Abstract
Introduction and Objectives
The frequency of copper deficiency and clinical manifestations following roux-en-y gastric bypass (RYGB) surgery is not yet clear. Objectives were to determine the prevalence and incidence of copper deficiency in patients who have undergone RYGB.
Design and Methods
We sought to determine the number of RYGB patients undergoing medical and nutritional follow-up visits at the Emory Bariatric Center who experienced copper deficiency and associated hematological and neurological complaints (n = 136). Separately, in patients followed longitudinally before and during 6 and 24 months following RYGB surgery, we obtained measures of copper status (n = 16). Systemic blood cell counts and measures of copper, zinc and ceruloplasmin were determined using standardized assays in reference laboratories including atomic absorption spectrometry and immunoassays.
Results
Thirteen patients were identified to have copper deficiency suggesting a prevalence of copper deficiency of 9.6%, and the majority of these had concomitant complications including anemia, leukopenia and various neuro-muscular abnormalities. In the longitudinal study, plasma copper concentrations and ceruloplasmin activity decreased over 6 and 24 months following surgery, respectively (P<0.05), but plasma zinc concentrations did not change. A simultaneous decrease in white blood cells was observed (P<0.05). The incidence of copper deficiency in these subjects was determined to be 18.8%.
Conclusions
The prevalence and incidence of copper deficiency following RYGB surgery was determined to be 9.6% and 18.8%, respectively, with many patients experiencing mild-to-moderate symptoms. Given that copper deficiency can lead to serious and irreversible complications if untreated, frequent monitoring of the copper status of RYGB patients is warranted.
Keywords: copper deficiency, bariatric surgery, nutritional complications
Introduction
Copper is an essential nutrient for humans and is a cofactor for several metalloenzymes that participate in critical body functions, including the mitochondrial respiratory chain (cytochrome c oxidase), elastin synthesis (lysyl oxidase), neurotransmitter synthesis (dopamine β-monooxygenase), protection from oxidative stress (Cu/Zn superoxide dismutase), iron absorption in enterocytes (hephaestin) and pigmentation (tyrosinase). The function of these and other cuproenzymes has been studied for many decades.1 Newer research suggests that the copper proteome in eukaryotes is much more expansive, about 1% of all proteins.2 Also, new physiological roles for copper are emerging in the literature; for example, in cell signaling,3 oncogenesis4 and in immune function.5
Copper deficiency is thought to be rare in the general US population because copper is found in commonly consumed foods, such as shellfish, whole grains, beans, nuts, dark leafy green vegetables and organ meats. The median dietary intake of copper in the US diet, is 1.0 to 1.6 mg day−1, which is usually sufficient to meet nutrient requirements (recommended dietary allowance = 0.9 mg Cuday−1 for adults).6 For this reason, copper is sometimes not added to multi-vitamin/multimineral supplements and is rarely measured by clinicians. Although not common, copper absorption can be impaired in some circumstances; copper uptake is limited by high dietary Zn2+,7 and particularly following partial or complete gastric resection because bioavailability of copper requires an acidic gastric environment.8 Also, because copper absorption primarily occurs in the duodenum9 patients whom have had intestinal resection bypass (for example, malabsorptive bariatric surgery)10,11 are at risk. These facts strongly suggest that patients who have undergone gastrectomy and gastrointestinal bariatric surgery are particularly susceptible to copper deficiency.
Bariatric surgery is an uniquely effective treatment for individuals with severe obesity, and is associated with alleviation of major comorbidities, and reduced risk of mortality.12,13 Given its effectiveness, bariatric surgery is gaining popularity as the number of procedures performed in the United States each year has increased, and at least 1.2 million individuals are currently affected.14,15 Currently in the United States, the roux-en-y gastric bypass (RYGB) is the most popular surgery, and considered to be the gold-standard bariatric procedure.16 The RYGB surgery procedure surgically alters the gastrointestinal tract by dramatically reducing stomach capacity, thereby restricting food intake and by re-routing ingested food to bypass the duodenum and the proximal jejunum, the main sites of copper absorption.17
Along with others, we have documented severe copper deficiency following gastric bypass surgery in several case-reports18–22 and in a cross-sectional study.23 Features of copper deficiency in individuals with documented depletion included hematologic abnormalities (anemia with or without leukopenia, neutropenia and/or thrombocytopenia) and various neuropathies (unsteady gait, muscle weakness and fatigue, extremity numbness and/or painful extremity paresthesias). In many cases, these symptoms initially were believed to be attributable to iron or vitamin B12 deficiency, but when patients did not respond to conventional treatments, further investigation revealed severely low copper status.18,20,22 Following copper supplementation, hematologic abnormalities are rapidly resolved, but improvement in myeloneuropathic signs and symptoms can be variable, suggesting that long-term deterioration may be irreversible.18,24,25 These studies suggest that copper deficiency following gastric bypass surgery may be under-diagnosed, which may lead to serious morbidity.
There is a lack of information about the prevalence and incidence of copper deficiency following gastric bypass surgery. In a cross-sectional analysis of 78 patients who had undergone gastric bypass surgery, the prevalence of copper deficiency was recently reported to be 15.4%.23 In this study, we sought to confirm this finding in a larger population of bariatric surgery patients. A second and more important objective was to determine the true incidence of copper deficiency following gastric bypass surgery by longitudinally measuring changes in indicators of copper status before and during 24 months following RYGB.
Patients and methods
The study objectives were: (1) to determine the prevalence of copper deficiency following RYGB; and (2) to determine the incidence of copper deficiency. These objectives were approached using two different patient populations as described below in study 1 and study 2.
Patients
Study 1
Objective 1 was assessed via a retrospective chart review of patients in the Emory Bariatric Center clinic population. Patients were eligible if they had undergone RYGB as well as postoperative follow-up medical evaluation and nutritional screening in the Emory Bariatric Center between March 2007 and March 2009. The RYGB surgical procedure has been described26 and the length of roux limb (100 or 150 cm) was recorded. During this period, 136 patients were screened. This included the analysis of copper, ceruloplasmin, zinc, and vitamin B12 as well as routine hematological studies. A detailed medical history and physical examination was also conducted on every patient. The Emory University Institutional Review Board approved the study (IRB 912-2003).
Study 2
Objective 2 was assessed in a prospective cohort study of 16 severely obese female patients who had been followed up for 24 months to assess the longer-term impact of RYGB on cardiometabolic and nutritional outcomes.27,28 Subjects were evaluated at baseline (before surgery) and again at 6 and 24 months post surgery. Exclusion criteria for this study were: (1) male gender; (2) age <18 years or >65 years; and (3) preoperative body mass index of <35 kg m−2. All patients were prescribed a multivitamin/multimineral regimen following surgery and patients were counseled by a registered dietician about these recommendations at each study visit. Dietary and supplement intake, using 3-day food records, was collected at the 24-month visit only (n = 10), and were analyzed using Food Processor SQL (ESHA Research, Salem, OR, USA). The Emory University Institutional Review Board approved the study and all patients signed informed consent before enrollment (IRB 333-2002).
Metabolic measures
Plasma samples were obtained at baseline (before surgery), 6 and 24 months following surgery and stored at −80 °C. For study 1, serum copper and zinc were commercially measured by ARUP Laboratories, Salt Lake City, UT, USA using inductively coupled plasma/mass spectrometry. The limit of sensitivity and coefficient of variation were stated by the company as 0.157 µmol l−1 and 7% for copper and 0.08 µmol l−1 and 5% for zinc, respectively. Hematological measures, including hemoglobin, hematocrit and automated cell counts were determined using standard methods at the Emory Medical Laboratory, a fully certified reference laboratory.29 In a subsample of patients, serum vitamin B12 and ferritin were measured at the Emory Medical Laboratory using a standard chemiluminescent immunoassay.
For study 2, ceruloplasmin activity and immunoreactive protein in plasma were determined by established protocols.30 The human ceruloplasmin enzyme assay uses o-dianisidine as substrate under standard conditions developed earlier.31 Plasma copper and zinc were determined by flame atomic absorption spectroscopy after dilution with metal-free water as described previously.32 Standards were prepared in 5% glycerol to mimic the viscosity of diluted plasma.
Statistical analysis
The statistical software STATISTICA (StatSoft Inc., Tulsa, OK, USA) was used for study analysis. For both study 1 and study 2, descriptive statistics were assessed using parametric test and expressed as mean ± s.e. of the mean. Prevalence of copper deficiency was determined in study 1 and was defined as the number of cases of copper deficiency during the period from March 2007 to March 2009; the denominator was 136 subjects. Incidence of copper deficiency was defined as the new development of copper deficiency during the 24-month period following surgery, in 16 subjects who had normal copper status at baseline; this was determined in study 2. Differences between population frequencies were assessed using χ2 analysis. For study 2, changes over time from baseline to 24 months following surgery were analyzed using repeated-measures analysis of variance. Relationships between plasma copper concentrations and secondary variables, including plasma ceruloplasmin activity were examined using Pearson correlations. In a subsample of five patients within study 2, who experienced the greatest baseline to post-surgery change in ceruloplasmin activity, changes in variables were assessed using paired t-tests. The significance level for the study was set at P<0.05.
Results
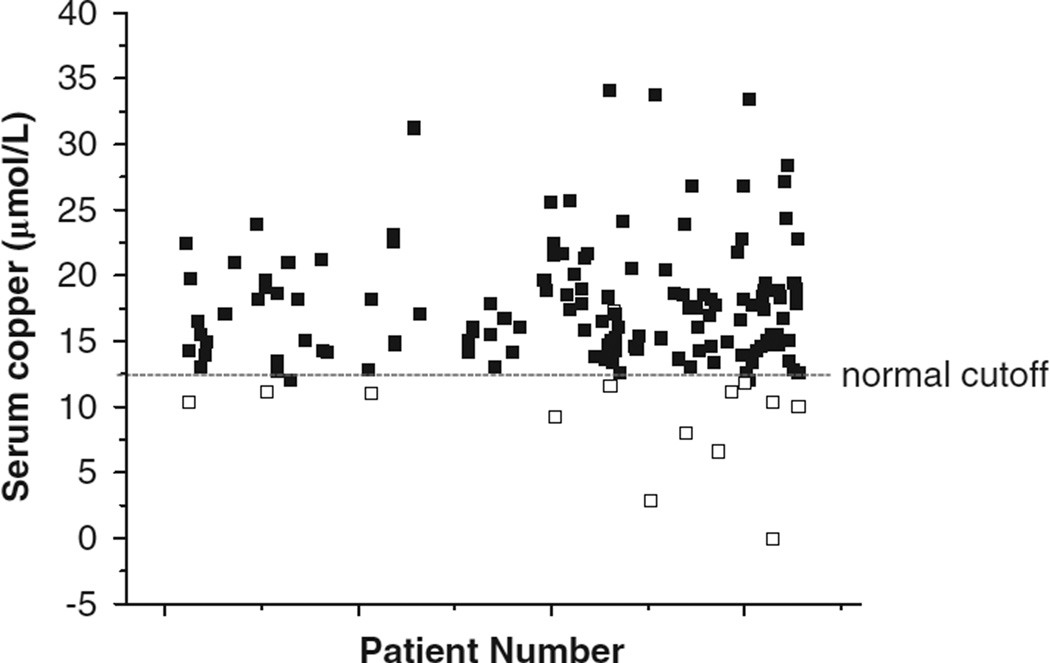
In the cross-sectional study, study 1, 136 eligible patients were assessed. The population was 89% female, 70% Caucasian and 28% African American. At the time of the chart review, (March 2009), subjects were 42.9 ± 0.8 years of age and were 33 ± 2 months post RYGB surgery. Out of these 136 eligible patients, 13 were found to be deficient in copper, defined as a value of <11 µmol l−1 in males and 12.6 µmol l−1 in females, a prevalence of 9.6% (Figure 1). Of the copper-deficient patients, all were female, six were African American and seven were Caucasian, and the average age was 44.2 ± 2.3 years (Table 1). Copper deficiency was observed equally in patients who were within the first year of surgery, or who were within years 1 to 3, or within years 3 to 5 post surgery. There were no significant differences between copper-deficient and non-deficient groups in age, gender or race/ethnicity.
Figure 1.
Serum copper in a RYGB clinic population. Serum copper concentrations were determined in patients (n = 136) who had undergone RYGB surgery at the Emory Bariatric Center and had visited the clinic for medical and nutritional follow-up during March 2007 and March 2009. Each patient number and their copper measurement(s) is (are) plotted; some patients had multiple clinic visits. Closed squares are values in the normal range, (12.6–24.3 µmol l−1)29, 13 samples are below the normal cutoff and considered deficient (open squares).
Table 1.
Prevalence and symptoms of copper deficiency in roux-en-y gastric bypass patients at the Emory Bariatric Center
| Characteristic | Number (percent) |
|---|---|
| N | 13 |
| Gender | |
| Female | 13 |
| Ethnicity | |
| African American | 6 |
| Caucasian | 7 |
| Months post surgery | |
| 0 to 11.9 months | 5 (38%) |
| 12 to 35.9 months | 5 (38%) |
| 36 to 60 months | 3 (23%) |
| Serum copper (µg dl−1) | 59.2 ± 4.6 |
| Hematological symptoms | |
| Anemia | 6 (46%) |
| Leukopenia | 2 (15%) |
| Fatigue | 5 (38%) |
| Neurological symptoms | 5 (38%) |
The demographic and symptom data of 13 patients in study 1 with deficient copper status is presented. Anemia was defined as hemoglobin <114 g l−1; leukopenia was defined as white blood cell count <3.6 × 109 l−1. When present, fatigue and neurological symptoms including weakness, not ambulatory, paraesthesia, carpal tunnel syndrome, tingling, joint pain and myalgias were noted in the patient’s charts.
Demographic and clinical data of study 1 patients with low copper status is given in Table 1. Of interest is that development of clinical symptoms occurred in a third of patients within the first 5 years of surgery, which indicates that copper deficiency can occur within a relatively short time frame. Most (9 out of 13) individuals with low copper status also exhibited sequelae that could be secondary to copper deficiency including anemia (46%) and leukopenia (15%), as well as fatigue (38%), and various neuropathies (38%). Chart review revealed reported symptoms as not ambulatory, unsteady gait, muscle weakness and pain, extremity numbness, and painful extremity paresthesias. Anemia, the most prevalent of all the abnormalities noted, can result from copper deficiency as well as deficiencies in other nutrients including iron and vitamin B12. Iron stores, assessed by serum ferritin values, were below normal in three out of seven patients for whom values could be obtained. In contrast, none of the eight patients with recorded cobalamin values were in the deficient range.
In the longitudinal study 2, 16 patients were assessed from baseline to 24 months following RYGB surgery. The mean age was 33.1 ± 2.0 years. In all, 4 women were self-described as African American in descent, 11 were Caucasian and 1 was Hispanic. Body mass index decreased from 49.0 ± 0.9 kg m−2 at baseline to 35.7 ± 1.2 kg m−2 at 6 months and 31.6 ± 1.6 kg m−2 at 24 months following surgery; other changes in adiposity have been published in greater detail elsewhere.28
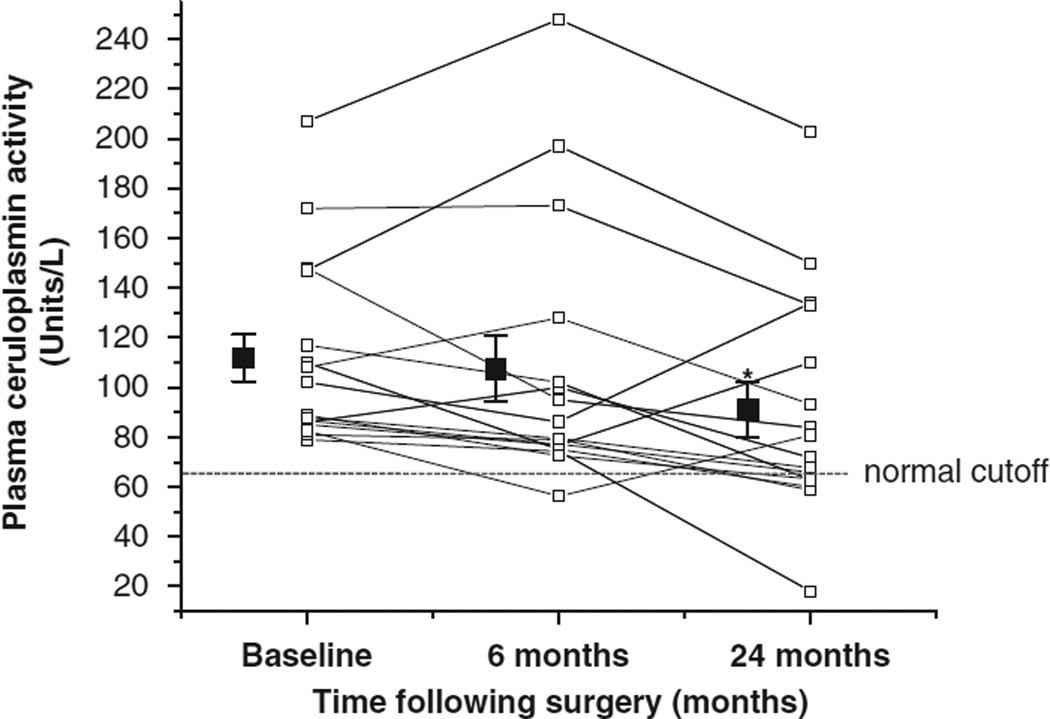
Changes in plasma concentrations of copper, zinc and ceruloplasmin activity during the 24 months following RYGB surgery are presented in Table 2. Compared with baseline concentrations, plasma copper decreased (effect of time, P = 0.019), by 10.8% at 6 months compared with baseline values (P = 0.03) and 10.1% at 24 months (P = 0.04) following surgery. There was no change in plasma zinc concentrations following surgery. Plasma ceruloplasmin activity also decreased following surgery (effect of time, P = 0.019), and at 24 months, activity had decreased by 18.6% compared with baseline values (P = 0.016) (Figure 2). Also at 24 months following surgery, plasma ceruloplasmin activity below the normal cutoff of 62 units l−1(ref. 31) was found in three subjects who each had normal activity at baseline. This corresponds to an incidence of copper deficiency following gastric bypass surgery of 18.8%.
Table 2.
Longitudinal changes in copper status and hematology following roux-en-y gastric bypass surgery
| Baseline | 6 Month | 24 Months | |
|---|---|---|---|
| Copper (µmol l−1) | 24.6 ± 1.6 | 22.0 ± 1.8* | 22.2 ± 1.8* |
| Zinc (µmol l−1) | 13.0 ± 0.5 | 11.8 ± 0.5 | 13.1 ± 0.6 |
| Ceruloplasmin activity (units l−1) | 111.7 ± 9.4 | 107.5 ± 13.3 | 91.0 ± 11.3* |
| Hemoglobin (g l−1) | 125.3 ± 3.5 | 122.4 ± 2.9 | 112.9 ± 2.9 |
| White blood cells (× 109 l−1) | 8.84 ± 0.87 | 6.46 ± 0.52* | 5.83 ± 0.35* |
Copper and zinc concentrations and ceruloplasmin activity, as well as hemoglobin and white blood cells were measured in plasma samples obtained from 16 patients who underwent roux-en-y gastric bypass surgery as described in Patients and methods for study 2. An asterisk (*) depicts significant decrease compared with the measure at baseline, by post hoc analysis, P < 0.05.
Figure 2.
Changes in plasma ceruloplasmin activity during 24 months following RYGB surgery. Ceruloplasmin activity was serially determined in study 2 subjects (n = 16) before (baseline), and 6 and 24 months following RYGB surgery and each individual data point is plotted (open squares). The mean and standard error of the mean for each timepoint is represented by closed squares. At 24 months following surgery, 3 ceruloplasmin activity values dropped below the normal cutoff.31 An asterisk (*) depicts values significantly different from baseline, P = 0.016.
To determine clinical manifestations of decreasing copper status following RYGB, we assessed corresponding changes in plasma hemoglobin and total white blood cells (Table 2). Compared with baseline values, a dramatic decrease in concentrations of white blood cells was observed as early as 6 months (P = 0.006) and also at 24 months (P = 0.000) following surgery (effect of time, P = 0.0001).29 At 24 months, anemia was found in 50% of subjects. We did not collect information on neurological complaints for subjects in the longitudinal study.
Data regarding iron and vitamin B12 status were pooled from those subjects in studies 1 and 2 who were copper deficient and who had normal copper status (Table 3). Subjects who had copper deficiency had lower blood hemoglobin (118.1 ± 3.6 versus 129.6 ± 1.1 g l−1) and ferritin (84.4 ± 21.8 versus 213.3 ± 42.3 pmol l−1) concentrations compared with those with normal copper status (P<0.05). However, blood concentrations of vitamin B12 were similar between copper-deficient and copper-sufficient groups (447.0 ± 55.2 and 497.5 ± 35.1 pmol l−1, respectively).
Table 3.
Assessment of iron status in copper-deficient and -sufficient subjects
| Copper deficient | Copper sufficient | |
|---|---|---|
| Hemoglobin (g l−1) | 118.1 ± 3.6* | 129.6 ± 1.1 |
| Ferritin (pmol l−1) | 84.4 ± 21.8* | 213.3 ± 42.3 |
| Vitamin B12 (pmol l−1) | 447.0 ± 55.2 | 497.5 ± 35.1 |
Blood concentrations of hemoglobin, ferritin and vitamin B12 were obtained from patients undergoing roux-en-y gastric patients in study 1 (retrospective chart review) and study 2 (longitudinal study) who had normal copper status (n = 110) and those who developed copper deficiency (n = 25). An asterisk (*) depicts values that were significantly decreased compared with the value in copper-sufficient subjects, P < 0.05.
In order to determine whether copper measures at 24 months following surgery were altered by the length of the malabsorptive roux limb, we compared plasma concentrations of copper and ceruloplasmin activity in patients who had obtained a 100 cm versus those with a 150 cm roux-limb. In both groups, comparable plasma copper concentrations (22.2 ± 2.3 and 22.6 ± 2.8 µmol l−1, respectively) and ceruloplasmin activity (91.3 ± 16.2 and 98.9 ± 15.3 units l−1, respectively) were found.
Dietary intake of copper and zinc are associated with copper status. Copper and zinc intake from food or supplements was obtained from ten patients (in study 2) at 24 months following surgery and was found to be 1.09 ± 0.3 and 8.8 ± 2.4 mg day−1, respectively. There were no correlations between dietary intake of copper and zinc and circulatory concentrations at the 24-month timepoint. There was also no difference in copper intake between copper-deficient and copper-sufficient subjects (1.59 ± 0.85 and 0.92 ± 0.29 mg day−1, respectively).
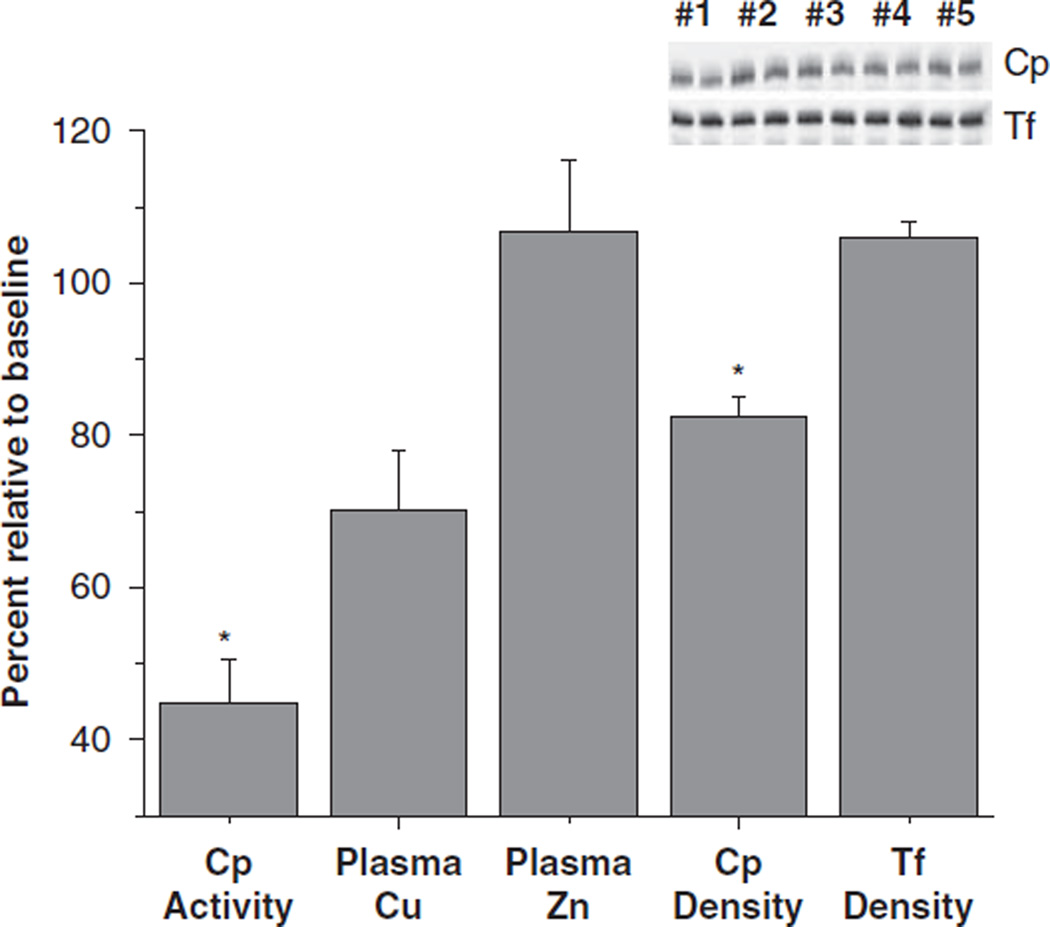
Plasma copper concentrations and plasma ceruloplasmin activity values were strongly correlated at each timepoint (baseline, 0.93; 6 months, 0.97; and 24 months, 0.91, P<0.000 for each). This suggests that circulatory ceruloplasmin activity responds to decreases in plasma copper concentrations, thus we wanted to determine whether corresponding decreases in plasma content of ceruloplasmin could also be observed. Using western blotting analysis, immunoreactive ceruloplasmin was detected in plasma of a subsample of five patients (in study 2) who were observed to have the greatest change in ceruloplasmin activity following surgery (decreased to 44% of baseline, P = 0.004); transferrin protein was used as a loading control (Figure 3). In this subsample of patients, plasma copper concentrations following surgery were decreased to 70% of baseline (P = 0.09), but zinc concentrations were unchanged. Plasma ceruloplasmin concentrations following surgery were decreased to 82% of baseline (P = 0.004) but transferrin concentrations were unchanged.
Figure 3.
Changes in ceruloplasmin activity and concentration following RYGB surgery. Changes in ceruloplasmin (Cp) density following surgery, relative to baseline levels, detected by western immunoblots in a subgroup of study 2 subjects who exhibited the greatest decrease in ceruloplasmin activity (mean = 44% of baseline) is shown (n = 5). Transferrin (Tf) density, used as a loading control, was not altered post surgery. Plasma copper (Cu) and zinc (Zn) concentrations were determined by flame atomic absorption spectrometry. An asterisk (*) depicts significant reductions compared with baseline measures, P<0.05.
Discussion
In this study, we demonstrate the impact of RYGB surgery on copper nutritional status using a cross-sectional and longitudinal analysis of two separate patient populations. In a cross-sectional study of a clinic population of RYGB patients, we found the prevalence of copper deficiency to be 9.6%. We show for the first time, in roux-en-y patients who were followed longitudinally for 24 months following surgery, that the incidence of copper deficiency is 18.8%. A majority of the copper-deficient subjects in both populations exhibited symptoms that may have been secondary to copper deficiency, including anemia, leukopenia and various neuropathies.
Copper deficiency following gastric surgeries including RYGB has been described, but much of the literature comprises case-reports.18,21,33,34 In a recent retrospective review of 78 patients who had gastric bypass surgery (average 1.3-year post surgery), Ernst et al.23 reported a deficiency of 15.4%. Other retrospective reviews of copper deficiency in patients who had underwent RYGB35 or the more malabsorptive bariatric procedures, jejunoileal bypass10,11 or biliopancreatic diversion procedure36 have been published but there was insufficient data to determine prevalence statistics. This study reviewed a larger series of patients (n = 136) and found the prevalence of copper deficiency to be 9.6%. Our laboratory used a lower cutoff than the former study (12.6 µg dl−1 versus 13.3 µmol l−1); given that the cutoffs from one laboratory to the next are not transferrable,37 both prevalence rates are within range. Interestingly, there did not seem to be a time period following surgery during which patients were more likely to develop low copper. Although copper deficiency has been reported in many patients who have undergone RYGB several years earlier,18,35 we observed copper deficiency in patients within the first year following surgery, and this has been reported elsewhere in a case report.21
Micronutrient depletion following bariatric surgery has been demonstrated38 but only by a few longitudinal studies.28,36,39 The novelty of this study is that it describes the impact of RYGB by measuring systemic copper concentrations before and during the 2 years after surgery. Although the study population is small, we found an incidence of 19% copper deficiency, as defined by ceruloplasmin activity.31 Although the cutoff defining deficiency may be arbitrary,37 a steady decrease in both copper concentrations and ceruloplasmin activity was observed (in the case of copper as early as 6 months following surgery). This finding suggests that for individuals, copper status may worsen following RYGB. The same phenomenon was not observed for zinc, which remained stable following surgery. The discrepancy between the incidence and prevalence rates of copper deficiency observed in this study is not clear. It is possible that copper deficiency may be transient in some individuals and may resolve over the long-term because of increased dietary intake40 or improved copper absorption because of gut adaptation.41 Larger prospective studies with longer follow-up are needed to assess these issues.
In this study, below normal blood concentrations of copper were associated with symptoms of copper deficiency including hematological and neurological abnormalities. Copper-deficient patients had concomitant anemia and lower blood hemoglobin and ferritin concentrations but normal vitamin B12 concentrations. Recognition of anemia in humans with copper deficiency has been known since the nineteenth century, but the mechanism of a copper–iron interaction is not clear.42 Pernicious anemia, which occurs with vitamin B12 deficiency, is not as likely to occur because most patients adhere to periodic cobalamin administration following RYGB.43 Other than hematological abnormalities, many patients experienced putative copper deficiency-related complaints including fatigue, poor wound healing, hair loss, paresthesia, carpal tunnel syndrome, and muscle and joint pain. Although the effects of copper supplementation were not determined in this study, several other studies have shown that with copper repletion many of the hematological abnormalities are rapidly resolved,10 and improvement in myeloneuropathy is seen, although long-term deterioration may be irreversible.18,24 An additional concern is the effect of copper deficiency in the growing fetus.44 These findings taken together with this study highlight the short- and long-term risks of symptomatic copper deficiency following RYGB.
The relatively high frequency of symptomatic copper malnutrition following RYGB suggests that greater screening of patients for copper depletion serially would be prudent. A lack of sensitive and specific biomarkers for assessing copper status makes it difficult to monitor and treat patients at risk for deficiency.45 Blood copper levels are tightly regulated, thus normal copper levels may falsely indicate adequate copper status.46 As has been described, we demonstrated strong correlations between concentrations of plasma copper and ceruloplasmin activity in this study. Moreover, in a preliminary analysis, plasma ceruloplasmin content (by western blotting assay) decreased in parallel with plasma copper and further testing should determine whether blotting for plasma ceruloplasmin is a simple and low cost way to assess copper status.
An explanation for the negative impact of RYGB surgery on copper absorption, is the exclusion of most of the stomach and duodenum, which are the principal areas of copper digestion and uptake.8,9 Another possibility is that surgery promotes food restriction and many patients experience food intolerances.40 Although some patients do not adhere to a daily regimen of micronutrient supplementation post surgery,47 our assessment of dietary copper intake at 24 months following surgery showed that it was adequate with regard to daily recommendations for the general population. It will be important for future studies to determine whether current dietary copper recommendations meet the nutritional requirements of RYGB patients, in whom copper malabsorption is likely due to exclusion of the duodenum and proximal jejunum after the bypass.
The limitations of this paper is that the chart review in study 1, and dietary and neurological information aspects of the longitudinal analysis were obtained retrospectively, thus some variables had missing data for some subjects. It is therefore possible that the number of patients dealing with neurological complaints is underestimated. The number of patients in the longitudinal study was small, and although it was adequately powered for changes in copper concentrations, it may not have been powered to detect changes in secondary outcomes including hemoglobin concentrations. Also the majority of study participants were female, and although this is reflective of the bariatric surgery population generally, there may be important gender differences in copper metabolism following RYGB that were not uncovered.9
Despite the limitations of this study, our findings suggest that copper status is impaired within months following RYGB and this has mild-to-moderate clinical manifestations in many patients. We found that the prevalence of copper deficiency in a RYGB clinic population was 9.6%, which is similar to that reported earlier. We also found an incidence of 18.8% in copper deficiency following RYGB surgery, which could lead to serious complications if left undetected and untreated. This study demonstrates that copper malnutrition following RYGB is not uncommon. Greater efforts for screening of this patient population are warranted since the number of patients undergoing this bariatric procedure is increasing.
Acknowledgements
This work was supported by National Institute of Health Grants R03 DK067167 and R21 DK 075745 (to NGM), K24 RR023356 (to TRZ), RO1 HD 39078 and a National Research Initiative Grant 2006-35200-17378 from the USDA National Institute for Food and Agriculture (to JRP), the General Clinical Research Center Grant M01 RR00039 and the Atlanta Clinical and Translational Science Institute Grant UL1 RR025008 and the International Copper Association. The authors have nothing to disclose. We graciously thank all the study participants. Adeola T Ayeni, MD assisted with clinical research coordination of the study participants and Amareshwar Chiruvella, MD assisted with data collection.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Failla ML, Johnson MA, Prohaska JR. Copper. In: Bowman BA, Russell RM, editors. Present Knowledge in Nutrition. 8th ed. Washington, DC: ILSI Press; 2001. pp. 373–383. [Google Scholar]
- 2.Andreini C, Banci L, Bertini I, Rosato A. Occurrence of copper proteins through the three domains of life: a bioinformatics approach. J Proteome Res. 2008;7:209–216. doi: 10.1021/pr070480u. [DOI] [PubMed] [Google Scholar]
- 3.Haremaki T, Fraser ST, Kuo YM, Baron MH, Weinstein DC. Vertebrate Ctr1 coordinates morphogenesis and progenitor cell fate and regulates embryonic stem cell differentiation. Proc Natl Acad Sci USA. 2007;104:12029–12034. doi: 10.1073/pnas.0701413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 5.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Food and Nutrition Board Institute of Medicine. Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 7.McClain CJ, Shedlofsky SI. Copper toxicity in Wilson’s disease: an absorbing problem. J Lab Clin Med. 1988;111:261–262. [PubMed] [Google Scholar]
- 8.Fields N, Craft N, Lewis C, Holbrook J, Rose A, Reiser S, et al. Contrasting effects of stomach and small intestine of rats on copper absorption. J Nutr. 1986;116:2219–2228. doi: 10.1093/jn/116.11.2219. [DOI] [PubMed] [Google Scholar]
- 9.Mason KE. A conspectus of research on copper metabolism and requirements of man. J Nutr. 1979;109:1979–2066. doi: 10.1093/jn/109.11.1979. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson RL, Dahms WT, Bray GA, Jacob R, Sandstead HH. Plasma zinc and copper in obesity and after intestinal bypass. Ann Intern Med. 1978;89:491–493. doi: 10.7326/0003-4819-89-4-491. [DOI] [PubMed] [Google Scholar]
- 11.Faber J, Randolph JG, Robbins S, Smith JC. Zinc and copper status in young patients following jejunoileal bypass. J Surg Res. 1978;24:83–86. doi: 10.1016/0022-4804(78)90078-1. [DOI] [PubMed] [Google Scholar]
- 12.Sjostrom L, Lindroos A-K, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 13.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 14.Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075–1079. doi: 10.1056/NEJMp048029. [DOI] [PubMed] [Google Scholar]
- 15.American-Society-for-Metabolic-and-Bariatric-Surgery. [cited on 06 September 2010];Fact sheet: metabolic and bariatric surgery. Available from http://www.asmbs.org/Newsite07/media/asbs_presskit.htm.
- 16.DeMaria EJ, Pate V, Warthen M, Winegar DA. Baseline data from American Society for Metabolic and Bariatric Surgery-designated Bariatric Surgery Centers of Excellence using the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis. 2010;6:347–355. doi: 10.1016/j.soard.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008;93:S89–S96. doi: 10.1210/jc.2008-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith DP, Liff DA, Ziegler TR, Esper GJ, Winton EF. Acquired copper deficiency: a potential serious and preventable complication following gastric bypass surgery. Obesity (Silver Spring) 2009;17:827–831. doi: 10.1038/oby.2008.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naismith RT, Shepherd JB, Weihl CC, Tutlam NT, Cross AH. Acute and bilateral blindness due to optic neuropathy associated with copper deficiency. Arch Neurol. 2009;66:1025–1027. doi: 10.1001/archneurol.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pineles SL, Wilson CA, Balcer LJ, Slater R, Galetta SL. Combined optic neuropathy and myelopathy secondary to copper deficiency. Surv Ophthalmol. 2010;55:386–392. doi: 10.1016/j.survophthal.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Rounis E, Laing CM, Davenport A. Acute neurological presentation due to copper deficiency in a hemodialysis patient following gastric bypass surgery. Clin Nephrol. 2010;74:389–392. [PubMed] [Google Scholar]
- 22.O’Donnell KB, Simmons M. Early-onset copper deficiency following roux-en-y gastric bypass. Nutr Clin Pract. 2011;26:66–69. doi: 10.1177/0884533610392921. [DOI] [PubMed] [Google Scholar]
- 23.Ernst B, Thurnheer M, Schultes B. Copper deficiency after gastric bypass surgery. Obesity (Silver Spring) 2009;17:1980–1981. doi: 10.1038/oby.2009.237. [DOI] [PubMed] [Google Scholar]
- 24.Kumar N, McEvoy KM, Ahlskog JE. Myelopathy due to copper deficiency following gastrointestinal surgery. Arch Neurol. 2003;60:1782–1785. doi: 10.1001/archneur.60.12.1782. [DOI] [PubMed] [Google Scholar]
- 25.Goodman BP, Bosch EP, Ross MA, Hoffman-Snyder C, Dodick DD, Smith BE. Clinical and electrodiagnostic findings in copper deficiency myeloneuropathy. J Neurol Neurosurg Psychiatry. 2009;80:524–527. doi: 10.1136/jnnp.2008.144683. [DOI] [PubMed] [Google Scholar]
- 26.Lin E, Gletsu N, Fugate K, McClusky D, Gu LH, Zhu J-L, et al. The effects of gastric surgery on systemic ghrelin levels in the morbidly obese. Arch Surg. 2004;139:780–784. doi: 10.1001/archsurg.139.7.780. [DOI] [PubMed] [Google Scholar]
- 27.Lin E, Liang Z, Frediani J, Davis SS, Sweeney JF, Ziegler TR, et al. Improvement in beta-cell function in patients with normal- and hyper-glycemia following roux-en-y gastric bypass surgery. Am J Physiol Endocrinol Metab. 2010;299:E706–E712. doi: 10.1152/ajpendo.00405.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin E, Armstrong-Moore D, Liang Z, Sweeney JF, Torres WE, Ziegler TR, et al. Contribution of adipose tissue to plasma 25-hydroxyvitamin D concentrations during weight loss following gastric bypass surgery. Obesity (Silver Spring) 2011;19:588–594. doi: 10.1038/oby.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Healthcare E. [Cited on March 31];Emory Medical Laboratory Services. Available from http://www.emoryhealthcare.org/medical-labservices/index.html.
- 30.Broderius M, Mostad E, Wendroth K, Prohaska JR. Levels of plasma ceruloplasmin protein are markedly lower following dietary copper deficiency in rodents. Comp Biochem Physiol C Toxicol Pharmacol. 2010;151:473–479. doi: 10.1016/j.cbpc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schosinsky KH, Lehmann HP, Beeler MF. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin Chem. 1974;20:1556–1563. [PubMed] [Google Scholar]
- 32.Butrimovitz GP, Purdy WC. The determination of zinc in blood plasma by atomic absorption spectrometry. Anal Chim Acta. 1977;94:63–73. doi: 10.1016/S0003-2670(01)83632-1. [DOI] [PubMed] [Google Scholar]
- 33.Kumar N, Ahlskog JE, Gross B., Jr Acquired hypocupremia after gastric surgery. Clin Gastroenterol Hepatol. 2004;2:1074–1079. doi: 10.1016/s1542-3565(04)00546-4. [DOI] [PubMed] [Google Scholar]
- 34.Tan JC, Burns DL, Jones HR. Severe ataxia, myelopathy, and peripheral neuropathy due to acquired copper deficiency in a patient with history of gastrectomy. J Parenter Enteral Nutr. 2006;30:446–450. doi: 10.1177/0148607106030005446. [DOI] [PubMed] [Google Scholar]
- 35.Halfdanarson TR, Kumar N, Li CY, Phyliky RL, Hogan WJ. Hematological manifestations of copper deficiency: a retrospective review. Eur J Haematol. 2008;80:523–531. doi: 10.1111/j.1600-0609.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 36.de Luis DA, Pacheco D, Izaola O, Terroba MC, Cuellar L, Martin T. Clinical results and nutritional consequences of bioliopancreatic diversion: three years of follow-up. Ann Nutr Metab. 2008;53:234–239. doi: 10.1159/000185641. [DOI] [PubMed] [Google Scholar]
- 37.Twomey PJ, Wierzbicki AS, Reynolds TM, Viljoen A. The copper/caeruloplasmin ratio in routine clinical practice in different laboratories. J Clin Pathol. 2009;62:60–63. doi: 10.1136/jcp.2007.055111. [DOI] [PubMed] [Google Scholar]
- 38.Xanthakos SA, Inge TH. Nutritional consequences of bariatric surgery. Curr Opin Clin Nutr Metab Care. 2006;9:489–496. doi: 10.1097/01.mco.0000232913.07355.cf. [DOI] [PubMed] [Google Scholar]
- 39.Aasheim ET, Bjorkman S, Sovik TT, Engstrom M, Hanvold SE, Mala T, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90:15–22. doi: 10.3945/ajcn.2009.27583. [DOI] [PubMed] [Google Scholar]
- 40.Trostler N, Mann A, Zilberbush N, Avinoach E, Charuzi I. Weight loss and food Intake 18 months following vertical banded gastroplasty or gastric bypass for severe obesity. Obes Surg. 1995;5:39–51. doi: 10.1381/096089295765558141. [DOI] [PubMed] [Google Scholar]
- 41.Chu KU, Tsuchiya T, Ishizuka J, Uchida T, Townsend CM, Jr, Thompson JC. Trophic response of gut and pancreas after ileojejunal transposition. Ann Surg. 1995;221:249–256. doi: 10.1097/00000658-199503000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prohaska JR. Impact of copper limitation on expression and function of multicopper oxidases (ferroxidases) Adv Nutr. 2011;2:129–137. doi: 10.3945/an.110.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasteyger C, Suter M, Gaillard RC, Giusti V. Nutritional deficiencies after roux-en-y gastric bypass for morbid obesity often cannot be prevented by standard multivitamin supplementation. Am J Clin Nutr. 2008;87:1128–1133. doi: 10.1093/ajcn/87.5.1128. [DOI] [PubMed] [Google Scholar]
- 44.Olivares M, Araya M, Uauy R. Copper homeostasis in infant nutrition: deficit and excess. J Pediatr Gastroenterol Nutr. 2000;31:102–111. doi: 10.1097/00005176-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Danzeisen R, Araya M, Harrison B, Keen C, Solioz M, Thiele D, et al. How reliable and robust are current biomarkers for copper status. Br J Nutr. 2007;98:676–683. doi: 10.1017/S0007114507798951. [DOI] [PubMed] [Google Scholar]
- 46.Milne DB. Assessment of copper nutritional status. Clin Chem. 1994;40:1479–1484. [PubMed] [Google Scholar]
- 47.Brolin RE, Gorman FH, Gorman RC, Petschenic AJ, Bradley LJ, Kenler HA, et al. Are vitamin B12 and folate deficiency clinically important after roux-en-y gastric bypass. J Gastrointest Surg. 1998;2:436–442. doi: 10.1016/s1091-255x(98)80034-6. [DOI] [PubMed] [Google Scholar]