Abstract
Background:
The most favorable method for cervical ripening is not fully agreed upon by practitioners; however, vaginal administration of isosorbide mononitrate (IMN) is considered a low-risk method of labor induction for pregnant women at full term. Our study was designed to assess the effect of IMN on cervical ripening and labor induction among primiparous women in Iran.
Materials and Methods:
A randomized, double-blind, placebo-controlled trial was conducted on 90 primiparous women with Bishop score ≤ 5, term pregnancy, and no sign of labor. The women were allocated into two groups to receive either a 40 mg (2 × 20 mg) IMN tablet vaginally (n = 45) or placebo (n = 45) at 0 and 12 h. If uterine contractions were not present after 24 h, women were sent to the labor section for induction of labor. Bishop score, reason for induction, duration of active, second, and third phase of labor, and mode of delivery were assessed.
Results:
There was a significant difference between the IMN group and the controls with respect to the Bishop score (4.92 vs. 4.03, P = 0.0.01), induction to active phase interval (387.6 vs. 520.4 min, P = 0.03), the length of induction (350 vs. 446 min, P = 0.03), and the drug administration to delivery interval (33.9 vs. 36.2 h, P = 0.03). The major side effect of IMN was headache, which responded to analgesia.
Conclusions:
IMN can be an effective alternative drug for cervical ripening and could decrease the labor interval. Further studies are necessary to fully address the benefits of IMN, especially in pregnancies of gestational age greater than 42 weeks.
Keywords: Bishop score, cervical ripening, Iran, isosorbide mononitrate, primiparous women
INTRODUCTION
At this point in time, the best method for cervical ripening has not been agreed by practitioners. Induction of labor with an unripe cervix is the main cause of induction failure.[1] The agents that is used to soften the cervix, should soften cervix with limited side effects for the woman (nausea and vomiting) and neonate (low APGAR score).[2] Medical and non-medical methods are used for labor induction. However, some agents for cervical ripening have many side effects and cannot be administered as outpatient treatment. The agents most commonly used in a hospital setting are prostaglandins which have some potential risks, e.g., hyperstimulation, that limit their usage in outpatient environment.[3]
An agent that ripens the cervix without stimulating uterine activity would be ideal for outpatient treatment. Nitric oxide (NO) is a free radical with a short half-life for cervical ripening.[4] Studies show that reduction of NO in the cervix may be a cause of post-term pregnancy,[5] because the main effect of NO is rearrangement of collagen, thereby allowing NO to soften the cervix without causing uterine contractions.[6] Isosorbide mononitrate (IMN) is a drug used principally in the treatment of angina pectoris, which acts by dilating the blood vessels so as to reduce blood pressure.[7,8] In animal studies, compounds like IMN and glyceryl trinitrate facilitate the production of NO to induce cervical ripening.[2,9] In a study by Ekerhoved et al., women received 40 mg IMN or placebo vaginally 4 h before elective cesarean, which induced a significant increase in cervical distensibility.[8] A study in which women with term pregnancy self-administered 40 mg IMN or placebo vaginally at 48 h, 32 h, and 16 h before scheduled hospital admission provides evidence on the efficacy of outpatient IMN for pre-induction cervical ripening.[10]
There are very few studies across the world that have evaluated the effect of IMN on cervical ripening, and to date, there are no studies that have evaluated this effect in Iran. Furthermore, other cervical ripeners like prostaglandins have certain side effects, and also using prostaglandins is not routinely followed in Iran. Our study aimed at to assess the effect of IMN on cervical ripening and labor induction among primiparous women in Iran.
MATERIALS AND METHODS
This was a randomized, double-blind, placebo-controlled trial. The study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences. All women gave signed written informed consent prior to the study. Sample size calculation was carried out by comparing the means among two groups (intervention and control). Mean ± SD of Bishop scores for the two groups were 6 ± 4.1 and 4 ± 1.78, respectively.[11] Power was set at 0.8, alpha level at 0.05, and the confidence interval (CI) at 95%. A total sample size 40 was needed in each group, which was increased to 45 per group to account for an additional 15% in attrition size.
Participants
Participants in this study consisted of 90 primiparous women who came to the Sina hospital (an educational hospital in Ahvaz, Iran), presenting with any signs of labor at term pregnancy from June to October 2010. The participants were selected from 114 women according to the inclusion/exclusion) criteria. Inclusion criteria were: Primiparous women of age 18-35 years, Bishop score ≤5, confirmed gestational age with a sonogram in the early weeks of gestation, body mass index (BMI) in the first trimester between 19.8 and 26, cephalic presentation, singleton fetus, having a normal non-stress test or biophysical test in the past 48 h, and gestational age of 40-42 weeks. Women with history of headache, alcohol abuse, polyhydramnios, placenta previa, or probability of placenta abruption, or any contraindication for induction of labor were excluded.
Randomization
Randomization to IMN or placebo was done in 1:1 ratio using a table of random numbers generated by Microsoft Excel. For example, if the sequence of the list generated by the computer was ABBAA, the first subject was randomized to the intervention group (A), followed by the second subject to the control group (B). The process continued until 90 subjects were enrolled into the two study groups. IMN and placebo were put into 90 individual pharmaceutical packages so that researchers and participants were not aware of the allocated treatment. None of the researchers had access to the information about allocation to the control or IMN group until the completion of the study.
Intervention
All women were examined at the first visit by one of the researchers (HY) and their Bishop score was measured. Each woman underwent a non-stress test and biophysical profile test (if they did not have it done in the past 48 h). A questionnaire was prepared for gathering socio-demographic data. A checklist was designed for measuring Bishop scores, blood pressure, length of first, second, and third stage of labor, and neonatal outcomes (APGAR in the 1st and 5th minutes after birth).
Women were permitted to go home and instructed to come to the hospital immediately if they had any sign of dangerous symptoms (leakage of amniotic fluid, vaginal bleeding, reduction in fetal movements); otherwise they were asked to come back to the hospital after 12 h. The IMN group received 40 mg IMN (2 × 20 mg) in the posterior fornix of the vagina, and the control group received placebo in the same site at 0 and 12 h. IMN tablets were prepared in Dubai (Neopharma, Abu Dubai, UAE) and the placebo tablets were prepared by a pharmacist in Ahvaz to have an identical appearance to IMN. A medical, demographic, and detailed obstetric history was taken and a physical examination performed for all participants by one of the researchers. After 12 h, the women were examined vaginally by the researcher and, if their labor had not started, a second dose of IMN or placebo was administered. All women at this stage were admitted to the labor section of the hospital and monitored until delivery.
If the labor did not start 24 h after first administration of the drug, labor induction was performed using classic induction of oxytocin, 10 IU in 1000 Ringer's solution starting at 4 microunits/min.
Outcome measures
Primary outcome measures included changes in Bishop score, length of the drug administration to active phase of labor, length of induction, the induction to delivery interval, the drug administration to delivery interval, the amount of oxytocin that was used, and the length of the second and third stages of labor. Mother or neonate complications, newborns’ APGAR scores at the 1st and 5th minutes after birth, and cesarean rates were recorded as secondary outcomes.
Statistical analysis
Data entry and analysis was carried out using SPSS version 16 (2006, SPSS Inc., Chicago, IL, USA). Between-group differences were assessed using an independent t-test for continuous variables, Chi-square test for categorical variables, and General Linear Model Repeated Measure for testing Bishop scores in different times. The third stage of labor was not normally distributed and the Mann–Whitney U test was used for between-group differences. P <0.05 was considered statistically significant.
RESULTS
The flow diagram in Figure 1 includes detailed information on the recruitment and retention of participants. Eighty women completed the study. The two groups did not have any significant difference in socio-demographic and obstetric history at baseline [Table 1].
Figure 1.
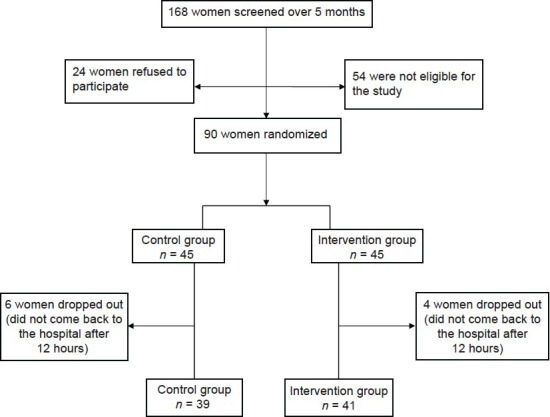
Flow diagram of recruitment and retention of participants in the study
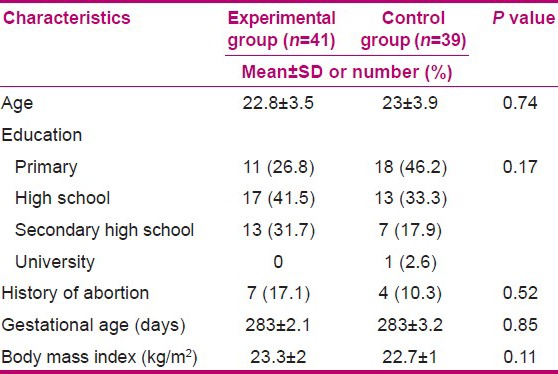
Table 1.
Socio-demographic and obstetric characteristics of participants at baseline
The mean first Bishop score was 2.8 ± 1.07 and 3.1 ± 1.3 for the intervention and control groups, respectively. The Bishop scores 12 h after intervention were 3.1 ± 1.5 and 3.2 ± 1.3, respectively. The scores 24 h after intervention were 4.9 ± 1.5 and 4.03 ± 1.5 in the intervention and control groups, respectively. The General Linear Model Repeated Measure showed a significant difference between groups regarding Bishop scores (P = 0.008) [Table 2].
Table 2.
Bishop score and labor progress (mean and standard deviation) in the intervention (IMN) and control groups
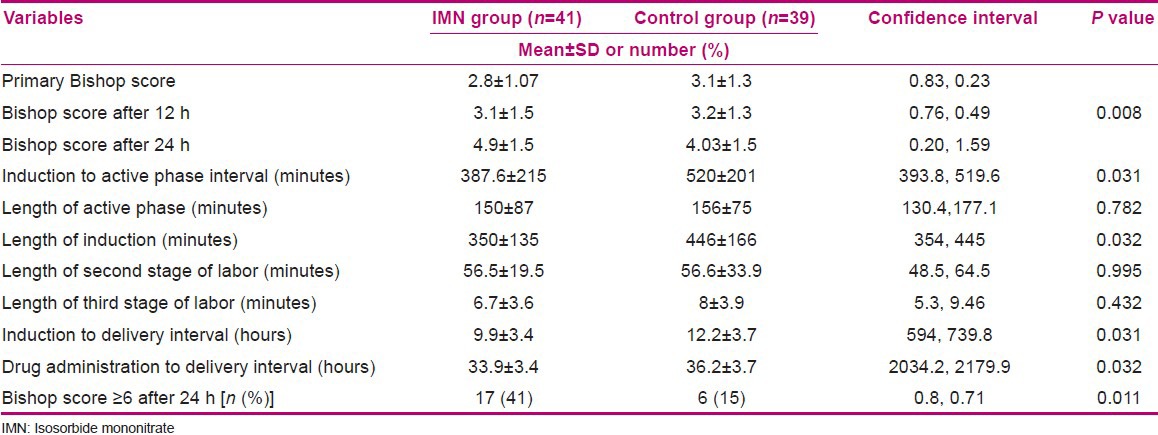
The duration of induction to active phase interval was 387.6 ± 215 and 520 ± 201 min in the intervention and control groups, respectively (95% CI = 393.8, 519.6, P = 0.031). The drug administration to delivery interval was significantly shorter in the IMN group compared with the control group (33.9 ± 3.4 vs. 36.2 ± 3.7 h, 95% CI = 2034.2, 2179.9, P = 0.032). Uterine contractions started in 10 women in the intervention group and 4 women in the control group without using induction drugs. There was no significant difference between the two groups regarding the length of the active phase, the amount of oxytocin required for induction, and the length of the second and third stages of labor [Table 2]. Overall, 75.6% of participants in the intervention group and 89.7% in the control group needed induction (P > 0.05). In the intervention group, 24.4% of women underwent cesarean because of meconium passage versus 25.6% in the control group. Ninety-five percent of the IMN group and 100% of the placebo group had neonates with APGAR ≥7 at the 1st minute after birth. The two groups did not have any significant differences in the mode of delivery, reason for cesarean, or neonatal outcomes (P > 0.05). Headache was the most common side effect of IMN (56.1% vs. 7.7%, P < 0.001); however, only three women needed analgesia for headache.
DISCUSSION
Our study aimed to evaluate the impact of IMN on cervical ripening and labor induction in primiparous women. All women recruited to the study had Bishop score ≤5. We observed uterine contractions starting in 10 (24.4%) women in the intervention group using only IMN treatment without the need of induction drugs. Other studies that have used IMN showed similar changes in the Bishop score.[11,12] However, some previous researches are not consistent with the present study. In a study by Bullarbo et al., the mean Bishop score was not significantly different between the intervention and placebo groups. This discrepancy is likely because in their study, the intervention group received only a single dose of IMN (40 mg), 24 h before admission to the hospital.[13] In our study, the proportion of women with Bishop score ≥6 after 24 h in the IMN group was significantly higher than that in the placebo group. In a study conducted by Eddama et al., the proportion of women with an unripe cervix after 24 h of outpatient treatment was significantly lower in the IMN group as compared with the placebo group (64% vs. 77%, P = 0.02).[14]
The induction to active phase interval and the drug administration to delivery interval were reduced significantly in the intervention group. In studies comparing IMN with misoprostol or dinoprostone, IMN was not as effective as these two drugs for cervical ripening.[11,15] However, in research evaluating the pure effect of IMN in comparison to placebo, IMN has been shown to be an effective means of ripening the cervix.[10]
In the present study, the length of active phase, and that of second and third stages of labor did not change. It appears that IMN as a cervical ripener only reduces the induction to active phase interval. These findings are in the line with those of other studies.[11,15,16]
The induction to delivery interval was significantly different between the two groups (P = 0.032). The duration between active phase and delivery is an important predictor for adverse pregnancy outcomes.[17] Prolonged labor is a cause of 8% of maternal mortality in developing countries[18] and can increase maternal morbidity in the short term e.g., infection and long term such as obstetric fistula[19] The drug administration to delivery interval was significantly shorter in the intervention group. The results of other studies are not similar to those of our study in this regard.[12,15] However, this is because in the other studies, if the Bishop score was ≤7, prostaglandin or cervical catheter was used for cervical ripening. The effect of prostaglandin on the Bishop score has been well documented.[20,21] The use of prostaglandin or cervical catheter for cervical ripening is not common in Iran. The results of Bollapragada et al.'s study refute any significant difference between IMN and placebo groups regarding the admission to delivery interval.[10] This discrepancy might be explained by the fact that the women in the Bollapragada et al.'s study administered IMN and placebo themselves at home, while in our study the drugs or placebo were administered in-hospital by one of the researchers. The insertion method could influence the effectiveness of IMN, and we recruited primiparous women (17.1 of IMN group and 10.3% of placebo group had a history of abortion) versus nulliparous women.
In our study, the fetal heart rate abnormalities, cesarean delivery rate, APGAR scores, and neonatal outcomes were similar in the two groups. However, IMN was associated with a significant increase in headache, but only three women needed analgesic drugs. This rate is comparable to the incidence observed in other studies of IMN used for cervical ripening at term.[13,17]
This is the first study conducted in Iran to assess the effect of IMN on cervical ripening and labor induction among primiparous women. Because other cervical ripeners are not routinely used in Iran, e.g., prostaglandin in term pregnancies, IMN has the potential to be a safe and cost-effective alternative for cervical ripening.
Our study has some limitations. As the purpose of the study was to assess the impact of IMN on primiparous women, the results are specific to primiparous women only and may not be easily generalized to other women. The administration of IMN and placebo was performed in the hospital setting owing to the uncertainty of correct use at home. There is a need for further investigation into home administration of the drug. We also did not compare the cost effectiveness of IMN before induction in this study. Future studies should consider an economic evaluation in the context of these results.
CONCLUSIONS
IMN can be an effective alternative drug for cervical ripening and could decrease the labor interval. Further studies are necessary to fully address the benefits of IMN, especially in pregnancies of gestational age greater than 42 weeks.
ACKNOWLEDGMENT
This study was a Mater thesis of HY. All expenses of this study were provided by Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. We hereby like to thank all women and also staffs of Sina hospital for their co-operation during data collection
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
REFERENCES
- 1.Abdellah MS, Hussein M, Aboalhassan A. Intravaginal administration of isosorbide mononitrate and misoprostol for cervical ripening and induction of labor: A randomized controlled trial. Arch Gynecol Obstet. 2011;284:25–30. doi: 10.1007/s00404-010-1572-4. [DOI] [PubMed] [Google Scholar]
- 2.Shi L, Shi SQ, Saade GR, Chawalisz K, Garfield RE. Studies of cervical ripening in pregnant rats: Effect of various treatments. Mol Hum Reprod. 2000;6:382–9. doi: 10.1093/molehr/6.4.382. [DOI] [PubMed] [Google Scholar]
- 3.Hofmeyr GJ, Gülmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010;10:CD000941. doi: 10.1002/14651858.CD000941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawalisz K, Garfield RE. Nitric oxide as the final mediator of cervical ripening. Hum Reprod. 1998;13:245–8. doi: 10.1093/humrep/13.2.245. [DOI] [PubMed] [Google Scholar]
- 5.Väisänen-Tommiska M. Nitric Oxide in Human Uterine Cervix: Role in Cervical Ripening. Academic Dissertation, Department of Obstetrics and Gynecology, Faculty of Medicine, Institute of Clinical Medicine, University of Helsinki. Finland. 2006:19–23. [Google Scholar]
- 6.Ledingham MA, Denison FC, Kelly RW, Young A, Norman JE. Nitric oxide donors stimulate prostaglandin F (2 alpha) and inhibit thromboxane B (2) production in the human cervix during the first trimester of pregnancy. Mol Hum Reprod. 1999;5:973–82. doi: 10.1093/molehr/5.10.973. [DOI] [PubMed] [Google Scholar]
- 7.Nicoll AE, Machenzie F, Greer IA, Norman JE. Vaginal application of the nitric oxide donor isosorbide mononitrate for preinduction cervical ripening: A randomized controled trial to determine effects on maternal and fetal hemodynamics. Am J Obstet Gynecol. 2001;184:958–64. doi: 10.1067/mob.2001.111797. [DOI] [PubMed] [Google Scholar]
- 8.Ekerhovd E, Bullarbo M, Andersch B, Norström A. Vaginal administration of the nitric oxide donor isosorbide mononitrate for cervical ripening at term: A randomized controled study. Am J Obstet Gynecol. 2003;189:1692–7. doi: 10.1016/s0002-9378(03)00865-2. [DOI] [PubMed] [Google Scholar]
- 9.Chawalisz K, Garfield RE. Regulation of the uterus and cervix during pregnancy and labor. Role of progesterone and nitric oxide. Ann N Y Acad Sci. 1997;26:238–53. doi: 10.1111/j.1749-6632.1997.tb48545.x. [DOI] [PubMed] [Google Scholar]
- 10.Bollapragada S, Mackenzie F. Randomized placebo-controlled trial of outpatient (at home) cervical ripening with Isosorbide Mononitrate (IMN) prior to induction of labor-clinical trial with analyses of efficacy and acceptability: The IMOP study. Obstet Gynecol Surv. 2009;64:699–700. doi: 10.1111/j.1471-0528.2009.02216.x. [DOI] [PubMed] [Google Scholar]
- 11.Habib SM, Emam SS, Saber AS. Outpatient cervical ripening with nitric oxide donor isosorbide mononitrate prior to induction of labor. Int J Gynaecol Obstet. 2008;101:57–61. doi: 10.1016/j.ijgo.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Rameez MF, Goonewardene IM. Nitric oxide donor isosorbide mononitrate for pre-induction cervical ripening at 41 weeks’ gestation: A randomised controlled trial. J Obstet Gynaecol Res. 2007;33:452–6. doi: 10.1111/j.1447-0756.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 13.Bullarbo M, Orrskog ME, Andersch B, Granström L, Norström A, Ekerhovd E. Outpatient vaginal administration of the nitric oxide donor isosorbide mononitrate for cervical ripening and labor induction postterm: A randomized controled study. Am J Obstet Gynecol. 2007;196:e1–5. doi: 10.1016/j.ajog.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 14.Eddama O, Petrou S, Schroeder L, Bollapragada SS, Mackenzie F, Norrie J, et al. The cost-effectiveness of outpatient (at home) cervical ripening with isosorbide mononitrate prior to induction of labour. BJOG. 2009;116:1196–203. doi: 10.1111/j.1471-0528.2009.02236.x. [DOI] [PubMed] [Google Scholar]
- 15.Chanrachakul B, Herabutya Y, Punyavachira P. Randomized trial of isosorbide mononitrate versus misoprostol for cervical ripening at term. Int J Gynaecol Obstet. 2002;78:139–45. doi: 10.1016/s0020-7292(02)00128-5. [DOI] [PubMed] [Google Scholar]
- 16.Collingham JP, Fuh KC, Caughey AB, Pullen KM, Lyell DJ, EI-Sayed YY. Oral misoprostol and vaginal isosorbide mononitrate for labor induction: A randomized controlled trial. Obstet Gynecol. 2010;116:121–6. doi: 10.1097/AOG.0b013e3181e408f2. [DOI] [PubMed] [Google Scholar]
- 17.Littleton LY, Engebretson J. 1st ed. USA: Delmar Cengage Learning; 2002. Maternal and neonatal and women's health nursing. [Google Scholar]
- 18.Samizadeh T. Iran: Mashhad School of Nursing and Midwifery; Mashhad University of Medical Sciences, Mashhad; 1999. Effect of relative presence at bedside of primiparous woman on labor duration. Master Thesis. [Google Scholar]
- 19.Neilson JP, Lavender T, Quenby S, Wray S. Obstructed labor reducing maternal dealth and disability during pregnancy. Br Med Bull. 2003;67:191–204. doi: 10.1093/bmb/ldg018. [DOI] [PubMed] [Google Scholar]
- 20.Hofmeyr GJ, Gulmezoglu AM. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2003;1 doi: 10.1002/14651858.CD000941. CD000941. [DOI] [PubMed] [Google Scholar]
- 21.Keirse M. Oxford: Cochrane Collaboration; 1995. Any Prostaglandin/Any Route for Cervical Ripening. [Google Scholar]


