Abstract
Voltage-gated L-type Ca2+ (Cav1.2) channels in vascular smooth muscle cells (VSMCs) are a predominant Ca2+ influx pathway that mediates arterial tone. Channel biogenesis is accomplished when the pore-forming α1C subunit co-assembles with regulatory Cavβ subunits intracellularly, and the multi-protein Cav1.2 complex translocates to the plasma membrane to form functional Ca2+ channels. We hypothesized that the main Cavβ isoform in VSMCs, Cavβ3, is required for the upregulation of arterial Cav1.2 channels during the development of hypertension, an event associated with abnormal Ca2+-dependent tone. Cav1.2 channel expression and function was compared between 2nd order mesenteric arteries (MA) of C57BL/6 wild-type (WT) and Cavβ3-/- mice infused with saline (control) or angiotensin II (Ang II) for 2 weeks to induce hypertension. The MA of Ang II-infused WT mice showed increased Cav1.2 channel expression and accentuated Ca2+-mediated contractions compared to saline-infused WT mice. In contrast, Cav1.2 channels failed to upregulate in MA of Ang II-infused Cavβ3-/- mice and Ca2+-dependent reactivity was normal in these arteries. Basal systolic blood pressure (SBP) was not significantly different between WT and Cavβ3-/- mice (98±2 mm Hg and 102±3 mm Hg, respectively), but the Cavβ3-/- mice showed a blunted pressor response to Ang II infusion. Two weeks after the start of Ang II administration, the SBP of Cavβ3-/- mice averaged 149±4 mm Hg compared to 180±5 mm Hg in WT mice. Thus, the Cavβ3 subunit is a critical regulatory protein required to upregulate arterial Cav1.2 channels and fully develop angiotensin II –dependent hypertension in C57BL/6 mice.
Keywords: Calcium channel, Cav1.2, β3 subunit, hypertension, mesenteric arteries
Introduction
During the development of hypertension, the L-type Ca2+ (Cav1.2) channel encoded by the Cav1.2 gene is upregulated in vascular smooth muscle cells (VSMCs).1-3 An over-abundance of vascular Cav1.2 channels has been documented in rats with genetic, renal and salt-dependent forms of hypertension [for review, see 4]. At least one study suggests that even a short-term elevation of intravascular pressure increases expression of arterial Cav1.2 channels in vivo, implicating the upregulation of Cav1.2 channels as an early event in the pathogenesis of hypertension.1 The increased expression of Cav1.2 channels enhances voltage-dependent Ca2+ influx resulting in abnormal vasoconstriction, which can be reversed by pharmacological antagonists of the Cav1.2 channel.1,2,5-7 Correspondingly, an increased Cav1.2 channel –mediated arterial tone in hypertensive animals and humans with essential hypertension may partly explain their sensitivity to the antihypertensive effect of clinical Ca2+ channel antagonists that block Cav1.2 channels. The same drugs only mildly lower blood pressure in normotensive subjects.8-11
The mechanisms that mediate the upregulation of arterial Cav1.2 channels during hypertension are unclear, but appear to primarily rely on post-transcriptional events since the increased expression of the Cav1.2 pore-forming α1C subunit has not been linked to a corresponding increase of α1C transcript.2,3 In the present study, we hypothesized that the regulatory β3 subunit (Cavβ3) of the Cav1.2 channel, which acts post-transcriptionally to promote surface expression of the α1C pore, may increase Cav1.2 channel abundance during hypertension. It is presumed that in VSMCs, similar to other cell types, the α1C subunit co-assembles with smaller β and α2δ subunits to form functional Ca2+ channels.12-15 Four gene families (β1, β2, β3, β4) encode Cavβ subunits in a tissue-specific manner. Cavβ3 is the principal subtype in VSMCs,16,17 where it is presumed to bind to α1C in the endoplasmic reticulum (ER) to enhance its trafficking to the plasma membrane (PM). At the cell surface, the multi-protein Cav1.2 channel assumes its critical function of mediating voltage-dependent Ca2+ influx during membrane depolarization.
The precise domains that permit interaction between α1C and Cavβ3 in the ER and the sequence of events that promote Cav1.2 expression on the surface of VSMCs remain elusive. However, Murakami et al16,17 reported that the aortae of Cavβ3-/- mice express fewer Cav1.2 channels compared to wild-type (WT) mice, implying that Cavβ3 contributes to the basal expression of Cav1.2 channels in VSMCs in vivo. Unexpectedly, despite the loss of arterial Cav1.2 channels, the Cavβ3-/- mice exhibited normal systolic blood pressure (SBP).16,17 This seeming dichotomy may indicate that blood pressure was sustained by compensatory mechanisms and/or indicate that Ca2+ influx through Cav1.2 channels contributes only minimally to basal blood pressure in normotensive subjects.8-11
Here, we considered the hypothesis that the regulatory Cavβ3 subunit promotes vascular Cav1.2 channel expression in hypertension, a disease in which this channel is pathogenically over-expressed. Our studies utilized Cavβ3-/- mice to explore if deletion of the Cavβ3 gene attenuates the upregulation of arterial Cav1.2 channels and the development of hypertension. The findings demonstrate that although Cavβ3-/- mice show normal resting SBP, they fail to fully develop angiotensin II (Ang II)-dependent hypertension and this loss of function is associated with an inability to upregulate arterial Cav1.2 channels. Thus, Cavβ3 may be a previously unrecognized but critical protein that contributes to the vascular Cav1.2 channel abnormalities that elevate blood pressure.
Methods
Blood pressure measurement
Procedures using animals were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. SBP was recorded using tail-cuff plethysmography before and after implanting subcutaneous osmotic minipumps containing either saline or Ang II (infused at 2 ng/g/min).
Vessel perfusion assays
Second order mesenteric arteries (MA) were isolated from saline-infused (SAL) and Ang II-infused hypertensive (AHT) mice, and used to perform vessel perfusion assays.
Patch clamp studies
The VSMCs were enzymatically isolated from 2nd order MA branches and whole-cell Ca2+ channel currents were recorded using Ba2+ (10 mmol/L) as a charge carrier with standard pulse protocols, solutions and a patch-clamp station described previously.1
Quantitative real-time PCR
Whole tissue RNA was isolated from 2nd order branches of MA pooled from two mice. Then qRT-PCR was performed using primers specific for α1C, Cavβ3 and GAPDH.
Western blotting
Protein lysates were prepared by pooling MA from two mice.1 Specific polyclonal antibodies directed against epitopes specific to α1C and Cavβ3 were used as probes.
Statistics
Data represent mean ± standard error of the mean for the number (n) of animals indicated in parentheses. Student's t-test was used to compare two data sets and one-way ANOVA with Bonferroni's post-hoc test was used for multiple group comparisons. P ≤ 0.05 was considered significant.
Results
Cav1.2 channel α1C and β3 subunits upregulate in MA of AHT mice
Initially we determined whether vascular Cav1.2 channels upregulate in Ang II-infused C57BL/6 mice, since previous studies demonstrating Cav1.2 upregulation were performed only in hypertensive rat models. Figure S1 shows that 14 days of subcutaneous Ang II infusion (2 ng/g/min) resulted in SBP values of 106±2, 155±5 and 172±8 mm Hg at 0, 7 and 14 days, respectively (n=10 each). The SBP values in SAL mice did not significantly differ from a basal value of 104±3 mm Hg. Subsequent studies were performed using mice infused with saline or Ang II for two weeks.
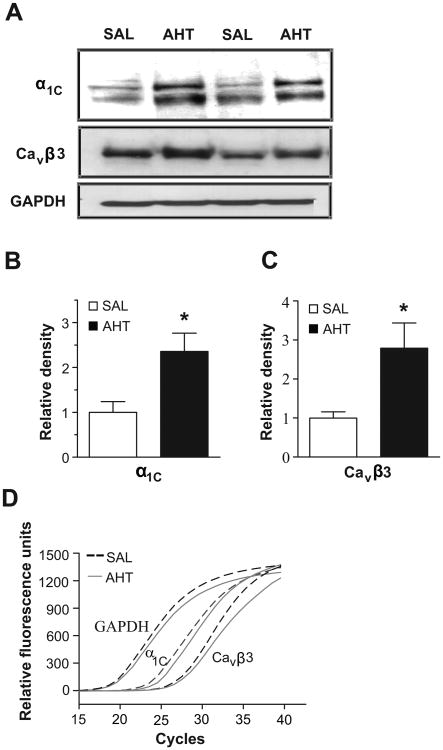
Next, we determined if the MA of AHT mice showed an increased expression of the α1C and Cavβ3 subunits that co-assemble to form functional Cav1.2 channels. Adjacent lanes on Western blot (WB) were loaded with MA lysate from SAL or AHT mice. The α1C protein was upregulated 2.4±0.4 fold in the MA of AHT mice compared to SAL mice (Fig. 1A,B). The abundance of the Cavβ3 subunit also increased during the development of hypertension. The immunodensity of the Cavβ3 band was 2.8±0.6 fold higher in MA lysate from AHT mice compared to SAL mice (Fig. 1A,C). Interestingly, qRT-PCR experiments using total RNA isolated from MA of SAL and AHT mice revealed that the transcript levels of both α1C (ΔCT values SAL 24.2±0.3, AHT 24.1±0.3) and Cavβ3 (ΔCT values SAL 25.1±0.5, AHT 25.1±0.6) were similar between the two isolates (Fig. 1D). The CT values were normalized to GAPDH, which was used as an amplification standard.
Figure 1.
A. WB showing adjacent lanes loaded with 40 μg of MA protein lysate pooled from either two SAL or AHT mice and probed with anti- α1C and Cavβ3. GAPDH was a loading control. B, C. Densitometric analyses of α1C and Cavβ3 immunoreactivity (n=6; * = p<0.05). D. qRT-PCR amplification corresponding to α1C, Cavβ3 and GAPDH transcripts averaged from 4 MA samples from SAL and AHT mice.
Increased Cav1.2 current and vascular reactivity in AHT mice
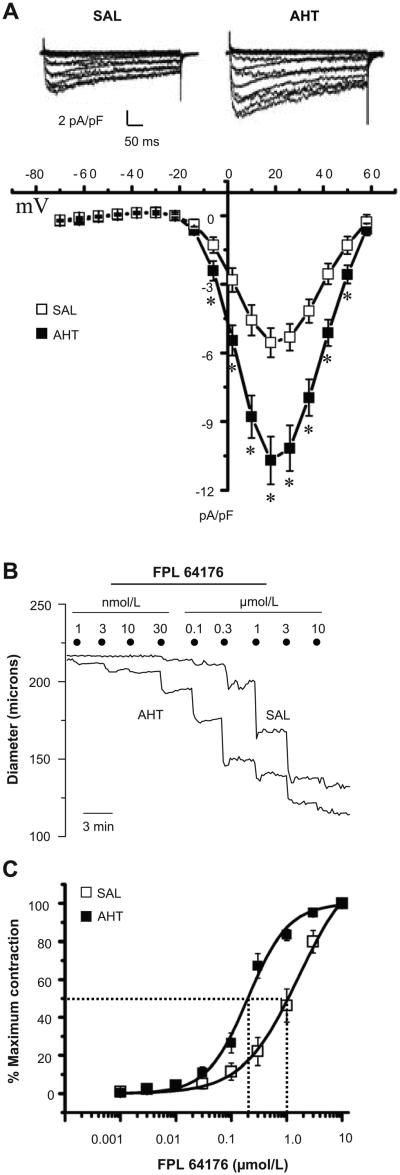
We confirmed that the over-abundance of Cav1.2 channels in MA of AHT mice was associated with enhanced channel function. Patch-clamp studies revealed increased Cav1.2 channel -mediated current in mesenteric VSMCs of AHT compared to SAL animals (Fig. 2A, top traces), which were blocked by nifedipine (Fig. S2). Current-voltage relationships suggested a 1.9±0.2 fold increase in peak current density in the VSMCs of AHT mice (-10.7±1.0 pA/pF) compared to SAL mice (-5.6±0.7 pA/pF) (Fig. 2A, n=23 each). Voltage-sensitive activation and inactivation were not different between groups (Fig. S3), revealing no abnormalities of Cav1.2 channel properties in VSMCs of AHT mice. The MA from AHT mice also exhibited enhanced reactivity to the Cav1.2 channel opener, FPL 64176 (Fig. 2B) resulting in a 5.1 -fold lower EC50 value of 224±37 nmol/L compared to 1144±331 nmol/L in arteries of SAL mice (Fig. 2C).
Figure 2.
A. Cav1.2 channel current elicited by 8 mV steps from -70 mV to +58 mV in mesenteric VSMCs of SAL and AHT mice (upper panel) and averaged I-V curves (n=23 each). B. On-line diameter responses of MA from SAL and AHT mice to increasing concentrations of FPL 64176 (1 nmol/L to 10 μmol/L, half-log units). C. Concentration-response relationships to FPL 64176 reveal a lower EC50 value for MA from AHT mice compared to SAL mice (n=7 each). * = p<0.05.
Cavβ3-/- mice show normal resting SBP
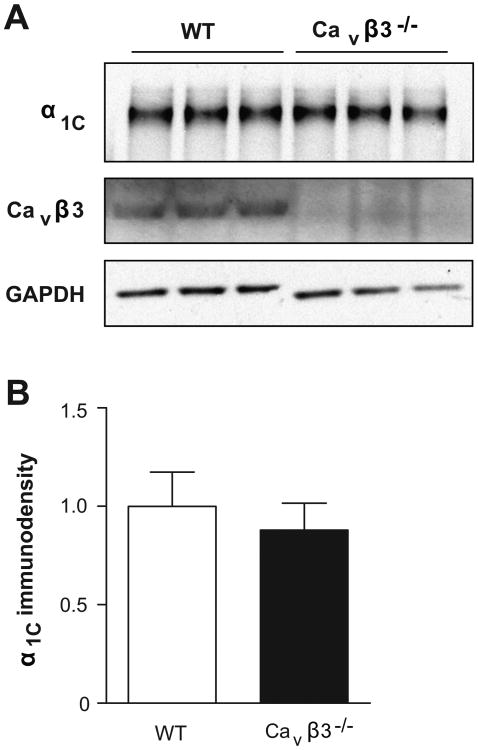
After linking an overabundance of arterial Cav1.2 channels in AHT mice to increased Cavβ3 expression for the first time, we directly tested if Cavβ3 subunits are required for Cav1.2 channel expression by employing WT and Cavβ3-/- mice. We observed no difference in α1C expression between MA from WT and Cavβ3-/- mice (Fig. 3A-B, n=12 each). Resting SBP levels also were similar between WT mice (98±2 mm Hg) and Cavβ3-/- mice (102±3 mm Hg) (Fig. S4A, n=10). The resting heart rate of 555±8 beats/min in WT mice (n=10) and 563±10 beats/min in Cavβ3-/- mice (n=11) also was not different (Fig. S4B).
Figure 3.
A. WB showing adjacent lanes loaded with 40 μg of MA protein lysate each pooled from two WT or Cavβ3-/- mice and probed with anti-α1C or Cavβ3. GAPDH was a loading control. B. Average densitometric values for α1C (n=12).
Cavβ3-/- mice fail to fully develop hypertension
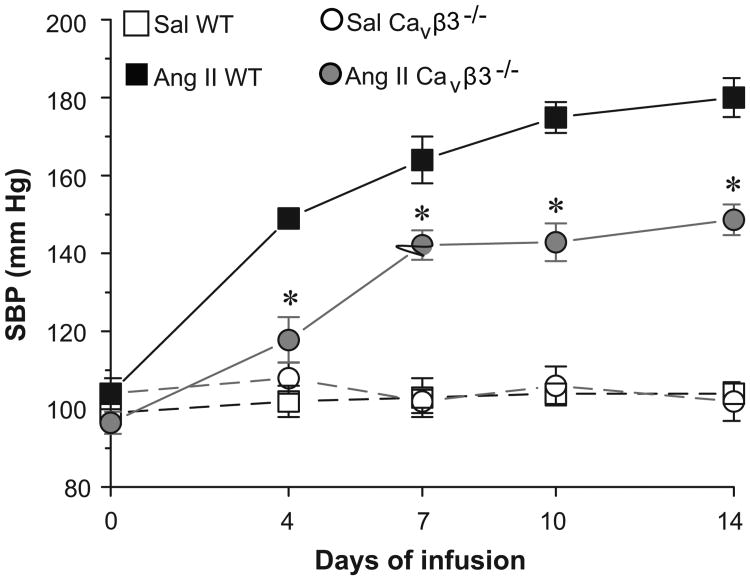
Subsequently, we explored if the Cavβ3 subunit contributes to the development of Ang II-dependent hypertension, which we postulated may partly rely on upregulation of Cav1.2 channels. WT and Cavβ3-/- mice were infused with saline (Sal) or Ang II for two weeks, and SBP was recorded for 3 days before infusion (day 0) and on days 4, 7, 10 and 14 after infusion. As expected, WT and Cavβ3-/- mice infused with saline maintained normal SBP that averaged 104±2 mm Hg and 102±5 mm Hg on day 14, respectively (Fig. 4, n=7 each). The WT mice infused with Ang II showed steadily rising SBP reaching an average value of 180±5 mm Hg on day 14 (Fig. 4, n=7). In contrast, the SBP response to Ang II infusion was significantly blunted in Cavβ3-/- mice, averaging only 149±4 mm Hg on day 14 (Fig. 4, n=7).
Figure 4.
The SBP response to two weeks of saline (Sal) or Ang II infusion in WT and Cavβ3-/- mice. Sal-infused WT and Cavβ3-/- mice showed normal SBP during the infusion period. SBP progressively increased in Ang II-infused WT mice and reached 180±5 mm Hg at 14 days. Cavβ3-/- mice showed a blunted blood pressure response to Ang II infusion and SBP at 14 days averaged only 149±4 mm Hg (n=7 each). * = p<0.05, significant difference between Ang II-infused WT and Cavβ3-/-.
MA of Cavβ3-/- mice fail to upregulate Cav1.2 channels
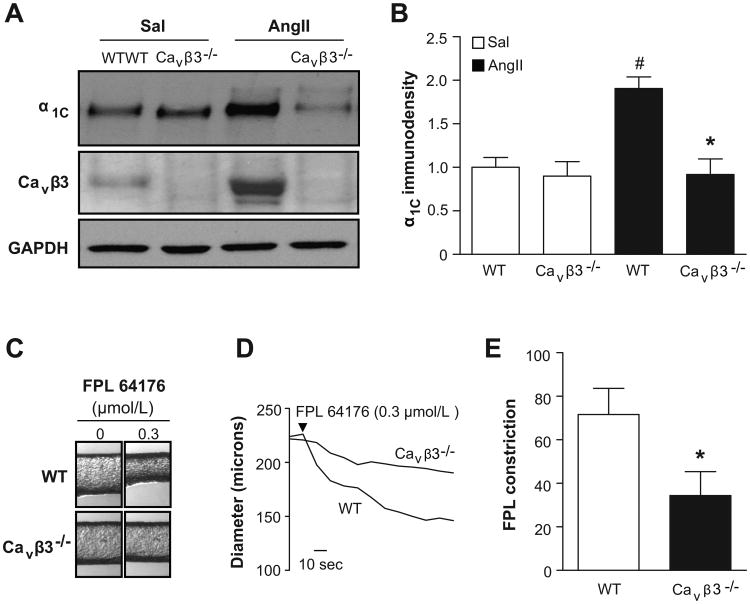
Next, we compared the expression level of the Cav1.2 channel α1C subunit between MA of WT and Cavβ3-/- mice infused with saline or Ang II. As expected, Ang II infusion was associated with an upregulation of the α1C and Cavβ3 subunits in MA of WT mice (Fig. 5A, lanes 1 and 3), which correlated to a 1.90±0.1 fold increase in Cav1.2 α1C (Fig. 5B, n=6) and a 2.65±0.7 fold increase in Cavβ3 immunosignal. In contrast, α1C failed to upregulate in MA of Ang II –infused Cavβ3-/- mice (Fig. 5A, lanes 2 and 4), showing only a 2.3% average increase compared to Sal-infused Cavβ3-/- animals (Fig. 5B, n=6).
Figure 5.
A. WB probed with antibodies directed against α1C and Cavβ3. Each lane was loaded with 40 μg of protein lysate pooled from the MA of two WT or Cavβ3-/- mice infused with either saline (Sal) or Ang II for two weeks. GAPDH was a loading control. B. Densitometric analyses revealed that only MA of WT mice upregulated α1C in response to Ang II (n=6). C, D. On-line picture and diameter recordings, respectively, of MA from Ang II-infused WT or Cavβ3-/- mice. The contraction to FPL 64176 (0.3 μmol/L) was blunted in arteries of Cavβ3-/- mice. E. Average diameter responses to FPL 64176 in MA from Ang II-infused WT and Cavβ3-/- mice (n=7 each). *, # = p<0.05, # indicates significant difference between Sal-infused mice and Ang II-infused WT mice. * indicates value is significantly different than Ang II -infused WT mice.
MA of Cavβ3-/- mice fail to show abnormal Cav1.2 channel–mediated reactivity
Finally, we explored whether the failure of Cav1.2 channels to upregulate in MA of Ang II-infused Cavβ3-/- mice also attenuated the heightened Ca2+-dependent reactivity observed in MA of Ang II-infused WT mice (Figs. 2B-C). To test this hypothesis, an ∼EC50 of FPL 64176 (0.3 μmol/L) was used to activate Cav1.2 channels in isolated MA. The diameter response to FPL 64176 in MA from Ang II-infused Cavβ3-/- of 34±11 μm was markedly less compared to a 72±12 μm reduction in MA from Ang II-infused WT mice (Fig. 5C-E; n=7).
Discussion
Our findings demonstrate that the MA of Cavβ3-/- mice infused with Ang II fail to upregulate Cav1.2 channels or show enhanced Ca2+-dependent reactivity, which are abnormalities observed in MA of Ang II-infused WT mice. Correspondingly, Cavβ3-/- mice fail to fully develop Ang II-dependent hypertension compared to WT mice. Collectively our findings draw attention to the Cavβ3 subunit in VSMCs as a critical protein necessary to upregulate Cav1.2 channels and elevate blood pressure in Ang II-dependent hypertension. The residual rise in SBP in response to Ang II infusion in Cavβ3-/- mice infers that factors in addition to the upregulation of Cav1.2 channels contribute to the elevated blood pressure in this form of hypertension, reflecting its complex pathogenesis as reviewed by Hall.18
Many studies during the past decade have shown that hypertension is associated with an elevated Ca2+ influx through arterial Cav1.2 channels.14 At least three rat models of hypertension show an increased density of functional Cav1.2 channels in the VSMCs of the aorta and in vascular beds involved in blood pressure regulation.1-3,5,6 In the renal circulation of aortic-banded rats, an increased Cav1.2 expression occurs by 48 hours after blood pressure elevation.1 Thus, an upregulation of Cav1.2 channels, the opening of which may be driven by the depolarized membrane potential of VSMCs exposed to high intravascular pressure, appears to contribute to the elevated vascular tone of hypertension.1,2 An increased Ca2+-dependent reactivity, and an enhanced vasodilator sensitivity to nifedipine, also has been reported in MA segments from hypertensive patients.7 These findings, combined with the observation that the blood pressure lowering effect of calcium channel blockers is exaggerated in hypertensive patients,8,9 raise the possibility that Cav1.2 channel abnormalities extend to essential hypertension in humans.
Here, we extend earlier findings in rats to the Ang II –infused hypertensive mouse by confirming that MA from AHT mice show increased Cav1.2 expression and Ca2+-dependent reactivity compared to arteries from SAL mice with normal blood pressure. Similar to earlier studies in rats,1-3 we failed to detect a major increase of α1C transcript in arteries of AHT mice that could account for the marked over-abundance of the α1C protein. Thus, the upregulation of arterial Cav1.2 channels in AHT mice appears to rely on post-transcriptional mechanisms that could include increases in translational efficiency and/or channel biogenesis, trafficking or surface stability.
In this regard, Cavβ subunits are known to increase the surface density of Ca2+ channels, although most studies have been performed in heterologous expression systems19,20 or nonvascular cell types.21,22 The Cavβ subunits co-assemble with pore-forming α-subunits during channel biogenesis to promote the trafficking of the Ca2+ channel complex from the ER to the PM.14,19 Although Cavβ are postulated to mask an ER retention signal in α-subunits as a mechanism to target Ca2+ channels to the cell surface, no specific motif has been identified. Altier et al.23 has alternatively demonstrated in HEK293 cells that the interaction between Cavβ and α1C prevents ubiquitination of the α1C subunit by ubiquitin ligase, thereby rescuing Cav1.2 channels from the ER-associated protein degradation (ERAD) complex that marks the channel for proteasomal degradation. Waithe et al.24 reported a similar interaction between Cavβ and Cav2.2 channels in neurons and concluded that Cavβ increases Cav2.2 surface density by conferring protection from proteosomal degradation. Finally, by co-expressing Nedd4 ubiquitin ligases and Cav1.2 channels in tsA-201 cells, Rougier et al.25 showed that Nedd4-1 ubiquitin ligase directly interferes with the Cavβ-mediated delivery of Cav1.2 channels to the PM.
The precise physiological role of Cavβ3 in VSMCs is unknown. Murakami et al.16,17 reported that α1C expression and Cav1.2 -mediated Ca2+ current were reduced in aortae from Cavβ3-/- mice, although SBP was normal. Here, we also observed comparable basal SBP between WT and Cavβ3-/- mice, and additionally, the MA of these mice exhibited similar basal Cav1.2 channel expression. However, MA of Cavβ3-/- mice failed to upregulate Cav1.2 channels or show accentuated Ca2+-dependent reactivity in response to Ang II infusion, which were abnormalities consistently observed in arteries of hypertensive WT mice. The absence of Cav1.2 channel upregulation in MA of Ang II –infused Cavβ3-/- mice was associated with a blunted blood pressure response to Ang II. Thus, our findings suggest an association between the Cavβ3 subunit, Cav1.2 upregulation and hypertension. Notably, the deletion of Cavβ3 was not limited to VSMCs in the Cavβ3-/- mice of our study, and thus we cannot state with certainty that the failure of Cavβ3-/- mice to fully develop Ang II –dependent hypertension relied on Cavβ3 deletion in VSMCs rather than nonvascular cell types. Although the Cavβ3-/- mouse has no gross phenotype, the Cavβ3 subunit also is extensively expressed in the brain and may negatively regulate NMDA receptors and potentially affect other neuronal functions.26 For this reason, the design of new Cavβ3-/- mice showing conditional and SMC-specific Cavβ3 gene deletion will be required to directly test the hypothesis that arterial Cavβ3 subunits contribute to experimental hypertension.
Notably, Fan et al.22 designed mutant Cavβ subunits that interact with α1C intracellularly, but lack the motifs required to target the Cav1.2 channel complex to the PM. These β subunit “decoys” represent potential therapeutics to reduce Cav1.2 surface expression in cardiovascular pathologies characterized by an overabundance of Cav1.2 channels.22,27,28 It may be advantageous to ameliorate anomalous Ca2+ influx by preventing the upregulation of Cav1.2 channels rather than using pharmacological blockers to reduce the activity of over-expressed channels. Our findings provide proof-of-principle for this concept by showing that Cavβ3 deletion can normalize Cav1.2 channel expression in VSMCs of hypertensive mice in which Cav1.2 over-expression is a contributing defect.
Perspectives
To our knowledge, the present study provides the first evidence to implicate a distinct Ca2+ channel β subunit in the development of hypertension. It has been proposed that small molecule inhibitors of Cavβ subunits represent future therapies to lower Ca2+ channel abundance in conditions associated with excessive Ca2+ influx including hypertension. Our findings support the concept that strategies to knockdown or inhibit the Cavβ3 subunit in VSMCs and thereby reduce Cav1.2 channel expression in the arterial circulation may represent novel therapeutic approaches for lowering blood pressure in hypertensive subjects.
Supplementary Material
Figure S1. The SBP profiles of C57BL/6 mice infused with isotonic saline (vehicle, SAL) or Ang II (2 ng/g/min, AHT) respectively, for two weeks. Values of SBP at weeks 1 and 2 in SAL mice were not significantly different from baseline at week 0 (n=10). However, SBP was significantly increased at weeks 1 and 2 after the start of Ang II infusion in AHT mice (n=10). * = p<0.05.
Figure S2. Representative traces of families of Cav1.2 channel currents elicited by 8 mV steps from -70 mV to +58 mV in mesenteric VSMCs of SAL and AHT mice. The control currents (top traces) were blocked by 1 μmol/L nifedipine (lower traces).
Figure S3. Boltzmann fits to voltage-dependent activation and inactivation relationships. There was no difference in half-activation and inactivation voltages between SAL and AHT mice. The V1/2 values for activation were 5.2±0.4 mV (SAL, n=11) and 4.0±0.4 mV (AHT, n=10). The V1/2 values for inactivation were -2.4±0.7 mV (SAL, n=8) and -4.6±0.7 mV (AHT, n=7).
Figure S4. Resting SBP was similar between WT and Cavβ3-/- mice (n=10 each). B. Resting heart rate also was not significantly different between WT and Cavβ-/- mice (n=10, 11).
Novelty and Significance.
What is New?
A β3 protein increases the number of voltage-gated Ca2+ channels in arteries during hypertension.
Mice in which the β3 protein is deleted fail to fully develop hypertension.
What Is Relevant?
The abnormal contraction of small arteries during hypertension is partly caused by the presence of too many Ca2+ channels.
Here, we report that a β3 protein is required for Ca2+ channels to increase in arteries during hypertension and lack of this protein reduces the level of blood pressure elevation.
Summary.
The β3 subunit is a critical protein in arteries that contributes to the upregulation of voltage-gated Ca2+ channels and the development of hypertension.
Acknowledgments
We are grateful to Dr. Hee-Sup Shin of the Korea Institute of Science and Technology for designing and sharing the Cavβ3-/- mice. We also thank Dr. William Guido from Virginia Commonwealth University for directly providing the mice from which on-site colonies were generated.
Sources of Funding: This work was supported by R01 HL064806-12 from the National Institutes of Health (NJR, SWR) and predoctoral grant PRE 092250224 from the South Central Affiliate of the American Heart Association (SVK).
Footnotes
Conflict of interest/disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pesic A, Madden JA, Pesic M, Rusch NJ. High blood pressure upregulates arterial L-type Ca2+ channels: is membrane depolarization the signal? Circ Res. 2004;94:e97–104. doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- 2.Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertens. 2002;40:214–219. doi: 10.1161/01.hyp.0000025877.23309.36. [DOI] [PubMed] [Google Scholar]
- 3.Wang WZ, Saada N, Dai B, Pang L, Palade P. Vascular-specific increase in exon 1B-encoded Cav1.2 channels in spontaneously hypertensive rats. Am J Hypertens. 2006;19:823–831. doi: 10.1016/j.amjhyper.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Sonkusare S, Fraer M, Marsh JD, Rusch NJ. Disrupting calcium channel expression to lower blood pressure: new targeting of a well-known channel. Mol Interv. 2006;6:304–310. doi: 10.1124/mi.6.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi K, Epstein M, Loutzenhiser R. Enhanced myogenic responsiveness of renal interlobular arteries in spontaneously hypertensive rats. Hypertens. 1992;19:153–160. doi: 10.1161/01.hyp.19.2.153. [DOI] [PubMed] [Google Scholar]
- 6.Simard JM, Li X, Tewari K. Increase in functional Ca2+ channels in cerebral smooth muscle with renal hypertension. Circ Res. 1998;82:1330–1337. doi: 10.1161/01.res.82.12.1330. [DOI] [PubMed] [Google Scholar]
- 7.Hutri-Kahonen N, Kahonen M, Wu X, Sand J, Nordback I, Taurio J, Pörsti I. Control of vascular tone in isolated mesenteric arterial segments from hypertensive patients. Br J Pharmacol. 1999;127:1735–1743. doi: 10.1038/sj.bjp.0702716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulthen UL, Bolli P, Amann FW, Kiowski W, Buhler FR. Enhanced vasodilatation in essential hypertension by calcium channel blockade with verapamil. Hypertens. 1982;4:26–31. [PubMed] [Google Scholar]
- 9.MacGregor GA, Rotellar C, Markandu ND, Smith SJ, Sagnella GA. Contrasting effects of nifedipine, captopril, and propranolol in normotensive and hypertensive subjects. J Cardiovasc Pharmacol. 1982;4:S358–S362. [PubMed] [Google Scholar]
- 10.Ishii H, Itoh K, Nose T. Different antihypertensive effects of nifedipine in conscious experimental hypertensive and normotensive rats. Eur J Pharmacol. 1980;64:21–29. doi: 10.1016/0014-2999(80)90365-9. [DOI] [PubMed] [Google Scholar]
- 11.Takata Y, Hutchinson JS. Exaggerated hypotensive responses to calcium antagonists in spontaneously hypertensive rats. Clin Exp Hypertens A. 1983;5:827–847. doi: 10.3109/10641968309081811. [DOI] [PubMed] [Google Scholar]
- 12.Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell α2δ1 subunits are essential for vasoregulation by Cav1.2 channels. Circ Res. 2009;105:948–955. doi: 10.1161/CIRCRESAHA.109.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. Structural basis of the α1-β subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- 14.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol. 2006;44:131–142. doi: 10.1016/j.vph.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van PF, Clark KA, Chatelain FC, Minor DL., Jr Structure of a complex between a voltage-gated calcium channel β-subunit and an α-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami M, Yamamura H, Murakami A, Okamura T, Nunoki K, Mitui-Saito M, Muarki K, hano T, Imaizumi Y, Flockerzi T, Yanagisawa T. Conserved smooth muscle contractility and blood pressure increase in response to high-salt diet in mice lacking the β3 subunit of the voltage-dependent calcium channel. J Cardiovasc Pharmacol. 2000;36:S69–S73. doi: 10.1097/00005344-200000006-00015. [DOI] [PubMed] [Google Scholar]
- 17.Murakami M, Yamamura H, Suzuki T, Kang MG, Ohya S, Murakami A, Miyoshi I, Sasano H, Muraki K, Hano T, Kasai N, Nakayama S, Campbell KP, Flockerzi V, Imaizumi Y, Yanagisawa T, Iijima T. Modified cardiovascular L-type channels in mice lacking the voltage-dependent Ca2+ channel β3 subunit. J Biol Chem. 2003;278:43261–43267. doi: 10.1074/jbc.M211380200. [DOI] [PubMed] [Google Scholar]
- 18.Hall JE. Historical perspective of the renin-angiotensin system. Mol Biotechnol. 2003;24:27–39. doi: 10.1385/MB:24:1:27. [DOI] [PubMed] [Google Scholar]
- 19.Chien AJ, Gao T, Perez-Reyes E, Hosey MM. Membrane targeting of L-type calcium channels. Role of palmitoylation in the subcellular localization of the β2a subunit. J Biol Chem. 1998;273:23590–23597. doi: 10.1074/jbc.273.36.23590. [DOI] [PubMed] [Google Scholar]
- 20.Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different β subunits in the membrane expression of the α1A and α2 calcium channel subunits: studies using a depolarization-sensitive α1A antibody. Eur J Neurosci. 1997;9:749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- 21.Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG. Role of the β2 subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci. 2002;43:1595–1603. [PubMed] [Google Scholar]
- 22.Fan QI, Vanderpool KM, O'Connor J, Marsh JD. Decoy calcium channel β subunits modulate contractile function in myocytes. Mol Cell Biochem. 2003;242:3–10. [PubMed] [Google Scholar]
- 23.Altier C, Garcia-Caballero A, Simms B, You H, Chen L, Walcher J, Tedford HW, Hermosilla T, Zampani GW. The Cavβ subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat Neurosci. 2011;14:173–180. doi: 10.1038/nn.2712. [DOI] [PubMed] [Google Scholar]
- 24.Waithe D, Ferron L, Page KM, Chaggar K, Dolphin AC. β-subunits promote the expression of Cav2.2 channels by reducing their proteasomal degradation. J Biol Chem. 2011;286:9598–9611. doi: 10.1074/jbc.M110.195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rougier JS, Albesa M, Abriel H, Viard P. Neuronal precursor cell-expressed developmentally down-regulated 4-1 (NEDD4-1) controls the sorting of newly synthesized Cav2 calcium channels. J Biol Chem. 2011;286:8829–8838. doi: 10.1074/jbc.M110.166520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Cingolani E, Ramirez Correa GA, Kizana E, Murata M, Cho HC, Marban E. Gene therapy to inhibit the calcium channel β subunit: physiological consequences and pathophysiological effects in models of cardiac hypertrophy. Circ Res. 2007;101:166–175. doi: 10.1161/CIRCRESAHA.107.155721. [DOI] [PubMed] [Google Scholar]
- 28.Telemaque S, Sonkusare S, Grain T, Rhee SW, Stimers JR, Rusch NJ, Marsh JD. Design of mutant β2 subunits as decoy molecules to reduce the expression of functional Ca2+ channels in cardiac cells. J Pharmacol Exp Ther. 2008;325:37–46. doi: 10.1124/jpet.107.128215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The SBP profiles of C57BL/6 mice infused with isotonic saline (vehicle, SAL) or Ang II (2 ng/g/min, AHT) respectively, for two weeks. Values of SBP at weeks 1 and 2 in SAL mice were not significantly different from baseline at week 0 (n=10). However, SBP was significantly increased at weeks 1 and 2 after the start of Ang II infusion in AHT mice (n=10). * = p<0.05.
Figure S2. Representative traces of families of Cav1.2 channel currents elicited by 8 mV steps from -70 mV to +58 mV in mesenteric VSMCs of SAL and AHT mice. The control currents (top traces) were blocked by 1 μmol/L nifedipine (lower traces).
Figure S3. Boltzmann fits to voltage-dependent activation and inactivation relationships. There was no difference in half-activation and inactivation voltages between SAL and AHT mice. The V1/2 values for activation were 5.2±0.4 mV (SAL, n=11) and 4.0±0.4 mV (AHT, n=10). The V1/2 values for inactivation were -2.4±0.7 mV (SAL, n=8) and -4.6±0.7 mV (AHT, n=7).
Figure S4. Resting SBP was similar between WT and Cavβ3-/- mice (n=10 each). B. Resting heart rate also was not significantly different between WT and Cavβ-/- mice (n=10, 11).