Abstract
Importance
Worldwide, the nonnucleoside reverse transcriptase inhibitors (NNRTIs) efavirenz and nevirapine are commonly used in first-line antiretroviral regimens in both adults and children with human immunodeficiency virus (HIV) infection. Data on the comparative effectiveness of these medications in children are limited.
Objective
To investigate whether virological failure is more likely among children who initiated 1 or the other NNRTI-based HIV treatment.
Design, Setting, and Participants
Retrospective cohort study of children (aged 3–16 years) who initiated efavirenz-based (n=421) or nevirapine-based (n=383) treatment between April 2002 and January 2011 at a large pediatric HIV care setting in Botswana.
Main Outcomes and Measures
The primary outcome was time from initiation of therapy to virological failure. Virological failure was defined as lack of plasma HIV RNA suppression to less than 400 copies/mL by 6 months or confirmed HIV RNA of 400 copies/mL or greater after suppression. Cox proportional hazards regression analysis compared time to virological failure by regimen. Multivariable Cox regression controlled for age, sex, baseline immunologic category, baseline clinical category, baseline viral load, nutritional status, NRTIs used, receipt of single-dose nevirapine, and treatment for tuberculosis.
Results
With a median follow-up time of 69 months (range, 6–112 months; interquartile range, 23–87 months), 57 children (13.5%; 95% CI, 10.4%–17.2%) initiating treatment with efavirenz and 101 children (26.4%; 95% CI, 22.0%–31.1%) initiating treatment with nevirapine had virological failure. There were 11 children (2.6%; 95% CI, 1.3%–4.6%) receiving efavirenz and 20 children (5.2%; 95% CI, 3.2%–7.9%) receiving nevirapine who never achieved virological suppression. The Cox proportional hazard ratio for the combined virological failure end point was 2.0 (95% CI, 1.4–2.7; log rank P<.001, favoring efavirenz). None of the measured covariates affected the estimated hazard ratio in the multivariable analyses.
Conclusions and Relevance
Among children aged 3 to 16 years infected with HIV and treated at a clinic in Botswana, the use of efavirenz compared with nevirapine as initial antiretroviral treatment was associated with less virological failure. These findings may warrant additional research evaluating the use of efavirenz and nevirapine for pediatric patients.
More than 2 million children worldwide are infected with human immunodeficiency virus (HIV), approximately 90% of whom live in sub-Saharan Africa.1,2 The scale-up of pediatric antiretroviral treatment in Africa has been lifesaving for children.3,4 Critical review of long-term outcomes from the first children to receive HIV treatment in this region may help to guide treatment program improvements.
The World Health Organization (WHO) recommends that children older than 3 years receive 1 of 2 nonnucleoside reverse transcriptase inhibitors (NNRTIs), nevirapine or efavirenz, and 2 drugs in the NRTI class.5 Most countries favor nevirapine-based regimens for the majority of children due to perceived comparable effectiveness at lower cost.6
Adult studies comparing efavirenz with nevirapine have shown mixed results.6–9 However, a pooled analysis of 1688 patients in 7 randomized controlled trials showed no difference in virological outcomes between the 2 medications.10
Limited data are available regarding the relative effectiveness of the 2 NNRTI options in children. The only published outcomes study comparing efavirenz and nevirapine-based therapies in children included those younger than 3 years, for whom first-line NNRTI-based therapy is no longer the standard of care in some parts of the world.11,12 Those children in the study receiving a generic fixed-dose combination of nevirapine, stavudine, and lamivudine had worse 1-year outcomes than those receiving efavirenz-based treatment (odds ratio, 2.5; 95% CI, 1.2–4.9).13
Botswana was one of the first African countries to offer treatment to children and thus has some of the longest-term treatment outcomes data. Until 2012, the Botswana National HIV/AIDS Treatment Guidelines recommended either nevirapine or efavirenz.14 Pediatric fixed-dose combinations have not been used. The NNRTI choice was determined by the clinician initiating treatment. We aimed to determine whether there was a difference in time to virological failure between children initiating nevirapine vs efavirenz-based treatment in Botswana.
METHODS
Study Design and Setting
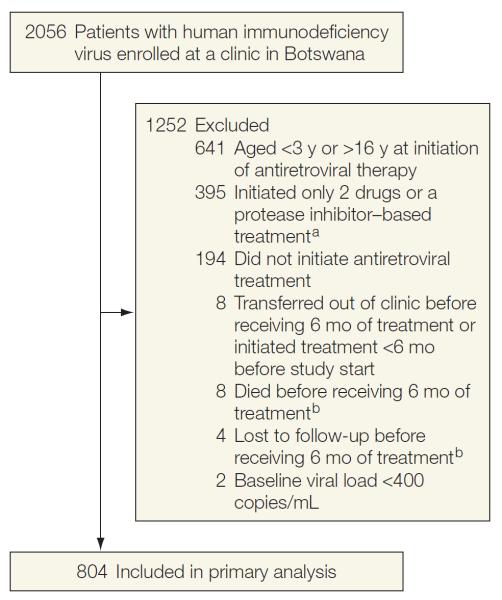
We conducted a retrospective cohort study of children who received treatment at the Botswana-Baylor Children's Clinical Centre of Excellence in Gaborone, Botswana, between April 2002 and January 2011.15 Virtually all the children are thought to have been perinatally infected. Eligibility criteria included confirmed HIV infection, age of 3 to 16 years at initiation of HIV treatment, and initiation of first-line NNRTI-based therapy. Some patients (n=45) were included in a prior study of genotypic resistance testing after virological failure.16 Children younger than 3 years were excluded because efavirenz is not recommended for that age group. The reasons for exclusion of clinic patients from the study cohort are indicated in Figure 1.
Figure 1.
Reasons for Exclusion of Clinic Patients From the Study Cohort
aThe patients who initiated a protease inhibitor–based treatment were enrolled in a clinical trial. A few patients began taking 2 nucleoside reverse transcriptase inhibitors before highly active antiretroviral therapy was available.
bIncluded in death and loss to follow-up counted as virological failure subanalysis.
A large number of patients with baseline characteristics similar to those of patients included in our cohort were enrolled in a clinical trial with first-line protease inhibitor–based treatment. As per the national treatment guidelines,14,17 most patients concurrently received the NRTIs zidovudine and lamivudine. Stavudine was substituted for zidovudine when baseline anemia was moderate or severe. Other NRTI substitutions were allowed, but were rarely used (Table 1).
Table 1.
Characteristics of Children Initiating Antiretroviral Therapy
| No. (%) of Patientsa |
|||
|---|---|---|---|
| Efavirenz (n = 421) | Nevirapine (n = 383) | P Valuesb | |
| Age, median (IQR), y | 8.1 (6.0–10.5) | 7.0 (4.8–9.8) | <.001 |
|
| |||
| Antiretroviral therapy initiated before 2008 | 299 (71.0) | 339 (88.5) | <.001 |
|
| |||
| Female sex | 175 (43.5) | 227 (56.5) | <.001 |
|
| |||
| Baseline CD4%, median (IQR) | (n = 409) 13 (8 to 20) | (n = 368) 15 (9 to 22) | .003c |
|
| |||
| Baseline CDC immunologic categoryd | |||
| 1 | 34 (8.1) | 51 (13.3) | .03c |
|
| |||
| 2 | 123 (29.2) | 126 (32.9) | |
|
| |||
| 3 | 262 (62.2) | 203 (53.0) | |
|
| |||
| Missing | 2 (0.5) | 3 (0.8) | |
|
| |||
| Baseline CDC clinical categorye | |||
| N | 29 (6.9) | 10 (2.6) | <.001c |
|
| |||
| A | 40 (9.5) | 44 (11.5) | |
|
| |||
| B | 84 (20.0) | 102 (26.6) | |
|
| |||
| C | 164 (39.0) | 173 (45.2) | |
|
| |||
| Missing | 104 (24.7) | 54 (14.1) | |
|
| |||
| Baseline HIV RNA, copies/mL | (n = 115) | (n = 175) | |
|
| |||
| Log10, median (IQR) | 5.2 (4.8 to 5.6) | 5.3 (4.9 to 5.7) | .32 |
|
| |||
| >750 000 | 15 (13.0) | 16 (9.1) | .35 |
|
| |||
| z Score, mean (SD) | |||
| Height for age | (n = 358) −2.2 (1.3) | (n = 341) −2.6 (1.7) | <.001 |
|
| |||
| Weight for height or body mass indexf | (n = 359) −1.0 (1.5) | (n = 340) −0.8 (1.4) | .16 |
| Nucleoside reverse transcriptase inhibitors | |||
| Zidovudine plus lamivudine | 380 (90.3) | 357 (93.2) | .18 |
|
| |||
| Stavudine plus lamivudine | 34 (8.1) | 24 (6.3) | |
|
| |||
| Tenofovir plus emtricitabine | 6 (1.4) | 1 (0.3) | |
|
| |||
| Abacavir plus lamivudine | 1 (0.2) | 0 | |
|
| |||
| Didanosine and stavudine | 0 | 1 (0.3) | |
|
| |||
| Single-dose nevirapine | |||
| Mother and/or baby received | 7 (1.7) | 11 (2.9) | .25 |
|
| |||
| None or unknown | 414 (98.3) | 372 (97.1) | |
|
| |||
| Treated for tuberculosis during antiretroviral therapy | |||
| Yes | 65 (15.4) | 31 (8.1) | .001c |
|
| |||
| No | 356 (84.6) | 348 (90.9) | |
|
| |||
| Missing | 0 | 4 (1.0) | |
|
| |||
| Pill count adherence, median (IQR), % doses takeng | (n = 287) 98 (97 to 99) | (n = 202) 98 (98 to 99) | .46 |
|
| |||
| Never achieved virological suppression | 11 (2.6) | 20 (5.2) | .06 |
|
| |||
| Died | 1 (0.2) | 1 (0.3) | .94 |
|
| |||
| Lost to follow-up | 0 | 9 (2.4) | .002 |
Abbreviations: CDC, Centers for Disease Control and Prevention; HIV, human immunodeficiency virus; IQR, interquartile range.
Unless otherwise indicated.
The t test was used for normally distributed continuous data, the Wilcoxon rank sum test for nonparametric continuous data, and the χ2 test for dichotomous data.
Based on comparisons of available data only. Inclusion of missing data as an additional category led to minimal changes for all variables.
Defined by age-specific classifications based on CD4 cell absolute counts and percentage12; discordance was classified as the lower of the 2. When the CD4 cell percentage was missing, the CDC immunologic category was determined based on the CD4 cell absolute count alone. Category 1 indicates mild or no apparent immunologic suppression; category 2, moderate immunologic suppression; and category 3, severe immunologic suppression. For those children enrolled in the study, the following cutoffs applied for CD4 cell count and CD4 cell percentage, respectively, for those aged 3 to 5 years: category 1,1000 cells/μL or greater (≥25%), category 2, 500 to 999 cells/μL (15%–24%), category 3, less than 500 cells/μL (<15%); and for those aged 6 years or older: category 1, 500 cells/μL or greater (≥25%), category 2, 200 to 499 cells/μL (15%–24%), category 3, less than 200 cells/μL (<15%).
Category N indicates that the patient has not been symptomatic or had a single category A symptom; category A, mild symptoms; category B, moderate symptoms; and category C, severe symptoms.18
Body mass index was calculated as weight in kilograms divided by height in meters squared.
Repeated measures for the same patient were averaged.
Drug doses were calculated based on national guidelines. Prior to 2008, nevirapine doses were based on the child's weight (7 mg/kg for age <8 years and 4 mg/kg for age ≥8 years) or body surface area (120–200 mg/m2) and efavirenz dosing charts were used.17 After 2008, WHO guidelines were used.5 Efavirenz became the guideline-preferred first-line NNRTI for adults after 2008, but efavirenz and nevirapine were still recommended equally as first-line therapy for children.14 Prior to 2008, adherence was not recorded; after 2008, pill counts were recorded at most clinical visits.
The study was reviewed and approved by the Botswana Health Research Development Committee and the institutional review boards of the University of Pennsylvania and Baylor College of Medicine. Informed consent was waived because the study provided no more than minimal risk to patients, the waiver did not adversely affect the rights and welfare of patients, and the research could not practicably be performed without having the waiver.
Data were obtained from electronic and paper medical records. A review of at least 10% of all nonprimary source data (ie, data in an electronic database that had been transcribed from paper records) demonstrated greater than 95% accuracy.
Variables of Interest
For the primary analysis, treatment was classified based on the starting regimen. The primary outcome was time from initiation of therapy to virological failure. Virological failure was defined by lack of plasma HIV RNA suppression to less than 400 copies/mL by 6 months or confirmed viral load of 400 copies/mL or greater after initial suppression. Most patients had viral load measurements at both 3 and 6 months of treatment.
Patients who did not achieve virological suppression at the 6-month measurement and who lacked a 3-month value had another viral load measurement to confirm lack of suppression. Patients withasingleviralloadofgreaterthan400 copies/mL preceding death or loss to follow-up were considered to have had virological failure. Patients who died or were lost to follow-up whose viral load was undetectable before death or loss to follow-up were not considered to have experienced virological failure. Patients were censored at the time of death, loss to follow-up, or at the end of observation, whichever came first.
Age was analyzed as a continuous variable. Baseline Centers for Disease Control and Prevention (CDC) immunologic category was defined by age-specific classifications based on CD4 cell absolute counts and percentage.12 Discordance between the classification based on absolute count or percentage was classified as the lower of the 2. The CDC clinical category was established based on the child's history of HIV-associated clinical symptoms.
Category C indicates severe symptoms, category B indicates moderate symptoms, category A indicates mild symptoms, and category N indicates no symptoms or a single category A symptom.18 Viral loads had an upper limit of 750 000 copies/mL; baseline viral load was log-transformed and analyzed both as a continuous variable and as having 750 000 copies/mL or greater or not.
Of note, the Botswana national guidelines mandated that baseline viral loads no longer be obtained after 2008. Exposure to single-dose nevirapine for prevention of mother-to-child transmission was based on receipt of nevirapine by the mother during labor or the infant after birth. The NRTI regimen was dichotomized as zidovudine and lamivudine or other. Tuberculosis treatment was defined as receipt of a rifampicin-containing regimen at any time following initiation of antiretroviral therapy.
Baseline acute and chronic nutritional status was calculated by determining z scores for body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) for age and height for age, respectively, using WHO AnthroPlus software version 3.2.2. For children younger than 5 years, weight-for-height z scores were substituted for BMI because BMI does not increase monotonically in young children. The reasons for switching regimens were abstracted from medical chart notes. Pill count adherence was averaged for each patient with adherence data.
Statistical Analysis
Characteristics of children initiating therapy with efavirenz- or nevirapine-based treatments were compared using t tests and rank sum tests for continuous data and χ2 tests for dichotomous or polytomous data. The primary end point was time to virological failure. Cox proportional hazards models were used to compare time to event and to control for covariates. All demographic and baseline clinical variables in Table 1 were evaluated as covariates. Time to virological failure was compared between groups univariately using Kaplan-Meier survival plots with the log-rank test. Covariates were considered for inclusion in the final hazard model on the basis of an unadjusted association with virological failure (P<.25).
Effect modification was tested by including main effects and interaction terms in the Cox models. Confounding was assessed by evaluating the association of each covariate with the exposure (drug) and the outcome (virological failure), as well as by assessing whether inclusion in the models affected the hazard ratio (HR). We also conducted analyses on the primary outcome using propensity scores to control for potential confounding, using a Cox model with 3 different propensity score models. The models were selected based on all potential confounders, and then the most plausible potential confounders.19,20 The proportional hazards assumption was assessed for all Cox models using Schoenfeld residuals.21 Missing data imputation was attempted for covariates as required, but the models used did not converge.22 Extensive sensitivity analyses were performed to assess the effect of missing data on the results observed with available data.
We used χ2 tests to compare the regimens with regard to the proportion with virological failure at any time point. Secondary analyses, otherwise identical to those described above, were performed to assess the effect of drug switches. These analyses assigned all patients who had a regimen switch to the NNRTI group to which they switched. Further secondary analyses characterized patients who died or were lost to follow-up with their last viral load undetectable as having virological failure. All analyses were 2-sided with a P value of less than .05 considered statistically significant. Analyses were performed with Stata version 11 software (StataCorp).
With a mean time to virological failure of 24 months in the reference group and a fixed sample size of 800 (380 on one regimen and 420 on the other), we had greater than 80% power to detect a HR for time to virological failure of less than 0.8 or greater than 1.2 among patients for each of the drug regimens and a 2-sided α level of .05.
RESULTS
There were 804 black African (Tswana) children who initiated treatment between April 2002 and January 2011 who were included in the primary analysis, with a median follow-up time of 69 months (range, 6–112 months; interquartile range, 23–87 months). The characteristics of the cohort are outlined in Table 1. After 2008, fewer patients received nevirapine and more patients taking nevirapine experienced treatment failure and thus were censored by 2008. More patients initiated treatment with efavirenz than nevirapine after 2008, resulting in more missing baseline viral loads in the efavirenz group.
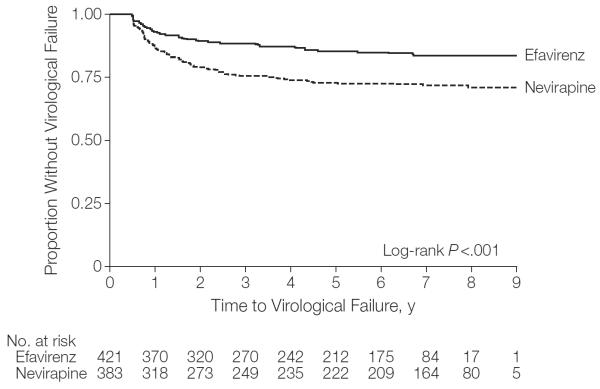
There were 158 children (19.7%; 95% CI, 17.0%–22.6%) who experienced virological failure; 57 children (13.5%; 95% CI, 10.4%–17.2%) in the efavirenz group and 101 children (26.4%; 95% CI, 22.0%–31.1%) in the nevirapine group (P<.001). The time to virological failure was shorter among patients taking nevirapine vs those taking efavirenz (Figure 2). The Cox proportional HR for the combined virological failure end point was 2.0 (95% CI, 1.4–2.7; log-rank P<.001). There were 11 children (2.6%; 95% CI, 1.3%–4.6%) receiving efavirenz and 20 children (5.2%; 95% CI, 3.2%–7.9%) receiving nevirapine who never achieved virological suppression.
Figure 2.
Kaplan-Meier Survival Estimate for Proportion Without Virological Failure in Total Cohort
Virological failure was defined by lack of plasma HIV RNA suppression to less than 400 copies/mL by 6 months or confirmed plasma HIV RNA of 400 copies/mL or greater after suppression. Time to virological failure was the time of the 6-month viral load for those who did not achieve virological suppression by 6 months. For those with subsequent virological rebound, time to virological failure was defined as the time of the first elevated viral load after initial virological suppression.
The virological failure rates at 1, 2, and 5 years with efavirenz were 6.7% (95% CI, 4.5%–9.5%), 10.2% (95% CI, 7.5%–13.5%), and 12.8% (95% CI, 9.8%–16.4%), respectively; and with nevirapine were 12.8% (95% CI, 9.6%–16.6%), 19.8% (95% CI, 16.0%–24.2%), and 25.1% (95% CI, 20.8%–29.7%), respectively. The absolute risk differences at the 3 time points were 6.1%, 9.6%, and 12.3%, all favoring efavirenz.
Adjusting for age, sex, baseline immunologic category, baseline clinical category, baseline viral load, nutritional status, NRTIs used, receipt of single-dose nevirapine, and tuberculosis treatment did not significantly change the HR. Similarly, the use of propensity scores did not appreciably change the results (eTable at http://www.jama.com).
When stratified by sex, time to virological failure was statistically significantly shorter for the nevirapine group vs the efavirenz group in both males (HR, 2.5 [95% CI, 1.6–4.0]; P<.001) and females (HR, 1.8 [95% CI, 1.1–2.8]; P=.02) (Figure 3). Although the shorter time to virological failure with nevirapine was more pronounced in males than in females, the medication group×sex interaction term in the Cox model was not statistically significant (P=.25).
Figure 3.
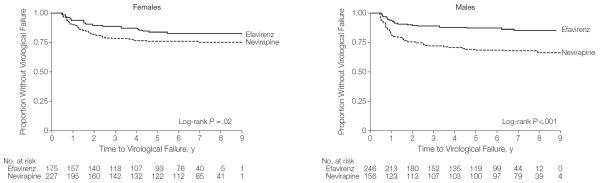
Kaplan-Meier Survival Estimates for Proportion Without Virological Failure by Sex
Virological failure was defined by lack of plasma HIV RNA suppression to less than 400 copies/mL by 6 months or confirmed plasma HIV RNA of 400 copies/mL or greater after suppression. Time to virological failure was the time of the 6-month viral load for those who did not achieve virological suppression by 6 months. For those with subsequent virological rebound, time to virological failure was defined as the time of the first elevated viral load after initial virological suppression.
The virological failure rates at 1, 2, and 5 years among males receiving efavirenz were 6.9% (95% CI, 4.1%–10.8%), 10.2% (95% CI, 6.7%–14.6%), and 11.8% (95% CI, 8.0%–16.5%), respectively; and among males receiving nevirapine were 18.0% (95% CI, 12.3%–24.9%), 24.4% (95% CI, 17.9%–31.9%), and 30.1% (95% CI, 23.1%–38.0%), respectively. The absolute risk difference at the 3 time points in males was 11.1%, 14.2%, and 18.3%, respectively.
The failure rates at 1, 2, and 5 years among females receiving efavirenz were 6.3% (95% CI, 3.2%–11.0%), 10.3% (95% CI, 6.2%–15.8%), and 14.3% (95% CI, 9.5%–20.4%), respectively; and among females receiving nevirapine were 9.3% (95% CI, 5.8%–13.8%), 16.7% (95% CI, 12.1%–22.2%), and 21.6% (95% CI, 16.4%–27.5%), respectively. The absolute risk difference at the 3 time points in females was 3.0%, 6.4%, and 7.3%, respectively. Of the 214 children (26.6%) who initiated treatment after their 10th birthday, 105 (49.1%) were female.
For those aged 10 years or older at initiation of antiretroviral treatment, more girls than boys initiated nevirapine (68 [65%] vs 22 [20%], respectively; P<.001). However, a similar percentage experienced treatment failure who were younger than 10 years (112 patients; 19.0% [95% CI, 15.9%–22.4%]) and aged 10 years or older (46 patients; 21.5% [95% CI, 16.2%–27.6%]) (P=.43). Older children did not experience treatment failure statistically significantly more rapidly than younger children (HR, 1.4 [95% CI, 1.0–1.9]; P=.09).
In a subanalysis of children younger than 10 years, 12.6% experienced efavirenz-based treatment failure vs 25% of children receiving nevirapine-based treatment (P<.001). For the children who were aged 10 years or older, 15.1% experienced efavirenz-based treatment failure vs 29% receiving nevirapine-based treatment (P=.01). The HR for treatment failure in children younger than 10 years was 2.0 (95% CI, 1.4–2.9;P<.001). The HR for treatment failure in the children aged 10 years or older was 2.3 (95% CI, 1.3–4.0; P=.003).
Sex-specific differences regarding choice of NNRTI for the 590 children who initiated treatment when younger than 10 years were much less pronounced, with nevirapine used in 159 girls (54%) and 134 boys (46%) (P=.06). A higher proportion of patients began efavirenz after 2008 (Table 1); however, the significant difference in virological failure rates between groups persisted after excluding those who initiated treatment with efavirenz after 2008 (16.7% [95% CI, 12.7%–21.4%] receiving efavirenz vs 29.2% [95% CI, 24.4%–34.4%] receiving nevirapine; P<.001).
Eight patients who died during the first 6 months and 2 patients who died during the follow-up interval were not included in the primary analysis. There were 13 patients who were lost to follow-up; 4 patients prior to receiving 6 months of treatment. Counting death and loss to follow-up with undetectable viral loads as having virological failure had little effect on the failure risk rate (HR, 2.1 [95% CI, 1.5–2.9] vs HR, 2.0 [95% CI, 1.4–2.7] in the primary analysis). The absolute risk when death and loss to follow-up were counted as failure was 13.5% (95% CI, 10.4%–17.2%) of those in the efavirenz group and 27.9% (95% CI, 23.5%–32.7%) of those in the nevirapine group. Age at time of virological failure was similar for males and females (mean age, 9.5 vs 10.3 years; P=.14). The virological failure rate among patients receiving tuberculosis treatment (23/96; 24.0% [95% CI, 15.8%–33.7%]) was also similar to the rate in those not receiving it (135/704; 19.1% [95% CI, 16.3%–22.3%]) (P=.27). All Cox models satisfied the proportional hazards assumption.
Regimen Switches
There were 39 patients (5%) who switched NNRTIs during the follow-up period and 3 patients (7.7%; 95% CI, 1.6%–20.9%) who experienced virological failure after switching regimens. Of those with virological failure, 2 patients switched from efavirenz to nevirapine and 1 patient switched from nevirapine to efavirenz. There was no significant change in the hazard of virological failure by regimen when exposure was classified based on the final NNRTI received rather than the initial NNRTI (HR, 1.9; 95% CI, 1.4–2.7). The reasons for the NNRTI switches are outlined in Table 2. The most common reasons for changing regimens were concern about the teratogenic effects of efavirenz in postpubertal girls and efavirenz-related gynecomastia.
Table 2.
Reasons for Switching Nonnucleoside Reverse Transcriptase Inhibitors
| No. (%) of Total Cohorta | |
|---|---|
| Switched from efavirenz to nevirapine (n = 26) | |
| Puberty | 13 (3.1) |
|
| |
| Gynecomastia | 7 (1.7) |
|
| |
| Neuropsychiatric effects | 4 (1.0) |
|
| |
| Out of stock | 2 (0.5) |
|
| |
| Switched from nevirapine to efavirenz (n = 13) | |
| Dermatologic reaction | 5 (1.3)b |
|
| |
| Regimen simplification | 4 (1.0) |
|
| |
| Hepatotoxic effects | 3 (0.8)b |
|
| |
| Emesis | 1 (0.3) |
|
| |
| Not documented | 1 (0.3) |
There were 421 patients who started taking efavirenz and 383 who started taking nevirapine.
One patient had 2 reasons for the treatment switch.
DISCUSSION
In this large cohort of children infected with HIV, time to virological failure was longer among children receiving efavirenz vs nevirapine. With the majority of the world's children receiving nevirapine-based antiretroviral therapy, these findings may have significant public health importance.23
Recent publications have suggested that wider use of efavirenz among adults in low-resource settings with high prevalence of HIV infection may improve outcomes and be cost-effective.24,25 Tuberculosis treatment has played an important role with better virological outcomes among adults with tuberculosis who are receiving efavirenz-based vs nevirapine-based antiretroviral therapy.26 This finding is of great importance for patients with HIV in sub-Saharan Africa where there is a high prevalence of co-infection.27 However, in our study, higher virological failure rates were not explained by children receiving tuberculosis therapy and nevirapine concurrently. Baseline resistance also seems an unlikely contributor because only 2.2% of our cohort were known to have received single-dose nevirapine. Therefore, other explanations must exist.
Adherence data for our cohort were limited, especially for those with virological failure. Available pill count adherence rates were very high and showed little variability. Therefore, we are unable to comment directly on the role of adherence in the observed outcomes.
We considered the potential for differences in NNRTI choice based on patient characteristics to be the greatest limitation of our study. The most likely source of important differential prescribing was expected to be disproportionate use of nevirapine for girls nearing the age of childbearing potential due to concerns for the teratogenic effects of efavirenz. We further expected that having a higher proportion of older girls taking nevirapine might bias in favor of efavirenz because adolescents may have worse adherence than younger children. However, regimen-specific differences were actually somewhat more pronounced among males than females, with males taking nevirapine having the worst outcomes.
Even though this study was limited to a single site, we believe that the findings will be generalizable to other settings with high HIV prevalence in most respects. Patients studied are similar to those in other high-prevalence African countries. Although Botswana is a moderate-income, rather than a low-income nation, children receiving care in the national antiretroviral treatment program are those whose families do not have private insurance and primarily represent lower socioeconomic strata. In our study, 43% of patients had height-forage z scores of less than − SDs on the WHO growth curves, indicating chronic malnutrition.28 Subtype of HIV may have an effect on treatment outcomes. In Botswana, most people with HIV have subtype Cinfection, which is one of the most common subtypes worldwide and the most common in Southern Africa.29 The HIV subtype C and D infections have been noted to be more virulent than other common viral subtypes.30,31 Although data on subtype-specific treatment outcomes are scarce, there is evidence that patients with subtype D virus have worse outcomes than patients with less virulent subtypes.30,31 More potent drugs may be necessary to ensure optimal treatment outcomes in patients with more aggressive virus.
The greatest limitation regarding generalizability of our cohort probably relates to the clinical site's active follow-up program for patients who miss clinic appointments. Due in large part to these efforts, the loss to follow-up rate in this cohort was less than 2% over the almost 10-year period of observation. Unmeasured potential confounding variables must be considered as limitations of all retrospective cohort studies. At our study site, there are multiple clinicians; patients do not routinely see the same clinicians at follow-up visits. Thus, clinician-specific differences should have minimal effects on treatment outcomes.
In other settings in which both efavirenz and nevirapine are being used for pediatric treatment, additional studies should be conducted to confirm or refute these results. Further studies assessing reasons for increased virological failure rates should also be undertaken to help provide insight into mechanisms of treatment failure in this setting. The finding of increased rates of virological failure among children receiving nevirapine suggests that more work should be done to make efavirenz a cost-effective option for pediatric antiretroviral treatment programs in resource-limited settings.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported in part by Health Resources and Services Administration grant T32 HP10026 (funding to Dr Lowenthal), funding from Bristol-Myers Squibb (awarded to Dr Lowenthal and provided funding to Drs Lowenthal and Gross), and grant P30 AI 045008 from the Penn Center for AIDS Research (awarded to Dr Gross). Dr Lowenthal is currently receiving research career development support from National Institute of Mental Health grant K23 MH095669.
Role of the Sponsor: The sponsors of the study had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Lowenthal had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lowenthal, Ellenberg, Steenhoff, Anabwani, Gross.
Acquisition of data: Lowenthal, Machine, Sagdeo, Boiditswe, Rutstein, Anabwani.
Analysis and interpretation of data: Lowenthal, Ellenberg, Steenhoff, Rutstein, Gross.
Drafting of the manuscript: Lowenthal.
Critical revision of the manuscript for important intellectual content: Ellenberg, Machine, Sagdeo, Boiditswe, Steenhoff, Rutstein, Anabwani, Gross.
Statistical analysis: Lowenthal, Ellenberg, Gross.
Obtained funding: Lowenthal, Gross.
Administrative, technical, or material support: Lowenthal, Machine, Sagdeo, Boiditswe, Steenhoff, Rutstein, Anabwani.
Study supervision: Lowenthal, Steenhoff, Gross.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Lowenthal reported receiving grant support from Bristol-Myers Squibb. Dr Ellenberg reported serving on data and safety monitoring boards and mock advisory committees for Bristol-Myers Squibb, Eli Lilly, Pfizer, EMD Serono, and Roche; and receiving grants from the National Institutes of Health. Dr Anabwani reported receiving research support from Boehringer Ingelheim. Dr Gross reported receiving grant support from the National Institutes of Health; serving on a data and safety monitoring board for Pfizer; providing expert testimony to Lewis Saul LLP; receiving payment for lectures from Viral Ed; and receiving honoraria and reimbursement for travel expenses from the International Association of Providers of AIDS Care. No other author reported disclosures.
Additional Contributions: We thank Heather Draper, MPH (Baylor International Pediatric AIDS Initiative), for assistance with responding to the initial review of this article; Margaret Tshokonego, MD (Botswana-Baylor Children's Clinical Centre of Excellence), for data entry support; and Jennifer Chapman, MPH (Children's Hospital of Philadelphia), for verifying the accurate coding and transcription of the data presented. Ms Draper, Dr Tshokonego, and Ms Chapman did not receive financial compensation for their contributions.
Online-Only Material: The eTable is available at http://www.jama.com.
REFERENCES
- 1.UNAIDS [Accessed November 21, 2010];AIDS epidemic update. 2009 http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf.
- 2.UNICEF [Accessibility verified March 28, 2013];Children and AIDS: fifth stocktaking report. 2010 http://www.unicef.org/publications/files/Children_and_AIDS-Fifth_Stocktaking_Report_2010_EN.pdf.
- 3.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298(16):1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 4.Little K, Thorne C, Luo C, et al. Disease progression in children with vertically-acquired HIV infection in sub-Saharan Africa: reviewing the need for HIV treatment. Curr HIV Res. 2007;5(2):139–153. doi: 10.2174/157016207780077002. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access: Recommendations for a Public Health Approach. World Health Organization Press; Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 6.Nachega JB, Hislop M, Dowdy DW, et al. Efavirenz versus nevirapine-based initial treatment of HIV infection. AIDS. 2008;22(16):2117–2125. doi: 10.1097/QAD.0b013e328310407e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann M, Witte S, Brust J, et al. Comparison of efavirenz and nevirapine in HIV-infected patients (NEEF Cohort) Int J STD AIDS. 2005;16(6):404–409. doi: 10.1258/0956462054094060. [DOI] [PubMed] [Google Scholar]
- 8.Lapphra K, Vanprapar N, Chearskul S, et al. Efficacy and tolerability of nevirapine- versus efavirenz-containing regimens in HIV-infected Thai children. Int J Infect Dis. 2008;12(6):e33–e38. doi: 10.1016/j.ijid.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Patel AK, Pujari S, Patel K, et al. Nevirapine versus efavirenz based antiretroviral treatment in naive Indian patients. J Assoc Physicians India. 2006;54:915–918. [PubMed] [Google Scholar]
- 10.Mbuagbaw LC, Irlam JH, Spaulding A, et al. Efavirenz or nevirapine in three-drug combination therapy with two nucleoside-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviralnaÄve individuals. Cochrane Database Syst Rev. 2010;12:CD004246. doi: 10.1002/14651858.CD004246.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Southern African Journal of HIV Medicine. [Accessed March 16, 2013];Guidelines for antiretroviral therapy in children— November 2009 version. http://www.sahivsoc.org/upload/documents/guidelines_Guidelines_for_Antiretroviral_Therapy_in_Children_November_2009.pdf.
- 12.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children [Accessed March 16, 2013];Guidelines for the use of antiretroviral agents in pediatric HIV infection. http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf.
- 13.Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46(2):187–193. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 14.Botswana Ministry of Health . Botswana National HIV/AIDS Treatment Guidelines. Botswana Ministry of Health; Gaborone: 2008. [Google Scholar]
- 15.Lowenthal ED, Anabwani GM, Jibril HB, et al. Botswana-Baylor Children's Clinical Centre of Excellence. South Afr J HIV Med. 2005;6(2):42–46. [Google Scholar]
- 16.Tolle M, Howard L, Kirk B, et al. Reverse transcriptase genotypes in pediatric patients failing initial antiretroviral therapy in Gaborone, Botswana. J Int Assoc Physicians AIDS Care (Chic) 2012;11(4):260–268. doi: 10.1177/1545109711422273. [DOI] [PubMed] [Google Scholar]
- 17.Botswana Ministry of Health . Botswana Guidelines on Anti-Retroviral Treatment. Botswana Ministry of Health; Gaborone: 2005. [Google Scholar]
- 18.Centers for Disease Control and Prevention 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Recomm Rep. 1994;43(RR-12):1–10. [Google Scholar]
- 19.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 pt 2):757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 21.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 22.Carlin J, Galati J, Royston P. A new framework for managing and analyzing multiply imputed data in Stata. Stata J. 2008;8(1):49–67. [Google Scholar]
- 23.World Health Organization [Accessibility verified March 28, 2013];Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. http://www.who.int/hiv/pub/progress_report2011/en/.
- 24.Uthman O, Mofenson LM, Nachega JB. Safety and effectiveness of efavirenz versus nevirapine-based regimens in resource-limited settings. AIDS. 2012;26(5):639–641. doi: 10.1097/QAD.0b013e3283509a40. [DOI] [PubMed] [Google Scholar]
- 25.Ouattara EN, Anglaret X, Wong AY, et al. Projecting the clinical benefits and risks of using efavirenz-containing antiretroviral therapy regimens in women of childbearing age. AIDS. 2012;26(5):625–634. doi: 10.1097/QAD.0b013e328350fbfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manosuthi W, Sungkanuparph S, Tantanathip P, et al. A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse-transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin. Clin Infect Dis. 2009;48(12):1752–1759. doi: 10.1086/599114. [DOI] [PubMed] [Google Scholar]
- 27.Walters E, Cotton MF, Rabie H, et al. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti-retroviral therapy. BMC Pediatr. 2008;8:1. doi: 10.1186/1471-2431-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Onis M, Onyango AW, Borghi E, et al. Comparison of the World Health Organization (WHO) Child Growth Standards and the National Center for Health Statistics/WHO International Growth Reference: implications for child health programmes. Public Health Nutr. 2006;9(7):942–947. doi: 10.1017/phn20062005. [DOI] [PubMed] [Google Scholar]
- 29.Geretti AM. HIV-1 subtypes. Curr Opin Infect Dis. 2006;19(1):1–7. doi: 10.1097/01.qco.0000200293.45532.68. [DOI] [PubMed] [Google Scholar]
- 30.Easterbrook PJ, Smith M, Mullen J, et al. Impact of HIV-1 viral subtype on disease progression and response to antiretroviral therapy. J Int AIDS Soc. 2010;13:4. doi: 10.1186/1758-2652-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baeten JM, Chohan B, Lavreys L, et al. HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J Infect Dis. 2007;195(8):1177–1180. doi: 10.1086/512682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.