Abstract
Evidence on the effectiveness of insecticide-treated curtains (ITCs) for reducing densities of Aedes mosquitoes, the principal vectors of dengue, is scarce. In Laem Chabang southeast of Bangkok, Thailand, the Breteau Index (BI) (number of positive containers/100 houses) was 45 in October 2006. In March 2007, we distributed long-lasting ITCs in 22 clusters (2,032 houses) and selected 66 control clusters (661 houses). Routine control activities continued in all clusters. Six months after distribution, the BI was 25.8 and 77.6 in intervention and control areas, respectively (P < 0.001). Eighteen months after distribution, the BI was 21.8 and 23.8, respectively (P = 0.28). The average number of ITCs/house at cluster level was associated with the BI (P < 0.01) after six months, when 70.5% of households still used ITCs, but not at 18 months, when ITC coverage had decreased to 33.2%. Deployment of ITCs can result in considerable reductions in Aedes infestation levels, but the effect is coverage dependent.
Introduction
Almost 50% of the world's population lives at risk of contracting dengue, a viral vector-borne disease transmitted primarily by Aedes aegypti and, to a lesser extent, Aedes albopictus mosquitoes. Annually, 50–100 million infections occur globally with an estimated 24,000 deaths, and incalculable costs of treating the large numbers of infected persons.1–3 There is no specific antiviral treatment. The prevention of dengue by immunization appears to be technically feasible: the leading candidate vaccine is a chimeric vaccine, which appeared to be safe, showing an effect of reducing at least 80% cases of infection with dengue virus type 3 (DENV3) and DENV4, but with only a partial effect on DENV1 and no significant effect on DENV2.4 Until an effective vaccine is available, and most likely even after then, vector control remains essential for dengue prevention.
In contrast to other major vector-borne diseases, such as malaria, leishmaniasis, and Chagas disease, in which most vector control and prevention activities target the adult stages of the vector, the prevention of Aedes sp. infestation has typically been directed against the immature stages of the mosquito. Larval control intents to contain the infestation levels all over the year or during the rainy season. Its effectiveness is clearly limited because dengue incidence continues to increase and outbreaks continue to occur in areas where such methods have been implemented for many years. Consequently, the potential of adult mosquito control measures for prevention and control of dengue, beyond their use in response to outbreaks, is being reconsidered.5 Luz and others6 concluded in a modeling exercise comparing larval and adult Aedes sp. control at different intensities that six high-efficacy adult vector control applications per year could be the most cost-effective dengue control option.
The most frequently used Aedes sp. adult control measures during epidemics are outdoor and/or indoor space spraying or fogging with insecticide. Indoor spraying with portable equipment can significantly reduce Aedes sp. density by levels of 80–100% in the first five days after application, but the effect wanes quickly over the next five weeks.7,8 There is much controversy over the efficacy of adulticide space spraying from aircraft or truck-mounted equipment for Aedes sp. control and experts conclude that its impact is, at best, limited and of short duration.9,10 Lethal ovitraps11,12 and insecticide-treated materials13–15 are tools that have been investigated in recent years for targeting the adult mosquito. Both approaches offer simple, affordable, low-tech, long-lasting, and potentially acceptable (to communities) solutions for household level dengue vector control. In response to a global dengue research agenda setting from the World Health Organization (WHO) in 2006,16 numerous field studies of both tools were conducted, and results of some of the insecticide-treated curtain (ITC) trials are now reaching the publication stage.
In Latin America, early studies indicated that ITCs had potential to reduce domestic vector infestations,13 although a subsequent study demonstrated that 50% ITC coverage was needed to reduce entomologic infestation by 50%.15 However, the first randomized controlled trial in Asia (in southern Thailand) suggested that the efficacy of ITCs may be limited to houses with complete outer walls and fewer or smaller outside doors and windows,17 and that the potential of ITCs for dengue vector control may not be universal. Clearly, further data are needed from trials in other locations before any judgment can be made. To that end, we report a controlled trial of ITCs at a site located southeast of Bangkok, Thailand.
Materials and Methods
Study setting.
The study was conducted in 2006–2008, 100 km southeast of Bangkok, in the large port city of Laem Chabang (13°6′N, 100°54′E, altitude = 25 meters), Thailand. In July 2005, 42,480 households were registered in Laem Chabang, but many seasonal workers from other regions regularly arrive in the city. The climate is tropical, with an average annual rainfall of 1,600 mm and the heaviest rains occur during May–October (75–340 mm/month). The average daily temperature ranges from 26°C to 29°C. Dengue is endemic to the area. During August 2006–July 2007, a total of 90 hospitalized dengue cases were reported by the local health authorities (approximately 112 cases/100,000 inhabitants). Aedes aegypti is the main Aedes species in environments such as Laem Chabang,18,19 and no Ae. albopictus mosquitoes were captured in adult traps during a mid-2006 survey in the area (11/2005–10/2006, DENCO project INCO-CT-2004-5177085, unpublished data).
In the baseline household survey (September 2006, 1,050 houses) in the intervention clusters20 approximately one-fifth of the households reported having had historically at least one case of dengue fever in the family. The average Breteau Index (BI) was 45 infested containers per 100 houses (95% confidence interval [CI] = 29–64) and the pupae per inhabitant index was 0.5 (95% CI = 0.2–0.7). The main methods applied by the households to decrease all nuisance mosquitoes were electric fans (50%) and space-spraying with locally purchased commercial insecticide aerosols (47%). Abate® larvicide (temephos) was rarely used and was previously recorded in only 12.6% of containers.20 In Laem Chabang, as in other areas of Thailand, indoor toilet water tanks are important household A. aegypti breeding sites.21
In this port city, the routine Aedes sp. vector control activities of the ministry of health were conducted by a team of five persons from the municipal government, together with 110 village health volunteers and with occasional support of the municipal hospital team. Year-round routine activities are limited: inhabitants can procure the larvicide Abate®, which is available for free from the village health volunteers' houses. When a clinical dengue case is reported, all houses within a 100-meter radius of the home of the positive case are fogged with deltamethrin by using portable equipment indoors and outdoors. In addition, two campaigns of intensified routine activities are planned every year, consisting of more active distribution of Abate® and deltamethrin space-spraying with truck-mounted equipment in all streets throughout Laem Chabang municipality.
Study design.
In this community intervention study, we provided ITCs to clusters of houses. The study was set up as a controlled trial. We randomly selected 22 clusters (80–110 houses/cluster, total = 2,032 households) defined by infrastructural boundaries in four urban subdistricts of Laem Chabang, and ensured that there was at least one street width distance between clusters. Sixty-six clusters of 10 houses were used as control clusters, which were randomly selected from the same four urban subdistricts as the intervention clusters but at a distance of at least 75 meters from the intervention houses. The control clusters were chosen in this way to ensure that the vector populations within were beyond the influence of the intervention clusters, and thus expected to exhibit natural seasonal fluctuations, whether influenced by routine vector control interventions.13 We determined the sample size and number of clusters as proposed by Hayes and Bennett,22 which had a power of 80% to detect in intervention clusters a two-fold decrease in the BI at an alpha error level of 0.05 (assuming a between-cluster coefficient of variation of 0.50).
In March 2007, we distributed ITCs to all households in the 22 intervention clusters that had agreed to use them and had given informed consent. The ITCs were distributed by the village health volunteers and distribution was supervised by the municipal vector control program and a team from the Chonburi Regional Disease Control office. The ITCs were made from PermaNet® polyester netting (Vestergaard-Frandsen, Lausanne, Switzerland), treated with a long-lasting formulation of deltamethrin (55 mg/m2) and coated with a protectant (no details disclosed by the manufacturer) to prevent degradation of the insecticide when exposed to ultraviolet light. The manufacturer stated at the time of the trial that this material did not require re-treatment and that its insecticidal effect was expected to last for up to two years or six standard washes (www.vestergaard-frandsen.com/permanet-curtain-e-brochure.pdf). The number of ITCs distributed per house depended on the number of windows in the living area and bedrooms (up to a maximum of five curtains/house: four window curtains and one door curtain). During distribution, information leaflets were distributed and at least one person in every household received instructions on the use and maintenance of the ITC through person-to-person communication. In intervention and control clusters, all routine dengue-related activities performed by the local health authorities continued without interference.
Data collection.
Entomologic surveys were conducted 6 months and 18 months after ITC distribution. In intervention clusters, half of the houses were selected, at each data collection point, through systematic random sampling for entomologic surveys and assessment of ITC use, and all houses in control clusters were sampled for entomologic monitoring. Each entomologic survey was conducted for nine days by 10 teams of two persons already involved in the routine vector control actions. These persons received additional training before executing the study surveys and were supervised by an entomologist. In all houses, containers were inspected for the presence of larvae and pupae. For larvae, only presence or absence was recorded, but if pupae were found they were counted. In October 2008, all pupae were collected, transported to the laboratory, and allowed to emerge for genus identification. The number of ITCs in use in each household sampled was observed and recorded on the data-collection form of the entomologic surveys in intervention clusters.
Aedes sp. eggs collected in indoor and outdoor ovitraps in field sites in January 2007 (before ITC were distributed) and October 2008 were reared to adults in the insectary (Entomology Laboratory of the Department of Medical Entomology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand). Adult mosquitoes (1,200 at both times of collection) were screened for deltamethrin (0.05%) susceptibility by using the standard WHO tube bioassay protocol.23
Data analysis.
Aedes sp. infestation levels were the outcome measures. We calculated per cluster and survey round the House Index (HI, number of houses with at least 1 container with immature Aedes sp. stages/100 inspected houses), the BI (number of containers with immature Aedes sp. stages/100 inspected houses) and Pupae per Person Index (PPI, number of Aedes sp. pupae/inhabitant). We also calculated 95% confidence intervals (CIs) around the average estimates per study arm at each time point and used a negative binomial regression model, which took into account the cluster design.
We developed three indicators per intervention cluster to describe ITC coverage: the percentage of houses with at least one ITC, the mean number of ITC for houses with at least one ITC, and the mean number of ITCs per house. The third variable was used in multivariate analysis and categorized (at the cluster level) into an average of 0 ITCs/house (i.e., control clusters), an average of > 0 but < 2 ITCs/house, and an average of ≥ 2 ITCs/house.
To estimate the effect of ITC deployment on Aedes sp. infestation and the influence of coverage at the cluster level, we constructed generalized linear random effect regression models with a negative binomial link function that took into account the cluster design, cluster sizes, and the dependence of observations. The BI and PPI were the dependent variables. Each of the 22 intervention clusters and the 66 control clusters contributed 1 data point at each of the entomologic survey rounds. The models included the presence or absence of intervention or the mean number of ITCs/house as the independent categorical variable. The P values of likelihood-ratio tests are reported.
From the October 2008 survey data, the proportion of immature stages belonging to the genus Aedes was calculated. For the deltamethrin susceptibility testing pre-intervention and post-intervention, the observed percentage of dead mosquitoes after 24 hours was calculated and classified according WHO interpretation: susceptible (mortality rate = 98–100%), tolerant (mortality rate 80–97%), and resistant (mortality rate < 80%).24
Data were analyzed by using Stata version 10.0 (StataCorp LP, College Station, TX).
Ethics.
This study was approved by the Institutional Review Board of the Institute of Tropical Medicine (Antwerp, Belgium) and the ethics committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok. Community representatives from each participating cluster approved the intervention and written informed consent was obtained from every household included in the study. The ITCs were made from material that has been approved for use as insecticide on bed nets by the WHO Pesticide Evaluation Scheme. The manufacturer (Vestergaard-Frandsen) donated the ITCs for the study, but the company was not involved in the study design, data collection, and analysis or interpretation and reporting of results. The trial was registered at ClinicalTrials.gov (no. NCT 00883441).
Results
The bioassays conducted to determine deltamethrin susceptibility demonstrated consistently that the mosquitoes collected in the study clusters before and after the intervention were tolerant to deltamethrin (mortality rates = 87% and 84%, respectively). Of the 1,266 pupae and larvae collected in the October 2008 survey, 96.9% were Aedes sp. and the remainder were Culex sp. Given the high percentage of Aedes sp. collected, we assumed that all container-breeding species in the study larval surveys were Aedes sp.25
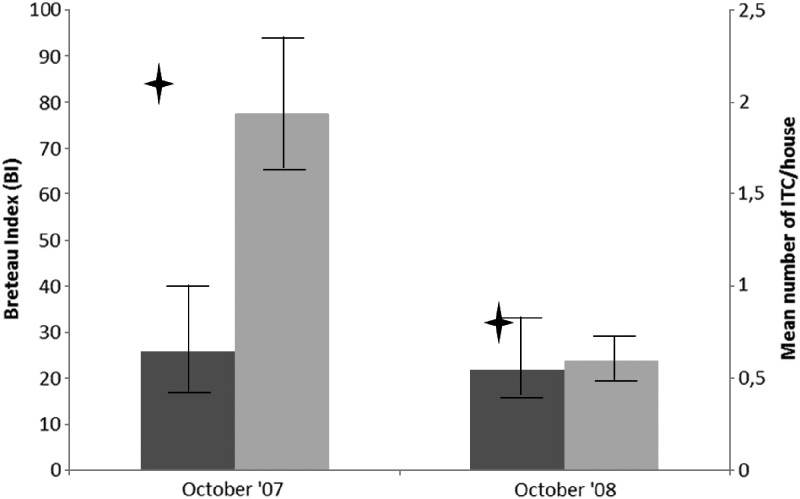
In October 2007 and October 2008, 6 and 18 months after ITC distribution, 1,101 and 1,013 houses were surveyed in the intervention clusters and 661 and 659 houses were surveyed in the control clusters, respectively. In October 2007, 6 months after ITC distribution, the proportion of households with at least 1 ITC hanging was 70.5% (95% CI = 59.6–81.5%), and the mean number of curtains (all houses included) was 2.17 ITCs/house (95% CI = 1.70–2.64) (Figure 1). Eighteen months after distribution, the corresponding figures were 33.2% (95% CI = 22.1–44.3%) and 0.8 ITCs/house (95% CI = 0.52–1.17). In households that continued using ITC, the mean number of ITC/house stayed relatively stable over time: 3.0 ITCs/house (95% CI = 2.7–3.3) at 6 months after distribution and 2.3 (95% CI = 2.0–2.6) at 18 months after distribution.
Figure 1.
Breteau indices (BI) in intervention and control clusters and insecticide-treated curtain (ITC) coverage in the intervention clusters, Laem Chabang, Thailand, 2007–2008. Error bars indicate 95% CI. Grey bars = control area; black bars = intervention area; cross = mean number of ITC/house.
In October 2007, the BI was 25.8 (95% CI = 15.4–40.1) in the intervention clusters and 77.6 (95% CI = 64.1–93.4) in the control clusters (Figure 1), the HI was 17.5% (95% CI = 11.3–27.2%) and 39.5% (95% CI = 34.0–45.8%), and the PPI was 0.21 (95% CI = 0.13–0.46) and 0.57 (95% CI = 0.37–0.91), respectively. The indoor/outdoor distribution of containers positive for pupae was similar in intervention and control areas (75.6% and 67.7% indoor respectively; P = 0.11). At that time point, when ITC coverage was on average still high, the effect of ITC deployment (using or not using ITCs in the clusters) on the BI and PPI was significant: the incidence rate ratio was 0.32 (95% CI = 0.21–0.49, P < 0.001) and 0.42 (95% CI = 0.18–0.98, P = 0.04), respectively.
In October 2008, when ITC coverage had decreased drastically, the BI was 21.8 (95% CI = 12.2–29.4) and 23.8 (95% CI = 19.8–28.6) in intervention and control clusters, respectively (Figure 1); the HI was 14.2% (95% CI = 9.6–21.1%) and 19.1% (95% CI = 16.0–22.8%); and the PPI was 0.19 (95% CI = 0.08–0.35) and 0.09 (95% CI = 0.03–0.14), respectively. The indoor/outdoor distribution of containers found positive for pupae was similar in intervention and control areas (66.1% and 54.8% indoor, respectively, P = 0.24). At that time point, the deployment of ITCs (using or not using ITCs in the clusters) was not significantly associated with entomologic infestation: the incidence rate ratio for BI was 0.80 (95% CI = 0.53–1.20, P = 0.28).
We further analyzed the relationship between mean number of ITCs/house and BI/PPI values (Table 1). The BI was coverage dependent (P < 0.001) at six months post-distribution and a similar trend, although not significant, was observed for the PPI. At 18 months after ITC distribution, when on average < 1 ITC/house was still in use, the presence of an ITC no longer affected entomologic indices, and there was no detectable trend in function of coverage (Table 1).
Table 1.
Aedes aegypti infestation ratios (Breteau Index and pupae per person index) in function of the mean number of ITCs/house in periods with high and low ITC coverage, Laem Chabang, Thailand 2007–2008*
| October 2007 (ITC coverage = 70.5%) | October 2008 (ITC coverage = 33.2%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breteau index | Pupae per person index | Breteau index | Pupae per person index | |||||||||
| Mean no. ITCs/house | IRR | 95% CI | P | IRR | 95% CI | P | IRR | 95% CI | P | IRR | 95% CI | P |
| 0† | 1 | Ref. | 0.001 | 1 | Ref. | 0.09 | 1 | Ref. | 0.32 | 1 | Ref. | 0.23 |
| > 0 and < 2 ‡ | 0.37 | 0.21–0.65 | 0.62 | 0.19–1.99 | 0.91 | 0.59–1.40 | 2.72 | 0.79–9.28 | ||||
| ≥ 2‡ | 0.28 | 0.16–0.49 | 0.25 | 0.08–0.73 | 0.45 | 0.17–1.21 | 1.72 | 0.11–26.2 | ||||
ITC = insecticide-treated net; IRR = incidence rate ratio; CI = confidence interval; Ref. = referent. P values were estimated by using the likelihood-ratio test.
Control clusters.
Intervention clusters.
Discussion
The presence of ITCs can decrease the BI and PPI in a setting in which the tools are well accepted and largely used by the households, but the scale of effect depends on the coverage and the number of curtains per house attained. The outcomes of this study also demonstrated that when the ITCs are only used in a modest proportion of houses, their deployment does not affect Aedes sp. infestations.
The controlled trial design is a major strength of this study. It enabled us to control for seasonal and temporal trends in vector density and influencing extraneous factors, such as routine vector control interventions. From a public health perspective, it is promising that results were obtained using an implementation model that mimics the reality of routine operational conditions, in which village health volunteers, which form part of the routine vector control program, distributed the ITCs. However, it is a limitation that we could not directly monitor adult Aedes sp. populations because of operational reasons and resource constraints, and we could not measure dengue transmission. In addition to the BI, we demonstrated an effect on the PPI, which is considered a more accurate proxy for adult mosquito abundance and thus dengue transmission risk.26 An unfortunate event was the loss of entomologic information from the control area at baseline, which precluded us from including pre-intervention infestation levels in the analysis, but this limitation did not invalidate observed results at 6 and 18 months post-intervention.
Although it was not the objective of this study, nor always possible, to provide barriers at all entry points of the houses in the study site, most houses were small and up to five ITCs were sufficient to cover all main windows and the main entry door. In addition, the numbers provided were based on the windows and doors that householders accepted to have covered. The decrease in ITC coverage and the determinants for uptake and continued use in this study site in Thailand had already been discussed, specifically for resident population, elsewhere.20 This finding highlights that additional promotional activities or community involvement needs to take place to sustain ITC coverage at a high level over time.
The difference in BI and PPI in the intervention and control areas six months after distribution can most likely be attributed to the effect of high coverage of ITCs. By design, we can rule out a differential influence on the intervention and control clusters of temperature, rainfall, or other environmental factors. There were no differences in routine vector control actions between intervention and control clusters. The local Aedes sp. mosquitoes were not resistant to deltamethrin, and ITCs still had a residual insecticidal effect > 98% at 12 months post-distribution, as was reported for this intervention project.27 The magnitude of ITC effect we observed, showing a relative effectiveness of 0.32 for the BI, is within the range of effect of other dengue vector control interventions,28 such as integrated vector management, which showed a relative effectiveness of 0.33 and environmental management of 0.71. Although a correlation between density of pupae and dengue virus–infected mosquitoes at the household level (which is correlated with dengue infection in children) has recently been demonstrated in a rural area of north central Thailand,29 there is no known critical threshold below which entomologic indices need to fall to achieve impact on transmission. Consequently, it is not possible to conclude whether the effects shown by this or other studies assessing the deployment of ITC will be sufficient to have an effect on dengue.
The absence of an effect in intervention clusters 18 months after ITC distribution was most likely caused by low coverage with ITCs. Their coverage-dependent effectiveness has been described,15 but the lack of an effect has not been described. At that time, local Aedes sp. strains were still susceptible to deltamethrin, and although it is possible that the residual insecticidal activity of the insecticide on ITCs had decreased over time, we do not suspect, which is consistent with observations on long-lasting insecticidal nets,30 that this activity decreased dramatically between 12 (when the effect was 98%27) and 18 months of use. The absence of an effect is unlikely to be caused by a spillover effect13 because control clusters were situated at a minimum of 75 meters from intervention clusters, and this phenomenon was not observed in October 2007, when coverage was still high and ITCs were already used for six months. We observed that Aedes sp. infestation levels at the last survey in intervention and control areas were below the October 2007 levels for the control area. Infestation levels are known to have a high variability over time, as can be observed in published reports on longitudinal follow-up of entomologic indices over multiple years.31–34 These levels depend on various factors, such as temperature, atmospheric moisture, rainfall, socioeconomic and environmental risk factors, besides control interventions.16 We cannot rule out that other control interventions were implemented around this last survey time, but if this was true, they were implemented in a similar way in the entire city.
The outcomes of this trial demonstrate that ITCs had a coverage-dependent effect on Aedes sp. infestations in a setting in southeast Asia in which a closed house design predominated and where Aedes sp. infestation levels were moderate. However, although this effect may be an optimistic finding at a time when the spread of dengue seems unstoppable, a number of important issues remain. This control method can be costly35 and until it is possible to deploy insecticides other than pyrethroids on netting, it will not provide a solution to emerging pyrethroid resistance in dengue vectors. What effect of ITC can be expected in areas with low indices and with existing intensive routine vector control programs? In such situations, could ITC be combined with other adulticidal interventions or would this lead to disappointing results, as in a recent trial combining long-lasting insecticide treated bed nets and indoor residual spraying for malaria control,36 or could such combinations be effective in targeting multiple vectors in future integrated control program design?
ACKNOWLEDGMENTS
We thank the municipality and population of Laem Chabang for participating in the study and using ITC, and Vestergaard-Frandsen for providing ITCs free of charge for the study. This study reports partial results of Workpackage 4 of the project DENCO-Towards Successful Dengue Prevention and Control.
Footnotes
Financial support: This study was supported by the European Union project DENCO: INCO-CT-2004-517708.
Authors' addresses: Veerle Vanlerberghe, Institute of Tropical Medicine, Nationalestraat 155, 2000 Antwerp, Belgium, E-mail: vvanlerberghe@itg.be. Yuwadee Trongtokit and Chamnarn Apiwathnasorn, Mahidol University, Thailand, E-mails: ytrongtokit@gmail.com and tmcaw@mahidol.ac.th. Somchai Jirarojwatana and Ravisara Jirarojwatana,Regional Office of Disease Prevention and Control, Chon Buri, Thailand, E-mails: sjiraroj@yahoo.com and rjiraroj@hotmail.com. Audrey Lenhart and Philip J. McCall, Liverpool School of Tropical Medicine, Liverpool L3 5QA, United Kingdom, E-mails: ajl8@cdc.gov and mccall@liv.ac.uk. Patrick Van der Stuyft, Institute of Tropical Medicine and University of Ghent, Ghent, Belgium, E-mail: pvdstuyft@itg.be.
References
- 1.Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, Dung NM, Hung NT, Hien TT, Farrar JJ. The WHO dengue classification and case definitions: time for a reassessment. Lancet. 2006;368:170–173. doi: 10.1016/S0140-6736(06)69006-5. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ. 2002;324:1563–1566. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Global Strategy for Dengue Prevention and Control, 2012–2020. Geneva: World Health Organization; 2012. [Google Scholar]
- 4.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenoughe A, Viviani S, Torniporth NG, Lang J. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 5.Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008;5:e68. doi: 10.1371/journal.pmed.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luz PM, Vanni T, Medlock J, Paltiel AD, Galvani AP. Dengue vector control strategies in an urban setting: an economic modelling assessment. Lancet. 2011;377:1673–1680. doi: 10.1016/S0140-6736(11)60246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro M, Quintana N, Quinones PM. Evaluating two pyrethroids in dengue vector control in Putumayo, Colombia [in Spanish] Rev Salud Publica (Bogota) 2007;9:106–116. doi: 10.1590/s0124-00642007000100011. [DOI] [PubMed] [Google Scholar]
- 8.Mani TR, Arunachalam N, Rajendran R, Satyanarayana K, Dash AP. Efficacy of thermal fog application of deltacide, a synergized mixture of pyrethroids, against Aedes aegypti, the vector of dengue. Trop Med Int Health. 2005;10:1298–1304. doi: 10.1111/j.1365-3156.2005.01522.x. [DOI] [PubMed] [Google Scholar]
- 9.Reiter P, Gubler DJ. Surveillance and control of urban dengue vectors. In: Gubler DJ, Kuno G, editors. Dengue and Dengue Hemorrhagic Fever. New York: CAB International; 1997. pp. 425–463. [Google Scholar]
- 10.Esu E, Lenhart A, Smith L, Horstick O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop Med Int Health. 2010;15:619–631. doi: 10.1111/j.1365-3156.2010.02489.x. [DOI] [PubMed] [Google Scholar]
- 11.Perich MJ, Kardec A, Braga IA, Portal IF, Burge R, Zeichner BC, Brogdon WA, Wirtz RA. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med Vet Entomol. 2003;17:205–210. doi: 10.1046/j.1365-2915.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- 12.Williams CR, Ritchie SA, Long SA, Dennison N, Russell RC. Impact of a bifenthrin-treated lethal ovitrap on Aedes aegypti oviposition and mortality in north Queensland, Australia. J Med Entomol. 2007;44:256–262. doi: 10.1603/0022-2585(2007)44[256:ioablo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Kroeger A, Lenhart A, Ochoa M, Villegas E, Levy M, Alexander N, McCall PJ. Effective control of dengue vectors with curtains and water container covers treated with insecticide in Mexico and Venezuela: cluster randomised trials. BMJ. 2006;332:1247–1252. doi: 10.1136/bmj.332.7552.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seng CM, Setha T, Nealon J, Chantha N, Socheat D, Nathan MB. The effect of long-lasting insecticidal water container covers on field populations of Aedes aegypti (L.) mosquitoes in Cambodia. J Vector Ecol. 2008;33:333–341. doi: 10.3376/1081-1710-33.2.333. [DOI] [PubMed] [Google Scholar]
- 15.Vanlerberghe V, Villegas E, Oviedo M, Baly A, Lenhart A, McCall PJ, Van der Stuyft P. Evaluation of the effectiveness of insecticide treated materials for household level dengue vector control. PLoS Negl Trop Dis. 2011;5:e994. doi: 10.1371/journal.pntd.0000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.TDR . Report of the Scientific Working Group Meeting on Dengue. Geneva, October 1–5, 2006. Geneva: World Health Organization; 2006. TDR/SWG/08. [Google Scholar]
- 17.Lenhart A, Trongtokit Y, Alexander N, Apiwathnasorn C, Satimai W, Vanlerberghe V, Van der Stuyft P, McCall PJ. A cluster randomized trial of insecticide treated curtains for dengue vector control in Thailand. Am J Trop Med Hyg. 2012;88:254–259. doi: 10.4269/ajtmh.2012.12-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponlawat A, Scott JG, Harrington LC. Insecticide susceptibility of Aedes aegypti and Aedes albopictus across Thailand. J Med Entomol. 2005;42:821–825. doi: 10.1603/0022-2585(2005)042[0821:ISOAAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Tsuda Y, Suwonkerd W, Chawprom S, Prajakwong S, Takagi M. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban-rural gradient and the relating environmental factors examined in three villages in northern Thailand. J Am Mosq Control Assoc. 2006;22:222–228. doi: 10.2987/8756-971X(2006)22[222:DSDOAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Vanlerberghe V, Villegas E, Jirarojwatana S, Santana N, Trongtorkit Y, Jirarojwatana R, Srisupap W, Lefevre P, Van der Stuyft P. Determinants of uptake, short-term and continued use of insecticide-treated curtains and jar covers for dengue control. Trop Med Int Health. 2011;16:162–173. doi: 10.1111/j.1365-3156.2010.02668.x. [DOI] [PubMed] [Google Scholar]
- 21.Tun-Lin W, Lenhart A, Nam VS, Rebollar-Tellez E, Morrison AC, Barbazan P, Cote M, Midega J, Sanchez F, Manrique-Saide P, Kroeger A, Nathan MB, Meheus F, Petzold M. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Trop Med Int Health. 2009;14:1143–1153. doi: 10.1111/j.1365-3156.2009.02341.x. [DOI] [PubMed] [Google Scholar]
- 22.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28:319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization . Instructions for Determining the Susceptibility or Resistance of Adult Mosquitoes to Organochlorine, Organophosphate and Carbamate Insecticides - Diagnostic Test. Geneva: World Health Organization; 2001. WHO/VBC/81.806. [Google Scholar]
- 24.World Health Organization . Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bioefficacy and Persistence of Insecticides on Treated Surfaces. Geneva: World Health Organization; 1998. Report of the WHO Informal Consultation. [Google Scholar]
- 25.Arredondo-Jimenez JI, Valdez-Delgado KM. Aedes aegypti pupal/demographic surveys in southern Mexico: consistency and practicality. Ann Trop Med Parasitol. 2006;100((Suppl 1)):S17–S32. doi: 10.1179/136485906X105480. [DOI] [PubMed] [Google Scholar]
- 26.Focks DA, Chadee DD. Pupal survey: an epidemiologically significant surveillance method for Aedes aegypti: an example using data from Trinidad. Am J Trop Med Hyg. 1997;56:159–167. doi: 10.4269/ajtmh.1997.56.159. [DOI] [PubMed] [Google Scholar]
- 27.Vanlerberghe V, Trongtokit Y, Cremonini L, Jirarojwatana S, Apiwathnasorn C, Van der Stuyft P. Residual insecticidal activity of long-lasting deltamethrin-treated curtains after 1 year of household use for dengue control. Trop Med Int Health. 2010;15:1067–1071. doi: 10.1111/j.1365-3156.2010.02582.x. [DOI] [PubMed] [Google Scholar]
- 28.Erlanger TE, Keiser J, Utzinger J. Effect of dengue vector control interventions on entomological parameters in developing countries: a systematic review and meta-analysis. Med Vet Entomol. 2008;22:203–221. doi: 10.1111/j.1365-2915.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 29.Yoon, IK, Getis A, Aldstadt J, Rothman AL, Tannitisupawong D, Koenraadt CJ, Fansiri T, Jones JW, Morrison AC, Jarman RG, Nisalak A, Mammen MP, Jr, Thammapolo S, Srikiatkhachorn A, Green S, Libraty DH, Gibbons RV, Endy T, Pimgate C, Scott TW. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis. 2012;6:e1730. doi: 10.1371/journal.pntd.0001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilian A, Byamukama W, Pigeon O, Atieli F, Duchon S, Phan C. Long-term field performance of a polyester-based long-lasting insecticidal mosquito net in rural Uganda. Malar J. 2008;7:49. doi: 10.1186/1475-2875-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro M, Sanchez L, Perez D, Carbonell N, Lefevre P, Vanlerberghe V, Van der Stuyft P. A community empowerment strategy embedded in a routine dengue vector control programme: a cluster randomised controlled trial. Trans R Soc Trop Med Hyg. 2012;106:315–321. doi: 10.1016/j.trstmh.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Morrison AC, Gray K, Getis A, Astete H, Sihuincha M, Focks D, Watts D, Stancil JD, Olson JG, Blair P, Scott TW. Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J Med Entomol. 2004;41:1123–1142. doi: 10.1603/0022-2585-41.6.1123. [DOI] [PubMed] [Google Scholar]
- 33.Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Zhou H, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J Med Entomol. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- 34.Toledo Romani ME, Vanlerberghe V, Perez D, Lefevre P, Ceballos E, Bandera D, Baly A, Van der Stuyft P. Achieving sustainability of community-based dengue control in Santiago de Cuba. Soc Sci Med. 2007;64:976–988. doi: 10.1016/j.socscimed.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 35.Baly A, Flessa S, Cote M, Thiramanus T, Vanlerberghe V, Villegas E, Jirarojwatana S, Van der Stuyft P. The cost of routine Aedes aegypti control and of insecticide-treated curtain implementation. Am J Trop Med Hyg. 2011;84:747–752. doi: 10.4269/ajtmh.2011.10-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbel V, Akogbeto M, Damien GB, Djenontin A, Chandre F, Rogier C, Moiroux N, Chabi J, Banganna B, Padonou GG, Henry MC. Combination of malaria vector control interventions in pyrethroid resistance area in Benin: a cluster randomised controlled trial. Lancet Infect Dis. 2012;12:617–626. doi: 10.1016/S1473-3099(12)70081-6. [DOI] [PubMed] [Google Scholar]