Abstract
The tourniquet test (TT) is a physical examination maneuver often performed on patients suspected of having dengue. It has been incorporated into dengue diagnostic guidelines and is used in clinical studies. However, little is known about TT performance characteristics in different patient types or epidemiologic conditions. In the dengue-endemic city of Iquitos, Peru, we performed TTs and dengue laboratory assays on 13,548 persons with febrile disease, recruited through either active (n = 1,095) or passive (n = 12,453) surveillance. The sensitivity was 52% and 56%, the specificity was 58% and 68%, the positive predictive value was 45% and 55%, and the negative predictive value was 64% and 69% for persons enrolled in active and passive surveillance, respectively. We demonstrated that the TT was more sensitive identifying dengue disease in women and those of younger age and that sensitivity increased the later a person came to a medical clinic for care.
Introduction
Dengue fever (DF), a mosquito-borne viral disease, threatens nearly four billion persons in at least 128 countries, and no effective vaccine or antiviral drug is currently licensed.1 Infection with any of the four dengue virus serotypes 1–4 (DENV-1, DENV-2, DENV-3, and DENV-4) can cause outcomes that range from asymptomatic or mild disease to fatal dengue hemorrhagic fever (DHF)/dengue shock syndrome. Typical signs and symptoms associated with most symptomatic infections include fever, headache, malaise, and body aches. Because of these often non-specific manifestations, dengue is frequently mistaken for other endemic febrile diseases, including typhus, leptospirosis, and malaria.2–4 Early diagnosis of DENV infection provides many benefits, including the timely application of management algorithms and supportive care.5 Furthermore, the exclusion of dengue from the differential diagnosis may help identify other infections with similar presentations. Although no antiviral drug is available to treat DENV infection, various antibacterial and antiparasitic medicines are available to treat diseases with manifestations similar to dengue.
A handful of assays that rapidly diagnose dengue exist, including immunochromatographic assays and polymerase chain reaction.6,7 Nevertheless, cost remains a hurdle in the wide-scale adoption of these assays in poor and under-served areas that are often disproportionally affected by dengue. Therefore, diagnosis and classification often rely on more readily available and affordable means.
The tourniquet test (TT) is a physical examination technique that can identify and stratify dengue disease. Infection with DENV may result in increased capillary permeability, a physiological state that the TT exploits by applying sustained pressure to these small vessels.8 The resulting petechiae (cutaneous pinpoint, non-raised, purplish-red spots) can be found in patients with DF and DHF. The TT is performed by inflating a blood pressure cuff midway between the systolic and diastolic blood pressure on a person's upper arm. After five minutes, if the number of petechiae counted in an area exceeds a certain number (i.e., ≥ 20 petechiae in a one square inch area), then the test result is considered positive. In 1997, the World Health Organization created guidelines for the identification and management of DHF.9 These included a positive TT result as a criterion, along with other findings, such as mucosal bleeding, in the diagnosis of DHF. The 2009 World Health Organization Dengue Guidelines for Diagnosis, Treatment, Prevention, and Control listed a positive TT result as a criterion for the diagnosis of probable dengue.5
Despite the widespread use of the TT in dengue clinical and research settings, little is known about the performance of this test across demographic groups and epidemiologic conditions. The objectives of this study were to assess TT use in discriminating dengue from non-dengue acute febrile illnesses in Iquitos, Peru, and to compare TT performance among different age groups, sexes, days of illness, and epidemiologic conditions.
Methods
Two separate recruitment strategies were used in this study, a clinic-based passive febrile surveillance component and a community-based active febrile surveillance component. Passive surveillance was covered by two protocols, protocol #2000.0006, approved by the U.S. Naval Medical Research Center (Silver Spring, MD) Institutional Review Board, and protocol #2010.0010, approved by the U.S. Naval Medical Research Unit No. 6 (NAMRU-6, Lima, Peru) Institutional Review Board. All persons < 18 years of age required consent from a parent or legal guardian, and all persons 8–17 years of age were also required to give written assent. Active surveillance was covered by a separate protocol, approved by institutional review boards at the University of California at Davis (protocol #2007-15244), Naval Medical Research Center (protocol #2005.0009), and NAMRU-6 (protocol #2007.0007). Written consent was obtained from all participating adults and the parents of participants 3–18 years of age; written assent was also obtained from persons 7–18 years of age. Each protocol was in compliance with all U.S. Federal regulations governing the protection of human subjects. All protocols were reviewed by the Ministry of Health of Peru, as well as local public health officials in Iquitos, Peru.
The study took place in Iquitos, a tropical city with approximately 400,000 persons located in the northeast part of Peru that experiences yearly surges in dengue cases.10,11 A TT was performed at each acute-phase visit by healthcare personnel in the clinic or home for the passive or active surveillance, respectively. This procedure was performed by inflating a blood pressure cuff to a point midway between the systolic and diastolic blood pressures. The cuff remained inflated for five minutes on a person's upper arm. Petechiae were then counted on the volar area of a person's forearm distal to the blood pressure cuff. A test result was considered positive if ≥ 20 petechiae were counted in a one square inch area. Standardized training in administering the TT was offered to all study personnel by one of three physicians (co-authors SV, CR, and IB).
Passive surveillance.
Passive surveillance was conducted during June 2002–June 2011 in 13 outpatient health clinics throughout the city of Iquitos. At each site, participants were enrolled with the same inclusion criteria used in prior clinical and epidemiologic studies involving these protocols.10,12 These criteria were 1) presence of fever ≥ 38.0°C occurring for ≤ 7 days and 2) no obvious source of infection such as cellulitis, dental abscess, influenza-like illness, or urinary tract infection. In addition to the acute-phase visit, persons were asked to return for a follow-up visit, usually 10–21 days later. Serum samples were collected at both the acute-phase and follow-up visit.
Active surveillance.
Two separate active surveillance cohort populations were monitored for dengue-like illness during the course of the study. The first cohort study (approximately 4,500 participants) was initiated in geographically diverse neighborhoods across Iquitos during September 2006–March 2011.13 The second cohort study (approximately 6,000 participants) was initiated in the Maynas and Tupac Amaru neighborhoods in April 2008 and is ongoing. This study uses data collected before June 2011. The surveillance structures for the two cohorts were similar. Participants were visited three times a week in their residence and asked about the occurrence of fever or other symptoms indicative of DENV infection. If a person affirmed the presence of fever and lacked an obvious source of infection (such as cellulitis, dental abscess, influenza-like illness, or urinary tract infection), a history, physical examination (including TT), and serum sample were obtained. Persons with and without DENV infection received subsequent daily medical examinations (including TTs) until two days after symptom abatement. A follow-up serum sample was obtained 14–21 days after the initial visit and compared with the acute-phase sample by enzyme-linked immunosorbent assay (ELISA) to assess for seroconversion.
Laboratory testing and determination of infection.
At NAMRU-6, acute samples were evaluated for infectious DENV by using culture on Vero and C6/36 cells and a standard indirect immunofluorescence assay as described.12 In addition, culture and indirect immunofluorescence assay were also performed for the following arboviruses known to cause undifferentiated fever in the region: Mayaro virus (MAYV), Oropouche virus (OROV), the group C arboviruses (GRCV), and Venezuelan equine encephalitis virus (VEEV).12 A subset of acute-phase samples was also evaluated by using reverse transcription–polymerase chain reaction during outbreak situations or if requested by the site clinician.7 Serologic testing was performed by using an ELISA for DENV IgM on acute-phase and follow-up samples as described.12 In addition to DENV, IgM ELISAs were also performed on the acute-phase and follow-up samples for MAYV, OROV, GRCV, and VEEV.
Based on the aforementioned assays, each participant was assigned one of the following diagnoses: DENV, MAYV, OROV, GRCV, VEEV, or negative. Detection of virus or RNA was considered definitive for the viruses listed above, and for cases diagnosed by ELISA, seropositivity was defined as the presence of IgM in either the acute-phase or follow-up sample. If a sample had IgM to more than one arbovirus, the following criteria were used to define a diagnosis: 1) an increase in IgM titer between acute-phase and convalescent-phase samples was considered more definitive than merely the presence of IgM in one or both samples; 2) if titers did not show an increase, but one arbovirus possessed a > 2-fold titer than all others, it was considered to be the cause of the infection; and 3) when titers were similar between two or more arboviruses, the sample was excluded from analysis.
Data analysis.
Exploratory data analysis (Fisher's exact test and Pearson's chi-square test) was conducted by using SAS version 9.314 and R version 2.15.15 Positive predictive value (PPV) was defined as the proportion of true positives to all positives; negative predictive value (NPV) as the proportion of true negatives to all negatives; and accuracy as the proportion of true positives and true negatives to all samples. To assess the relationship between TT positivity and various demographic variables, a mixed effects logistic regression model was constructed using the lme4 package in R version 2.15. Fixed effects included participant age (in 10-year intervals, e.g., 0–10, 11–20), sex, and day of illness at presentation, and year was included as a random effect. A separate model was developed to determine if patient attributes (age, sex, and day of illness) could be used to better predict dengue from non-dengue. Age, sex, day of illness, and TT result, as well as interaction terms between TT result and the other variables, were included in the initial model. Based on the Akaike information criterion, a model that included TT and the interaction terms (TT with age, sex, and day of illness) was a better fit than the full model.
Results
In total, 12,453 and 1,095 persons with TT results during an acute febrile illness participated in the passive and active surveillance portions of the study, respectively. These two groups yielded 5,103 (41%) and 439 (40%) persons with dengue, respectively. Characteristics of the two surveillance methods and the persons recruited in each method are shown in Table 1.
Table 1.
Characteristics of the active and passive surveillance networks, Peru*
| Variable | Type of surveillance | |
|---|---|---|
| Active | Passive | |
| Site of participant evaluation | Home | Medical clinic |
| Study years | 2006–2011 | 2002–2011 |
| TT performed on consecutive days | Yes | No |
| No. persons recruited | 1,095 | 12,453 |
| No. (%) persons without dengue | 656 (60) | 7,350 (59) |
| No. (%) persons with dengue | 439 (40) | 5,103 (41) |
| Method of dengue diagnosis, no. (%) | ||
| Culture+ (regardless of PCR/ELISA results) | 217 (49) | 2,693 (53) |
| PCR+/culture– (regardless of ELISA results) | 95 (22) | 183 (3) |
| ELISA+/culture–/PCR– | 127 (29) | 2,227 (44) |
| Serotype, no. (%)† | ||
| DENV-1 | 5 (2) | 12 (1) |
| DENV-2 | 84 (27) | 325 (11) |
| DENV-3 | 29 (9) | 1728 (60) |
| DENV-4 | 194 (62) | 811 (28) |
| Age, years, no. (%)‡ | ||
| 0–10 | 131 (12) | 992 (8) |
| 11–20 | 378 (35) | 3,990 (32) |
| 21–30 | 221 (20) | 3,348 (27) |
| 31–40 | 116 (11) | 1,975 (16) |
| 41–50 | 111 (10) | 1,221 (10) |
| 51–60 | 80 (7) | 657 (5) |
| > 60 | 55 (5) | 266 (2) |
| Sex, no. (%) | ||
| M | 454 (41) | 6,166 (50) |
| F | 641 (59) | 6,287 (50) |
TT = tourniquet test; PCR = polymerase chain reaction; ELISA = enzyme-linked immunosorbent assay; DENV = dengue virus.
Serotype information was available only for persons given a diagnosis by culture or PCR.
Age information was not available for 3 active surveillance and 4 passive surveillance persons.
Overall TT performance and performance during high-transmission periods.
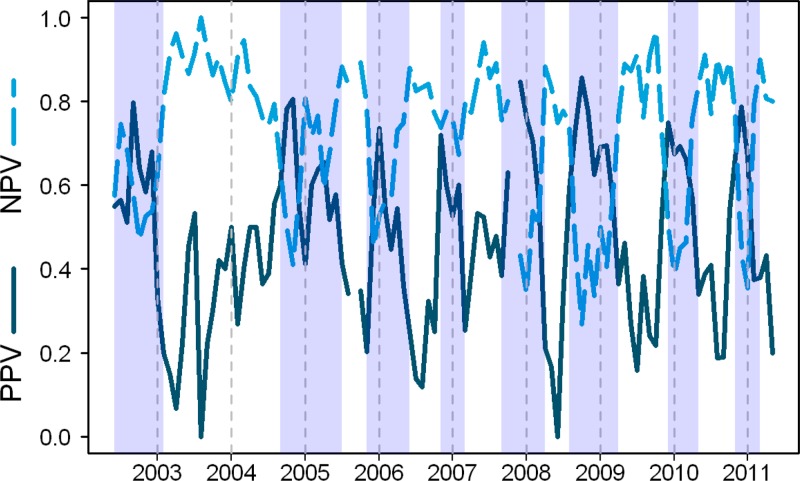
Sensitivity, specificity, PPV, and NPV of the TT obtained through active and passive surveillance are shown in Table 2. Tourniquet test sensitivity (Spearman's correlation coefficient = 0.25, 95% confidence interval [CI] = 0.05–0.44) and specificity (–0.33, 95% CI = –0.53 to –0.14]) were correlated with monthly dengue incidence. The variation in NPV and PPV over time and during varying transmission periods is shown in Figure 1.
Table 2.
Performance characteristics of the tourniquet test (TT) in each of two surveillance methods, Peru*
| Variable | Passive surveillance, no. persons | Active surveillance,† no. persons | ||
|---|---|---|---|---|
| TT+ | TT– | TT+ | TT– | |
| DENV+ | 2,852 | 2,251 | 226 | 213 |
| DENV– | 2,359 | 4,991 | 273 | 383 |
| Sensitivity, % | 56 | 52 | ||
| Specificity, % | 68 | 58 | ||
| PPV, % | 55 | 45 | ||
| NPV, % | 69 | 64 | ||
DENV = dengue virus; PPV = positive predictive value; NPV = negative predictive value.
At presentation.
Figure 1.
Monthly tourniquet test positive predictive value (PPV, solid line) and negative predictive value (NPV, dashed line) for dengue, Peru, 2002–2011. Vertical dashed lines demarcate the start of each year. Shaded areas indicate time periods of elevated dengue transmission (two or more consecutive months where dengue cases [absolute cases or as a proportion of all febrile cases] exceed the median for the study period). Breaks indicate months (September 2005 and November 2007) where no data were available.
Tourniquet test performance by day of presentation, age, sex, and DENV serotype.
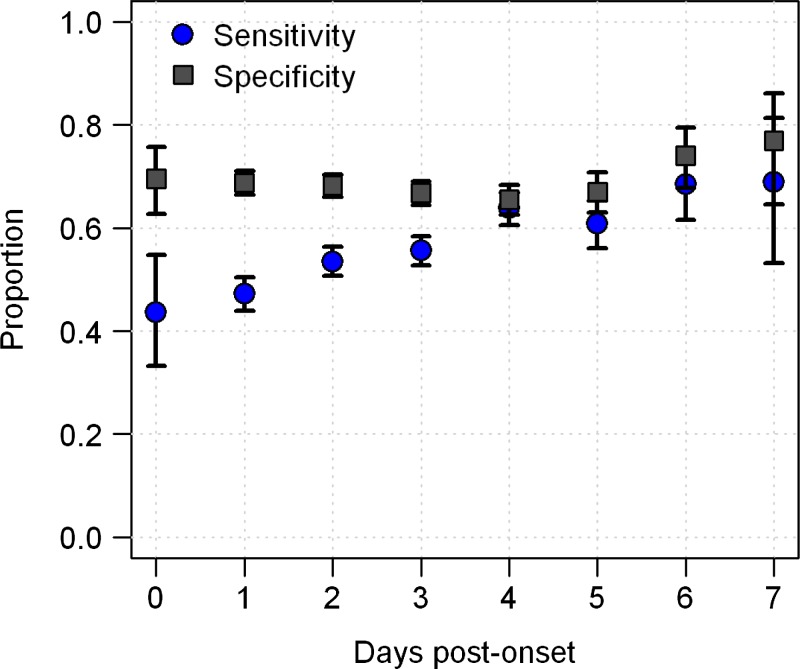
In passive surveillance, TT sensitivity was lowest for persons reporting on onset day 0 and greatest for those reporting on day 7 (45% versus 69%; P < 0.0001, by χ2 test for trend) (Figure 2). Specificity did not vary significantly in relation to day of presentation (P = 0.70. by χ2 test for trend).
Figure 2.
Sensitivity and specificity of the tourniquet test in diagnosing dengue based on which day a person with febrile disease initially came for care at a medical clinic (passive surveillance), Peru. Error bars indicate 95% confidence intervals for tourniquet test sensitivity and specificity at each day post-onset.
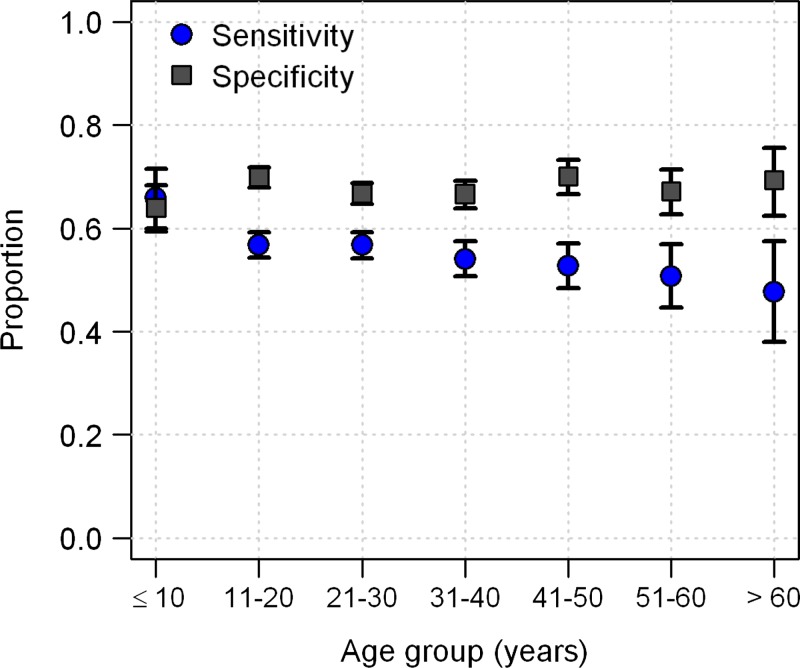
The sensitivity of the TT depended on the age of the person, ranging from 65% in participants ≤ 10 years of age to 46% in participants > 60 years of age (P < 0.001, by χ2 test for trend) (Figure 3). In contrast, specificity did not differ with age (P = 0.83, χ2 test for trend). The TT had higher sensitivity for females (59% sensitivity in females versus 53% in males; P < 0.0001, by Pearson's χ2 test), but a higher specificity for males (73% specificity in males versus 63% in females; P < 0.0001, by Pearson's χ2 test). These sex differences in sensitivity and specificity were consistent across almost all years; only in 2003 was sensitivity higher in males.
Figure 3.
Sensitivity and specificity of the tourniquet test in diagnosing dengue disease in febrile persons seeking care at medical clinics (passive surveillance) for different age groups, Peru. Error bars indicate 95% confidence intervals for tourniquet test sensitivity and specificity for each age group.
To address the possibility that TT outcome was influenced by DENV serotype, we compared TT sensitivity across serotypes. To control for potential biases across the course of the study, we only compared DENV serotypes during periods of co-circulation. The TT sensitivities were similar for DENV-3 (195 of 309, 63.1%) and DENV-4 (201 of 319, 63.0%) in 2008. However, TT sensitivity was significantly higher for DENV-2 (237 of 324, 73.1%) than DENV-4 (51 of 84, 60.7%) during an epidemic in 2010–2011 (P = 0.0326, by Pearson's χ2 test).
To adjust for potential confounders, we developed a logistic regression model of DENV-infected participants that included sex, age, and day of illness at presentation, with TT result as the response variable. In this model, sex (males, adjusted odds ratio [aOR] = 0.77, 95% CI = 0.69–0.87), age (aOR per 10-year category = 0.91, 95% CI = 0.88–0.95), and day of illness at presentation (aOR per day = 1.21, 95% CI = 1.17–1.26) were significantly associated with a positive TT result. To determine whether we could use these patient characteristics to develop an algorithm for improving TT diagnostic use, we developed a second logistic regression model of all participants (dengue and non-dengue). Tourniquet test, along with interactions of TT with age, sex, and day of illness were included in the model as independent variables. Tourniquet test (odds ratio [OR] = 1.57, 95% CI = 1.33–1.86) and each of the interaction terms with TT were significantly associated with dengue, albeit with relatively modest effect sizes (sex [reference female], OR = 1.31, 95% CI = 1.17–1.47; day of illness, OR = 1.18, 95% CI = 1.13–1.22; age [per 10-year category], OR = 0.94, 95% CI = 0.90–0.98). This model provided only a marginal improvement in diagnostic capacity (accuracy = 65.4%) over a model that included TT result alone (accuracy = 64.1%).
Tourniquet test performance on repeat testing.
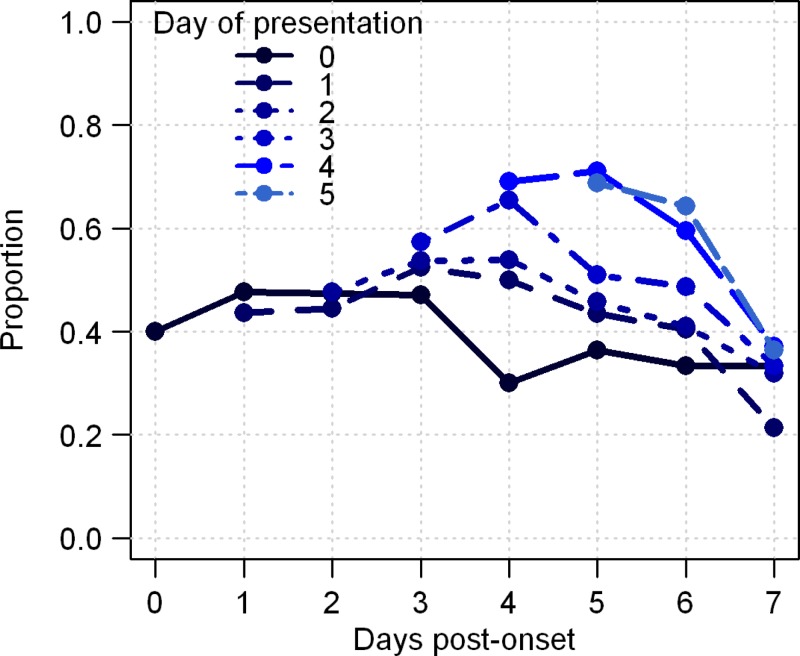
In contrast to passive surveillance, participants in active surveillance often received a TT on multiple successive days. The average number of total TTs performed on these persons was 3.6. On the day of presentation, sensitivity was greater for persons detected later in their illness, with a sensitivity of 40% for persons presenting on day 0 and 69% on post-onset day 5 (Figure 4). Nevertheless, sensitivity did not increase substantially over time for repeated measures on the same person. Of the 263 persons with DENV infections who had a positive TT result on any day, 226 (86%) had a positive test result the first time it was performed, and an additional 13 (9%) were captured on a second test performed the next day. Overall, repeat testing resulted in a moderate increase in sensitivity to 60% (263 of 439) and a moderate decrease in specificity to 56% (370 of 656).
Figure 4.
Sensitivity of tourniquet test in persons with dengue detected through active surveillance and tested on multiple consecutive days. Each curve represents a tourniquet test result on the first day of presentation and subsequent days. Results are organized based on day of presentation.
Tourniquet test performance in the presence of other endemic arboviruses.
After excluding 54 persons in passive surveillance who had equivocal serologic results, 311 persons had laboratory evidence of infection with a non-DENV arbovirus: VEEV (175 persons), OROV (62), MAYV (55), and GRCV (19). The TT result was positive in 22%, 37%, 33%, and 42% of these persons, although the PPV was < 1% for each of the four viruses.
Discussion
This study showed that the TT had a sensitivity of 52–56% and a specificity of 58–68% when used at an initial visit at our study sites in northeastern Peru, but performed differently depending on sex and age of participants. Although the TT tended to be more sensitive as persons presented later in the disease, the clinical utility of performing repeated TTs was minimal.
Filtration capacity, a marker for capillary permeability, has been shown to be higher in healthy children than adults, a phenomenon attributed to loss of microvasculature permeability as surrounding muscle fibers grow with age.16 In addition to the age findings, the same study showed greater permeability in adult females than males. These physiological factors may explain why we found the TT to be more sensitive in those of younger age and in females.
We observed an increase in TT sensitivity when the initial test was performed more days after the onset of illness. Increases in microvascular permeability over the course of dengue disease, as noted in a group of Vietnamese children, may account for this finding.8 In contrast, when following the same person over multiple days, we noted a slight increase in sensitivity between the first and second days of observation, but not after that. Eighty-six percent of those who had a positive TT result sometime during their illness also had a positive TT result during their first examination.
These seemingly contradictory findings, increasing TT sensitivity with later day of initial testing yet decreasing TT sensitivity with subsequent testing, may be explained by two potential mechanisms. Those presenting early in the course of the illness, even if they are experiencing the peak of their symptoms, may not have had sufficient time for the physiological mediators of vascular permeability to fully manifest compared with those presenting later in the course of their disease. If persons tend to seek care at the peak of their illness, then subsequent decreases in sensitivity may occur concomitantly with physiological changes associated with recovery. Conversely, the decrease in sensitivity with subsequent testing may be attributable to something inherent to the testing process, such as changes in the microvasculature, skin, or TT procedure that could occur with re-testing. Because of the limited added benefit with re-testing, and because the TT is not always a well-tolerated procedure, we recommend against performing TTs multiple times throughout a patient's disease course.17
Because NPV and PPV are calculated based on underlying transmission dynamics, both varied substantially over the 10-year time span; NPV was > 80% for most low-transmission periods and PPV was > 80% during high-transmission periods (Figure 1). One of these high-transmission periods occurred in late 2010 and early 2011, during the introduction of a new DENV lineage (lineage 2 of American/Asian genotype DENV-2) associated with an increase in quantity and severity of disease.18 Prior to this time, DHF had scarcely been reported in the city, but during this period one large Iquitos hospital reported more than seven times the number of dengue-related hospitalizations in just one month (January 2011) compared with previous 12-month periods. As expected during a time of increased disease activity, PPV increased and NPV decreased, but TT sensitivity exceeded 70%, the highest value during consecutive months over the study period. This increase in TT sensitivity concomitant with a period of severe disease has not been reported. Previous studies showed no difference in sensitivity between persons with DF compared with those with DHF.3,19,20
We were unable to compare those with DF to those with DHF because our studies did not collect all the data necessary (i.e., platelet levels or liver function test results) to differentiate between these two entities. In addition to our sensitivity results, we also found that specificity decreased during times of increased transmission. These results suggest that in addition to potential biological factors, operator-related factors may influence TT results. For example, study personnel may have been attuned to recent dengue transmission reports and may have subtly biased the TT reading.
In addition to the TT, other methods exist for diagnosis of dengue at the bedside, including rapid diagnostic assays and clinical scoring systems. A study that evaluated IgM detection with four rapid diagnostic tests showed sensitivities of 21–98% and specificities of 77–91%.21 Another study evaluating four rapid IgM assays, found a more consistent range of sensitivities (71–80%) but had a wider range of specificities (46–89%).6 Non-structural protein 1 assays performed comparably, with sensitivities of 49–59% and specificities of 93–99%.6 Although rapid assays may possess slightly better outcome measures than the TT, the cost of rapid diagnostic assays must be considered in countries such as Peru, where the average annual per capita health expenditures are US $269.22 Another approach to dengue diagnosis uses data normally collected during a patient's admission. This data includes basic laboratory results (e.g., leukocyte count, liver function test results), physical examination findings (e.g., rash), and symptoms (e.g., retro-orbital pain, arthritis) with and without the TT to predict who will have dengue versus another febrile illness.23,24 Such strategies may result in enhanced sensitivity but introduce added complexity in the form of complicated algorithms.
Our findings are consistent with those of other reports showing that a positive test result may occur in the absence of DENV infection.25 Other investigators have shown that scrub typhus and influenza may cause a positive TT result, and our study adds VEEV, MAYV, OROV, and GRCV infections to this list, although the TT in all of these entities had a lower sensitivity than that for dengue fever.3,20
A strength of our findings was that this study was conducted at 13 sites over the course of 10 years and used many study personnel to perform the TT. Although training and periodic oversight was offered by three local physicians over the course of the study, slight variation in technique and method may have occurred among sites. Nevertheless, our data reflect what would be expected from a wide array of healthcare personnel, thereby attenuating any solitary deviation of technique that may have been practiced. A limitation is that specific data about the number of petechiae observed was not recorded. Other investigators have assessed different cut-off levels for petechiae number and the subsequent impact on sensitivity and predictive value of the TT.25 We preferred to adhere to a value of 20 petechiae in an area measuring one square inch, the approach described in previous dengue guidelines and used by many but not all previous studies evaluating TT performance.3,9,19,20,23,26,27
Like many other physical examination signs and laboratory assays, the TT remains a tool in the diagnostic armamentarium against dengue. Its variable performance characteristics for different ages, sexes, and epidemiologic conditions must be weighed against the relative simplicity and speed in which this procedure yields a result with a sensitivity rivaling some rapid diagnostic assays. Although insufficient to provide definitive dengue diagnostic information, the greatest value of the TT may be in quickly indicating dengue disease during high-transmission periods and rapidly excluding disease during low-transmission periods.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Ministries of Health of Peru or Department of the Navy, Department of Defense, or the U.S. Government.
Footnotes
Financial support: This study was supported by the Armed Forces Health Surveillance Center Global Emerging Infections Systems Research Program (847705.82000.25GB.B0016), the Military Infectious Disease Research Program (S0263_10_LI and S0216_09_LI), the National Institute of Allergy and Infectious Disease (grant AI-42332), and the Innovative Vector Control Consortium (http://www.ivcc.com/).
Disclosure: The authors have declared that no competing interests exist. The corresponding author had full access to all data in the study and final responsibility for the decision to submit this publication. Eric S. Halsey and Tadeusz J. Kochel are military service members and Stalin Vilcarromero, Isabel Bazan, and Claudio Rocha are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. § 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. § 101 defines a U.S. Government work as a work prepared by a military service members or employees of the U.S. Government as part of those persons' official duties.
Authors' addresses: Eric S. Halsey, Stalin Vilcarromero, Brett M. Forshey, Claudio Rocha, and Isabel Bazan, U.S. Naval Medical Research Unit No. 6, Washington, DC, E-mails: eric.halsey@med.navy.mil, stalinf@yahoo.com, brett.forshey@gmail.com, claudio.rocha@med.navy.mil, and isabelbazana@gmail.com. Steven T. Stoddard, Thomas W. Scott, and Amy C. Morrison, Department of Entomology, University of California, Davis, CA, E-mails: ststoddard@gmail.com, twscott@ucdavis.edu, and amy.aegypti@gmail.com. Tadeusz J. Kochel, Viral and Rickettsial Diseases Department, Naval Medical Research Center, Silver Spring, MD, E-mail: tad.kochel@med.navy.mil. Martin Casapia, Asociacion Civil Selva Amazonica, Iquitos, Loreto, Peru, E-mail: mcasapia@acsaperu.org.
References
- 1.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health. 2008;13:1328–1340. doi: 10.1111/j.1365-3156.2008.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregory CJ, Lorenzi OD, Colon L, Garcia AS, Santiago LM, Rivera RC, Bermudez LJ, Baez FO, Aponte DV, Tomashek KM, Gutierrez J, Alvarado L. Utility of the tourniquet test and the white blood cell count to differentiate dengue among acute febrile illnesses in the emergency room. PLoS Negl Trop Dis. 2011;5:e1400. doi: 10.1371/journal.pntd.0001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruce MG, Sanders EJ, Leake JA, Zaidel O, Bragg SL, Aye T, Shutt KA, Deseda CC, Rigau-Perez JG, Tappero JW, Perkins BA, Spiegel RA, Ashford DA. Leptospirosis among patients presenting with dengue-like illness in Puerto Rico. Acta Trop. 2005;96:36–46. doi: 10.1016/j.actatropica.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.WHO, TDR . Dengue Guidelines for Diagnosis, Treatment, Prevention and Control: New edition. 2009. http://www.who.int/rpc/guidelines/9789241547871/en/ Available at. [PubMed] [Google Scholar]
- 6.Blacksell SD, Jarman RG, Bailey MS, Tanganuchitcharnchai A, Jenjaroen K, Gibbons RV, Paris DH, Premaratna R, de Silva HJ, Lalloo DG, Day NP. Evaluation of six commercial point-of-care tests for diagnosis of acute dengue infections: the need for combining NS1 antigen and IgM/IgG antibody detection to achieve acceptable levels of accuracy. Clin Vaccine Immunol. 2011;18:2095–2101. doi: 10.1128/CVI.05285-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bethell DB, Gamble J, Pham PL, Nguyen MD, Tran TH, Ha TH, Tran TN, Dong TH, Gartside IB, White NJ, Day NP. Noninvasive measurement of microvascular leakage in patients with dengue hemorrhagic fever. Clin Infect Dis. 2001;32:243–253. doi: 10.1086/318453. [DOI] [PubMed] [Google Scholar]
- 9.WHO . Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Second edition. Geneva: World Health Organization; 1997. Dengue: Clinical diagnosis. Chapter 2. [Google Scholar]
- 10.Forshey BM, Morrison AC, Cruz C, Rocha C, Vilcarromero S, Guevara C, Camacho DE, Alava A, Madrid C, Beingolea L, Suarez V, Comach G, Kochel TJ. Dengue virus serotype 4, northeastern Peru, 2008. Emerg Infect Dis. 2009;15:1815–1818. doi: 10.3201/eid1511.090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison AC, Minnick SL, Rocha C, Forshey BM, Stoddard ST, Getis A, Focks DA, Russell KL, Olson JG, Blair PJ, Watts DM, Sihuincha M, Scott TW, Kochel TJ. Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis. 2010;4:e670. doi: 10.1371/journal.pntd.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forshey BM, Guevara C, Laguna-Torres VA, Cespedes M, Vargas J, Gianella A, Vallejo E, Madrid C, Aguayo N, Gotuzzo E, Suarez V, Morales AM, Beingolea L, Reyes N, Perez J, Negrete M, Rocha C, Morrison AC, Russell KL, Blair PJ, Olson JG, Kochel TJ. Arboviral etiologies of acute febrile illnesses in western South America, 2000–2007. PLoS Negl Trop Dis. 2010;4:e787. doi: 10.1371/journal.pntd.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, Reiner RC, Jr, Vilcarromero S, Elder JP, Halsey ES, Kochel TJ, Kitron U, Scott TW. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci USA. 2013;110:994–999. doi: 10.1073/pnas.1213349110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SAS OnlineDoc 9.3. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 15.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. http://www.R-project.org/ Available at. [Google Scholar]
- 16.Gamble J, Bethell D, Day NP, Loc PP, Phu NH, Gartside IB, Farrar JF, White NJ. Age-related changes in microvascular permeability: a significant factor in the susceptibility of children to shock? Clin Sci (Lond) 2000;98:211–216. [PubMed] [Google Scholar]
- 17.Kabra SK, Jain Y, Pandey RM, Madhulika, Singhal T, Tripathi P, Broor S, Seth P, Seth V. Dengue haemorrhagic fever in children in the 1996 Delhi epidemic. Trans R Soc Trop Med Hyg. 1999;93:294–298. doi: 10.1016/s0035-9203(99)90027-5. [DOI] [PubMed] [Google Scholar]
- 18.Durand Velazco S, Fiestas Solorzano V, Sihuincha Maldonado M, Chavez Lencinas C, Vasquez Vela V, Torrejon Flores C, Rodriguez Ferruchi H, Cabezas Sanchez C. Impact of the dengue epidemic due to a new lineage of DENV-2 American/ Asian genotype in the health services demand in hospital “Cesar Garayar Garcia”, Iquitos. Rev Peru Med Exp Salud Publica. 2011;28:157–159. doi: 10.1590/s1726-46342011000100027. [DOI] [PubMed] [Google Scholar]
- 19.Srikiatkhachorn A, Gibbons RV, Green S, Libraty DH, Thomas SJ, Endy TP, Vaughn DW, Nisalak A, Ennis FA, Rothman AL, Nimmannitaya S, Kalayanarooj S. Dengue hemorrhagic fever: the sensitivity and specificity of the world health organization definition for identification of severe cases of dengue in Thailand, 1994–2005. Clin Infect Dis. 2010;50:1135–1143. doi: 10.1086/651268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayxay M, Phetsouvanh R, Moore CE, Chansamouth V, Vongsouvath M, Sisouphone S, Vongphachanh P, Thaojaikong T, Thongpaseuth S, Phongmany S, Keolouangkhot V, Strobel M, Newton PN. Predictive diagnostic value of the tourniquet test for the diagnosis of dengue infection in adults. Trop Med Int Health. 2011;16:127–133. doi: 10.1111/j.1365-3156.2010.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunsperger EA, Yoksan S, Buchy P, Nguyen VC, Sekaran SD, Enria DA, Pelegrino JL, Vazquez S, Artsob H, Drebot M, Gubler DJ, Halstead SB, Guzman MG, Margolis HS, Nathanson CM, Rizzo Lic NR, Bessoff KE, Kliks S, Peeling RW. Evaluation of commercially available anti-dengue virus immunoglobulin M tests. Emerg Infect Dis. 2009;15:436–440. doi: 10.3201/eid1503.080923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministerio de Salud, Peru . Principales Indicadores del Gasto en Salud. http://www.minsa.gob.pe/transparencia/gi_docvar.asp# Available at. [Google Scholar]
- 23.Potts JA, Thomas SJ, Srikiatkhachorn A, Supradish PO, Li W, Nisalak A, Nimmannitya S, Endy TP, Libraty DH, Gibbons RV, Green S, Rothman AL, Kalayanarooj S. Classification of dengue illness based on readily available laboratory data. Am J Trop Med Hyg. 2010;83:781–788. doi: 10.4269/ajtmh.2010.10-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawasdivorn S, Vibulvattanakit S, Sasavatpakdee M, Iamsirithavorn S. Efficacy of clinical diagnosis of dengue fever in paediatric age groups as determined by WHO case definition 1997 in Thailand. Dengue Bull. 2001;25:56–64. [Google Scholar]
- 25.Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, Viramitrachai W, Ratanachu-eke S, Kiatpolpoj S, Innis BL, Rothman AL, Nisalak A, Ennis FA. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 26.Norlijah O, Khamisah AN, Kamarul A, Paeds M, Mangalam S. Repeated tourniquet testing as a diagnostic tool in dengue infection. Med J Malaysia. 2006;61:22–27. [PubMed] [Google Scholar]
- 27.Kalayanarooj S, Nimmannitya S, Suntayakorn S, Vaughn DW, Nisalak A, Green S, Chansiriwongs V, Rothman A, Ennis FA. Can doctors make an accurate diagnosis of dengue infections at an early stage? Dengue Bull. 1999;23:1–9. [Google Scholar]