Abstract
Evidence on the impact of using diagnostic tests in community case management of febrile children is limited. This effectiveness trial conducted in Burkina Faso, Ghana, and Uganda, compared a diagnostic and treatment package for malaria and pneumonia with presumptive treatment with anti-malarial drugs; artemisinin combination therapy (ACT). We enrolled 4,216 febrile children between 4 and 59 months of age in 2009–2010. Compliance with the malaria rapid diagnostic test (RDT) results was high in the intervention arm across the three countries, with only 4.9% (17 of 344) of RDT-negative children prescribed an ACT. Antibiotic overuse was more common: 0.9% (4 of 446) in Uganda, 38.5% (114 of 296) in Burkina Faso, and 44.6% (197 of 442) in Ghana. Fever clearance was high in both intervention and control arms at both Day 3 (97.8% versus 96.9%, P = 0.17) and Day 7 (99.2% versus 98.8%, P = 0.17). The use of diagnostic tests limits overuse of ACTs. Its impact on antibiotic overuse and on fever clearance is uncertain.
Introduction
Malaria and pneumonia are leading causes of morbidity and mortality among under-fives in sub-Saharan Africa,1–3 despite the availability of cost-effective interventions for both conditions. Community case management of malaria and pneumonia have both been shown to reduce under-five mortality,4,5 and both strategies are recommended by the World Health Organization (WHO).6–8
Parasitological confirmation before administration of antimalarial treatment has recently been recommended by WHO for everyone presenting with symptoms compatible with malaria at all levels of the health system.9 Such confirmation is increasingly important in the context of declining malaria transmission, when a decreasing proportion of fever cases is likely to be caused by malaria.10 Furthermore, given the overlap in symptoms between malaria and pneumonia,11 the WHO and the United Nations Children Fund (UNICEF) now recommend integrated community case management (iCCM) of malaria and pneumonia in endemic areas in low- and middle-income countries.8
Rapid diagnostic tests (RDTs) for malaria are now available with sensitivities comparable to routine microscopy in detecting malaria12–14 and offer a practical means15,16 to improve diagnosis and quality of care of febrile children in malarious areas. Several studies have shown that community health workers (CHWs) can use RDTs safely and effectively.14,17–20
Increased respiratory rate is one of the most specific symptoms of pneumonia21–23 and respiratory rate timers (RRTs)24 have been recommended by WHO and UNICEF as a diagnostic tool for pneumonia, with studies19,25,26 showing that CHWs can be successfully trained to use them.
We designed an integrated diagnostic and treatment package for malaria and pneumonia, which involves trained CHWs, equipped with RDTs and RRTs and supplied with anti-malarial drugs (artemisinin based combination therapy (ACTs)) and antibiotics, administering treatments based on the results of the two tests. We report here an effectiveness trial conducted in three African countries, Burkina Faso, Ghana, and Uganda, with differing national health systems. We evaluated the effect of the package on the clinical outcome of febrile episodes in children and on the use of anti-malarial and antibiotic drugs.
Materials and Methods
Study areas and populations.
We report this evaluation using the CONSORT statement extension to cluster randomized trials.27 The evaluation was conducted in Burkina Faso, Ghana, and Uganda, in the districts of Saponé, Kassena Nankana, and Iganga, respectively. Saponé and Kassena Nankana are situated in the Sudan-Sahelian eco-climatic zone, with a seasonal malaria transmission pattern. Iganga is situated in South Eastern Uganda with an equatorial climate and minimal seasonal variation in malaria transmission.28 In Saponé, malaria transmission is markedly seasonal, with most transmission occurring during the rainy season, and with an entomological inoculation rate (EIR) ranging from 50 to 200 infective bites/person/year. Plasmodium falciparum is the predominant malaria parasite accounting for more than 95% of infections in children < 5 years of age. The malaria burden is heaviest among children < 5 years of age, who experience an average of two clinical malaria episodes every transmission season. Iganga district has year-round malaria transmission, and over 90% of malaria cases are caused by P. falciparum infection.28 Transmission peaks are seen following the rains: i.e., April to June and September to December.29 The annual EIR is not known, but is reported to be > 500 infective bites/person/year in the neighboring district of Tororo.30 In Kassena Nankana, malaria transmission occurs during most months of the year; however, there is a distinct seasonal pattern with the peak of transmission coinciding with the period of the major rains (May–October) and the dry season (November–April) seeing very low rates of malaria infection. Transmission has been estimated to be 418 infective bites/person/year.31–33
Data were collected in Burkina Faso from August 2009 to June 2010, in Ghana from April 2009 to February 2010, and in Uganda from October 2009 to October 2010. The sites were selected by an advisory committee from those who responded to a call by TDR in 2006. Some variations in the methodology were unavoidable, because in each case the specific national health framework was followed. Because of varying sample size per country, and the ease of access to nationally available supplies, the studies started and ended at different times.
Twelve villages participated in the study in Burkina Faso, 16 in Ghana, and 14 in Uganda. In Burkina Faso, the 12 villages were selected from two community clinic catchment areas within the Saponé Health District with an estimated total population of 9,000. The 16 villages selected in the Kassena-Nankana districts were drawn from 47 villages with a total population of 150,000 under continuous demographic surveillance. The 14 villages in Iganga were all drawn from Namungalwe sub-county, which has a population of ∼38,100 in 19 villages. Malaria, pneumonia, and diarrheal diseases are the leading causes of ill-health among children 1–59 months of age in the three study areas.34–36
We included children 6–59 months of age (with the exception of Uganda where the lower limit was 4 months in line with national guidelines on the use of ACTs in children37,38) with measured fever or history of fever (last 24 hours), who presented to a CHW. Exclusion criteria included severe illness (according to Integrated Management of Childhood Illness [IMCI] guidelines), known chronic disease, reported anti-malarial or antibiotic treatment in the previous 2 weeks, or known sensitivity to the study medications.
Study design.
The study was designed to assess the effect of the use of a diagnostic and treatment package for iCCM, comprising RDTs and ACTs for malaria, RRTs and antibiotics for pneumonia, on recovery from fever, and the rational use of medicines.
We performed an open, cluster randomized two-arm trial in the three countries. Clusters were the villages (catchment populations) of individual CHWs. Within the study areas we excluded, for ethical reasons, clusters that were more than 5 km from a designated health facility where CHWs referred cases for special care to minimize non-completion of referral because of distance. A cluster randomized design was chosen over an individually randomized design to reduce contamination, facilitate supervision, reduce costs, and to ensure that the CHWs maintained the correct treatments based on the tests in the intervention arm and the presumptive treatment in the control arm.
In the intervention arm, CHWs assessed children with acute febrile illness for malaria using RDTs, and for pneumonia by counting their respiratory rate with RRTs. Treatment was then provided on the basis of the test results. Children with a positive RDT received artemether-lumefantrine in Burkina Faso and Uganda, and artesunate-amodiaquine in Ghana. Children with a high respiratory rate received amoxicillin in Ghana and Uganda, and cotrimoxazole in Burkina Faso. The criterion for antibiotic administration was the presence of a high respiratory rate, regardless of the presence of cough or difficult breathing, in contrast to WHO guidelines.39 Additionally, paracetamol (PCT) was provided to all children in whom both RDT and RRT were negative, and to children with an axillary temperature > 38.5°C.
In the control arm, all febrile children received ACTs based on a presumptive diagnosis of malaria as provided for in the current IMCI guidelines. In line with the protocol, CHWs in Burkina Faso and Uganda were not provided with antibiotics. In Ghana, in line with existing practice, CHWs in the control clusters were also supplied with amoxicillin that they could provide to children based on clinical judgment. Antibiotics and anti-malarial drugs were provided as 3-day treatment courses, whereas PCT was provided for 2 days, so that it did not interfere with the fever assessment on Day 3. The first dose of all treatments was administered under the supervision of the CHW, and if the child vomited within 30 minutes they were given another dose. The dosing schedule was explained to caregivers who then administered the remaining treatments at home.
Malaria RDTs used.
First Sign Malaria Pf Card Test (Unimed International, Inc., Santa Clara, CA), Paracheck Pf Rapid test for P. falciparum Malaria (Device) (Orchid Biomedical System, Goa, India), and ICT Malaria Pf Cassette test (ICT Diagnostics South Africa) were used in Burkina Faso, Ghana, and Uganda, respectively. First Sign, Paracheck, and ICT have panel detection scores (PDS) at parasite densities from 2,000 parasites/μL of 86.1%, 97.5%, and 97.5%, respectively, and PDSs at parasites densities of 200 parasites/μL of 31.7%, 54.4%, and 82.3%, respectively.40
Drugs used in the study.
The ACTs used in the study were Coartem manufactured by Novartis Pharma in Burkina Faso, Acumal (artesunate-amodiaquine) manufactured by JCPL Pharma PVT Ltd., India in Ghana, and Coartem manufactured by Novartis Pharmaceuticals Corporation Suffern, New York in Uganda. Antibiotics used in the study were cotrimoxazole in Burkina Faso manufactured by Medicamen Biotech Ltd., India, Kinamox (amoxicillin) in Ghana manufactured by Kinapharma Ltd., and in Uganda amoxicillin manufactured by Zhangjiakou Shengda Pharmaceutical Co. Ltd., China (re-packed by Kampala Pharmaceutical Industries, 1996 Ltd.).
Paracetamol used in the study was manufactured by Laborate Pharmaceutical (India) for Burkina Faso, Kinapharma Ltd. for Ghana, and Kampala Pharmaceutical Industries (1996) Ltd. for Uganda.
Community health workers.
A total of 57 CHWs were recruited (13 in Burkina Faso, 16 in Ghana, and 28 in Uganda), half of whom were randomized to the intervention arm. The CHWs were selected by their respective community, based on minimum criteria that included the ability to read and write clearly so that they would be able to complete the study Case Report form (CRF). In Burkina Faso new CHWs were selected, whereas in Uganda existing CHWs previously trained to provide anti-malarial drugs in the community as part of the community case management were used. In Ghana, existing community health nurses (living within the community and hired and trained by the Ghana Health Service to provide basic services) were used.
Sample size.
The estimated sample size for the study was 4,360 febrile children between 4 and 59 months of age, with Burkina Faso and Ghana contributing 1,200 each, and Uganda 1,960. The sample size was estimated using the simplified formula by Hayes and Bennett41 for cluster randomized trials, with a power of 80% to detect an absolute difference in fever clearance 72 hours after initiation of treatment of 10% (85% against 75%) between the two arms with a two-sided alpha of 0.05. The estimation took seasonality into account, and accounted for loss to follow-up. We assumed a coefficient of variation between clusters of 0.12.
Training.
The CHWs were taught how to take a history, recognize clinical features of uncomplicated malaria, and signs of severe illness requiring referral; preparation of thick blood films for malaria microscopy; the use of classification and treatment algorithms for malaria and pneumonia (intervention arm only); use of simple dosing guidelines based on age for ACTs and PCT; managing drug supplies; obtaining informed consent; and completing CRFs including documentation of reported signs and symptoms, physical examination results, and medications administered to the child. In cases where informed consent was declined, the child received standard presumptive management of fever with an ACT.
In addition, CHWs in the intervention arm were taught the clinical features of non-severe pneumonia; use of malaria RDTs; infection control measures; how to count respiratory rate; and the use of simple dosing guidelines based on age for antibiotics.
There was interactive training consisting of oral presentations, discussions, role play, and supervised hands-on practice for all the study CHWs. At the end of the training, facilitators assessed the competency of the CHWs to follow the algorithm, complete study forms, and for CHWs in the intervention arm the appropriate use of RDTs and RRTs, as reported elsewhere.19
At the health facility level, health personnel were oriented on the treatment strategies in the two arms, and received refresher training on IMCI. These staff provided care to children referred from the study and provided supportive supervision to the CHWs.
Quality assurance plan.
The CHWs were supervised weekly by field supervisors to detect and correct any deviations from the protocol. During these visits, the following were monitored: completeness of data captured in the CRFs, respect of inclusion/exclusion criteria, drug administration, drug and RDT storage conditions, and assessment and follow-up of enrolled children.
At each supervisory visit three samples of RDTs were collected from the batch in use. The RDT samples were tested against a sample of blood confirmed positive by microscopy to ensure the RDTs were still functioning with adequate sensitivity.42
At the end of each month all CHWs attended a review meeting at the health center at which the assessment, treatment, and follow-up algorithms were reviewed, and problem areas were identified and discussed. The CHWs brought their registers and CRFs. The CHWs identified as having problems with a particular part of the algorithm were followed up and were provided with support and retraining by the study team. The accuracy of CHWs in performing the RDTs was assessed as part of each supervisory visit. When there were no patients at the time of the visit, CHWs were asked to demonstrate how they perform the procedure, including how they do the finger prick and how they read the RDT result. The CHWs were observed and reminded to perform the RDT in a well-lighted place.
Refresher training was provided to the health facility microscopists on malaria microscopy before enrollment started. Ten slides per month were selected for quality control (5 low density and 5 negative slides) using a random number system recommended by WHO43 and sent to a reference laboratory for rereading on a “blinded” basis.
Data collection methods.
All children who were tested for malaria on Day 0 also had a thick blood film prepared by the CHWs. The blood films were collected within 24 hours in all three sites by a fieldwork supervisor with a motorbike. Blood films were stained with 10% Giemsa stain for 10–15 minutes and screened microscopically under a ×100 oil immersion lens using a light microscope by a microscopist trained by the study teams and based at the referral center. A blood slide was taken at Day 0 in the control arm to enable a comparison of the prevalence of malaria in the two arms (in Uganda this was only done in a sub-sample of enrolled children across both dry and rainy seasons).
The number of parasites present per white blood cell was counted, and the figure multiplied by 8,000 (an average white blood cell count per μL) to give the parasite density.44 Slides were double read, and when there was a discrepancy between the two readings the slide was read again by an experienced/senior microscopist who was independent of this study and whose reading was considered final. The microscopy results were used to establish the accuracy of the RDTs when used in the communities. Children with a negative RDT, but with a positive slide reading were traced at home and treated with ACTs. All patient data were recorded on CRFs at all three sites.
Patient management and follow-up.
The CHWs reviewed children and completed the CRF on Days 0, 3, 7, and on unscheduled visit days. A review on Day 3 determined if a child had recovered from fever (temperature below 37.5°C as measured by a digital thermometer). Children in either arm who had not recovered were referred to a designated health center. All children not referred at Day 3 (clinically recovered) were reviewed on Day 7, and any fever relapse cases were referred to the health center. All children referred (Day 3 or 7) were examined by a trained project nurse, and managed according to IMCI guidelines. Children who did not come for scheduled visits were traced and assessed at home.
Study outcomes.
The primary outcome was resolution of fever at Day 3, whereas resolution of fever at Day 7, and the use of anti-malarial and antibiotic drugs were secondary outcomes. Outcomes were measured and analyzed at the individual level.
Data analyses.
Data were entered into microcomputers and analyzed using Epi-Info 6.0 (CDC, Atlanta, GA) and STATA 9.0 (College Station, TX). We compared proportions of the study outcomes between the two groups. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using random effects logistic regression analysis with the treatment arm and country as fixed effects, and cluster as a random effect. Analysis was by intention-to-treat.
Ethical approval.
Ethical approval for these studies was granted by the WHO Ethics Review Committee and by the appropriate national and Institutional Ethical Review Boards of each participating country. Approval was obtained from district, local, and community leaders as well as household heads. Informed consent was obtained from caregivers of children who participated in the studies. The WHO TDR project numbers are A60486 for Burkina Faso, A60490 for Ghana, and A60487 for Uganda. The trial was registered online at http://register.clinicaltrials.gov with the registration number NCT00720811.
Results
Baseline characteristics.
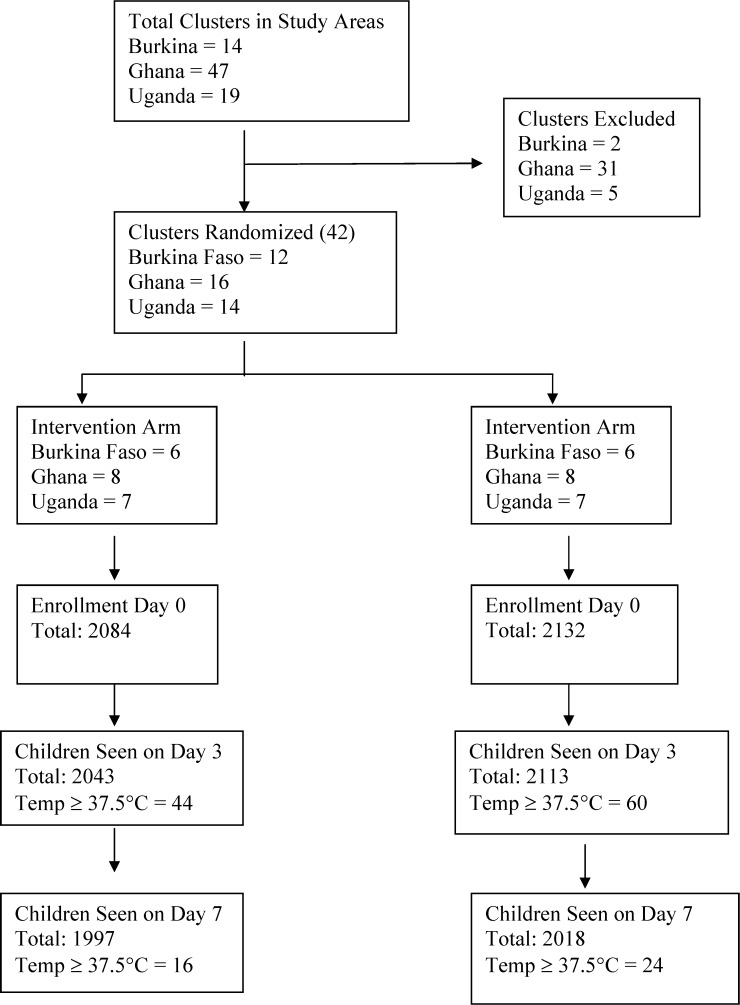
Overall, 4,216 children between 4 and 59 months of age were enrolled in Burkina Faso, Ghana, and Uganda. The number of children enrolled in intervention and control arms respectively were 525 and 576 in Burkina Faso, 584 and 591 in Ghana, and 975 and 965 in Uganda (Figure 1). With the exception of reported cough, baseline characteristics were comparable across the two arms (Table 1). In total, 64.5% (2,691 of 4,216) had temperatures ≥ 37.5°C at enrollment and 69.5% (2,729 of 3,925) had microscopically confirmed malaria.† The RDT positivity rate was 74.5% (391 of 525) in Burkina Faso (Table 2A), 84.2% (492 of 584) in Ghana (Table 2B), and 87.9% (857 of 975) in Uganda (Table 2C).
Figure 1.
Study profile.
Table 1.
Baseline characteristics of children at enrollment
| Burkina Faso | Ghana | Uganda | ||||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| Number of children enrolled | 525 | 576 | 584 | 591 | 975 | 965 |
| Number (%) with measured temperature ≥ 37.5 | 436 (83.0%) | 457 (79.3%) | 351 (60.1%) | 372 (62.9%) | 563 (57.7%) | 512 (53.1%) |
| Mean age (in months) | 28.7 | 30.3 | 24.6 | 26.0 | 27.8 | 27.3 |
| Number (%) of females | 277 (52.8%) | 276 (47.9%) | 277 (47.4%) | 317 (53.6%) | 451 (46.3%) | 478 (49.5%) |
| P. f. asexual parasitemia prevalence (by microscopy) | 284 (54.1%) | 313 (54.3%) | 439 (75.2%) | 435 (73.6%) | 783 (80.3%) | 475/674* (70.5%) |
| Geometric mean parasite density in positives | 11,841 | 10,505 | 15,320 | 12,350 | 7,663 | 7,318 |
| Number (%) of children with cough | 252 (48.0%) | 241 (41.8%) | 123 (21.1%) | 226 (38.2%) | 555 (56.9%) | – |
| Number (%) of children with diarrhea | 134 (25.5%) | 154 (26.7%) | 208 (35.6%) | 208 (35.2%) | – | – |
Blood smear for microscopy done in only a sub-sample of children in the control arm in Uganda.
Table 2A.
Assessment classification and treatment in Burkina Faso
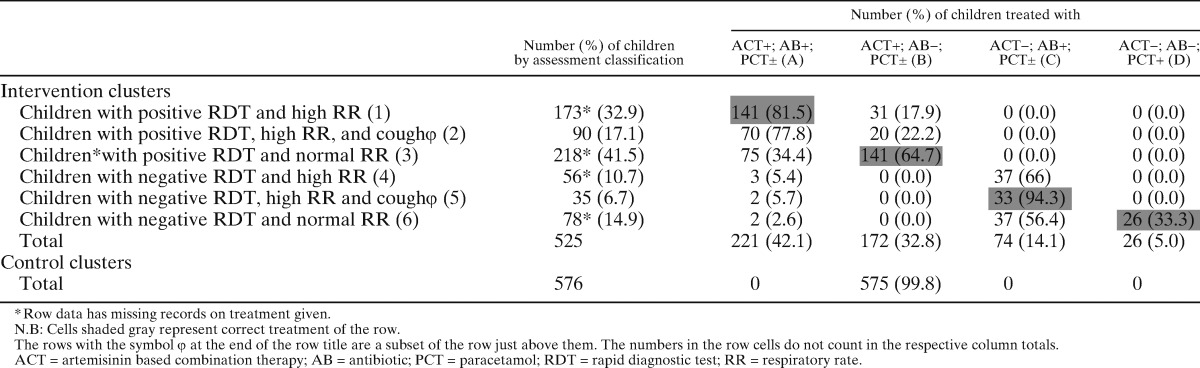
Table 2B.
Assessment classification and treatment in Ghana
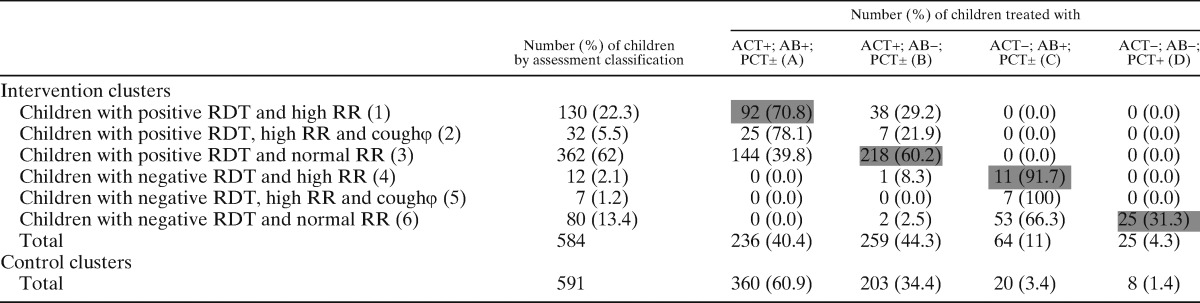
N.B: Cells shaded gray represent correct treatment of the row.
The rows with the symbol φ at the end of the row title are a subset of the row just above them. The numbers in the row cells do not count in the respective column totals.
ACT = artemisinin based combination therapy; AB = antibiotic; PCT = paracetamol; RDT = rapid diagnostic test; RR = respiratory rate.
Table 2C.
Assessment classification and treatment in Uganda
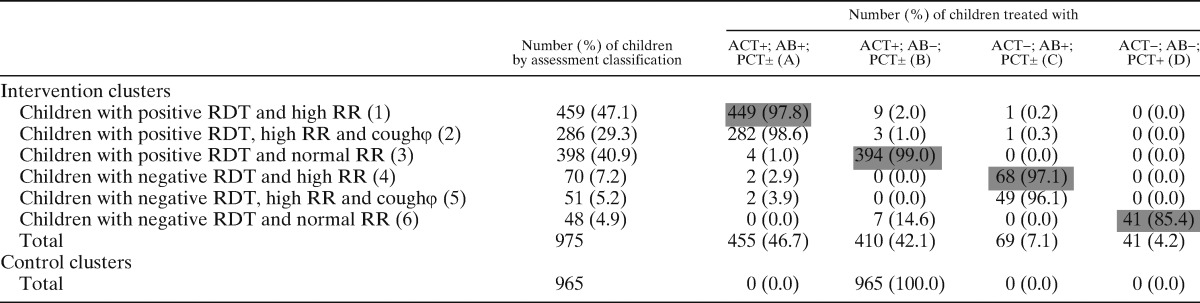
N.B: Cells shaded gray represent correct treatment of the row.
The rows with the symbol φ at the end of the row title are a subset of the row just above them. The numbers in the row cells do not count in the respective column totals.
ACT = artemisinin based combination therapy; AB = antibiotic; PCT = paracetamol; RDT = rapid diagnostic test; RR = respiratory rate.
Use of medicines.
Use of medicines by assessment classification is summarized by country in Table 2A–C. In the intervention clusters, there was good compliance with RDT results by CHWs across the three countries with minimal overuse of ACTs. Only 1 case out of 1,740 RDT-positive children did not receive an ACT, whereas only 4.9% (17 of 344) of RDT-negative children were prescribed an ACT.
With regard to antibiotics, there were varying degrees of overuse (prescription to a child with a normal respiratory rate) in Burkina Faso, Ghana, and Uganda, with 38.5% (114 of 296), 44.6% (197 of 442), and 0.9% (4 of 446) of children with a normal respiratory rate receiving an antibiotic, respectively. Conversely, some children with high respiratory rates did not receive an antibiotic: 13.5% (31 of 29) in Burkina Faso, 27.5% (39 of 142) in Ghana, and 1.7% (9 of 529) in Uganda. Among children with high respiratory rates, we analyzed data within the subgroup that also had a cough; 16% (20 of 125) in Burkina Faso, 17.9% (7 of 39) in Ghana, and 0.9% (3 of 337) in Uganda of these children did not receive an antibiotic. The overall rate of antibiotic underuse in this subgroup was 6.0% (30 of 501).
In the control clusters, ACTs were given to all children in all countries, leading to a potential unnecessary prescription of ACTs in 25.6%, 15.8%, and 12.1% of cases in Burkina Faso, Ghana, and Uganda, respectively (assuming a similar proportion of RDT negative cases in the control and intervention clusters). In Ghana (where antibiotics were available in control clusters as well) 51.4% (300 of 584) of all children were prescribed antibiotics in the intervention clusters compared with 64.3% (380 of 591) in the control clusters (OR 1.7, 1.34–2.17; P < 0.001), suggesting less overprescription of antibiotics in the intervention arm.
Impact on fever clearance.
Fever clearance results are summarized in Table 3. There were high fever clearance rates across the countries and arms. Fever clearance rates at Day 3 and Day 7 were 97.8% (95% CI 97.0, 98.7) and 99.2% (95% CI 98.7, 99.8) in intervention clusters, and 96.9% (95% CI 96.1, 97.6) and 98.8% (95% CI 98.3, 99.3) in control clusters. The estimated ORs for failure to clear fever (intervention versus control) were 0.69 (95% CI 0.41, 1.16; P = 0.17) at Day 3 and 0.62 (95% CI 0.32, 1.22; P = 0.17) at Day 7.
Table 3.
Fever persistence on study Days 3 and 7 after the onset of treatment
| Cluster | # of ch. with Temp ≥ 37.5 and/or reported hot body at D0 | # of ch. seen at D3 | # of ch. with Temp ≥ 37.5 at D3 (% of ch. Seen at D3) | #. of ch. seen at D7 | # of ch. with Temp ≥ 37.5 at D7 (% of ch. Seen at D7) |
|---|---|---|---|---|---|
| Intervention clusters | |||||
| Burkina Faso | 525 (436) | 507 | 11 (2.2) | 485 | 2 (0.4) |
| Ghana | 584 (351) | 578 | 10 (1.7) | 561 | 6 (1.1) |
| Uganda | 975 (563) | 958 | 23 (2.4) | 951 | 8 (0.8) |
| Total | 2084 | 2043 | 44 (2.2) | 1997 | 16 (0.8) |
| Control clusters | |||||
| Burkina Faso | 576 (457) | 563 | 21 (3.7) | 527 | 5 (0.9) |
| Ghana | 591 (372) | 591 | 8 (1.4) | 573 | 10 (1.7) |
| Uganda | 965 (512) | 959 | 37 (3.9) | 918 | 9 (1.0) |
| Total | 2132 | 2113 | 66 (3.1) | 2018 | 24 (1.2) |
A subgroup analysis was done to explore if the use of antibiotics in the control arm in Ghana may have diluted the effect of the intervention, with Ghana data excluded. Fever clearance was marginally better in the intervention arm at 97.7% (95% CI 96.8, 98.6: 1,431 of 1,465) compared with 96.2% (95% CI 95.0, 97.3: 1,464 of 1,522) in the control arm at Day 3, with a 41% reduction in odds of having fever at Day 3 (OR = 0.59, 95% CI 0.38–0.93). At Day 7, fever clearance was 99.4% in the intervention arm (95% CI 98.8, 99.99) compared with 99.0% (95% CI 98.7, 99.4) in the control arm (OR = 0.64, 95% CI 0.28, 1.49).
Although no formal pharmacovigilance system had been put into place, there were no passive reports of severe adverse events or deaths at any of the three sites.
Discussion
We performed an effectiveness trial in three countries, examining the effects on fever clearance and rational use of medicines of a diagnostic and treatment package, comprising RDTs and ACTs for malaria, respiratory rate (RR) timers, and antibiotics for pneumonia, implemented at the community level to treat children < 5 years of age with fever episodes. In each country, we adopted a pragmatic approach, following existing national policies. This resulted in some variations in what was actually done in each country. The package led to a clear improvement in the appropriate use of ACTs, and in Ghana, the only site in which antibiotics were available in the control arm, to fewer prescriptions of antibiotics. The vast majority of children recovered from fever in both the intervention and control groups, and no effect of the intervention on the clinical outcome (recovery from fever) were detected. The microscopy positivity rates reported from the three sites (54–80%) are not for the general population, but for children who had been sick and were taken to CHWs for care. These rates are much higher than what would have been expected in a population-based sample.28,32
Inappropriate use of ACTs is a major concern, because it may lead to the development of resistance to these highly effective drugs.45 The potential for misuse of ACTs could be particularly high at the community level, where ACTs are distributed by non-professional staff.46,47 The latest WHO malaria treatment guidelines9 recommend parasitological confirmation before administering anti-malarial drugs to a patient presenting with fever in all areas, including highly malaria-endemic settings. The shift from symptom-based to RDT-based treatment with ACTs has major implications. It limits the over-diagnosis of malaria and thus the inappropriate use of ACTs and expenditure,48 and reduces missed diagnosis of other causes of fever49,50 at a time when a declining proportion of fevers in Africa are attributable to malaria.10 Administering ACTs only to patients with a positive RDT has led to dramatic reductions in the use of ACTs in Cambodia,18 mainlandTanzania,51 and Zanzibar.52 Nevertheless, in some health facility settings substantial proportions of patients with negative test results were reported to receive ACTs,53–56 although improvements in adherence to treatment guidelines were achieved after intensive training in some cases.51,57
Poor adherence to RDT results has also been reported when febrile episodes are managed at the community level by CHWs, with up to 58% of patients with a negative RDT result being treated with an antimalarial.58 Poor compliance with referral advice by patients with fever but with a negative RDT result has also been reported.59 It is noteworthy that in both these studies there was no alternative diagnostic test, nor treatment of RDT-negative patients. However, very high levels of appropriate prescription of ACTs after rapid malaria testing were achieved in Chikankata, Zambia in a program in which febrile children were assessed for pneumonia, and antibiotic treatment was provided as appropriate25; similar findings were reported more recently in another study in Zambia that used an intensive training and supervision model.60
The results of our study in three sub-Saharan African countries, conducted with a similar design to the one in Chikankata, confirm that inappropriate use of ACTs is extremely rare (< 5% of cases) when alternative diagnostic tests and treatment of other conditions are provided to patients with negative RDT results (in our study children with fever and a negative RDT were prescribed antibiotic treatment if there were signs of pneumonia, and PCT if there were none). On the contrary, when anti-malarial treatment is administered to all febrile children, without prior parasitological confirmation, as in the control clusters of our study, an important proportion of cases are treated with ACTs unnecessarily. This study was conducted in areas of high malaria prevalence, confirmed both by microscopic examination and RDT (Tables 1 and 2). It is important to note that the use of RDTs to guide treatment decisions in these high transmission settings resulted in substantial overprescription of ACTs, compared with treatment based on the results of blood slide microscopy. Nonetheless, based on the proportion of negative RDT results in the intervention clusters of the study sites, the proportion of unnecessary use of ACTs was estimated to vary between 12% and 26% across the three sites. This finding has public health implications, suggesting that a disease management approach based on a diagnostic and treatment package improves the appropriate use of ACTs, and provides alternative treatment of other causes of febrile illness.
Inappropriate use (non-compliance with study guidelines) of antibiotics was high in two study sites. More than a third of children with a normal RR received antibiotic treatment in Ghana and Burkina Faso, whereas 27% and 14% of children with a high RR did not receive any antibiotics in the two countries, respectively. In Ghana, however, where CHWs could prescribe antibiotics in control clusters based on their clinical judgment, 64.3% of children were treated with antibiotics in control clusters compared with 51.4% in intervention clusters. In this study, we used only a high respiratory rate as the criterion for antibiotic use, whereas the WHO39 recommends the presence of cough or difficulty breathing together with high respiratory rate as the criteria. This is a limitation of this study, and fewer children would have been treated with antibiotics under WHO guidelines than were treated using a criterion of rapid breathing alone. Overuse of antibiotics is a well-known phenomenon at all levels of the health system in low-, middle-, and high-income countries, as reported by the WHO,61 and has been recently reported to be aggravated by the introduction of RDT in the decision algorithm.51 In Uganda, over- or under-prescription of antibiotics was rare, accounting for only 0.9% and 1.7% of cases. This difference between Uganda and the other sites may be explained by the more intensive supervision program of the CHWs, and by local treatment practices. It is possible that local antibiotic prescription practices influenced behavior in the intervention clusters. Vialle-Valentin and colleagues62 have reported higher antibiotic use among under-five children in Ghana compared with other countries including Uganda.
The study was conducted in areas with microscopic malaria prevalence ranging between 54% and 80%. Cost-effectiveness of the integrated package compared with presumptive malaria treatment may be lower in areas with high rather than low malaria transmission, if judged only by savings in anti-malarial treatments. However, the WHO now recommends that all suspected cases of malaria be confirmed with a diagnostic test before treatment9 in all settings; furthermore, the development of an integrated package fitting all epidemiologic settings, regardless of the relative prevalence of malaria and pneumonia, has obvious advantages in terms of translation into policy. It is also possible that the overuse of medicines may be higher in settings with lower malaria prevalence, in which many of the RDTs would be negative. It will be important to explore this issue further in these lower transmission settings.
The primary endpoint of our study was fever clearance 3 days from initiation of treatment. One might expect a higher proportion of children with fever to be afebrile after a few days if treated more rationally, with different drugs based on the results of diagnostic tests. Consistently across the study sites, over 96% of the children were afebrile at the follow-up visits in both intervention and control arms. Although a smaller proportion of children in the intervention arm remained febrile at Days 3 and 7 compared with the control arm, the numbers of such children were small and the difference observed could be caused by chance. This result is not easy to interpret. One possibility is that the frequency of minor, self-limiting viral infections as the cause of fevers was high, and diluted the effect of specific anti-malarial and antibacterial treatment. Furthermore, in a context of high parasitemia, it is possible that antibiotics might not have a measurable effect on fever clearance. Our findings are consistent with those from a study in Zambia25 that found no evidence that the risk of persistence of fever differed in intervention and control clusters after 5 and 7 days of treatment. Adding cough/difficulty breathing to our treatment algorithm would not have altered this because most under-five children who needed an antibiotic received one in the intervention arm.
The subgroup analysis that was conducted suggests that the use of antibiotics in the control arm in Ghana may have diluted the effect of the intervention, as there was some evidence of an effect of the intervention on fever clearance when data from only Burkina Faso and Uganda were used. However, visual inspection of the data from these two countries does not suggest a large difference in fever clearance rates at Day 3 in the intervention compared with the control arm.
Other aspects of feasibility, and acceptability of the approach based on the diagnostic and treatment package was assessed in all sites and will be reported in detail elsewhere. Overall, the CHWs were found to be able to perform their tasks satisfactorily, including RDTs for malaria and respiratory rate counting. A study in Uganda found that when RDT results and RR were double-checked by laboratory technicians and pediatricians there was a concordance rate of 100% for RDTs and 93% for RR.19 The acceptability of CHWs performing RDTs and RR counting was also high among communities, CHWs, and health staff. The majority of interviewed community members were satisfied with CHWs treating children according to the package.19
Though the study was not designed to evaluate RDT performance, the positivity rates for microscopy were consistently lower than those for RDTs across the three sites. This may be explained by the fact that all three RDTs used HRP2 as the target antigen, which can persist in the blood stream after parasite clearance,63–67 suggesting that some positive RDTs were indicative of past rather than current infection. The choice of which RDT to use was made for one of the following reasons: availability, resistance to high storage temperature (40°C), delivery time, and national policy. Burkina Faso received their supply from TDR as their original supplier failed to deliver. The RDTs used were FirstSign in Burkina Faso, Paracheck pf in Ghana, and ICT in Uganda. It should also be noted that the RDTs were selected before the publication of “Malaria Rapid Diagnostic Test Performance – Results of WHO product testing of malaria RDTs Round 2”40 in which RDT performance was recorded for 67 RDTs. The ranking of the PDS for the RDTs was 28 for ICT, 49 for Paracheck, and 59 for First Sign,40 indicating that none really achieved a high combined measure positivity rate, along with inter-test and inter-lot consistency, although their PDSs were above 70/100.
The study shows that an iCCM approach based on the use of diagnostics and medicines for malaria and pneumonia by CHWs improves the rational use of anti-malarial drugs, and may reduce the inappropriate use of antibiotics at the community level in settings where they are already available. Fever clearance was very high in both intervention and control arms at both Day 3 and Day 7 and when all three countries were included in the analysis there was no clear evidence of a clinical benefit of the intervention. These findings add to the evidence base for iCCM as a public health strategy.
ACKNOWLEDGMENTS
We thank the community members, opinion leaders, CHOs, the health workers, research assistants, field supervisors, and workers in the districts of Sapone in Burkina Faso, Kassena Nankana in Ghana, and Iganga in Uganda; this study would not have been possible without their co-operation. We also acknowledge the technical and financial support from the UNICEF/UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases.
Disclaimer: The authors declare that they have no competing interests.
Footnotes
Financial support: This study was funded by UNICEF/UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR) through project numbers A60486 for Burkina Faso, A60490 for Ghana, and A60487 for Uganda.
Authors' addresses: David Mukanga and Karin Källander, School of Public Health, Makerere University College of Health Sciences Kampala, Uganda/Division for Global Health Department of Public Health Sciences, Karolinska Institutet, Stockholm Sweden, E-mails: dmukanga@afenet.net and Karin.kallander@ki.se. Alfred B. Tiono, Amadou T. Konaté, and Sodiomon B. Sirima, Centre National de Recherche et de Formation sur le Paludisme, Ouagadougou, Burkina Faso, E-mails: t.alfred@fasonet.bf, a.konate.cnlp@fasonet.bf, and s.sirima.cnlp@fasonet.bf. Thomas Anyorigiya, Abraham R. Oduro, and Lucas Amenga-Etego, Navrongo Health Research Centre, Navrongo, Ghana, E-mails: tanyorigiya@navrongo.mimcom.org, aoduro@navrongo.mimcom.org, and lamenga-etego@navrongo.mimcom.org. James K. Tibenderana, Malaria Consortium Africa, Kampala, Uganda, E-mail: j.tibenderana@malariaconsortium.org. Simon Cousens, London School of Hygiene and Tropical Medicine, London, England, E-mail: simon.cousens@lshtm.ac.uk. Guy Barnish, le Moncheny, Limousin, France, E-mail: gbarnish@liv.ac.uk. Franco Pagnoni, Evidence for Antimalarial Policy and Access Unit, UNICEF/UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR), Geneva, Switzerland, E-mail: pagnonif@who.int.
Reprint requests: David Mukanga, School of Public Health, Makerere University College of Health Sciences, PO Box 7072, Kampala, Uganda, E-mail: dmukanga@afenet.net.
Blood smear for microscopy done in only a sub-sample of children in the control arm in Uganda.
References
- 1.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–2234. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 2.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 3.Kinney MV, Kerber KJ, Black RE, Cohen B, Nkrumah F, Coovadia H, Nampala PM, Lawn JE, Axelson H, Bergh AM, Chopra M, Diab R, Friberg I, Odubanjo O, Walker N, Weissman E. Sub-Saharan Africa's mothers, newborns, and children: where and why do they die? PLoS Med. 2010;7:e1000294. doi: 10.1371/journal.pmed.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidane G, Morrow RH. Teaching mothers to provide home treatment of malaria in Tigray, Ethiopia: a randomized trial. Lancet. 2000;356:550–555. doi: 10.1016/S0140-6736(00)02580-0. [DOI] [PubMed] [Google Scholar]
- 5.Sazawal S, Black RE. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect Dis. 2003;3:547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 6.WHO . Evidence Base for Community Management of Pneumonia. Geneva: WHO; 2002. WHO/FCH/CAH/02.23. [Google Scholar]
- 7.WHO . Scaling up home-based management of malaria - from research to implementation. In: Eckert E, editor. Geneva: WHO; 2004. WHO/HTM/MAL/2004.1096, WHO/IDE/HMM/04.1. [Google Scholar]
- 8.WHO/UNICEF . Joint Statement: Management of Pneumonia in Community Settings. Geneva/New York: WHO/UNICEF; 2004. WHO/FCH/CAH/04.06. [Google Scholar]
- 9.WHO . Guidelines for the Treatment of Malaria. Second edition. Geneva: World Health Organization; 2010. [Google Scholar]
- 10.D'Acremont V, Lengeler C, Genton B. Reduction in the proportion of fevers associated with Plasmodium falciparum parasitemia in Africa: a systematic review. Malar J. 2010;9:240. doi: 10.1186/1475-2875-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Dempsey TJ, McArdle TF, Laurence BE, Lamont AC, Todd JE, Greenwood BM. Overlap in the clinical features of pneumonia and malaria in African children. Trans R Soc Trop Med Hyg. 1993;87:662–665. doi: 10.1016/0035-9203(93)90279-y. [DOI] [PubMed] [Google Scholar]
- 12.Murray CK, Bell D, Gasser RA, Wongsrichanalai C. Rapid diagnostic testing for malaria. Trop Med Int Health. 2003;8:876–883. doi: 10.1046/j.1365-3156.2003.01115.x. [DOI] [PubMed] [Google Scholar]
- 13.Bell D, Wongsrichanalai C, Barnwell JW. Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat Rev Microbiol. 2006;4:682–695. doi: 10.1038/nrmicro1474. [DOI] [PubMed] [Google Scholar]
- 14.Harvey SA, Jennings L, Chinyama M, Masaninga F, Mulholland K, Bell DR. Improving community health worker use of malaria rapid diagnostic tests in Zambia: package instructions, job aid and job aid-plus-training. Malar J. 2008;7:160. doi: 10.1186/1475-2875-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins H, Bebell L, Kambale W, Dokomajilar C, Rosenthal PJ, Dorsey G. Rapid diagnostic tests for malaria at sites of varying transmission intensity in Uganda. J Infect Dis. 2008;197:510–518. doi: 10.1086/526502. [DOI] [PubMed] [Google Scholar]
- 16.Bisoffi Z, Gobbi F, Angheben A, Van den Ende J. The role of rapid diagnostic tests in managing malaria. PLoS Med. 2009;6:e1000063. doi: 10.1371/journal.pmed.1000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmardi KA, Malik EM, Abdelgadir T, Ali SH, Elsyed AH, Mudather MA, Elhassan AH, Adam I. Feasibility and acceptability of home-based management of malaria strategy adapted to Sudan's conditions using artemisinin-based combination therapy and rapid diagnostic test. Malar J. 2009;8:39. doi: 10.1186/1475-2875-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuoka J, Poudel KC, Poudel-Tandukar K, Nguon C, Ly P, Socheat D, Jimba M. Assessing the quality of service of village malaria workers to strengthen community-based malaria control in Cambodia. Malar J. 2010;9:109. doi: 10.1186/1475-2875-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukanga D, Babirye R, Peterson S, Pariyo GW, Ojiambo G, Tibenderana JK, Nsubuga P, Kallander K. Can lay community health workers be trained to use diagnostics to distinguish and treat malaria and pneumonia in children? Lessons from rural Uganda. Trop Med Int Health. 2011;16:1234–1242. doi: 10.1111/j.1365-3156.2011.02831.x. [DOI] [PubMed] [Google Scholar]
- 20.Mubi M, Janson A, Warsame M, Martensson A, Kallander K, Petzold MG, Ngasala B, Maganga G, Gustafsson LL, Massele A, Tomson G, Premji Z, Bjorkman A. Malaria rapid testing by community health workers is effective and safe for targeting malaria treatment: randomized cross-over trial in Tanzania. PLoS ONE. 2011;6:e19753. doi: 10.1371/journal.pone.0019753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berman S, Simoes EA, Lanata C. Respiratory rate and pneumonia in infancy. Arch Dis Child. 1991;66:81–84. doi: 10.1136/adc.66.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolstad PR, Burnham G, Kalter HD, Kenya-Mugisha N, Black RE. The integrated management of childhood illness in western Uganda. Bull World Health Organ. 1997;75((Suppl 1)):77–85. [PMC free article] [PubMed] [Google Scholar]
- 23.Weber MW, Mulholland EK, Jaffar S, Troedsson H, Gove S, Greenwood BM. Evaluation of an algorithm for the integrated management of childhood illness in an area with seasonal malaria in the Gambia. Bull World Health Organ. 1997;75((Suppl 1)):25–32. [PMC free article] [PubMed] [Google Scholar]
- 24.WHO . Product Information Sheets 2000 Edition. Geneva: World Health Organization; 2000. WHO/V&B/00.13. [Google Scholar]
- 25.Yeboah-Antwi K, Pilingana P, Macleod WB, Semrau K, Siazeele K, Kalesha P, Hamainza B, Seidenberg P, Mazimba A, Sabin L, Kamholz K, Thea DM, Hamer DH. Community case management of fever due to malaria and pneumonia in children under five in Zambia: a cluster randomized controlled trial. PLoS Med. 2010;7:e1000340. doi: 10.1371/journal.pmed.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kallander K, Tomson G, Nsabagasani X, Sabiiti JN, Pariyo G, Peterson S. Can community health workers and caretakers recognize pneumonia in children? Experiences from western Uganda. Trans R Soc Trop Med Hyg. 2006;100:956–963. doi: 10.1016/j.trstmh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomized trials. BMJ. 2004;328:702–708. doi: 10.1136/bmj.328.7441.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uganda Bureau of Statistics . Uganda Demographic and Health Survey 2006. Calverton, MD: ORC Macro and Kampala, Uganda: UBOS; 2006. [Google Scholar]
- 29.Kamugisha E, Bujila I, Lahdo M, Pello-Esso S, Minde M, Kongola G, Naiwumbwe H, Kiwuwa S, Kaddumukasa M, Kironde F, Swedberg G. Large differences in prevalence of Pfcrt and Pfmdr1 mutations between Mwanza, Tanzania and Iganga, Uganda: a reflection of differences in policies regarding withdrawal of chloroquine? Acta Trop. 2011;121:148–151. doi: 10.1016/j.actatropica.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D'Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 31.Binka FN, Morris SS, Ross DA, Arthur P, Aryeetey ME. Patterns of malaria morbidity and mortality in children in northern Ghana. Trans R Soc Trop Med Hyg. 1994;88:381–385. doi: 10.1016/0035-9203(94)90391-3. [DOI] [PubMed] [Google Scholar]
- 32.Koram KA, Owusu-Agyei S, Fryauff DJ, Anto F, Atuguba F, Hodgson A, Hoffman SL, Nkrumah FK. Seasonal profiles of malaria infection, anemia, and bednet use among age groups and communities in northern Ghana. Trop Med Int Health. 2003;8:793–802. doi: 10.1046/j.1365-3156.2003.01092.x. [DOI] [PubMed] [Google Scholar]
- 33.Appawu M, Owusu-Agyei S, Dadzie S, Asoala V, Anto F, Koram K, Rogers W, Nkrumah F, Hoffman SL, Fryauff DJ. Malaria transmission dynamics at a site in northern Ghana proposed for testing malaria vaccines. Trop Med Int Health. 2004;9:164–170. doi: 10.1046/j.1365-3156.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- 34.Iganga District . Uganda: Iganga District Local Government; 2008. (Five-Year Orphans and other Vulnerable Children Strategic Plan 2008/2009–2012/2013). [Google Scholar]
- 35.Adjuik M, Smith T, Clark S, Todd J, Garrib A, Kinfu Y, Kahn K, Mola M, Ashraf A, Masanja H, Adazu K, Sacarlal J, Alam N, Marra A, Gbangou A, Mwageni E, Binka F. Cause-specific mortality rates in sub-Saharan Africa and Bangladesh. Bull World Health Organ. 2006;84:181–188. doi: 10.2471/blt.05.026492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkina Faso MOH . Annual Statistics Ouagadougou. Burkina Faso: Ministry of Health; 2010. [Google Scholar]
- 37.Uganda MOH . Implementation Guidelines for Home-Based Management of Fever Strategy in Children. Second edition. Kampala: Ministry of Health; 2005. [Google Scholar]
- 38.Uganda MOH . Integrated community case management of childhood malaria, pneumonia and diarrhoea. In: Community Health Department MoH, editor. Implementation Guidelines. Kampala: 2010. p. 50. [Google Scholar]
- 39.WHO . Integrated Management of Childhood Illness (IMCI) Handbook. Geneva: WHO: Department of Child and Adolescent Health and Development; 2005. [Google Scholar]
- 40.WHO . Malaria Rapid Diagnostic Test Performance: Results of WHO product testing malaria RDTs: Round 2. Geneva: UNICEF/UNDP/World Bank/WHO; 2009. Special Programme for Research and Training in Tropical Diseases, Centers for Disease Control (U.S.), and Foundation for Innovative New Diagnostics. [Google Scholar]
- 41.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28:319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 42.Lon CT, Alcantara S, Luchavez J, Tsuyuoka R, Bell D. Positive control wells: a potential answer to remote-area quality assurance of malaria rapid diagnostic tests. Trans R Soc Trop Med Hyg. 2005;99:493–498. doi: 10.1016/j.trstmh.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 43.WHO . Malaria Microscopy Quality Assurance Manual. Geneva: World Health Organization; 2009. http://www.who.int/malaria/publications/malaria_microscopy_QA_manual.pdf Version 1. Available at. Accessed July 2011. [Google Scholar]
- 44.Greenwood BM, Armstrong JR. Comparison of two simple methods for determining malaria parasite density. Trans R Soc Trop Med Hyg. 1991;85:186–188. doi: 10.1016/0035-9203(91)90015-q. [DOI] [PubMed] [Google Scholar]
- 45.Maude RJ, Woodrow CJ, White LJ. Artemisinin antimalarials: preserving the “magic bullet.”. Drug Dev Res. 2010;71:12–19. doi: 10.1002/ddr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Alessandro U, Talisuna A, Boelaert M. Editorial: Should artemisinin-based combination treatment be used in the home-based management of malaria? Trop Med Int Health. 2005;10:1–2. doi: 10.1111/j.1365-3156.2004.01375.x. [DOI] [PubMed] [Google Scholar]
- 47.Charlwood D. The paradox of home management of malaria with artemisinin combinations. Trends Parasitol. 2004;20:405–406. doi: 10.1016/j.pt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Hume JC, Barnish G, Mangal T, Armazio L, Streat E, Bates I. Household cost of malaria overdiagnosis in rural Mozambique. Malar J. 2008;7:33. doi: 10.1186/1475-2875-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, Saganda K, Shao J, Kitua A, Olomi R, Greenwood BM, Whitty CJ. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amexo M, Tolhurst R, Barnish G, Bates I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet. 2004;364:1896–1898. doi: 10.1016/S0140-6736(04)17446-1. [DOI] [PubMed] [Google Scholar]
- 51.D'Acremont V, Kahama-Maro J, Swai N, Mtasiwa D, Genton B, Lengeler C. Reduction of anti-malarial consumption after rapid diagnostic tests implementation in Dar es Salaam: a before-after and cluster randomized controlled study. Malar J. 2011;10:107. doi: 10.1186/1475-2875-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Msellem MI, Martensson A, Rotllant G, Bhattarai A, Stromberg J, Kahigwa E, Garcia M, Petzold M, Olumese P, Ali A, Bjorkman A. Influence of rapid malaria diagnostic tests on treatment and health outcome in fever patients, Zanzibar: a crossover validation study. PLoS Med. 2009;6:e1000070. doi: 10.1371/journal.pmed.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamer DH, Ndhlovu M, Zurovac D, Fox M, Yeboah-Antwi K, Chanda P, Sipilinyambe N, Simon JL, Snow RW. Improved diagnostic testing and malaria treatment practices in Zambia. JAMA. 2007;297:2227–2231. doi: 10.1001/jama.297.20.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandler CI, Whitty CJ, Ansah EK. How can malaria rapid diagnostic tests achieve their potential? A qualitative study of a trial at health facilities in Ghana. Malar J. 2010;9:95. doi: 10.1186/1475-2875-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reyburn H, Mbakilwa H, Mwangi R, Mwerinde O, Olomi R, Drakeley C, Whitty CJ. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomized trial. BMJ. 2007;334:403. doi: 10.1136/bmj.39073.496829.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lubell Y, Reyburn H, Mbakilwa H, Mwangi R, Chonya S, Whitty CJ, Mills A. The impact of response to the results of diagnostic tests for malaria: cost-benefit analysis. BMJ. 2008;336:202–205. doi: 10.1136/bmj.39395.696065.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ssekabira U, Bukirwa H, Hopkins H, Namagembe A, Weaver MR, Sebuyira LM, Quick L, Staedke S, Yeka A, Kiggundu M, Schneider G, McAdam K, Wabwire-Mangen F, Dorsey G. Improved malaria case management after integrated team-based training of health care workers in Uganda. Am J Trop Med Hyg. 2008;79:826–833. [PubMed] [Google Scholar]
- 58.Chinkhumba J, Skarbinski J, Chilima B, Campbell C, Ewing V, San Joaquin M, Sande J, Ali D, Mathanga D. Comparative field performance and adherence to test results of four malaria rapid diagnostic tests among febrile patients more than five years of age in Blantyre, Malawi. Malar J. 2010;9:209. doi: 10.1186/1475-2875-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomson A, Khogali M, de Smet M, Reid T, Mukhtar A, Peterson S, von Schreeb J. Low referral completion of rapid diagnostic test-negative patients in community-based treatment of malaria in Sierra Leone. Malar J. 2011;10:94. doi: 10.1186/1475-2875-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chanda P, Hamainza B, Moonga HB, Chalwe V, Pagnoni F. Community case management of malaria using ACT and RDT in two districts in Zambia: achieving high adherence to test results using community health workers. Malar J. 2011;10:158. doi: 10.1186/1475-2875-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.WHO . The World Medicines Situation 2011 Rational Use of Medicines. Geneva: World Health Organization; 2011. http://www.who.int/medicines/areas/policy/world_medicines_situation/WMS_ch14_wRational.pdf Available at. Accessed July 2011. [Google Scholar]
- 62.Vialle-Valentin CE, Lecates RF, Zhang F, Desta AT, Ross-Degnan D. Predictors of antibiotic use in African communities: evidence from medicines household surveys in five countries. Trop Med Int Health. 2011;17:211–222. doi: 10.1111/j.1365-3156.2011.02895.x. [DOI] [PubMed] [Google Scholar]
- 63.Tjitra E, Suprianto S, Dyer ME, Currie BJ, Anstey NM. Detection of histidine rich protein 2 and panmalarial ICT Malaria Pf/Pv test antigens after chloroquine treatment of uncomplicated falciparum malaria does not reliably predict treatment outcome in eastern Indonesia. Am J Trop Med Hyg. 2001;65:593–598. doi: 10.4269/ajtmh.2001.65.593. [DOI] [PubMed] [Google Scholar]
- 64.Mayxay M, Pukrittayakamee S, Chotivanich K, Looareesuwan S, White NJ. Persistence of Plasmodium falciparum HRP-2 in successfully treated acute falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:179–182. doi: 10.1016/s0035-9203(01)90156-7. [DOI] [PubMed] [Google Scholar]
- 65.Singh N, Shukla MM. Short report: field evaluation of posttreatment sensitivity for monitoring parasite clearance of Plasmodium falciparum malaria by use of the Determine Malaria pf test in central India. Am J Trop Med Hyg. 2002;66:314–316. doi: 10.4269/ajtmh.2002.66.314. [DOI] [PubMed] [Google Scholar]
- 66.WHO . Evaluating Diagnostics: The Malaria Guide. Geneva: WHO-Regional Office for the Western Pacific/TDR; 2006. Evaluation of rapid diagnostic tests: malaria. [Google Scholar]
- 67.Swarthout TD, Counihan H, Senga RK, van den Broek I. Paracheck-Pf accuracy and recently treated Plasmodium falciparum infections: is there a risk of over-diagnosis? Malar J. 2007;6:58. doi: 10.1186/1475-2875-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]