Abstract
Malaria and pneumonia are leading causes of childhood mortality. Home Management of fever as Malaria (HMM) enables presumptive treatment with antimalarial drugs but excludes pneumonia. We aimed to evaluate the impact of adding an antibiotic, amoxicillin (AMX) to an antimalarial, artesunate amodiaquine (AAQ+AMX) for treating fever among children 2–59 months of age within the HMM strategy on all-cause mortality. In a stepped-wedge cluster-randomized, open trial, children 2–59 months of age with fever treated with AAQ or AAQ+AMX within HMM were compared with standard care. Mortality reduced significantly by 30% (rate ratio [RR] = 0.70, 95% confidence interval [CI] = 0.53– 0.92, P = 0.011) in AAQ clusters and by 44% (RR = 0.56, 95% CI = 0.41–0.76, P = 0.011) in AAQ+AMX clusters compared with control clusters. The 21% mortality reduction between AAQ and AAQ+AMX (RR = 0.79, 95% CI = 0.56 –1.12, P = 0.195) was however not statistically significant. Community fever management with antimalarials significantly reduces under-five mortality. Given the lower mortality trend, adding an antibiotic is more beneficial.
Introduction
Mortality in under-five children remains a major concern. It is one of the key millennium development goals.1 Although some countries have seen reductions in under-five mortality rates, rates of decline have been slower and almost stagnant in Africa and Asia.2–4 In Ghana, under-five mortality increased from 108 of 1,000 live births in 1999 to 111 of 1,000 live births in 2003 and remained at that level in 2006.5 Globally, most post-neonatal deaths in under-five children are caused by only a handful of conditions: pneumonia, malaria, diarrhea, malnutrition, and acquired immunodeficiency syndrome (AIDs).1,6 Globally, pneumonia is the commonest cause of childhood death, but in developing countries, especially most of sub-Saharan Africa (sSA), malaria is often the commonest cause of death in under-five children.7,8 Malaria and pneumonia are responsible for 16% and 15% of under-five mortality, respectively, in sSA.9 In Ghana, malaria and pneumonia are the next most important causes of under-five mortality after early neonatal mortality, contributing 25% and 20% of under-five mortality, respectively.5 Early treatment has been shown to reduce morbidity and mortality for both diseases.10–12
In malaria-endemic Africa, children with pneumonia could easily be misdiagnosed and treated as having malaria. Similarities between symptoms have been well described and in their severe forms, they both present with fever, rapid breathing, and chest in-drawing, with a rapid decline to death, and co-morbidity is not uncommon.13–16
Given that these similarities provide opportunities for missed diagnosis, over-treatment and or under-treatment, appropriate diagnostic tools and effective medication are essential to enable health workers determine if a child has malaria, pneumonia or both in order to treat the child quickly and appropriately.15 Where resources are constrained this becomes even more complicated. Although many forms of health care exist in all societies, most of the African populace has access to orthodox health care mainly through public health centers. These provide primary health care but often lack the requisite equipment and trained staff to provide laboratory-assisted diagnosis of either condition. Indeed, for the majority of rural Africa, even this level of care may be unavailable and not always affordable. Children are thus often first treated at home or within communities.17,18 Health centers are a second or last resort and children often die at home or within the community.19
To address this issue of access to affordable and effective health care, the concept of using laypersons referred to as community health workers (CHWs), previously untrained in health to manage and report on simple ailments has been advocated.20 The home management for malaria (HMM) strategy is one such strategy that enables fever to be managed presumptively as malaria. Although the concept of home and community management of malaria is well established in Africa, unlike Asia, this is not so for pneumonia despite the co-morbidity reported by others.15 Community programs managing uncomplicated pneumonia reduced mortality of children in Nepal.8 In Ghana, earlier attempts to introduce community management of pneumonia where CHWs had to distinguish malaria from pneumonia were abandoned after CHWs consistently found it difficult to distinguish and manage these two illnesses separately.21 Following the call by the United Nations Children's Fund (UNICEF) and World Health Organization (WHO) in 2002 for pneumonia to be managed at the community level and integrated with ongoing community programs, and given the difficulty in distinguishing malaria from pneumonia, the problems of access, and the importance of reducing the current mortality rates in under-five children, we explored through a cluster-randomized open trial, the added survival benefit of including an antibiotic to the routine management of fever with an anti malarial drug in children under-five. We report on this trial using guidelines from the extension of the consort statement of pragmatic trials.22–25
Methods
Study site.
The trial was conducted between January 2006 and December 2009 in Dangme-West district in southern Ghana, a district with a population of about 110,000 people. Malaria transmission occurs throughout the year with peaks during the rainy seasons in April and October. Plasmodium falciparum constitutes 97–99% of parasitemia. Peak parasite rates occur in children 5–9 years of age but morbidity and mortality are highest in children younger than 5 years of age.26,27 At the time of the trial, caregivers could take their sick children to either of the four health centers or six community clinics in the district. Only one health center had a functioning laboratory microscopic testing for malaria parasites. Elsewhere, malaria was treated presumptively using artesunate amodiaquine. None of them had x-ray facilities. Pneumonia was treated with amoxicillin in line with the national drug guidelines. There was no district hospital and severely ill children were referred to hospitals of neighboring districts. There were two private clinics, two private maternity homes, two pharmacies, and 42 registered drug retail shops in the district from which caregivers were likely to seek care.28 Drugs used to treat children with fever and or fast breathing within communities was often inappropriate and included chloroquine and various antibiotics such as co-trimoxazole and metronidazole. Baseline data indicated that more than a third of caregivers treated their children themselves with left over drugs or stored drugs. (Results of care seeking and treatment received before the intervention was introduced will be presented elsewhere.) Dodowa Health Research Center (DHRC), a research site of the Ghana Health Service (GHS) is situated in the district, and collects information on vital events during biannual censuses (Health and Demographic Surveillance System [HDSS]).
Hypothesis.
The hypothesis for this trial was that early and appropriate management of fever in children under 5 years of age with an antimalarial and an antibiotic within the HMM strategy by CHWs would significantly reduce mortality when compared with only using an antimalarial. The primary outcome was all-cause mortality in the intervention arms (two-arm design), and the control arm (three-arm design). Secondary outcomes were to determine the impact of the intervention on some morbidity indicators (results to be presented elsewhere).
Trial design.
This was a cluster-randomized controlled open trial of an antimalarial versus an antimalarial plus an antibiotic for the treatment of fever among children 2–59 (inclusive) months of age in which the interventions were introduced in a stepped wedge manner. The cluster design was chosen over the individual randomized controlled design to reduce contamination that would be difficult to control, to improve supervision of CHWs, and to reduce the overall cost of implementing the trial. The stepped wedge design was chosen for pragmatic reasons, because it was impossible to introduce the interventions into all clusters simultaneously29; this also enabled the team to ensure that all children in the study area would eventually receive the intervention, avoiding the ethical issue of leaving children in a study area without the intervention (efficacious drugs) that work.
Randomization.
There were 376 communities scattered all over the district with populations of under-five children ranging between 0 and 153. Each community had their own local name and many had their own clan head or leader. Using HDSS data on the community populations, communities were grouped into clusters of ∼100 children under 5 years of age. In all, 114 clusters of about 100 children under 5 years of age were formed. Stratified randomization and cluster allocation were performed using computer generated randomization in “Microsoft Excel” 2007 (Microsoft, Redmond, WA) by an independent (visiting) statistician. Briefly, communities belonging to a cluster averaging 100 children were assigned a unique number. Clusters were then assigned random numbers using the Excel random function into the three arms, namely the control arm in which the standard care (usual care available for a sick child through a combination of orthodox and unorthodox drug providers and health facilities) available in the district before the intervention remained unchanged, the artesunate amodiaquine arm (AAQ) and the artesunate amodiaquine and amoxicillin (AAQ+AMX) arm. Because of the stepped wedge design, clusters randomized to the control arm were then subjected to another level of randomization and reassigned random numbers into AAQ and AAQ+AMX arms. No restriction, minimization or allocation sequences were applied.
The intervention.
The CHWs dispensed an antimalarial drug alone (artesunate amodiaquine [AAQ]), or together with an antibiotic (artesunate amodiaquine plus amoxicillin [AAQ+AMX]) to children with a fever. Communities were sensitized using various information, education, and communication (IEC) media including community meetings, audio messages, and drama.
Eligibility criteria.
Children, 2–59 months of age with fever as reported by the caregiver, born to mothers' resident at least 3 months in the district were eligible. Children as they turned 2 months and children 2–59 months of age who moved into the district during the study were included.
Exclusion criteria.
Severely ill children with danger signs (vomiting everything, unable to breastfeed or drink water, lethargy or coma, convulsions) were excluded and referred. Children > 59 months of age and children who turned 60 months of age during the trial were excluded.
Selection of CHWs.
The details of the selection process of CHWs are the same as those described in a related trial in which the feasibility of using CHWs in Ghana was explored.30 Briefly, lay persons resident in their various communities were selected through an interactive process based on criteria set by community members during an open community meeting or by their community leaders. Nominated CHWs were informed that their participation would be voluntary and without any financial remuneration.
Training of CHWs.
A CHW manual, relevant data capture tools such as data capture of the sick child consultation, referral forms, and drug recording forms were designed. Various IEC messages and an IEC video were developed. The CHWs were trained over 3 days using modified Integrated Management of Childhood Illnesses (IMCI) modules and video that had been developed with the Ministry of Health/Ghana Health Service for health workers.31 Training was conducted by experienced Ghana Health Service national IMCI trainers together with some study team members. Training was held separately for CHWs from different arms and was identical apart from one aspect: CHWs from AAQ+AMX clusters received additional training on how to administer amoxicillin and on how to teach caregivers from those clusters to administer amoxicillin at home.
Assessment of child by CHWs.
All CHWs were trained to receive clients and to enquire about fever. Fever was not assessed with a thermometer. The CHWs were trained to assess for signs of severe illness defined as difficulty in breastfeeding or drinking water, excessive vomiting, convulsions, lethargy, and coma and such children were referred. The CHWs were also trained to assess the child's respiration for fast breathing (respiratory rates were counted for one minute using a watch), chest in-drawing and stridor as well as other symptoms/signs such as palmer pallor, severe weight loss, diarrhea lasting more than 5 days, bloody diarrhea, and pedal edema. However, no criteria for pneumonia were applied; thus, the presence of any of these symptoms was not used to determine the type of treatment a sick child would receive within their cluster in line with our study design. These caregivers were provided the study drugs for their child's fever, counseled, and referred to the health center with a written referral form where they were told these other symptoms would be treated. Attempts were made to follow-up all referred children (results to be presented elsewhere). The CHWs were provided a list of children within their communities however treatment was not denied any non-resident sick child if they were taken to a CHW by their caregivers with a complaint of fever.
Drugs and dosage.
All drugs were produced in different strengths and color-coded for age. Drugs were provided at no cost to caregivers. Artesunate and amodiaquine were prescribed as follows: 25 mg artesunate plus 75 mg amodiaquine for children 2–11 months of age and 50 mg artesunate plus 150 mg amodiaquine for those 12–59 months of age, all once per day for 3 days. Dispersible amoxicillin tablets were prescribed as follows: 300 mg to children aged 2–11 months, 500 mg to those aged 12–35 months, and 650 mg to those 36–59 months of age, twice per day for 3 days32–34: the first dose was given under CHW supervision. The CHWs did not follow-up the children at home but caregivers were asked to return with their children after 2 days. Caregivers were also counseled to return if their child's condition worsened, if the child convulsed, developed a skin rash or began itching, became pale, weak, were unable to breastfeed or drink, began vomiting, or had diarrhea or bloody diarrhea after they took the study drug. Generally, caregivers were encouraged to return immediately if their child's condition changed or did not seem to improve. Adverse drug reactions were followed up passively through reports to CHWs by caregivers. These caregivers were followed up by field supervisors and interviewed.
Introduction of the intervention.
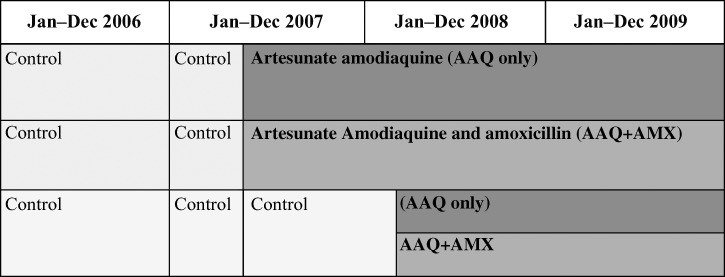
Over a 3-month period, the intervention was introduced into two-thirds of the 114 clusters (randomized to receive AAQ [37 clusters] and AAQ+AMX [39 clusters] and the remaining third of the clusters were left as control clusters (38 clusters) without any intervention. All three arms ran concurrently allowing the three-arm design to run for 6 months, after which the intervention was introduced into the remaining control clusters leading to a two-arm design (55 clusters in the AAQ arm and 59 clusters in the AAQ+AMX arm), which was maintained until the end of the trial resulting in the stepped wedge design (Figure 1).
Figure 1.
Overall timeline of the trial with a stepped wedge introduction of the interventions.
Quality assurance.
The CHWs were visited fortnightly by field supervisors who checked drug supplies and forms using a standard checklist. Two CHW review trainings were held during the period of the study. The drugs were tested to ensure drug quality during the trial period. The drugs met the minimum standards with a mean disintegration time of 2 min, a dissolution time ranging between 91.5% and 94.6%, and the percentage active ingredients ranging between 94.35% and 95.1%. Field workers who collected data during surveys were different from field supervisors who supervised CHWs and those who collected and validated mortality data.
Sample size for determining mortality rates.
The under-five mortality rate within the region was 80 of 1,000 live births at the time the study was initiated.35 To measure the difference in all-cause mortality between the two intervention arms, using a coefficient of variation of 0.25, a design effect factor of 1.68, an alpha of 0.05% and 80% power, and targeting an effect size of 20% 100 clusters (50 per intervention arm) of 100 children had to be followed up over 18 months. Sixty clusters (30 in each arm) were required to detect an effect size of 25% in all-cause mortality between the AAQ and control arms, and for AAQ+AMX and control arm, the number of clusters was 20 clusters/arm.
Data collection.
During the first year baseline information was collected. Mortality was assessed through bi-annual censuses conducted routinely by the HDSS team complimented by a Community Key Informant system (CKI). All selected CHWs were also trained to record any deaths, births, and pregnancies that occurred within their communities. Such information was collated monthly by the study team and reported to the HDSS team for verification.
Other surveys.
Twice per year 10 clusters were selected randomly for cross-sectional surveys to collect information on demography, height, weight, hemoglobin level, parasitemia, fever during the preceding 14 days, vitamin A and immunization status, and presence and use of insecticide-treated bed nets. This was to enable us to determine the impact of the trial on morbidity (results will be presented elsewhere).
Analysis.
The data set constituted all deaths collated by the HDSS and the CKI. Because of the stepped wedge nature of the trial, person-years of follow-up were used to calculate each child's contribution to the denominator. Person-time of follow-up for the control period was calculated using either January 1, 2006 (the start of the trial), date child attained 2 months, or date of entry if the child was born or moved in after the start of the trial but before the intervention began for that cluster. Person-time contributing to the intervention phase was calculated from the date that the last community in a cluster began its drug intervention. For most clusters, these dates did not vary much as CHWs were trained in clusters. Follow-up ended when children died, turned 60 months of age, exited the district, or at the end of the trial. Migration between clusters was reduced in the design by providing CHWs with a list of children in their communities and caregivers were requested to visit their own CHWs during sensitization meetings. Migration of children in and out of the district was taken into account during analysis in the following manner: All children each provided with unique identification numbers, were followed up by the HDSS as part of the household census carried out biannually. During a visit to a household, all household members are enumerated. If an eligible child had left the district, the date they left the district was noted; the child designated as “exited” on that date and no longer contributed person-years of follow-up. If the child was found to have come back during the next enumeration census, the dates of return were noted and that child would begin to contribute person-years of follow-up again. If the child had not returned, the event remained noted as “exited from the district,” not contributing any more person-years of follow-up. Using this method, multiple periods of follow-up were calculated for children where necessary, thus excluding from the follow-up period, any period for which they had been absent from the district. Migration between clusters could not be totally controlled, however our analysis was by intention to treat. The estimated effects of the intervention are presented as relative risks together with 95% confidence intervals (CI). Crude mortality rates were compared using the χ2 test for significance. Crude age-specific mortality rates were calculated. Adjusted analysis took into account gender, age groups, seasonality, and the cluster-randomized design using the random effects in a Poisson regression model.
Ethics.
The trial was approved by the ethics committees of the Ghana Health Services and WHO, through the Special Program for Research and Training in Tropical Diseases (TDR). District health and local government authorities provided approval for the study. Group consent was obtained from community leaders and members during community meetings that were held in the first year before the intervention begun. Written individual informed consent was obtained from all caregivers in the first year to determine their willingness to participate in the entire trial, but written informed consent was also obtained from parents or guardians for all subsequent surveys or interview(s).
Results
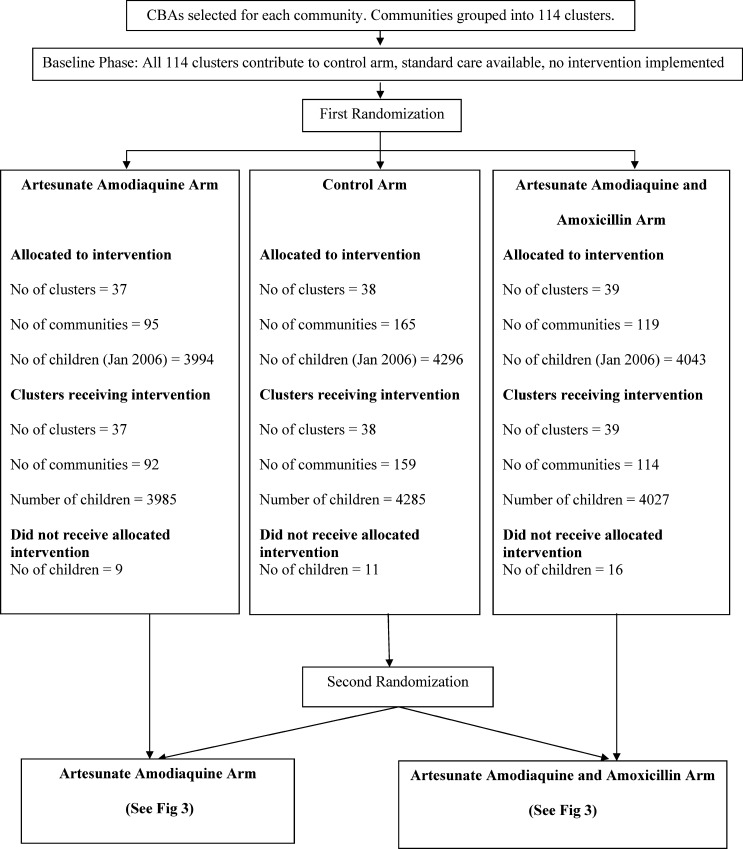
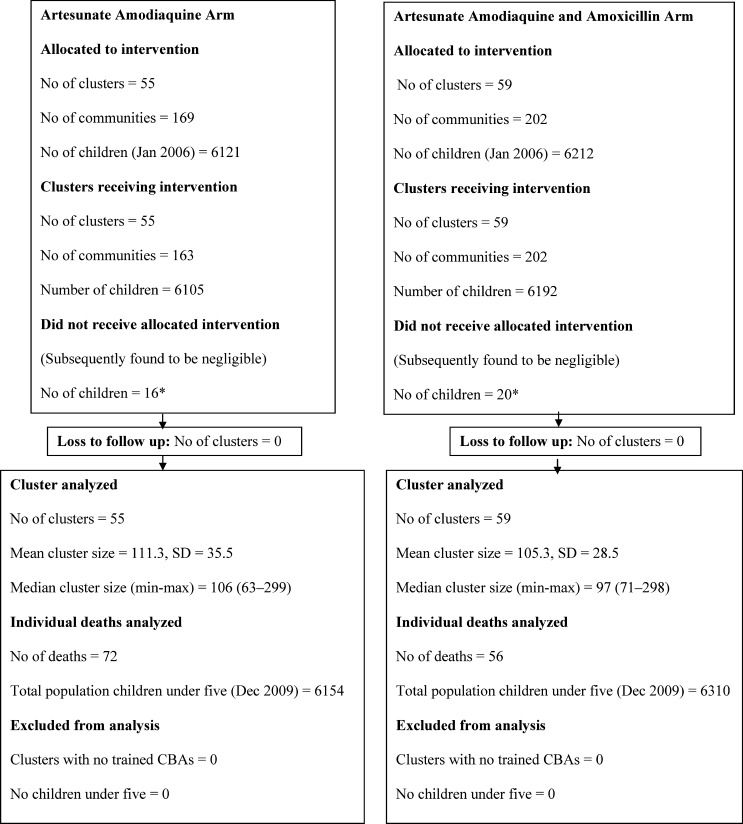
In January 2006, there were 12,333 children under 5 years of age resident in the district, a number that increased to 12,454 by December 2009. Almost all caregivers agreed to the general participation of their eligible children under 5 years of age in the trial. The decline rate was 0.2%. All 114 clusters contributed to the control arm until April 2007, when the 37 clusters with 3,994 children were randomized to receive AAQ, 39 clusters with 4,043 children to receive AAQ+AMX received the intervention and 38 clusters with 4,296 children continued to receive standard care (control arm). The control clusters randomized to either of the two interventions received their intervention 6 months later, resulting in two arms of 55 (AAQ) with 6,121 children and 59 (AAQ+AMX) clusters with 6,212 (Figure 1). Initiation of the intervention was delayed because of costs and logistical challenges causing the three-arm design to last for only 6 months instead of 1 year to allow sufficient time for follow-up of the two-arm design. Data on clusters, children included per arm, and deaths are shown in the participant flow charts (Figures 2 and 3). Details on almost 13,000 consultations by CHWs will be published elsewhere however, correct drugs per age group were provided in over 90% of consultations.
Figure 2.
Cluster and participant flow after first randomization.
Figure 3.
Cluster and participant flow after second randomization.
Table 1 provides the baseline characteristics of children 2–59 months of age collected during cross-sectional surveys from a total of 30 randomly selected clusters between April 2006 and April 2007 before the interventions were introduced. These surveys established that randomization of clusters to the three arms were similar for almost all relevant variables. The mean age of the children was 29.1 months, SD = 15.86, the male/female ratio was 51.1:48.9. Clusters randomized to the different arms were similar in all but two aspects: in the prevalence's of household ownership treated nets and parasitemia in the children. Clusters randomized to the AAQ arm had a significantly lower prevalence of the use of household ownership of treated nets (31.2%) compared with the clusters randomized to the AAQ+AMX arm (36.6%), and clusters randomized to remain the control arm (38.1%) (P = 0.005). The prevalence of parasitemia was also significantly lower in clusters randomized to both the AAQ arm (10.1%) and the AAQ+AMX arm (8.9%) compared with the control arm (14.7%) (P = 0.000).
Table 1.
Baseline variables established during 2-week morbidity recall surveys conducted in randomly selected clusters allocated to specific interventions in the pre-intervention phase of the trial*
| Variable | AAQ n/N (%) | AAQ+AMX n/N (%) | Control n/N (%) | Total n/N (%) |
|---|---|---|---|---|
| Children | 863 (32.0) | 826 (30.6) | 1012 (37.5) | 2701 (100.0) |
| Male/Female ratio | 49.4:50.6 | 52.3:47.7 | 51.6:48.4 | 51.1:48.9 |
| Mean age months (SD) | 29.4 (15.15) | 28.9 (16.60) | 29.7 (15.85) | 29.4 (15.86) |
| Treated nets* | 269/863 (31.2) | 302/826 (36.6) | 386/1012 (38.1) | 957/2701 (35.4) |
| Parasite count/μL < 5000† | 87/863 (10.1) | 73/826 (8.9) | 148/1012 (14.7) | 308/2701 (11.4) |
| Parasite count/μL ≥ 5000 | 6/863 (0.7) | 3/826 (0.4) | 6/1012 (0.6) | 15/2701 (0.6%) |
| Mean hemoglobin g/dL (SD) | 9.8 (1.65) | 9.9 (1.71) | 9.9 (1.57) | 9.9 (1.64) |
| Febrile episodes | 80/863 (9.3) | 96/826 (11.7) | 96/1012 (9.5) | 272/2701 (10.1) |
| Wt for age-underweight < 2 SD | 160/854 (18.7) | 132/817 (16.2) | 191/1008 (18.9) | 483/2679 (18.0) |
| Height for age-stunting < 2 SD | 156/850 (18.4) | 164/807 (20.3) | 197/998 (19.7) | 517/2655 (19.5) |
| Weight for height-wasted < 2SD | 61/850 (7.1) | 54/807 (6.7) | 57/998 (5.7) | 182/2637 (6.9) |
| Children 12–23 months fully immunized∞ | 144/198 (72.7) | 137/176 (77.8) | 158/215 (73.5) | 439/589 (74.5) |
| Vitamin A coverage* | 723/863 (87.3) | 694/826 (90.2) | 849/1012 (88.8) | 2266/2701 (88.8) |
AAQ = clusters randomized to AAQ artesunate amodiaquine arm; AAQ+AMX = clusters randomized to AAQ+AMX artesunate amodiaquine with amoxicillin arm. Control = clusters randomized to remain without any intervention in the first phase of randomization.
Statistically significant difference at P < 0.05; SD = Standard Deviation. ∞ one dose BCG, three doses DPT/HIPHEP, one dose measles and yellow fever.
There were 310 deaths during the trial, including 181 that occurred during the control period. During the intervention, 72 children died in AAQ clusters and 57 in AAQ+AMX clusters (Table 2). The number of deaths, person-years of follow-up, crude mortality rates within each arm, as well as mortality rates for the control period in the year 2006 and 2007 remained the same, although a decline in mortality was observed in the intervention arms from 2008 to 2009 (Table 2). Mortality rates in the two intervention arms declined significantly in comparison to the control arm but the difference between the two intervention arms was not statistically significant. The attributable risk indicates a difference between AAQ and AAQ+AMX of < 1/1,000. After adjusting for gender, age group, wet and dry seasons, and clustering, there was still a statistically significant reduction in all cause mortality between both intervention arms and the control arm (AAQ: reduction 30%, RR = 0.70, 95% CI = 0.53–0.92, P = 0.011 and AAQ+AMX: reduction 44%, RR = 0.56, 95% CI = 0.41–0.76, P = 0.011). However, the difference in the mortality reduction between the AAQ and AAQ+AMX arms of 21% was not significant (RR = 0.79, 95% CI = 0.56–1.12, P = 0.195) (Tables 3 and 4) Gender and seasonality had no effect on mortality rates. The mortality reduction was highest in the 36–47- and 48–59-month age groups compared with the 2–11 months age group (Tables 3 and 4). Reduction in all cause mortality between each intervention and the control arm was statistically significant for children in the 36–47 months and 48–59 months age groups (Table 3).
Table 2.
Crude mortality rates by intervention arm and year*
| Intervention arm | Deaths | Person years | Mortality rate/1,000 py | 95% confidence intervals | |
|---|---|---|---|---|---|
| Control | 181 | 26,760 | 6.76 | 5.85–7.82 | |
| AAQ | 72 | 15,109 | 4.77 | 3.78–6.00 | |
| AAQ+AMX | 57 | 14,968 | 3.81 | 2.94–4.94 | |
| Year | Intervention arm | Deaths | Person years | Mortality rate/1,000 py | 95% confidence intervals |
| 2006 | Control | 98 | 12,8504 | 7.64 | 6.27–9.31 |
| 2007 | Control | 64 | 10.1678 | 6.30 | 4.93–8.04 |
| AAQ | 8 | 1,7714 | 4.52 | 2.26–9.03 | |
| AAQ+AMX | 11 | 1.7614 | 6.24 | 3.46–11.28 | |
| 2008 | Control | 19 | 3.0324 | 6.27 | 3.00–9.82 |
| AAQ | 25 | 5.6979 | 4.39 | 2.97–6.49 | |
| AAQ+AMX | 20 | 5.5894 | 3.58 | 2.31–5.55 | |
| 2009 | Control | NA | NA | NA | NA |
| AAQ | 39 | 7.2293 | 5.40 | 3.94–7.38 | |
| AAQ+AMX | 26 | 7.2079 | 3.61 | 2.46–5.30 |
AAQ = artesunate amodiaquine only; AAQ+AMX = artesunate amodiaquine with amoxicillin; NA = not applicable.
Table 3.
Adjusted mortality rate ratios
| Initial three-arm design | Final two-arm design | |||
|---|---|---|---|---|
| Indicators | Rate ratio (95% confidence interval) | P value | Rate ratio (95% confidence interval) | P value |
| Arm | ||||
| Control* | 1 | N/A | ||
| AAQ | 0.70 (0.53–0.92) | 0.011 | 1 | |
| AAQ+AMX* | 0.56 (0.41–0.76) | < 0.001 | 0.79 (0.56–1.12) | 0.195 |
| Age group* | ||||
| 2–11 | 1 | 1 | ||
| 12–23 | 0.85 (0.62–1.15) | 0.282 | 0.67 (0.41–1.11) | 0.119 |
| 24–35 | 0.67 (0.49–0.93) | 0.016 | 0.79 (0.49–1.27) | 0.326 |
| 36–47 | 0.36 (0.24–0.53) | < 0.001 | 0.41 (0.23–0.73) | 0.003 |
| 48–59 | 0.35 (0.24–0.52) | < 0.001 | 0.34 (0.19–0.63) | 0.001 |
| Gender | ||||
| Male | 1 | 1 | ||
| Female | 1 (0.80–1.25) | 0.979 | 0.95 (0.67–1.34) | 0.758 |
| Dry season | 1 | 1 | ||
| 1.06 (0.84–1.33) | 0.627 | 0.99 (0.70–1.40) | 0.952 | |
Three arm: Likelihood-ratio test of alpha = 0: χ2(01) = 1.04; probability > = χ2 = 0.153; Two arm: Likelihood-ratio test of alpha = 0:χ2 (01) = 0.00; probability > = χ2 = 1.000. AAQ = artesunate amodiaquine only; AAQ+AMX = artesunate amodiaquine with amoxicillin.
Adjusted for age group, gender, season, clustering, and random effect.
Table 4.
Adjusted mortality rate ratios between the two intervention arms and the control arm
| AAQ vs. control | AAQ+AMX vs. control | |||
|---|---|---|---|---|
| Indicators | Rate ratio (95% confidence interval) | P value | Rate ratio (95% confidence interval) | P value |
| Arm | ||||
| Control* | 1 | 1 | ||
| AAQ | 0.70 (0.53–0.92) | 0.011 | NA | |
| AAQ+AMX* | NA | 0.56 (0.41–0.76) | < 0.001 | |
| Age group* | ||||
| 2–11 | 1 | 1 | ||
| 12–23 | 0.96 (0.68–1.34) | 0.800 | 0.83 (0.59–1.17) | 0.281 |
| 24–35 | 0.70 (0.49–1.00) | 0.052 | 0.59 (0.41–0.85) | 0.005 |
| 36–47 | 0.37 (0.24–0.58) | < 0.001 | 0.31 (0.20–0.49) | < 0.001 |
| 48–59 | 0.37 (0.24–0.57) | < 0.001 | 0.34 (0.22–0.53) | < 0.001 |
| Gender | ||||
| Male | 1 | 1 | ||
| Female | 0.99 (0.77–1.27) | 0.940 | 1.03 (0.80–1.33) | 0.813 |
| Dry season | 1 | 1 | ||
| 1.10 (0.85–1.40) | 0.459 | 1.05 (0.81–1.37) | 0.702 | |
Three arm: Likelihood-ratio test of alpha = 0: χ2(01) = 1.04; probability > = χ2 = 0.153;
AAQ = artesunate amodiaquine only; AAQ+AMX = artesunate amodiaquine with amoxicillin.
Adjusted for age group, gender, season, clustering, and random effect.
In total 5,818 and 6,601 eligible children were treated in the AAQ and the AAQ+AMX clusters respectively. The numbers of deaths averted were 109 and 124, respectively. The number of children that need to be treated to prevent one child death in both arms was 53. Seven adverse drug reactions were reported by caregivers however only one was assessed by the research team to be so. This child experienced itching after receiving the combination treatment.
Discussion
Treatment of uncomplicated fever episodes among children 2–59 months of age with the antimalarial (AAQ), or in combination with the antibiotic (AAQ+AMX) at community level by trained CHWs resulted in reduction of all-cause mortality in comparison to those treated with standard care that includes treatment at home, by traditional healers, care from drug retail shops, or from the formal health sector. The AAQ reduced mortality by 30% and AAQ+AMX by 44%, both significantly different in comparison to standard care but without significant difference between the two interventions. We aimed at assessing if adding an antibiotic to the antimalarial treatment as proposed by Home-based Management of Malaria would reduce mortality. Within HMM strategy, children in malaria-endemic areas with fever are considered to suffer from malaria and treated as such if diagnostic means are not available. This approach neglects other causes of fever, in particular respiratory infections, the next important cause of fever in children in Africa. Considerable overlap of symptoms between malaria and pneumonia has been reported such that clinical diagnosis was considered unreliable.13,15,16 A combination of very high respiratory rate in relation to age and chest in drawing indicates severe pneumonia, but also severe malaria, and is reason for referral. In line with the HMM program, our approach was practical; fever was noted by a caregiver, generally the mother, the CHW examined the child, assessed the need for referral and gave treatment. For examination and assessment a checklist was used. Information on symptoms and signs at baseline among sick children with a fever is being used to study overlap between malaria and pneumonia in our study population. This practical approach is likely to be common practice in many areas in the countryside of sSA.
We, like others before us, have shown that community management of fever by trained CWHs using antimalarials and antibiotics is feasible, even with artemisinin combination treatment with or without an antibiotic.36 Studies on community case management of pneumonia among 0–5-year-old children showed a 50–70% reduction of deaths.8,37 The 21% reduction between our intervention arms is likely caused by reduction of deaths because of bacterial infections, notably pneumonia. Pneumonia is a significant cause of childhood mortality in Ghana consistently ranking second or third as top causes of childhood mortality after malaria.
Several factors probably contributed to the lack of statistical significance for the difference between the two intervention arms. Only mortality rates for children 0–59 months of age in the study district were available and formed the basis for our calculations, however our intervention was limited to children aged 2–59 months. Neonatal and post-neonatal mortality have since been shown to contribute considerably to overall under-five mortality in Ghana.5 The mortality rate in our study population was probably lower, reducing the power of the study. By not targeting pneumonia specifically for treatment in the second arm, we incur a dilution effect, thereby reducing our ability to achieve significant differences. A post hoc calculation based on the actual prevailing mortality rate for this age group during the control period shows a power of 64%. Mortality declined in general (Table 2), which may be a reflection of the socio-economic development in the country and general improvements in health and health care, but major shifts in child health care within our study district and the country did not occur during the study period. Since the end of our study the Ghana Child Health policy supports management of malaria and pneumonia at community level by trained CHWs.
At the time of conceptualization of our study and during the study (2006–2010) RDTs had not yet been evaluated on large scale in the countryside let alone if used by CHWs in community projects. We acknowledge this as a limitation. Recent field trials have proved that RDT use by CHWs is feasible and improves the ability of these workers to distinguish between malaria and pneumonia.38–41 The use of RDTs in identifying children with fever not caused by malaria, may lead to better case management of other diseases and to further mortality reduction. However, RDTs have not always led to rational use of antimalarial drugs. Health workers have been reluctant to decline antimalarial treatment of RDT negative patients leading to over-prescription of ACTs.38–40,42,43 Current WHO malaria treatment guidelines (2010) recommend parasitological confirmation of malaria before treatment in all age groups and settings, including at the community level.44 Some authors have cautioned against a policy that abandons presumptive treatment for RDT use at all levels, however others insist the change must be made.45,46 In 2011, Ghana was unable to implement the use of RDTs even at health facility level. In its most current guidelines for case management of malaria, although the GHS recognized RDTs as being beneficial, health care workers were encouraged to consider laboratory confirmation of malaria in under-five children on a case-by-case basis, if their practice settings were favorable.”47
We acknowledge other limitations of our study. Children were assessed for criteria for referral (high respiration rate and chest in-drawing). Fever was not actually measured. The basis of our study was the HMM approach: fever was treated as malaria unless another cause is obvious. The WHO RDT-based policy at the time was restricted to adults and older children.48 Children under 5 years of age were still treated presumptively. This approach did not include respiratory infections, inclusive of pneumonia and other treatable bacterial infections. Furthermore, a previous trial in Ghana in which CHWs had to be trained to distinguish malaria from pneumonia using respiratory rates had faced major challenges resulting in that part of the trial being abandoned.5 With the well-described symptom overlap between malaria and pneumonia, our aim was not to distinguish malaria from pneumonia but to determine if there would be a reduction in under-five mortality sufficient enough for Ghana to review its HMM policy at the community level where diagnostic facilities are not available. Fever was used because it is the entry point for HMM. The caregiver's report of fever as a proxy for malaria has been found to be a reliable indicator of malaria in children in the same district, although fairly unreliable elsewhere.49 Differences in the reliability of fever as malaria may be caused by the differences in malaria transmission and general disease epidemiology of those countries. We also realized that large-scale use of antibiotics may have negative consequences. However, in a controlled community trial like this, compliance with antibiotic regimen and rational antibiotic use has been shown to improve rather than deteriorate (data not shown). In Ghana, access to antibiotics although regulated, is poorly controlled and caregivers can buy antibiotics over the counter from several sources. During baseline surveys, a third of caregivers reported storage of antibiotics including amoxicillin and co-trimoxazole at home (data not shown). Access to antibiotics in the AAQ arm could also have contributed to further reducing our effect size, however this cannot be confirmed. Caregiver compliance with treatment regimen was high (data to be presented elsewhere). A significant reduction in mortality between the two intervention arms could have helped in the discussion on the balance of positive and negative effects of this approach, however we were unable to show statistical significance. The cost-benefit analysis of this approach was investigated and found to be cost-effective both for the antimalarial arm and the antimalarial and antibiotic arm.50
The stepped wedge design has been described in Hussey and Huges as “particularly useful for evaluating the population-level impact of an intervention that has been shown to be effective in an individually randomized trial.”51 Introducing the intervention and disseminating information to a population of 110,000 could only be performed gradually, hence a phased design. The period of randomization into three arms was actually too short for a concurrent comparison. The two interventions are in fact compared without a concurrent control group, thus with a historical control group, which is not ideal. However, withholding treatment from children was considered unethical. A concurrent design would have meant implementing a HDSS in another district that would have required funds far beyond the funding for this trial. The alternative would have been to use panel surveys to access mortality in a neighboring district but the limitations of that, given our financial and logistic constraints made this unfeasible. Lack of a concurrent control group for the full duration of the study meant we compared mortality rates overlapping different years and this does not account for other factors that might have influenced mortality in one year but not another. Fortunately, there were no major epidemics or changes in health or economic policies over the study period that could have directly impacted child mortality in the district.
Mortality rates in the district were found to be lower than estimated by the DHS report for mortality among under-five children within the region and was not anticipated because the Dangme-West district is rural and mortality rates in rural districts tend to be higher than the reported regional average mortality rates in Ghana.35
The study findings are unlikely to be the same in areas with different malaria transmission patterns. If ARI are caused by viral infections, antibiotics are then unnecessary, however, if bacterial pneumonias contribute significantly to under-five mortality tthen the trend toward lower mortality is biologically plausible.
In conclusion, our study clearly shows that home management of fevers, a strategy in which lay persons are trained to assess and treat children 2–59 months of age reported to have fever with antimalarials with or without an antibiotic significantly reduces mortality. The trend toward lower mortality in the group receiving an antimalarial and an antibiotic, and evidence from other studies that show the benefits of treating pneumonia at the community level, suggests that integrated management of both malaria and pneumonia is preferable to therapy of malaria alone.
ACKNOWLEDGMENTS
The authors thank the caregivers, their children, and community members of the Dangme-West District who participated in this trial. We acknowledge the contribution of Dodowa Health Research Centre staff, especially the HDSS team, the malaria pneumonia team, and staff of the health centers who received our referrals. We are grateful for technical input from K. Koram of Noguchi Memorial Institute for Medical Research, Evelyn Ansah-The District Director of Health Services, Dangme West District, Ghana. Frank Cobelens from the Academic Medical Center Netherlands and Simon Cousens of the London School of Hygiene and Tropical Medicine who reviewed our analysis.
ICMJE criteria for authorship met: M. A. Chinbuah, P. A. Kager, M. Gyapong, J. O. Gyapong made primary contributions to the design and undertaking of the trial, analysis, interpretation of results and writing the manuscript. M. Abbey and J. Nonvignon contributed to the undertaking of the trial and edited written drafts. E. Awini contributed to the HDSS field work, analysis and edited written drafts. M. Adjuik conducted the randomization of clusters, contributed to analysis and edited written drafts. M. Aikins contributed to the overall design of the study and edited written drafts. F. Pagnoni contributed to the interpretation of the results and writing of the manuscript.
Footnotes
Financial support: This was provided by UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases and the Knowledge Enriches Programme of the Netherlands Organization for the Advancement of Tropical Research, WOTRO (NWO) in the Netherlands.
Disclosure: TDR/UNDP Trial registration. A: 20189: The trial protocol can be accessed from the WHO (Ethics Committee).
Authors' addresses: Margaret A. Chinbuah and Mercy Abbey, Research and Development Division, Ghana Health Service, Accra, Ghana, E-mails: Amanua.Chinbuah@ghsmail.org and Mercy.Abbey@ghsmail.org. Piet A. Kager, Department of Internal Medicine, Division of Infectious Diseases, Tropical Medicine and AIDS, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands, E-mail: p.a.kager@amc.uva.nl. Margaret Gyapong, Ghana Health Service, Dodowa, Dangme-West, Ghana, E-mail: Margaret.Gyapong@ghsmail.org. Elizabeth Awini, Health and Demographic Surveillance Team, Dodowa Health Research Center, Ghana Health Service, Dodowa, Dangme-West, Ghana, E-mail: Awini.Elizabeth@gmail.com. Justice Nonvignon and Moses Aikens, Department of Health Policy, Planning and Management, School of Public Health, College of Health Sciences, University of Ghana, Legon, Ghana, E-mails: Jnonvignon@ug.edu.gh and maikins@ug.edu.gh. Martin Adjuik, The International Network for the Demographic Evaluation of Populations and Their Health in Developing Countries (INDEPTH) Network, Kanda, Accra, Ghana, E-mail: info@indepth-network.org (www.indepth-network.org, E-mail: madjuik@gmail.com). Franco Pagnoni, UNDP/UNICEF/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, World Health Organization, Geneva, Switzerland, E-mail: pagnonif@who.int. John O. Gyapong, School of Public Health, University of Ghana, Accra, Ghana, E-mail: jgyapong@ug.edu.gh.
References
- 1.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–2234. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 2.UNICEF . Children and the Millennium Development Goals: Progress Towards a World Fit for Children. New York: United Nations Children's Fund; 2007. [Google Scholar]
- 3.UNICEF . State of the World's Children, 2008. New York: United Nations Children's Fund; 2007. [Google Scholar]
- 4.Schellenberg JA, Victora CG, Mushi A, de Savigny D, Schellenberg D, Mshinda H, Bryce J. For the Tanzania IMCI MCE baseline household survey study group. Inequalities among the very poor: health care for children in rural southern Tanzania. Lancet. 2003;361:561–566. doi: 10.1016/S0140-6736(03)12515-9. [DOI] [PubMed] [Google Scholar]
- 5.Family Health Division of the Ghana Health Service . Child Situation Analysis. Accra; Ghana: 2007. [Google Scholar]
- 6.Mulholland K. Commentary: co-morbidity as a factor in child health and child survival in developing countries. Int J Epidemiol. 2005;34:375–377. doi: 10.1093/ije/dyi028. [DOI] [PubMed] [Google Scholar]
- 7.Rudan I, Boschi-Pinto C, Biloglav Z, Muholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham SM, English M, Hazir T, Enarson P, Duke T. Challenges to improving case management of childhood pneumonia at health facilities in resource-limited settings. Bull World Health Organ. 2008;86:349–355. doi: 10.2471/BLT.07.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Prbhat J, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 10.Kidane G, Morrow RH. Teaching mothers to provide home treatment of malaria in Tigray, Ethiopia: a randomized trial. Lancet. 2000;356:550–555. doi: 10.1016/S0140-6736(00)02580-0. [DOI] [PubMed] [Google Scholar]
- 11.Sirima SB, Konate A, Tiono AB, Convelbo N, Cousens S, Pagnoni F. Early treatment of childhood fevers with pre-packed antimalarial drugs in the home reduces severe malaria morbidity in Burkina Faso. Trop Med Int Health. 2003;8:133–139. doi: 10.1046/j.1365-3156.2003.00997.x. [DOI] [PubMed] [Google Scholar]
- 12.Dawson P, Pradhan Y, Houston R, Karki S, Poudel D, Hodgins S. From research to national expansion: 20 years' experience of community-based management of childhood pneumonia in Nepal. Bull World Health Organ. 2008;86:339–343. doi: 10.2471/BLT.07.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odempsey TJ, McArdle TF, Laurence BE, Lamount AC, Todd JE, Greenwood BM. Overlap in the clinical features of pneumonia and malaria in African Children. Trans R Soc Trop Med Hyg. 1993;87:662–665. doi: 10.1016/0035-9203(93)90279-y. [DOI] [PubMed] [Google Scholar]
- 14.Kolstad PR, Burnham G, Kalter HD, Kenya-Mugisha N, Black RE. The integrated management of childhood illness in western Uganda. Bull World Health Organ. 1997;75:77–85. [PMC free article] [PubMed] [Google Scholar]
- 15.Gwer S, Newton CR, Berkley JA. Over diagnosis and co-morbidity of severe malaria in African children: A guide for Clinicians. Am J Trop Med Hyg. 2007;77((Suppl 6)):6–13. [PMC free article] [PubMed] [Google Scholar]
- 16.Källender K, Nsungwa-Sabiiti J, Peterson S. Symptom overlap for malaria and pneumonia-policy implications for home management strategies. Acta Trop. 2004;90:211–214. doi: 10.1016/j.actatropica.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Ahorlu C, Dunyo SK, Afari EA, Korma KA, Nkrumah FK. Malaria-related beliefs and behavior in southern Ghana: implications for treatment, prevention and control. Trop Med Int Health. 1997;2:488–499. [PubMed] [Google Scholar]
- 18.Dzator J, Asafu-Adjaye J. A study of malaria care provider choice in Ghana. Ghana Health Policy. 2004;69:389–401. doi: 10.1016/j.healthpol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 19.UNICEF . Malaria a Major Cause of Child Death and Poverty in Africa. New York: UNICEF; 2004. [Google Scholar]
- 20.WHO/UNICEF . Joint Statement: Management of Pneumonia in Community Settings. Geneva: World Health Organization; 2004. WHO/FCH/CAH/04.06. [Google Scholar]
- 21.Brown ENL. Early Appropriate Home Management of Fevers in Children Aged 6 Months to 6 Years in Ghana. Geneva; WHO-TDR: 2001. [Google Scholar]
- 22.Schultz KF, Altman DG, Moher D. For the CONSORT group CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. J Pharmacol Pharmacother. 2010;1:100–107. doi: 10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, Oxman AD, Moher D. CONSORT group; Pragmatic Trials in Healthcare(Practihc) group Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337:a2390. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomized trials. BMJ. 2006;328:702–708. doi: 10.1136/bmj.328.7441.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afari EA, Dunyo SK, Koram KA, Nkrumah FK. Epidemiology of Malaria with Special Emphasis on Transmission, Morbidity, Mortality and Disease Control in Ghana. Part 1. Malaria Transmission, Morbidity and Mortality in Coastal Savanna and Forest Areas in Southern Ghana. Legon, Ghana: Noguchi Memorial Institute for Medical Research. University of Ghana; 1992. [Google Scholar]
- 27.Koram K, Abuaku B, Duah N, Quashie N. Comparative efficacy of antimalarial drugs including ACTs in the treatment of uncomplicated malaria among children under 5 years in Ghana. Acta Trop. 2005;95:194–203. doi: 10.1016/j.actatropica.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Nonvignon J, Aikins MK, Chinbuah MA, Abbey M, Gyapong M, Garshong BN, Fia S, Gyapong JO. Treatment choices for fever for children under-five years in a rural Ghanaian district. Malar J. 2010;9:188. doi: 10.1186/1475-2875-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown CA, Lilford RJ. The stepped wedge trial design: a systematic review BMC Medical Research Methodology. BMC Med Res Methodol. 2006;6:54. doi: 10.1186/1471-2288-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinbuah AM, Gyapong JO, Pagnoni F, Wellington EK, Gyapong M. Feasibility and acceptability of the use of artemether-lumefantrine in the home management of uncomplicated malaria in children 6–59 months old in Ghana. Trop Med Int Health. 2006;11:1005–1016. doi: 10.1111/j.1365-3156.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization/United Nations Children's Fund/Ministry of Health/Ghana Health Service . Integrated Management of Childhood Illness: Sick Child Models and Video. Accra; Ghana: 2002. [Google Scholar]
- 32.Fonseca W, Hoppu K, Rey LC, Amaral J, Qazi S. Comparing pharmacokinetics of amoxicillin given twice per day to children older than 4 months with pneumonia. Antimicrob Agents Chemother. 2003;47:997–1001. doi: 10.1128/AAC.47.3.997-1001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.el Moussaoui R, de Borgie CA, van den Broek P, Hustinx WN, Bresser P, van den Berk GE, Poley J-W, van den Berg B, Krouwels FH, Bonten MJ, Weenink C, Bossuty PM, Speelman P, Opmeer BC, Prins JM. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate–severe community acquired pneumonia: randomized, double blind study. BMJ. 2006;332:1355. doi: 10.1136/bmj.332.7554.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO . Child Health and Adolescent Development (CAH) Technical Updates of the Guidelines on the Integrated Management of Childhood Illnesses. Evidence and Recommendations for Further Adaptation. Geneva: World Health Organization; 2005. [Google Scholar]
- 35.Ghana Statistical Service, Noguchi Memorial Institute for Medical Research and ORC Macro . Ghana Demographic and Health Survey 2003. Maryland: 2004. [Google Scholar]
- 36.Ajayi IO, Browne EN, Garshong B, Bateganya F, Yusuf B, Agyei-Baffour P, Doamekpor L, Balyeku A, Munguti K, Cousens S, Pagnoni F. Feasibility and acceptability of artemisinin–based combination therapy for the home management of malaria in four African sites. Malar J. 2008;7:6. doi: 10.1186/1475-2875-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theodoratou E, Al-Jilaihawi S, Woodward F, Ferguson J, Jhass A, Balliet M, Kolcic I, Sadruddin S, Duke T, Rudan I, Campbell H. The effect of case management on childhood pneumonia mortality in developing countries. Int J Epidemiol. 2010;39:i155–i171. doi: 10.1093/ije/dyq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeboah-Antwi K, Pilingana P, Macleod WB, Semrau K, Siazeele K, Kalesha P, Hamain B, Seidenberg P, Mazimba A, Sabin L, Kamholz K, Thea DM, Hamer DH. Community case management of fever due to malaria and pneumonia in children under five in Zambia: a cluster randomized controlled trial. PLoS Med. 2010;7:e1000340. doi: 10.1371/journal.pmed.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukanga D, Tibenderana JK, Kiguli J, Pariyo GW, Waiswa P, Bajunirwe F, Mutamba B, Counihan H, Ojiambo G, Kallander K. Community acceptability of use of rapid diagnostic tests for malaria by community health workers in Uganda. Malar J. 2010;9:203. doi: 10.1186/1475-2875-9-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chinkumba J, Skarbinski J, Chilima B, Campbell C, Ewing V, San Joaquin M, Sande J, Ali D, Mathanga D. Comparative field performance and adherence to test results of four malaria rapid diagnostic tests among febrile patients more than five year of age in Blantyre, Malawi. Malar J. 2010;9:209. doi: 10.1186/1475-2875-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bisofi Z, Gobbi F, Anghenben A, Van den Ende J. The role of rapid diagnostic tests in managing malaria. PLoS Med. 2009;6:e1000063. doi: 10.1371/journal.pmed.1000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamer DH, Ndhlovu M, Zurovac D, Fox M, Yeboah-Antwi K, Chanda P, Sipilinyambe N, Simon JL, Snow RW. Improved diagnostic testing and malaria treatment practices in Zambia. JAMA. 2007;297:2227–2231. doi: 10.1001/jama.297.20.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reyburn H, Mbakilwa H, Mwangi R, Mwerinde O, Olomi R, Darkeley C, Whitty CJ. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomized trial. BMJ. 2007;334:403. doi: 10.1136/bmj.39073.496829.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO . Guidelines for the Treatment of Malaria. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 45.English M, Reyburn H, Goodman C, Snow RW. Abandoning presumptive antimalarial treatment of febrile children aged less than five years. A case of running before we can walk? PLoS Med. 2009;6:e1000015. doi: 10.1371/journal.pmed.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Acremont V, Lengeler C, Mshinda H, Mtasiwa D, Tanner M, Genton B. Time to move from presumptive malaria treatment to laboratory-confirmed diagnosis and treatment in African children with fever. PLoS Med. 2009;6:e252. doi: 10.1371/journal.pmed.0050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ministry of Health, Republic of Ghana, Ghana Health Service, Presidents Malaria Initiative USAID/CDC, World Health Organization, Roll Back Malaria, Global Fund . Guidelines for the Case Management of Malaria in Ghana. Accra; Ghana: 2010. [Google Scholar]
- 48.WHO . Guidelines for Treatment of Malaria. Geneva: World Health Organization; 2006. [Google Scholar]
- 49.Dunyo SK, Koram KA, Nkrumah FK. Letter to editor: caregivers know when their children are hot. Lancet. 1997;350:1550. doi: 10.1016/S0140-6736(05)63981-5. [DOI] [PubMed] [Google Scholar]
- 50.Nonvignon J, Chinbuah MA, Gyapong M, Abbey M, Awini E, Gyapong JO, Aikins M. Is home management of fevers a cost-effective way of reducing under-five mortality in Africa? The case of a rural Ghanaian District. Trop Med Int Health. doi: 10.1111/j.1365-3156.2012.03018.x. doi:10111/j.1365-3156.2012.03018.x. [DOI] [PubMed] [Google Scholar]
- 51.Hussey MA, Huges JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28:182–191. doi: 10.1016/j.cct.2006.05.007. [DOI] [PubMed] [Google Scholar]