Abstract
Background
Segmental bone loss remains a challenging clinical problem. A frequent mitigating factor is inadequate blood supply. Small molecules that activate the hypoxia inducible factor pathway (HIF) can be used to stimulate angiogenesis. We investigated an approach to promote healing using angiogenic and osteogenic compounds in combination with a biodegradable, weight bearing scaffold.
Methods
Adult rats underwent removal of a 5mm segment of femur stabilized by a cylindrical biodegradable implant and intramedullary fixation. Treatment groups included (1) saline (negative control), (2) desferrioxamine ((DFO), a HIF activator), (3) low dose rhBMP-2 (5µg), (4) DFO and low dose rhBMP-2 (5µg) or (5) rh-BMP-2 (10µg). Angiography was used to evaluate vascularity. Bone healing was assessed by radiographs, µCT, histology and biomechanical testing.
Results
Increased vascularity was seen at 6 weeks in the DFO treatment group. There appeared to be increased bone bridging as assessed by radiographic scores and µCT in the BMP groups, although the quantification of bone volume did not show statistically significant differences. Biomechanical testing revealed improved stiffness in the treatment groups.
Conclusions
DFO improved angiogenesis and stiffness of bone healing in segmental defects. BMP improved radiographic scores and stiffness. Use of angiogenic compounds in segmental bone loss is promising.
Clinical Relevance
Activation of the HIF pathway may prove useful for bone defects, particularly where impaired blood supply exists. The low cost approach could be useful in segmental bone defects clinically.
Keywords: Hypoxia inducible factor, segmental defect, desferrioxamine, polypropylene fumarate
Introduction
Non-union of fractures remains a significant clinical problem with reported rates of 5–10% in routine fractures and as high as 80% in very challenging scenarios such as segmental bone loss1. The blood supply to an injured area plays a vital role in healing. Increased vascularity has been shown to improve healing in soft tissue wounds2 and in fractures3. In areas of bone loss, healing is a particular challenge due to loss of the periosteal sleeve and its blood supply function4.
It seems logical, therefore, that increasing vascularity would lead to increased bone healing. Such increase in fracture healing has been seen with angiogenic growth factors such as erythropoietin (EPO) and vascular endothelial growth factor (VEGF). An alternative approach to amplify neo-vascularization is activation of the hypoxia inducible factor (HIF) pathway, the master regulator of angiogenic and metabolic responses to low oxygen. We have previously shown that local application of the small molecule desferrioxamine (DFO), which blocks the degradation of HIF, leads to neo-angiogenesis and increased bone formation in fracture and distraction osteogenesis3,5.
A further challenge in segmental bone defects is the transportation and containment of osteoinductive compounds. The ideal carrier would not only deliver these compounds to the fracture site, but also serve as structural support to allow weightbearing, then resorb after it has served its function. We have investigated the use of biodegradable polypropylene fumarate (PPF) scaffolds to transport a variety of substances including bone morphogenetic protein-2 (BMP) and gentamicin6,7.
In the present study, we combined the HIF activation approach with the PPF scaffold for structural support and drug delivery. We hypothesized that delivery of DFO would increase vascularity in a critical sized segmental bone defect, thereby increasing bone healing. We utilized a rat femoral defect model and compared the approach to BMP-2, a commonly used clinical intervention, for stimulation of bone healing, especially across bone defects.
Materials and Methods
Surgical Procedure
A 5mm long cylindrical scaffold was manufactured from biodegradable polypropylene fumarate/tricalcium phosphate (PPF/TCP) with dicalcium phosphate dihydrate (DCPD) cement portals as previously described6. All animal procedures were approved by the institutional animal care and use committee. Thirteen week old Brown Norway rats were anesthetized with Isoflurane. Buprenorphine was administered intraoperatively and for three days post-operatively for analgesia. Segmental defects were created in the femur (5mm), bridged by the scaffold and fixed with an intramedullary Kirschner wire as previously described6. Normal saline or one of four treatments were applied to the dicalcium phosphate cement portals. 106 surgeries were performed, there were three surgical failures (comminution, improper implant placement) for a total of 103 specimens. Rats were divided into one of five treatment groups: [1] DFO (30µL of a 400 µM solution) (n = 23) (Sigma), [2] low dose (5µg) rh-BMP-2 (n=16) (Medtronic Sofamor Danek), [3] combined DFO (30µL of a 400 µM solution) and low dose rh-BMP-2 (5µg)(n=20), [4] 10µg rh-BMP-2 (n=21) (the dose shown effective in a prior study using same approach6), [5] saline control (n=23). Seven rats had early implant failure (2 from saline group, 3 DFO, 1 BMP-low dose, 1 BMP), and three were deemed to have developed infection (1 each in saline, DFO and DFO/BMP-L groups).
Radiographic analysis
X-rays were obtained at 0, 3, 6, and 12 weeks. Healing was graded at 12 weeks in a blinded fashion by 2 independent observers as 0=no healing, 1=partial spanning of the defect, 2=complete healing of defect. Micro computed tomography (µCT) analysis of bone healing was performed at 12 weeks using a Scanco 40 µCT (ScanCo Medical). A 16 micron isotropic voxel size was used and the volume of interest was defined as 500 slices centered on the bone defect. A segmentation threshold of 270 was used and direct calculation of bone parameters was performed.
Assessment of Vascularity
µCT angiography was performed as previously described3,5. Briefly, rats were injected with sodium pentobarbital, followed by thoracotomy and left ventricle cannulation. The circulation was perfused with heparin, then formalin fixative, then microFil© silicone lead contrast agent. The femur was removed and decalcified. The vessels were then analyzed using the Scanco µCT 40. The volume of interest was 400 slices centered on the bone defect at 16 micron resolution with a segmentation threshold of 132. Spatial segmentation of the contrast within the bone was performed and three-dimensional angiograms were reconstructed.
Biomechanical testing
The bones were tested to failure by four-point bending in an anterior-posterior direction using a custom made four-point bending fixture on an 858 MiniBionix Materials Testing System (MTS Systems). A cross head speed of 0.1mm/sec was used to break each femur-scaffold unit. Load displacement curves were generated. The failure load was the maximum force recorded during the test. Stiffness was the slope of the linear portion of the load-displacement graph. Several specimens from the saline group had insufficient healing to permit testing and results were considered to be zero.
Histology
Femora were harvested at 12 weeks, fixed and decalcified, embedded in plastic and sectioned. After Trichrome staining, sections were evaluated qualitatively for bone or cartilage formation, inflammatory response and incorporation or replacement of the scaffold.
Statistical Analysis
Micro-CT measurements were compared between treatment groups using analysis of variance (ANOVA) followed by Tukey’s HSD test to identify differences when the ANOVA was significant. The mechanical testing and X-ray scoring variables were not normally distributed. Kruskal-Wallis non-parametric ANOVA was used to analyze them followed by Mann Whitney U tests when the ANOVA was significant.
Results
Vascularity
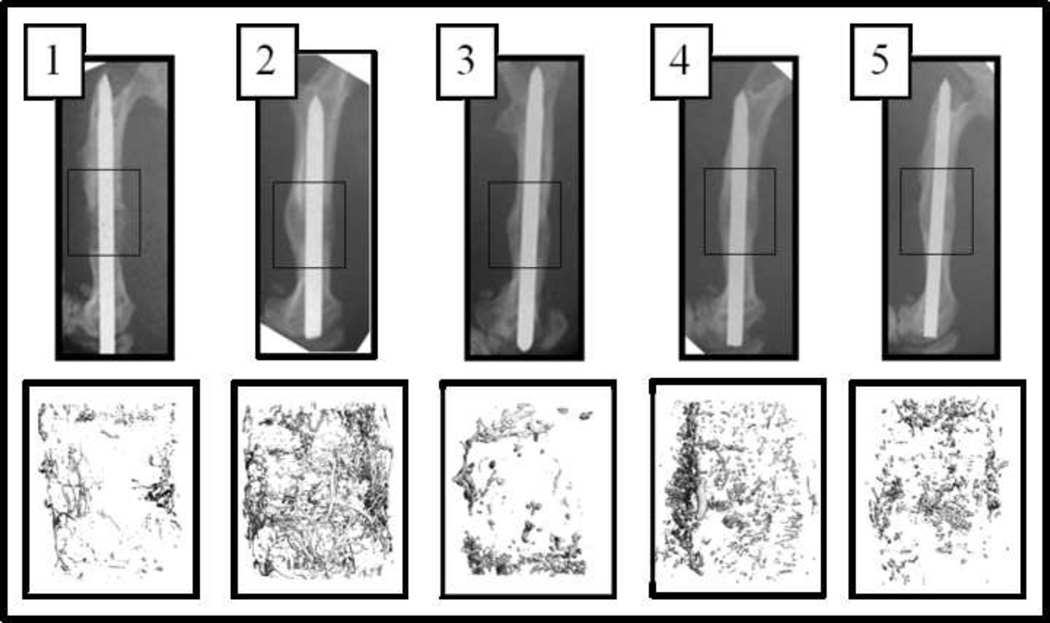
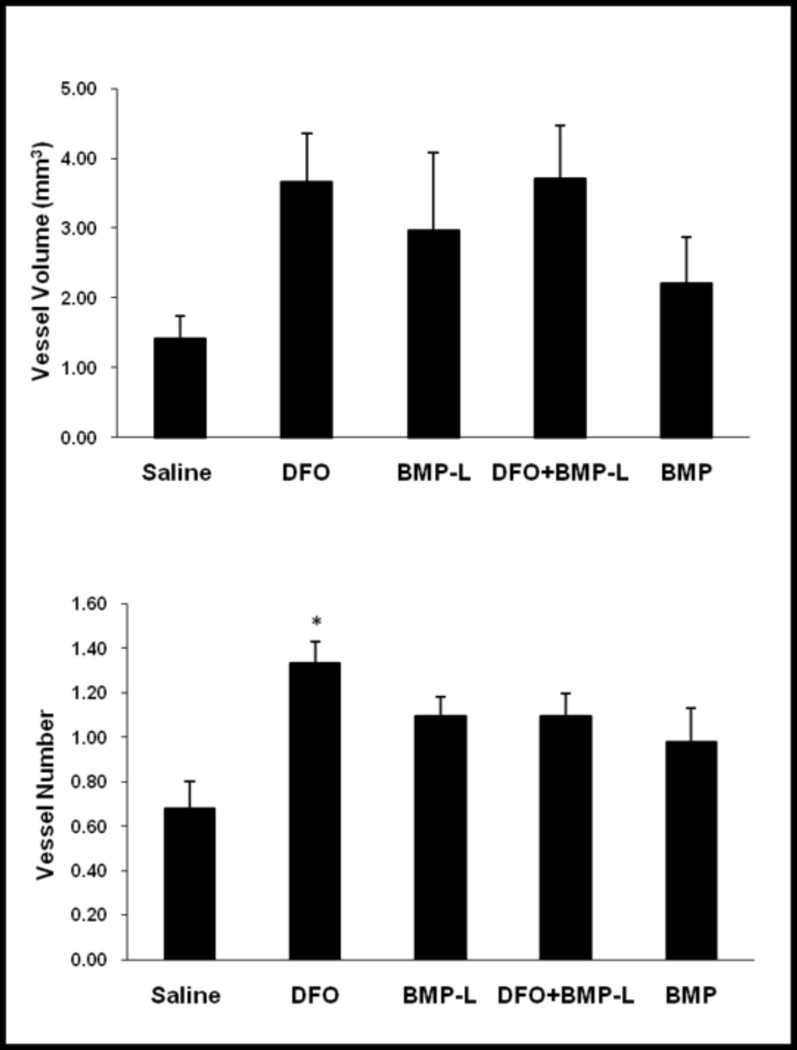
41 rats underwent perfusion and harvest. Three specimens were excluded due to insufficient perfusion (1 each in DFO, BMP-L and BMP groups), leaving 5–8 specimens for analysis for each group. µCT angiography results are summarized in Figure 1 with representative images (Figure 1A). Vessel number was approximately doubled in the DFO group compared to saline controls (p=0.01). The increases in vessel numbers in the other treatment group were not statistically significant (p=0.15). Similarly, the increases observed in vessel volume in the defect were not statistically significant (Figure 1B).
Figure 1.
(A) X-rays demonstrating segmental defect (boxed area) with scaffold in place at 6 weeks (top row). 3-D reconstructions of vascularity within the segmental defect from µCT angiography are shown (bottom row). 1-Saline, 2-DFO, 3-BMP-Low dose, 4-DFO + BMP-Low dose, 5- BMP. (B) Quantification of Vessel Volume (top) and Vessel Number (bottom) within the segmental defect by µCT angiography. Statistically significant increase in vessel number was seen in the DFO group compared to controls (* p<0.05 compared to saline control).
Radiographic Analysis of Bone Healing
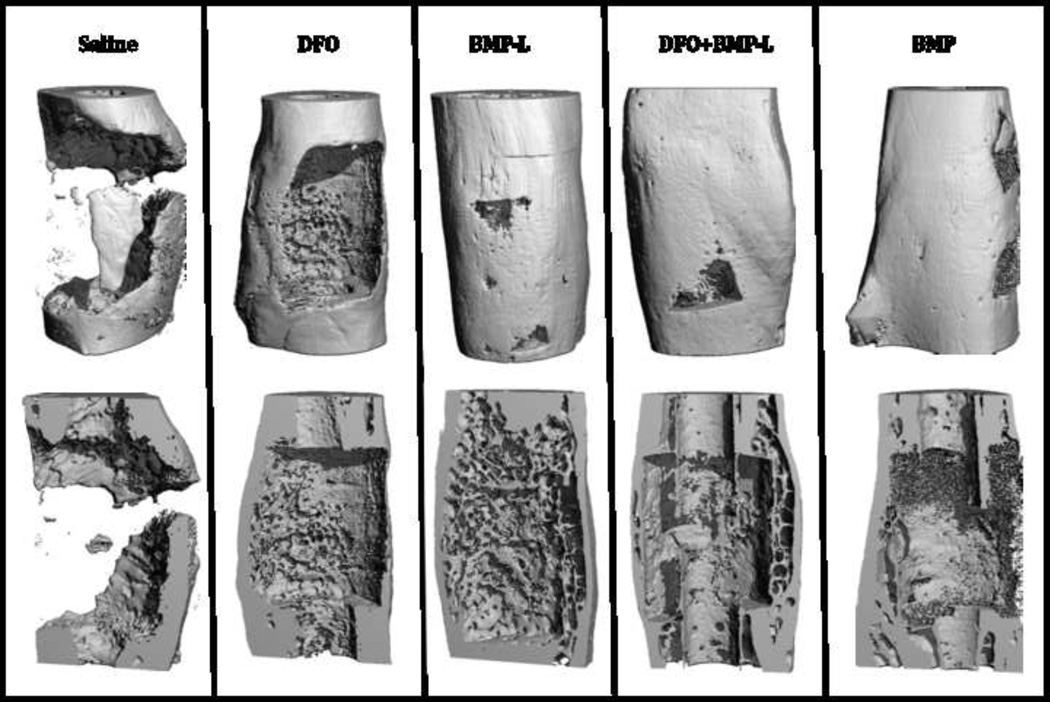
µCT analysis of bone healing was performed at 12 weeks. Representative images are shown in Figure 2. There appeared to be increased bone bridging noted on reconstructed images in the treatment groups. Quantification of bone volume showed that despite increases in the mean bone volumes in the treatment groups ranging from 18–37% compared to saline, the differences did not reach statistical significance (p= 0.18). Scoring of the radiographs at 12 weeks revealed a median score of 2 (complete healing) in all the BMP- containing groups compared to 1 for the DFO group and 0 for the saline group (p<0.001). Representative sequential x-rays are shown in Figure 3.
Figure 2.
µCT of bone healing at 12 weeks. Representative three dimensional reconstruction images are shown (top row). Sagittal cut-away reconstructed images (bottom row) show increased bone bridging in the treatment groups compared to no bony bridge noted in the saline group. The increases in bone volume in the treatment groups were not statistically significant.
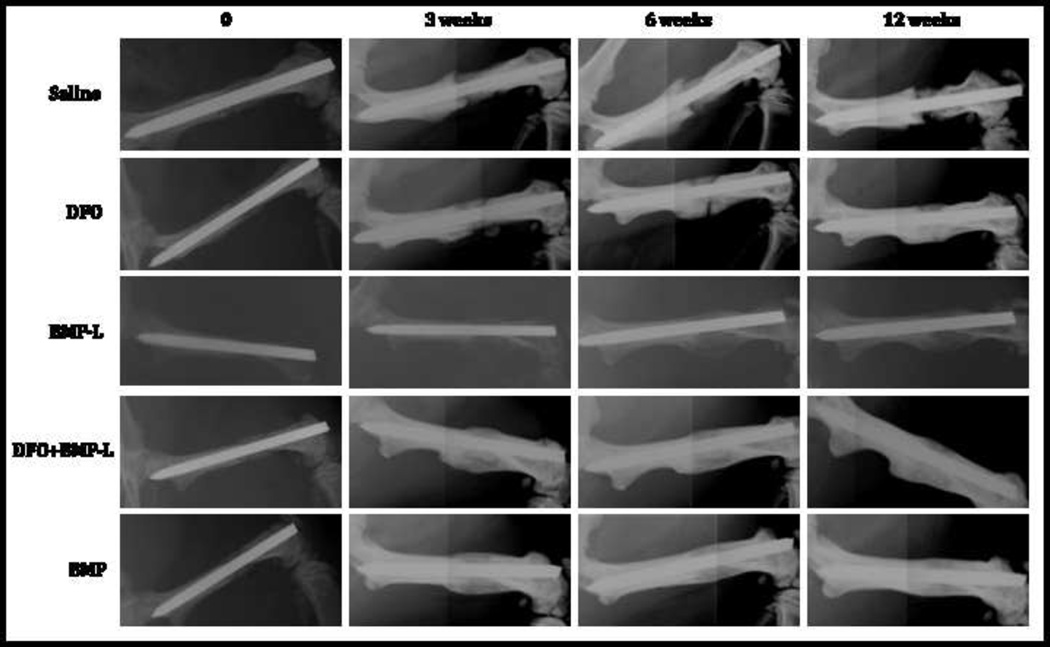
Figure 3.
Representative radiographs at 0,3,6 and 12 weeks from a single rat from each group. Note collapse of the implant with failure of healing in the saline group compared to varying degrees of bone healing in the treatment groups.
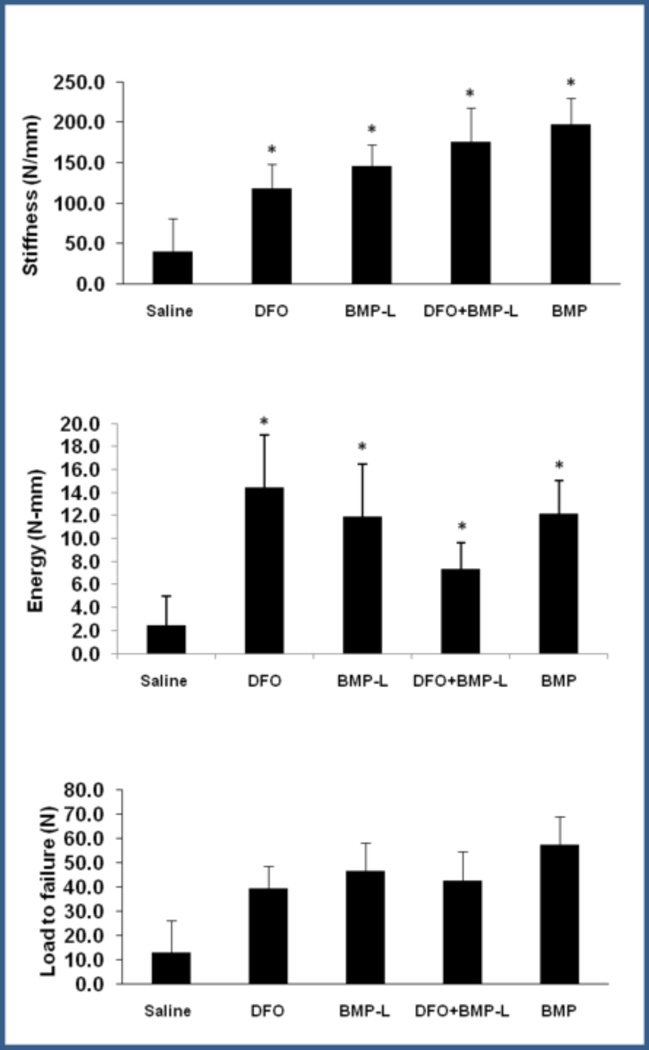
Biomechanical Testing
Specimens were analyzed by four point bending (saline n=7, 6/7 not tested due to insufficient healing, all other groups n=6–9). Results are represented graphically in Figure 4. Stiffness and energy to failure were increased in the all treatment groups compared to controls (p = 0.04 and 0.048, respectively). There were no statistically significant differences between the treatment groups. Load to failure was also increased in all treatment groups compared to controls, but did not reach statistical significance (p=0.08), For reference, mean values for contralateral femora are stiffness = 397 N/mm and load to failure = 226 N, with the highest value in the treatment groups reaching less than 50% of intact femora. The femora failed at the interface between the scaffold/healing bone and the cut ends of the femora.
Figure 4.
Biomechanical testing of femora following healing of segmental defects at 12 weeks. Results are shown graphically for Stiffness (top), Energy to Failure (middle), and Load to Failure (bottom). (* p<0.05 compared to control).
Histology
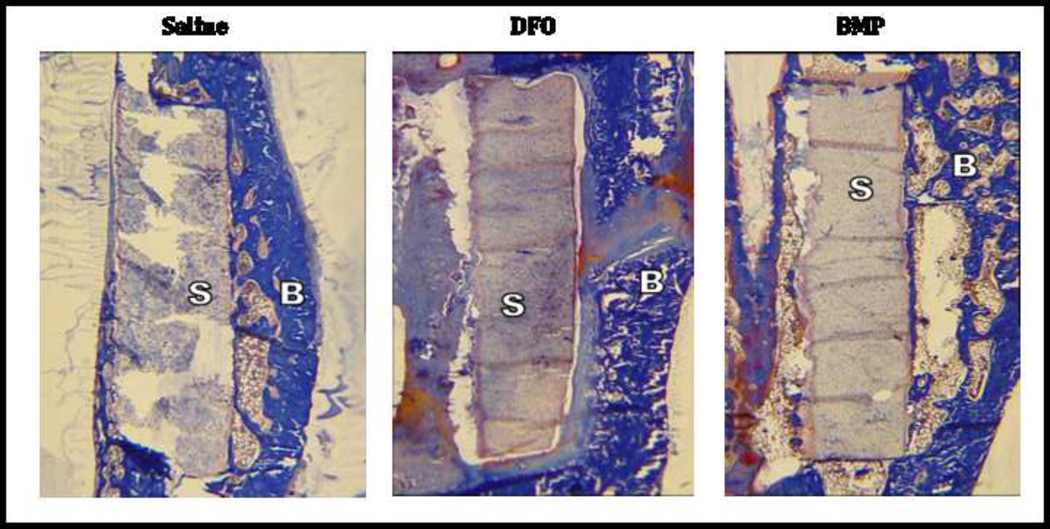
Qualitative evaluation of a single histologic specimen from saline, DFO and BMP (10µg) groups was performed. Bone growth was seen adjacent to, but not replacing the scaffold which was clearly visible at 12 weeks (Figure 5). No significant cartilage formation was seen. No significant inflammatory reaction was seen.
Figure 5.
Histology of healing segmental defects. Twelve weeks after creation of segmental defects. Femora were harvested, fixed and embedded. Longitudinal sections were cut and stained with Tri-Chrome. Representative 2× images are shown from Saline, DFO and BMP treated groups. Note the scaffolds (S) appear relatively intact with the exception of some artifact from sectioning. Bone (B) has formed around and adjacent to the scaffolds, but does not replace the scaffold. No inflammatory reaction is seen on these or higher power images (not shown).
Discussion
We have shown that the application of HIF activating small molecules to PPF scaffolds increases bone vascularity in our rat femoral defect model. This is associated with improved radiographic scores and increased stiffness and energy to failure compared to controls. The combination of DFO and a lower dose BMP-2 also increased stiffness and energy to failure compared to controls. An ideal solution has not yet been reached, as all treatment groups had inferior biomechanical testing results compared to normal femora at 12 weeks.
In clinical settings of bone loss, strategies to increase bone healing often include attempts to increase blood supply directly (e.g. vascularized tissue transfer) or indirectly (e.g. distraction osteogenesis). On a cellular/molecular level, previous attempts to increase vascularity have focused mainly on VEGF. Delivery of VEGF was effective in a segmental defect model, however use of VEGF or other growth factors is expensive because it requires manufacture of recombinant protein or a use of a vector 8,9. Stimulation of a master regulator such as HIF, however, can be achieved with use of an inexpensive small molecule, DFO. Further, HIF can activate not only VEGF, but also other components of the angiogenic cascade including VEGF receptors, and associated factors such as angiotensins which are required to destabilize and restabilize blood vessels10.
The use of HIF activators to increase vascularity is attractive. DFO has potential for accelerated translation to clinical application, as it is already in FDA approved clinical use as an iron chelator. Our study suggests that some barriers to application remain, in that an improved delivery system may be required. The present study demonstrates less increase in vascular response compared to our previous investigations in mouse models3,5, perhaps due to the increased volume and relative acellularity of the defect compared to fracture and distraction models, or simply the increased distance over which the small molecules must diffuse. We are currently investigating delivery systems that would increase the duration of HIF activator delivery, as well as investigating new potentially more effective small molecule activators of HIF.
There are recognized limitations to the present study. We did not specifically investigate mechanism of action, although previous work has demonstrated that DFO increases HIF activation, VEGF expression, and angiogenesis 5,11. Also, despite improved radiographic scores and biomechanical stiffness and energy to failure our µCT studies did not show significant increase in bone volume in the segmental defects at 12 weeks compared to controls. This may be related to limitations inherent to the µCT methodology. We selected a volume of interest of 500 slices for consistency to include the entire defect and a small length of surrounding bone. With non-locked intramedullary support, the defect is not length-stable, and partial collapse (as noted on some of the x-rays in the saline group) could result in more of the original bone outside the defect being included in the analysis. Additionally, although previous experience with the model suggested 5mm was a critical defect, some of our controls had greater than expected bone healing, suggesting that a larger size defect may have been necessary to eliminate partial healing with scaffold only. Finally, the number of groups and non-parametric nature of the data required use of more rigorous statistical tests, increasing the difficulty of reaching statistical significance.
In conclusion, the present study supports the applicability of small molecule activation of the HIF pathway to increase vascularity in the healing of segmental bone defects. This low cost approach cold be useful in segmental bone defects clinically.
Acknowledgements
We gratefully acknowledge the services of the University of Alabama at Birmingham Center for Metabolic Bone Disease Core Laboratories (NIH Grant P30-AR46031) including the Histomorphometry and Molecular Analyses Core (Director: Gene P. Siegal, MD, PhD) and the Small Animal Bone Phenotyping Core (Director: Timothy R. Nagy, PhD).
We also acknowledge support from the University of Alabama at Birmingham Biostatistics Consulting Center, in particular Director Naomi Fineberg.
Footnotes
Publisher's Disclaimer: Disclaimer: The authors received a research grant from the Kaul Center for Research and Innovation at the Children’s Hospital of Alabama. None of the authors or their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
REFERENCES
- 1.Tzioupis C, Giannoudis P. Prevalence of long-bone non-unions. Injury. 2007;38(Suppl 2):S3–S9. doi: 10.1016/s0020-1383(07)80003-9. [DOI] [PubMed] [Google Scholar]
- 2.Martin D, Semple J, Sefton M. Poly(methacrylic acid-co-methyl methacrylate) beads promote vascularization and wound repair in diabetic mice. J Biomed Mater Res A. 2010;93:484–492. doi: 10.1002/jbm.a.32528. [DOI] [PubMed] [Google Scholar]
- 3.Shen X, Wan C, Ramaswamy G, et al. Prolyl h ydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J Orthop Res. 2009;27:1298–1305. doi: 10.1002/jor.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia P, Holstein J, Maier S, et al. Development of a reliable non-union model in mice. J Surg Res. 2008;147:84–91. doi: 10.1016/j.jss.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Wan C, Gilbert S, Wang Y, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A. 2008 Jan;105(2):686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu T, Warden S, Turner C, et al. Segmental bone regeneration using a load-bearing biodegradable carrier of bone morphogenetic protein-2. Biomaterials. 2007;28:459–467. doi: 10.1016/j.biomaterials.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart RL, Cox JT, Volgas D, et al. The use of a biodegradable, load-bearing scaffold as a carrier for antibiotics in an infected open fracture model. J Orthop Trauma. 2010;24:587–591. doi: 10.1097/BOT.0b013e3181ed1349. [DOI] [PubMed] [Google Scholar]
- 8.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geiger F, Bertram H, Berger I, et al. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res. 2005;20:2028–2035. doi: 10.1359/JBMR.050701. [DOI] [PubMed] [Google Scholar]
- 10.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asikainen T, Ahmad A, Schneider B, et al. Stimulation of HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free Radic Biol Med. 2005;38:1002–1013. doi: 10.1016/j.freeradbiomed.2004.12.004. [DOI] [PubMed] [Google Scholar]