, Iturbe-Ormaetxe I, Yamada R, McGraw EA, O'Neill SL. Host adaptation of a Wolbachia strain after long-term serial passage in mosquito cell lines. Appl Environ Microbiol. 2008;74:6963–6969. doi: 10.1128/AEM.01038-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kambris Z, Blagborough AM, Pinto SB, Blagrove MS, Godfray HCJ, Sinden RE, Sinkins SP. Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles gambiae. PLoS Pathog. 2010;6:e1001143. doi: 10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu GJ, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O'Neill SL. A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 19.Pan XL, Zhou GL, Wu JH, Bian GW, Lu P, Raikhel AS, Xi ZY. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2012;109:E23–E31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang YF, O'Neill SL. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 21.Harrington LC, Connors KJ, Cator LJ, Helinski ME. Assortative mating in the dengue vector mosquito, Aedes aegypti. Am J Trop Med Hyg. 2009;81:1017. [PubMed] [Google Scholar]
- 22.Ponlawat A, Harrington LC. Factors associated with male mating success of the dengue vector mosquito, Aedes aegypti. Am J Trop Med Hyg. 2009;80:395–400. [PubMed] [Google Scholar]
- 23.Xue RD, Barnard DR, Muller GC. Effects of body size and nutritional regimen on survival in adult Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2010;47:778–782. doi: 10.1603/me09222. [DOI] [PubMed] [Google Scholar]
- 24.Armbruster P, Hutchinson RA. Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae) J Med Entomol. 2002;39:699–704. doi: 10.1603/0022-2585-39.4.699. [DOI] [PubMed] [Google Scholar]
- 25.Maciel-De-Freitas R, Codego CT, Lourenco-De-Oliveira R. Body size-associated survival and dispersal rates of Aedes aegypti in Rio de Janeiro. Med Vet Entomol. 2007;21:284–292. doi: 10.1111/j.1365-2915.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 26.Nasci RS. Relationship between adult mosquito (Diptera, Culicidae) body size and parity in field populations. Environ Entomol. 1986;15:874–876. [Google Scholar]
- 27.Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Zhou H, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J Med Entomol. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- 28.Breuker CJ, Brakefield PM, Gibbs M. The association between wing morphology and dispersal is sex-specific in the glanville fritillary butterfly Melitaea cinxia (Lepidoptera: Nymphalidae) Eur J Entomol. 2007;104:445–452. [Google Scholar]
- 29.Corbet SA. Butterfly nectaring flowers: butterfly morphology and flower form. Entomol Exp Appl. 2000;96:289–298. [Google Scholar]
- 30.Hassall C, Thompson DJ, Harvey IF. Latitudinal variation in morphology in two sympatric damselfly species with contrasting range dynamics (Odonata: Coenagrionidae) Eur J Entomol. 2008;105:939–944. [Google Scholar]
- 31.Kemp DJ. Butterfly contests and flight physiology: why do older males fight harder? Behav Ecol. 2002;13:456–461. [Google Scholar]
- 32.Hoffmann AA, Ratna E, Sgro CM, Barton M, Blacket M, Hallas R, De Garis S, Weeks AR. Antagonistic selection between adult thorax and wing size in field released Drosophila melanogaster independent of thermal conditions. J Evol Biol. 2007;20:2219–2227. doi: 10.1111/j.1420-9101.2007.01422.x. [DOI] [PubMed] [Google Scholar]
- 33.Santos M, Iriarte PF, Cespedes W. Genetics and geometry of canalization and developmental stability in Drosophila subobscura. BMC Evol Biol. 2005;5:7. doi: 10.1186/1471-2148-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritchie SA, Johnson PH, Freeman AJ, Odell RG, Graham N, Dejong PA, Standfield GW, Sale RW, O'Neill SL. A secure semi-field system for the study of Aedes aegypti. PLoS Negl Trop Dis. 2011;5:e988. doi: 10.1371/journal.pntd.0000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ball TS, Ritchie SR. Sampling biases of the BG-Sentinel trap with respect to physiology, age, and body size of adult Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2010;47:649–656. doi: 10.1603/me09218. [DOI] [PubMed] [Google Scholar]
- 36.Maciel-de-Freitas R, Eiras AE, Lourenco-de-Oliveira R. Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes aegypti (Diptera: Culicidae) Mem Inst Oswaldo Cruz. 2006;101:321–325. doi: 10.1590/s0074-02762006000300017. [DOI] [PubMed] [Google Scholar]
- 37.Williams CR, Long SA, Russell RC, Ritchie SA. Field efficacy of the BG-sentinel compared with CDC backpack aspirators and CO2-baited EVS traps for collection of adult Aedes aegypti in Cairns, Queensland, Australia. J Am Mosq Control Assoc. 2006;22:296–300. doi: 10.2987/8756-971X(2006)22[296:FEOTBC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Chadee DD, Ritchie SA. Efficacy of sticky and standard ovitraps for Aedes aegypti in Trinidad, West Indies. J Vector Ecol. 2010;35:395–400. doi: 10.1111/j.1948-7134.2010.00098.x. [DOI] [PubMed] [Google Scholar]
- 39.Chadee DD, Ritchie SA. Oviposition behavior and parity rates of Aedes aegypti collected in sticky traps in Trinidad, West Indies. Acta Trop. 2010;116:212–216. doi: 10.1016/j.actatropica.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Hiss EA, Fuchs MS. Effect of matrone on oviposition in mosquito, Aedes aegypti. J Insect Physiol. 1972;18:2217. doi: 10.1016/0022-1910(72)90250-8. [DOI] [PubMed] [Google Scholar]
- 41.Judson CL. Feeding and oviposition behavior in Aedes aegypti (L). I. Preliminary studies of physiological control mechanisms. Biol Bull. 1967;133:369–378. doi: 10.2307/1539832. [DOI] [PubMed] [Google Scholar]
- 42.Lavoipierre MMJ. Biting behavior of mated and unmated females of an African strain of Aedes aegypti. Nature. 1958;181:1781–1782. doi: 10.1038/1811781a0. [DOI] [PubMed] [Google Scholar]
- 43.Ritchie SA, Rapley LP, Williams C, Johnson PH, Larkman M, Silcock RM, Long SA, Russell RC. A lethal ovitrap-based mass trapping scheme for dengue control in Australia: I. Public acceptability and performance of lethal ovitraps. Med Vet Entomol. 2009;23:295–302. doi: 10.1111/j.1365-2915.2009.00833.x. [DOI] [PubMed] [Google Scholar]
- 44.Detinova TS. Age-grouping methods in Diptera of medical importance with special reference to some vectors of malaria. Monogr Ser World Health Organ. 1962;47:13–191. [PubMed] [Google Scholar]
- 45.Clements AN, Boocock MR. Ovarian development in mosquitoes: stages of growth and arrest and follicular resorption. Physiol Entomol. 1984;9:1–8. [Google Scholar]
- 46.Gwadz RW, Spielman A. Corpus allatum control of ovarian development in Aedes aegypti. J Insect Physiol. 1973;19:1441–1448. doi: 10.1016/0022-1910(73)90174-1. [DOI] [PubMed] [Google Scholar]
- 47.Lee SF, White VL, Weeks AR, Hoffmann AA, Endersby NM. High-throughput PCR assays to monitor Wolbachia infection in the dengue mosquito (Aedes aegypti) and Drosophila simulans. Appl Environ Microbiol. 2012;78:4740–4743. doi: 10.1128/AEM.00069-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohlf FJ. tpsUtil, File Utility Program, Version 1.26. Department of Ecology and Evolution; State University of New York at Stony Brook: 2004. [Google Scholar]
- 49.Rohlf FJ. tpsDig, Digitize Landmarks and Outlines, Version 2.16. Department of Ecology and Evolution; State University of New York at Stony Brook: 2010. [Google Scholar]
- 50.Yeap HL, Mee P, Walker T, Weeks AR, O'Neill SL, Johnson P, Ritchie SA, Richardson KM, Doig C, Endersby NM, Hoffmann AA. Dynamics of the “popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics. 2011;187:583–595. doi: 10.1534/genetics.110.122390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vargas RE, Ya-umphan P, Phumala-Morales N, Komalamisra N, Dujardin JP. Climate associated size and shape changes in Aedes aegypti (Diptera: Culicidae) populations from Thailand. Infect Genet Evol. 2010;10:580–585. doi: 10.1016/j.meegid.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Bookstein FL. Morphometric Tools for Landmark Data Geometry and Biology. New York: Cambridge University Press; 1991. [Google Scholar]
- 53.Jirakanjanakit N, Leemingsawat S, Thongrungkiat S, Apiwathnasorn C, Singhaniyom S, Bellec C, Dujardin JP. Influence of larval density or food variation on the geometry of the wing of Aedes (Stegomyia) aegypti. Trop Med Int Health. 2007;12:1354–1360. doi: 10.1111/j.1365-3156.2007.01919.x. [DOI] [PubMed] [Google Scholar]
- 54.Siegel JP, Novak RJ, Lampman RL, Steinly BA. Statistical appraisal of the weight wing length relationship of mosquitoes. J Med Entomol. 1992;29:711–714. doi: 10.1093/jmedent/29.4.711. [DOI] [PubMed] [Google Scholar]
- 55.Arnqvist G, Martensson T. Measurement error in geometric morphometrics: Empirical strategies to assess and reduce its impact on measures of shape. Acta Zoologica Academiae Scientiarum Hungaricae. 1998;44:73–96. [Google Scholar]
- 56.Klingenberg CP, McIntyre GS. Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with procrustes methods. Evolution. 1998;52:1363–1375. doi: 10.1111/j.1558-5646.1998.tb02018.x. [DOI] [PubMed] [Google Scholar]
- 57.Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Res. 2011;11:353–357. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- 58.Miller GE. Asymptotic test statistics for coefficients of variation. Comm Statist Theory Methods. 1991;20:3351–3363. [Google Scholar]
- 59.Schluter D. Estimating the form of natural-selection on a quantitative trait. Evolution. 1988;42:849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. [DOI] [PubMed] [Google Scholar]
- 60.Kozak M. dotplot.errors, a new R function to ease the pain of creating dotplots. Commun Biometry Crop Sci. 2010;5:69–77. [Google Scholar]
- 61.Padmanabha H, Lord CC, Lounibos LP. Temperature induces trade-offs between development and starvation resistance in Aedes aegypti (L.) larvae. Med Vet Entomol. 2011;25:445–453. doi: 10.1111/j.1365-2915.2011.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohammed A, Chadee DD. Effects of different temperature regimens on the development of Aedes aegypti (L.) (Diptera: Culicidae) mosquitoes. Acta Trop. 2011;119:38–43. doi: 10.1016/j.actatropica.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Bader CA, Williams CR. Mating, ovariole number and sperm production of the dengue vector mosquito Aedes aegypti (L.) in Australia: broad thermal optima provide the capacity for survival in a changing climate. Physiol Entomol. 2012;37:136–144. [Google Scholar]
- 64.Briegel H. Metabolic relationship between female body size, reserves and fecundity of Aedes aegypti. J Insect Physiol. 1990;36:165–172. [Google Scholar]
- 65.Naksathit AT, Scott TW. Effect of female size on fecundity and survivorship of Aedes aegypti fed only human blood versus human blood plus sugar. J Am Mosq Control Assoc. 1998;14:148–152. [PubMed] [Google Scholar]
- 66.Steinwascher K. Relationship between pupal mass and adult survivorship and fecundity for Aedes aegypti. Environ Entomol. 1982;11:150–153. [Google Scholar]
- 67.Briegel H, Knusel I, Timmermann SE. Aedes aegypti: size, reserves, survival, and flight potential. J Vector Ecol. 2001;26:21–31. [PubMed] [Google Scholar]
- 68.Nasci RS. Influence of larval and adult nutrition on biting persistence in Aedes aegypti (Diptera, Culicidae) J Med Entomol. 1991;28:522–526. doi: 10.1093/jmedent/28.4.522. [DOI] [PubMed] [Google Scholar]
- 69.Mogi M, Miyagi I, Abadi K, Syafruddin Inter- and intraspecific variation in resistance to desiccation by adult Aedes (Stegomyia) spp. (Diptera: Culicidae) from Indonesia. J Med Entomol. 1996;33:53–57. doi: 10.1093/jmedent/33.1.53. [DOI] [PubMed] [Google Scholar]
- 70.Helinski MEH, Harrington LC. Male mating history and body size influence female fecundity and longevity of the dengue vector Aedes aegypti. J Med Entomol. 2011;48:202–211. doi: 10.1603/me10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ponlawat A, Harrington LC. Age and body size influence male sperm capacity of the dengue vector Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2007;44:422–426. doi: 10.1603/0022-2585(2007)44[422:aabsim]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 72.Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J Med Entomol. 2000;37:89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- 73.Loeschcke V, Bundgaard J, Barker JS. Reaction norms across and genetic parameters at different temperatures for thorax and wing size traits in Drosophila aldrichi and D-buzzatii. J Evol Biol. 1999;12:605–623. [Google Scholar]
- 74.Reiskind MH, Zarrabi AA. Is bigger really bigger? Differential responses to temperature in measures of body size of the mosquito, Aedes albopictus. J Insect Physiol. 2012;58:911–917. doi: 10.1016/j.jinsphys.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 75.Hoffmann AA, Woods RE, Collins E, Wallin K, White A, McKenzie JA. Wing shape versus asymmetry as an indicator of changing environmental conditions in insects. Aust J Entomol. 2005;44:233–243. [Google Scholar]
- 76.Schneider JR, Morrison AC, Astete H, Scott TW, Wilson ML. Adult size and distribution of Aedes aegypti (Diptera: Culicidae) associated with larval habitats in Iquitos, Peru. J Med Entomol. 2004;41:634–642. doi: 10.1603/0022-2585-41.4.634. [DOI] [PubMed] [Google Scholar]
- 77.Tun-Lin W, Burkot TR, Kay BH. Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, Australia. Med Vet Entomol. 2000;14:31–37. doi: 10.1046/j.1365-2915.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- 78.Muir LE, Kay BH. Aedes aegypti as a vector of dengue viruses in northern Queensland: what have we learnt?. Arbovirus research in Australia Proceedings Seventh Arbovirus Research in Australia Symposium and Second Mosquito Control Association of Australia Conference; Surfers Paradise, Australia. 25–29 November, 1996; 1997. pp. 190–193. [Google Scholar]
- 79.Jirakanjanakit N, Dujardin J-P. Discrimination of Aedes aegypti (Diptera: Culicidae) laboratory lines based on wing geometry. Southeast Asian J Trop Med Public Health. 2005;36:858–861. [PubMed] [Google Scholar]
- 80.Jirakanjanakit N, Leemingsawat S, Dujardin JP. The geometry of the wing of Aedes (Stegomyia) aegypti in isofemale lines through successive generations. Infect Genet Evol. 2008;8:414–421. doi: 10.1016/j.meegid.2007.05.004. [DOI] [PubMed] [Google Scholar]