Abstract
Tibial compression can increase murine bone mass. However, loading protocols and mouse strains differ between studies which may contribute to conflicting results. We hypothesized that bone accrual is influenced more by loading history than by mouse strain or animal handling. The right tibiae of 4-month C57BL/6 and BALB/c mice were subjected to axial compression (10 N, 3 days/week, 6 weeks). Left tibiae served as contralateral controls to calculate relative changes [(Loaded-Control)/Control]. The WashU protocol applied 60 cycles/day, at 2 Hz, with 10 s rest-insertion between cycles; the Cornell/HSS protocol applied 1200 cycles/day, at 6.7 Hz, with 0.1 s rest-insertion. Because sham loading, sedation and transportation did not affect tibial morphology, unhandled mice served as age-matched controls (AC). Both loading protocols were anabolic for cortical bone, but Cornell/HSS loading elicited a more rapid response that was greater than WashU loading by 13%. By 6 weeks, cortical bone volume of each loading group was greater than of AC (avg. +16%) and not different from each other. Ultimate displacement and energy-to-fracture were greater in tibiae loaded by either protocol and ultimate force was greater with Cornell/HSS loading. At 6 weeks, independent of mouse strain, the WashU protocol produced minimal trabecular bone and the trabecular bone volume fraction of Cornell/HSS tibiae was greater than of AC by 65% and of WashU by 44%. We concluded that tibial adaptation to loading was more influenced by waveform than mouse strain or animal handling and therefore may have targeted similar osteogenic mechanisms in C57BL/6 and BALB/c mice.
Keywords: Biomechanics, Bone Architecture/Structure, Mechanical Loading, Exercise, Bone Strength
INTRODUCTION
In-vivo loading is a well established approach to increase bone mass and strength in pre-clinical studies [1]. In mice, axial tibial compression is a non-invasive loading method that can induce mechanical strains greater than those during habitual loading and thus stimulate cortical and trabecular bone changes [2,3]. As more studies using axial tibial compression in mice are published, it is apparent that differences in loading protocols between studies may contribute to differences in findings, especially with respect to trabecular bone. No studies have directly compared different loading protocols.
Studies in non-murine species have established the importance of several loading parameters, such as rest-insertion, frequency/strain-rate, and cycle number, in the bone adaptation response [4–7]. In particular, rest-insertion enhances the response of bone to in vivo mechanical loading [8–10]. With that understanding, we (WashU) [11,12] and others [2] have applied axial tibial compression to mice for 40–60 cycles/day, with 10 seconds of rest between each 0.5 s load-unload (2 Hz) ramp. This approach upregulates osteoblast/matrix genes in the tibial diaphysis and leads to increased cortical bone volume, predominately by periosteal expansion [12]. However, this same loading regime was reported to reduce trabecular bone volume in adult (3–7 months) mice [2,11,12]. In particular, we reported a 25% lower trabecular BV/TV in loaded limbs compared to contralateral controls after 2 weeks of loading in 7-months BALB/c mice [11]. By contrast, others, e.g., Cornell/HSS, have reported increased cortical and trabecular bone volume with another loading protocol using axial tibial compression in growing C57BL/6 [13,14]. Compared to the former loading protocol, the latter protocol applies 20-times more daily cycles (1200), uses a negligible rest insertion (0.1 s), and a faster loading rate (6.7 Hz or 0.15 s load-unload).
Although the different loading histories may have engendered the disparity in trabecular responses, several other factors may have also played a role. Bone adaptation to exogenous mechanical forces may differ with mouse strain. For instance, low-amplitude mechanical stimuli produce greater bone formation rates in C57BL/6 mice than BALB/c [15]. Similarly, C3H/He and C57BL/6 differ in their response to loading [16], but these differences appear to be age-dependent and largely explainable by differences in baseline bone formation between the two strains [17]. The importance of age is further highlighted by the finding that 4 weeks of aging (8 weeks to 12 weeks) in C57BL/6 mice completely alters the trabecular response to tibial loading from gain to loss [2]. Therefore, any comparisons between loading protocols will require age-matched animals. Lastly, differences in trabecular outcomes may relate to differences in animal handling. In particular, our practice is to transport animals to the lab for daily or alternate day loading, whereas others perform loading in their animal facility. Previous data indicated that 5 weeks of daily handling and sedation induced trabecular and cortical loss in the tibia of 6-month, male C57BL/6 mice [18].
Our goal in this study was to better understand the conditions needed to produce anabolic cortical and trabecular responses to axial tibial compression in mice. Our objectives were to: 1) determine the effects of animal transport and sedation on tibial morphology, and 2) compare the structure and strength of tibiae from two inbred mouse strains subjected to two different (and previously published) protocols over a course of 6 weeks. We hypothesized that bone accrual is influence more by loading history than by mouse strain or animal handling.
METHODS
Experimental Design
Four-month old female mice from two inbred strains, C57BL/6 and BALB/c (Charles River, Wilmington, MA), were used in the studies approved by the Animal Studies Committee of Washington University (Table 1). Right tibiae of mice were subjected to one of two non-invasive, axial compression loading protocols [3,11] using a materials testing machine (Instron ElectroPulse E1000, Norwood, MA, USA). While under anesthesia and for all loading groups, right tibiae (n = 8 for each mouse strain and loading group) were positioned vertically and subjected to 10 N (peak force) of tibial compression, 3 days/week for 6 weeks. The applied peak force was comparable to values used in recent studies by us and others [11–14]. The peak tibial force corresponds to a peak periosteal strain of −2800 µε in C57BL/6 (unpublished data) and −2350 µε in BALB/c [12]. (Note that these values represent the peak compressive values, which occur at the postero-lateral apex of the cross-section; tensile values reported by others [13,14] occur on the antero-medial surface and are less in magnitude for the same applied force.) The loading protocols were based on published reports from our group (WashU) and the Cornell/HSS group (Table 2). The left tibiae were not loaded and served as contralateral controls. Another set of C57BL/6 and BALB/c mice were not loaded and served as age-matched controls (AC: C57BL/6, n=8; BALB/c, n=9). Animals had access ad libitum to standard mouse chow and water.
Table 1.
Experimental design of animal handling and tibial compression over 6 weeks
| Mouse Strain | Group | Outcomes | |
|---|---|---|---|
| 0 & 3 week | 6 week | ||
| BALB/c | AC* | µCT | µCT/Mechanical Testing |
| Sham | µCT | µCT | |
| Sham+Trans | µCT | µCT | |
| C57BL/6 & BALB/c | AC | µCT | µCT/Mechanical Testing |
| WashU Loading [11,12] | µCT | µCT/Mechanical Testing | |
| Cornell/HSS Loading [3,13] | µCT | µCT/Mechanical Testing | |
Age-matched control animals (AC, n=8–9) were not handled, sedated or transported, except for µCT scanning. Sham-loaded animals (Sham, n=5) were sedated 3 days per week and right tibiae were placed in loading fixtures without load. Transport plus sham-loaded animals (Sham+Trans, n=5) received sham treatment and were transported (~150 m indoors) to the loading machine 3 days/week. The right tibia of WashU (n=8) and Cornell/HSS (n=8) animals were loaded 3 days/week. The left tibiae were not loaded and served as contralateral controls. All the animals were imaged by in vivo µCT; tibiae from loaded and AC animals were mechanically tested post mortem.
BALB/c age-matched control animals were also used as controls for loading.
Table 2.
Parameters for WashU and Cornell/HSS loading protocols.
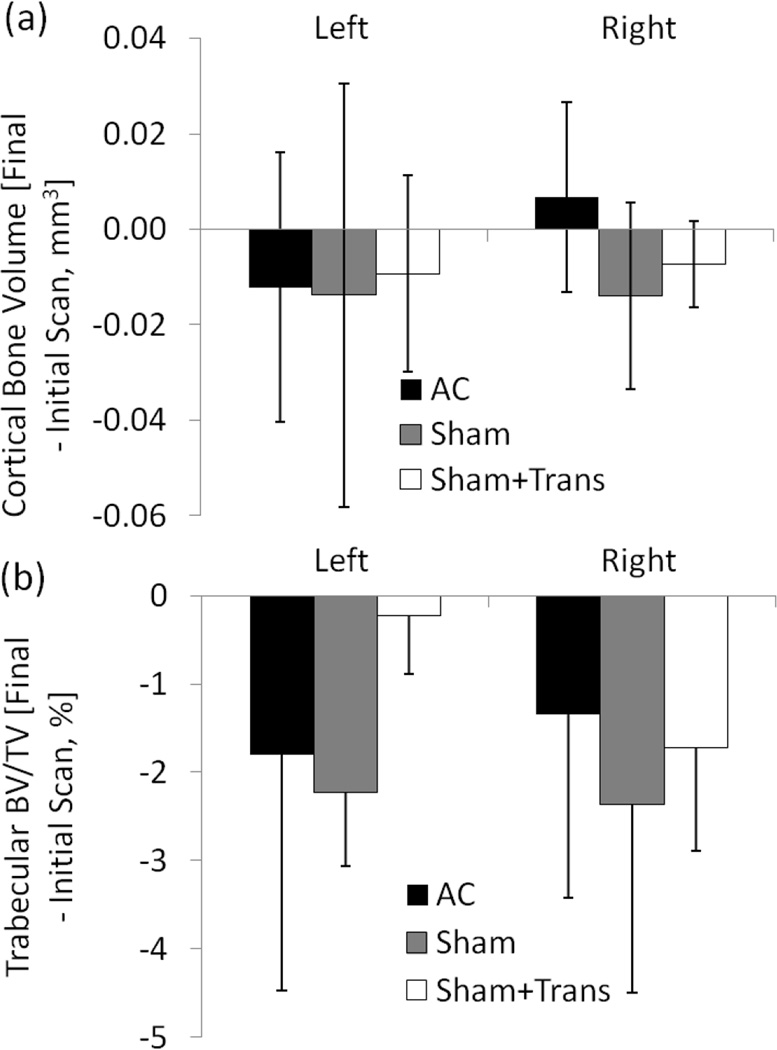
In order to assess the effects of animal handling, sedation and transport on tibial outcomes, a separate set of BALB/c mice was subjected to sham loading and sedation, with and without transport to the loading machine. Sham-loaded animals (Sham, n=5) were sedated 3 days per week and their right tibiae placed in loading fixtures without load for 10 minutes in our animal facility. Transport plus sham-loaded animals (Sham+Trans, n=5) received sham treatment and were transported (~150 m indoors) to the loading machine 3 days/week. A third set of BALB/c mice were not handled, sedated or moved (except for µCT scanning, described below) and served as age-matched controls (AC, n=9). Handling, sedation and transport did not affect cortical bone volume or trabecular bone volume fraction (Figure 1). Changes with time of cortical and trabecular bone of left and right tibiae in Sham and Sham+Trans animals were not different to age-matched animals (p>0.05). Some typical aging-related loss of trabecular bone was observed. For example, at 6 weeks, trabecular BV/TV tended to be slightly reduced in most groups, although the difference reached significance only in the right and left tibiae of Sham+Trans and Sham animals, respectively (Figure 1b). Importantly, the temporal change in BV/TV did not differ between right limbs from the AC, Sham and Sham+Trans groups. Therefore, handling, sedation and transport did not affect our outcomes of interest, and age-matched mice (AC) were judged to be suitable as controls for the subsequent loading experiment.
Figure 1.
In vivo µCT measured (a) cortical bone volume at the mid-diaphyseal tibia and (b) trabecular bone volume fraction at the proximal tibial metaphysis. Data represent the differences between the final (6 week) and initial (0 week) scan for right (“loaded”) and left (contralateral) tibia. Compared to age-matched controls (AC), neither sham loading nor sham loading with transport affected tibial morphology.
Micro Computed Tomography
Right and left tibiae were scanned in vivo at 0, 3 and 6 weeks by µCT (VivaCT 40, Scanco, Brüttisellen, Switzerland) to determine bone morphology and mineral density. Scan resolution was 21 µm (70 kV, 114 µA, 100 ms integration time). Cortical volume of interest was assessed at the mid-diaphysis, 5 mm proximal of the tibio-fibular junction, and spanned 34 slices (0.7 mm). Trabecular volume of interest was assessed at the proximal metaphysis, distal of the growth plate, and spanned 30 slices. Automatic contouring was used to analyze all slices with a lower/upper threshold of 260/1000 (340 mg HA/cm3) to segment bone from other tissue [19]. The outcomes for cortical bone included bone volume (BV), total area (Tt.Ar), medullary area (M.Ar), cortical thickness (Ct.Th) and tissue mineral density (TMD). For trabecular bone, outcomes included bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and volumetric bone mineral density (vBMD), consistent with reported guidelines [20].
Whole-Bone Mechanical Testing
Post mortem, left and right hindlimbs were excised en bloc and stored (−20°C) until mechanical testing, which was used to assess the functional consequence of putative changes in cortical bone structure. Immediately before testing, tibiae were excised and cleaned of soft tissue. Tibiae were placed axially in custom loading fixtures at room temperature, preloaded to a compression of 0.1 N and compressed at a rate of 0.03 mm/s until failure using a materials testing machine (Instron ElectroPulse E1000, Norwood, MA, USA). Axial compression tests the whole bone and replicates in vivo loading; failure occurred at the mid-diaphysis, which is entirely cortical bone. A custom LabVIEW (National Instruments, Austin, TX) program was used to determine ultimate force, ultimate displacement, stiffness and energy-to-fracture from force-displacement curves.
Statistics
Paired t-tests compared right (“loaded”) and time referent left (contralateral) tibiae. For all outcomes, the relative change due to loading was calculated as the percent difference between loaded and contralateral tibia [(Loaded-Contralateral)/Contralateral, %]. This new variable was used for further analysis because C57BL/6 and BALB/c mice differ in baseline morphology [21]. For morphological outcomes, three-way ANOVAs using loading group (AC vs. WashU vs. Cornell/HSS), duration of loading (0 vs. 3 vs. 6 wk) and mouse strain (C57BL/6 vs. BALB/c) as factors were used to determine main effects. One of three methods of post hoc testing was used for each significant main effect. For significant loading group effects, one-way ANOVAs with post-hoc Tukey tests compared groups at each time point. For significant time (duration of loading) effects, repeated measures with post-hoc Tukey tests compared timepoints within each group. For significant mouse strain effects, unpaired t-tests compared strains at each respective group/time point. For mechanical property outcomes, two-way ANOVAs using loading group and mouse strain as factors were used to determine main effects. Since previous data showed continual loss of trabecular BV/TV from contralateral tibiae over 6 weeks of loading [12], we also determined loading group effects on bone morphology and mechanical outcomes of contralateral tibiae using separate three-way and two-way ANOVAs and post-hoc Tukey tests. Statistical significance was considered at p < 0.05 (StatView 5.0, SAS Institute, Cary, NC, USA).
RESULTS
Tibial Loading Increased Cortical Bone Volume; Accrual was Faster with Cornell/HSS Loading
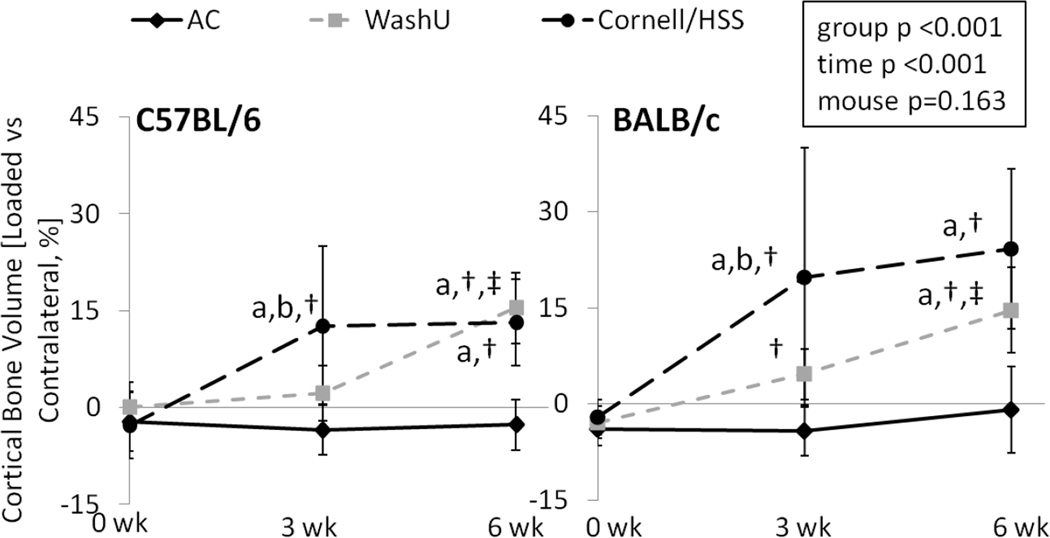
Independent of loading group or mouse strain, tibial loading increased cortical bone volume of the loaded side compared to the contralateral side, except after 3 weeks of WashU loading in C57BL/6 (Table 3). In C57BL/6, a difference in relative change of cortical bone volume between WashU loading and AC animals was not reached until 6 weeks of loading. By contrast, at 3 weeks, the relative change of cortical bone volume in C57BL/6 with Cornell/HSS loading was 20% greater versus AC and 13% greater versus WashU loading (Table 3, Figure 2). At 6 weeks, the relative change of cortical bone volume in C57BL/6 of both loading groups was 16% greater than of AC, with no difference between loading groups. BALB/c mice responded similarly, except that there was a significant increase in cortical volume of WashU loaded tibiae at 3 weeks.
Table 3.
Cortical bone morphology of left (contralateral) and right (“loaded”) tibia from C57BL/6 and BALB/c mice over 6 weeks
| Parameter | Strain | Time [week] |
AC | WashU | Cornell/HSS | |||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |||
| Cortical Bone Volume [mm3]G, T | C57BL/6 | 0 | 0.60±0.05 | 0.58±0.06 | 0.60±0.03 | 0.60±0.03 | 0.61±0.05 | 0.59±0.03 |
| 3 | 0.60±0.05 | 0.58±0.03 | 0.61±0.03 | 0.62±0.02 | 0.59±0.04 | 0.67±0.04*,a,b,† | ||
| 6 | 0.62±0.05 | 0.60±0.05 | 0.62±0.02 | 0.71±0.02*,a,†,‡ | 0.62±0.03 | 0.70±0.04*,a,† | ||
| BALB/c | 0 | 0.64±0.04 | 0.62±0.04* | 0.64±0.04 | 0.62±0.03* | 0.65±0.03 | 0.64±0.03 | |
| 3 | 0.62±0.05 | 0.60±0.06* | 0.64±0.03 | 0.67±0.03*,† | 0.62±0.04 | 0.74±0.10*,a,b,† | ||
| 6 | 0.63±0.06 | 0.62±0.06 | 0.62±0.04 | 0.71±0.03*,a,†,‡ | 0.62±0.04 | 0.77±0.07*,a,† | ||
| Tt.Ar [mm2]G, T | C57BL/6 | 0 | 1.42±0.15 | 1.40±0.19 | 1.43±0.15 | 1.47±0.14 | 1.50±0.21 | 1.45±0.16 |
| 3 | 1.31±0.10 | 1.30±0.09 | 1.39±0.08 | 1.52±0.06*,a,† | 1.37±0.12 | 1.58±0.09*,a,† | ||
| 6 | 1.32±0.11 | 1.29±0.12 | 1.36±0.07 | 1.43±0.09*,a | 1.34±0.11 | 1.52±0.09*,a,b,† | ||
| BALB/c | 0 | 1.40±0.11 | 1.36±0.12 | 1.40±0.14 | 1.37±0.11 | 1.43±0.08 | 1.43±0.06a | |
| 3 | 1.35±0.08 | 1.33±0.11 | 1.39±0.12 | 1.51±0.14*,a,† | 1.37±0.17 | 1.58±0.12*,a,b,† | ||
| 6 | 1.29±0.12 | 1.34±0.18 | 1.28±0.07 | 1.46±0.07*,†,‡ | 1.33±0.11 | 1.58±0.11*,a,† | ||
| M.Ar [mm2]G, T | C57BL/6 | 0 | 0.70±0.10 | 0.70±0.15 | 0.72±0.13 | 0.76±0.12 | 0.78±0.18 | 0.75±0.15 |
| 3 | 0.61±0.04 | 0.62±0.10 | 0.65±0.09 | 0.76±0.14*,a | 0.65±0.13 | 0.77±0.08*,a,† | ||
| 6 | 0.55±0.04 | 0.55±0.05 | 0.59±0.09 | 0.63±0.06*,a | 0.61±0.09 | 0.71±0.08*,a,b,† | ||
| BALB/c | 0 | 0.61±0.08 | 0.61±0.08 | 0.60±0.09 | 0.59±0.09 | 0.62±0.05 | 0.64±0.05 | |
| 3 | 0.55±0.06 | 0.55±0.05 | 0.57±0.05 | 0.60±0.10 | 0.57±0.06 | 0.59±0.17 | ||
| 6 | 0.51±0.06 | 0.56±0.11 | 0.51±0.05 | 0.56±0.08 | 0.56±0.07 | 0.60±0.06 | ||
| Ct.Th [mm]G, T, M | C57BL/6 | 0 | 0.16±0.01 | 0.16±0.01 | 0.16±0.01 | 0.15±0.01* | 0.16±0.01 | 0.15±0.01 |
| 3 | 0.17±0.01 | 0.17±0.01 | 0.17±0.01 | 0.16±0.01 | 0.17±0.01 | 0.17±0.02† | ||
| 6 | 0.18±0.01 | 0.18±0.01 | 0.17±0.01 | 0.20±0.01*,a,†,‡ | 0.18±0.01 | 0.19±0.01*,a,b,† | ||
| BALB/c | 0 | 0.20±0.01 | 0.19±0.01* | 0.20±0.01 | 0.19±0.01 | 0.20±0.01 | 0.19±0.01* | |
| 3 | 0.20±0.01 | 0.19±0.01* | 0.19±0.01 | 0.20±0.02 | 0.19±0.01 | 0.21±0.02a | ||
| 6 | 0.20±0.01 | 0.19±0.01* | 0.20±0.01 | 0.22±0.02*,a,†,‡ | 0.19±0.01 | 0.23±0.02*,a,†,# |
Data are presented as mean±SD.
: Difference between left (contralateral) and right (“loaded”) tibia;
: main effect of group,
: relative change (Loaded vs Contralateral, %) different from AC of same mouse strain and time,
: relative change different from WashU of same mouse strain and time;
: main effect of time,
: relative change different from 0 week of same group and mouse,
: relative change of 6 week different from 3 week of same group and mouse,
: main effect of mouse strain,
: relative change different from C57BL/6 of same group and time; p<0.05.
Figure 2.
In vivo µCT measured cortical bone volume at the mid-diaphyseal tibia of age-matched (AC), WashU loaded and Cornell/HSS loaded mice over 6 weeks. Independent of mouse strain, 6 weeks of WashU and Cornell/HSS loading increased cortical bone volume. Data represent differences [%] between right (loaded) and left (contralateral) tibia. Unlike WashU loading, Cornell/HSS loading increased bone volume at 3 weeks and produced greater changes in BALB/c mice than in C57BL/6. a: significant difference vs. AC, b: significant difference vs. WashU; †: significant difference vs 0 wk, ‡: significant difference between 6 and 3 wk; p<0.05.
While the net increase in cortical bone volume was not significantly different between mouse strains, the manner of cortical bone apposition was dependent on both mouse strain and loading history (Table 3). In C57BL/6 mice and for both loading protocols, greater cortical bone volume in loaded tibiae versus contralateral was due to greater relative total area (10%) despite greater relative medullary area (13%). On the other hand, loading of BALB/c tibiae increased total area by 17% and did not significantly change medullary area. Unlike the time-dependent differences in changes in cortical bone volume between loading groups, both loading protocols led to greater total area at 3 weeks as well as 6 weeks. Increases in cortical thickness between loaded and contralateral tibiae emerged with time and resembled changes to bone volume at 6 weeks; except for Cornell/HSS loading of C57BL/6 (Table 3, Figure 3a). The relative change of cortical thickening was less in C57BL/6 tibiae subjected to Cornell/HSS loading than WashU loading because the Cornell/HSS protocol prevented contraction of medullary area. By contrast, increases in cortical thickness in BALB/c tibias did not differ between loading protocols. There were no significant differences in cortical tissue mineral density between the loaded tibia and contralateral control of any group (data not shown). There was no effect of loading on cortical bone morphology of contralateral tibiae; contralateral tibiae maintained cortical bone volume over the 6-week study duration.
Figure 3.
At 6 weeks, in vivo µCT measured (a) cortical thickness at the tibial mid-diaphysis of age-matched control (AC), WashU loaded and Cornell/HSS loaded mice. From the same tibiae, (b) energy-to-fracture was determined by load-to-failure tests. Data represent differences [%] between right (“loaded”) and left (contralateral) tibia. Independent of mouse strain and waveform, 6 weeks of tibial loading produced greater cortical thickness. Cornell/HSS loading produced less relative cortical thickness than WashU loading in C57BL/6 but was not different than WashU loading in BALB/c. Energy-to-fracture was greater in tibiae of both mouse strains subjected to Cornell/HSS loading and BALB/c mice subjected to WashU loading. a: significant difference vs. AC, b: significant difference vs. WashU, # significant difference between C57BL/6 and BALB/c; p<0.05.
Tibial Loading Enhanced Load-to-Failure Mechanical Properties
Loading led to improvements in some mechanical properties of the loaded tibia compared to the contralateral side, with similar relative changes for both loading groups compared to AC (Table 4). Overall, compared to the contralateral tibia, ultimate displacement and energy-to-fracture were greater in tibiae loaded by both waveforms and in both mouse strains. Similarly, the relative changes of ultimate displacement and energy-to-fracture of loaded tibiae were greater than those of AC (Table 4, Figure 3b). Cornell/HSS loading increased the ultimate force of the loaded tibia by 18% in both mouse strains, whereas WashU loading did not significantly change ultimate force (8%, p≥0.10). On the other hand, in C57BL/6 mice, 6 weeks of Cornell/HSS loading resulted in lower stiffness of loaded tibiae versus contralateral controls, while stiffness did not change significantly with WashU loading (p≥0.19). In BALB/c mice, stiffness was not affected by loading. Post-yield displacement did not change with loading in any group or mouse strain (data not shown).
Table 4.
Mechanical properties of left (contralateral) and right (“loaded”) tibia from C57BL/6 and BALB/c mice at 6 weeks
| Parameter | Strain | AC | WashU | Cornell/HSS | |||
|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | ||
| Ultimate Force [N]G | C57BL/6 | 24.5±2.8 | 26.6±3.2 | 26.5±3.6 | 28.3±2.4 | 25.4±1.7 | 29.1±2.8* |
| BALB/c | 21.1±2.7 | 22.1±2.7 | 23.6±3.4 | 25.9±2.7 | 23.8±1.8 | 28.4±2.5*,a | |
| Ultimate Displacement [mm]G | C57BL/6 | 0.7±0.3 | 0.7±0.1 | 0.7±0.2 | 1.0±0.3*,a | 0.6±0.1 | 0.9±0.1*,a |
| BALB/c | 0.6±0.1 | 0.6±0.1 | 0.7±0.1 | 1.0±0.2*,a | 0.7±0.1 | 1.1±0.2*,a | |
| Stiffness [N·mm−1]G | C57BL/6 | 62±18 | 68±7 | 69±20 | 53±10 | 67±7 | 55±8* |
| BALB/c | 49±8 | 56±13 | 54±9 | 49±10 | 52±6 | 53±8 | |
| Energy-to-Fracture [N·mm]G | C57BL/6 | 7.5±0.6 | 8.2±2.3 | 8.8±1.7 | 10.7±2.2 | 7.3±1.2 | 12.1±2.7*,a |
| BALB/c | 6.3±0.8 | 6.8±1.4 | 7.4±1.0 | 10.0±1.3*,a | 7.9±1.2c | 10.9±1.7*,a |
Data are presented as mean±SD.
: Difference between left (contralateral) and right (“loaded”) tibia;
: main effect of group,
: relative change (Loaded vs Contralateral, %) different from AC of same mouse strain and time,
: absolute value of contralateral outcome different from contralateral of AC from same mouse; p<0.05.
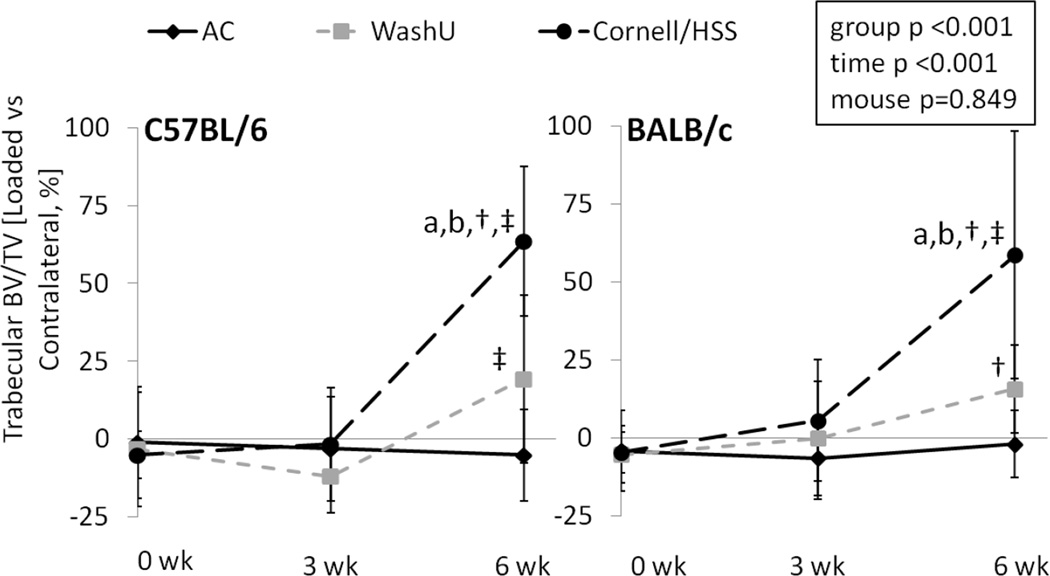
Cornell/HSS Loading was Strongly Anabolic to Trabecular Bone
After 6 weeks, tibiae from both mouse strains subjected to WashU loading displayed mildly greater trabecular BV/TV than the contralateral tibiae, but the relative change of trabecular BV/TV was not significantly different from AC animals (Table 5, Figure 4). By contrast, trabecular BV/TV of Cornell/HSS loaded tibiae from both mouse strains was greater than of contralateral tibiae. Moreover, the relative change of trabecular BV/TV of Cornell/HSS loaded tibiae was greater than of AC tibiae by 65% and of WashU tibiae by 44%. There were minimal differences in trabecular BV/TV between loaded and contralateral tibiae at 3 weeks, except trabecular BV/TV of WashU loaded tibiae was less than of contralateral tibiae of C57BL/6.
Table 5.
Trabecular bone morphology of left (contralateral) and right (“loaded”) tibia from C57BL/6 and BALB/c mice over 6 weeks
| Parameter | Strain | Time [wk] |
AC | WashU | Cornell/HSS | |||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |||
| Trabecular BV/TV [%]G, T | C57BL/6 | 0 | 12.1±0.9 | 11.8±1.5 | 11.5±1.3 | 11.1±2.5 | 11.2±1.6 | 10.6±1.3 |
| 3 | 11.2±1.1 | 10.8±1.3 | 11.4±1.5 | 10.1±2.3* | 11.8±1.2 | 11.5±1.6 | ||
| 6 | 10.1±0.9 | 9.5±1.3 | 9.8±1.1 | 11.6±2.0‡ | 10.3±1.1 | 16.8±2.1*,a,b,†,‡ | ||
| BALB/c | 0 | 19.4±3.7 | 18.6±4.3 | 21.1±2.0 | 20.0±2.6 | 19.8±2.4 | 18.9±2.7 | |
| 3 | 18.6±2.9 | 17.2±3.7 | 18.6±3.1 | 18.4±3.9 | 17.8±4.5 | 18.2±3.1 | ||
| 6 | 17.6±3.1 | 17.3±3.3 | 18.1±2.7 | 20.8±3.0*,† | 15.2±4.4 | 22.9±3.4*,a,b,†,‡ | ||
| Tb.Th [µm]G, T, M | C57BL/6 | 0 | 50±2 | 48±3 | 49±2 | 50±5 | 50±2 | 48±2* |
| 3 | 53±2 | 52±3 | 51±3 | 57±1*,a | 52±3 | 65±3*,a,b,† | ||
| 6 | 57±4 | 56±4 | 52±3 | 72±6*, a,†,‡ | 55±4 | 89±9*,a,b,†,‡ | ||
| BALB/c | 0 | 58±5 | 56±5 | 60±1 | 59±3 | 58±3 | 57±3* | |
| 3 | 59±4 | 56±5* | 58±2 | 57±5# | 56±3 | 61±4*,a,b,# | ||
| 6 | 62±3 | 58±4* | 59±3 | 69±6*,a,†,‡,# | 57±4c | 80±7*,a,b,†,‡,# | ||
| Tb.N [mm−1]T | C57BL/6 | 0 | 4.4±0.3 | 4.3±0.3 | 4.3±0.3 | 4.3±0.4 | 4.3±0.3 | 4.3±0.3 |
| 3 | 4.0±0.2 | 4.0±0.3 | 4.1±0.4 | 4.1±0.3 | 4.1±0.2 | 4.2±0.5 | ||
| 6 | 3.7±0.3 | 3.7±0.3 | 4.0±0.3 | 3.9±0.2 | 4.1±0.3 | 4.0±0.2 | ||
| BALB/c | 0 | 5.1±0.4 | 4.9±0.4 | 5.3±0.4 | 5.1±0.2 | 5.3±0.4 | 5.0±0.4 | |
| 3 | 4.7±0.4 | 4.6±0.3 | 4.8±0.4 | 4.9±0.4 | 5.1±0.8 | 4.9±0.5 | ||
| 6 | 4.3±0.4 | 4.3±0.4 | 4.6±0.5 | 4.7±0.4 | 4.3±0.8 | 4.7±0.6*,†,‡ | ||
| vBMD [mg HA·cm−3]G, T, M | C57BL/6 | 0 | 141±10 | 136±12 | 140±9 | 130±20 | 139±10 | 125±12* |
| 3 | 133±10 | 123±14 | 138±10 | 117±23* | 145±18 | 128±14 | ||
| 6 | 125±13 | 119±12 | 126±12 | 129±11 | 133±11 | 163±18*,a,b,†,‡ | ||
| BALB/c | 0 | 202±26 | 191±30 | 214±17 | 201±17 | 204±19 | 193±14* | |
| 3 | 195±20 | 178±30 | 195±21 | 189±28 | 190±36 | 192±18 | ||
| 6 | 187±21 | 180±23 | 190±19 | 208±23 | 168±35 | 224±22*,a,b,†,‡ |
Data are presented as mean±SD.
: Difference between left (contralateral) and right (“loaded”) tibia;
: main effect of group,
: relative change (Loaded vs Contralateral, %) different from AC of same mouse strain and time,
: relative change different from WashU of same mouse strain and time,
: absolute value of contralateral outcome different from contralateral of AC from same mouse;
: main effect of time,
: relative change different from 0 week of same group and mouse,
: relative change of 6wk different from 3 week of same group and mouse,
: main effect of mouse strain,
: relative change different from C57BL/6 of same group and time; p<0.05.
Figure 4.
In vivo µCT measured trabecular bone volume fraction at the proximal tibial metaphysis of age-matched (AC), WashU loaded and Cornell/HSS loaded mice over 6 weeks. Data represent differences [%] between right (“loaded”) and left (contralateral) tibia. Independent of mouse strain, 6 weeks of Cornell/HSS loading produced greater trabecular bone volume fraction than WashU loading or AC. These differences were evident at 6 weeks but not 3 weeks. a: significant difference vs. AC, b: significant difference vs. WashU, †: significant difference vs 0 week, ‡: significant difference between 6 and 3 weeks; p<0.05.
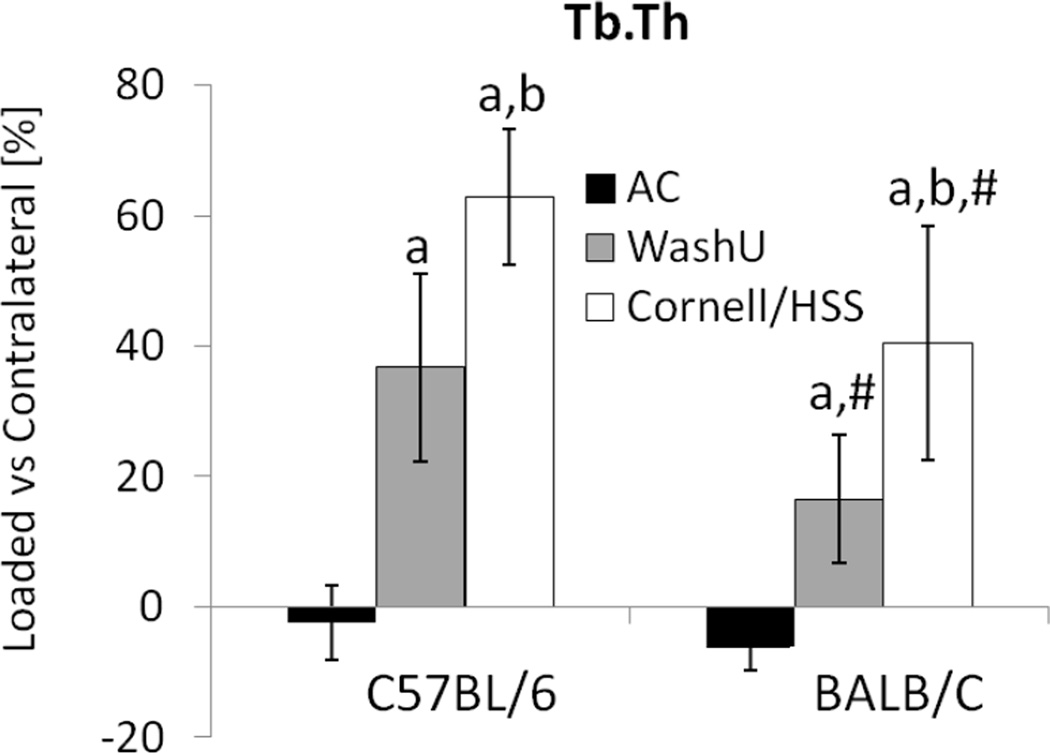
The increases in trabecular BV/TV with loading were largely due to changes in trabecular thickness, which demonstrated loading group-, time- and mouse strain-dependency. Three weeks of WashU loading increased trabecular thickness in C57BL/6, but BALB/c required 6 weeks to thicken trabeculae (Table 5, Figure 5). By contrast, Cornell/HSS loaded tibia from both mouse strains had greater trabecular thickness at 3 and 6 weeks. Comparing mouse strains, relative change of trabecular thickness at 3 and 6 weeks was greater in C57BL/6 than BALB/c mice. Unlike the potent loading effect on trabecular thickness, there were few changes in trabecular number. Cornell/HSS loading of BALB/c mice at 6 weeks was the only comparison between loaded and contralateral tibiae that showed a difference (increase) in trabecular number. Lastly, the relative changes in vBMD with loading generally mirrored the changes in BV/TV.
Figure 5.
At 6 weeks, in vivo µCT measured trabecular thickness at the proximal tibial metaphysis of age-matched (AC), WashU loaded and Cornell/HSS loaded mice. Data represent differences [%] between right (“loaded”) and left (contralateral) tibia. Independent of mouse strain and waveform, 6 weeks of tibial loading produced greater trabecular thickness. Cornell/HSS loading produced greater trabecular thickness than WashU loading in both mouse strains. a: significant difference vs. AC, b: significant difference vs. WashU, # significant difference between C57BL/6 and BALB/c; p<0.05.
DISCUSSION
We subjected tibiae of C57BL/6 and BALB/c mice to two unique loading protocols (WashU and Cornell/HSS) for 6 weeks. Of the two protocols, the Cornell/HSS protocol applied more daily cycles (1200 vs. 60), at a faster rate (0.15 s vs. 0.5 s load-unload), with a smaller rest insertion (0.1 s vs. 10 s) and over a shorter bout time (300 s vs. 630 s). We hypothesized that the loading protocol would influence tibial bone accrual more than mouse strain or animal handling. Strikingly, the Cornell/HSS waveform was more anabolic to trabecular bone than the WashU waveform at 6 weeks. The cortical bone response between the two waveforms was not significantly different after 6 weeks of loading, but the Cornell/HSS protocol induced cortical bone accrual faster than the WashU protocol. Accordingly, changes in most mechanical properties did not differ between protocols, although tibiae loaded by the Cornell/HSS waveform were stronger than the control while WashU loaded tibiae did not significantly change strength. Consistent with our hypothesis, mouse strain did not impact the overall tibial response to axial compression in terms of trabecular bone volume fraction and cortical bone volume, but influenced the manner of relative changes in trabecular thickness and cortical accrual, which were greater in C57BL/6 than BALB/c. Lastly, handling, sedation and transport did not affect mid-diaphyseal cortical bone volume or metaphyseal trabecular bone volume fraction. Together, these data demonstrate that the disparity in bone response to tibial compression between WashU and Cornell/HSS loading was elicited by loading history and not mouse strain or animal handling, and that the Cornell/HSS waveform was a more anabolic stimulus, especially with regard to trabecular bone.
In clear contrast to the minimal effect of WashU loading on trabecular bone, 6 weeks of alternate-day tibial compression using the Cornell/HSS protocol robustly increased trabecular BV/TV in both mouse strains by trabecular thickening as early as 3 weeks. Cornell/HSS loading increased trabecular bone outcomes similar to 4 weeks of climbing in mice [22] and 3 weeks of running in rats [23]. In other studies using physiologic, non-invasive tibial compression, lower magnitude compression (−3 N) applied 5 days/week from the Cornell/HSS laboratory also leads to a time-dependent increase in metaphyseal volume but relatively mild changes in trabecular BV/TV [3]. Increasing the tibial compression magnitude (−11.5 N) applied in growing mice stimulates a more robust osteogenic response of trabecular bone that is detectable at 2 weeks [13]. The earlier appearance of change in trabecular BV/TV in their 2-week study compared to our 3-week time point may have been due to the greater number of loading bouts per week (5 vs 3 days/week), triggering greater osteogenesis [24]. Similarly, Cornell/HSS loading engendered greater and earlier trabecular thickening than WashU loading, resulting in greater trabecular BV/TV in both mouse strains at 6 weeks. Between mouse strains, trabecular thickening was greater in C57BL/6 than BALB/c, perhaps due to fewer initial trabeculae in C57BL/6.
Unlike Cornell/HSS loading, tibial compression using the WashU protocol led to minor increases in trabecular bone and its impact on trabecular bone has been inconsistent. In the current study, 4-month old mice loaded to −10 N with the WashU protocol lost trabecular BV/TV at 3 weeks but had mildly increased trabecular BV/TV and significant trabecular thickening by 6 weeks. Previous studies from our group [11,12] and others [2] conflict with these results, where WashU-like tibial compression exacerbated age-related loss of tibial trabecular bone in young-adult mice (3–7 months old), regardless of mouse strain (C57BL/6 or BALB/c) or duration of loading (2–6 weeks). In the same studies [2,11,12], WashU-like loading of tibiae of growing and old mice (2-months and 22-months old) displayed mild osteogenic trabecular responses. The reasons for the inconsistencies are unclear, but knee trauma may influence the effect of loading on metaphyseal trabecular bone. Ko et al. [25] show that 6-months old C57BL/6 mice subjected to daily Cornell/HSS tibial compression for 6 weeks at a compression of −9 N leads to damage of the articular cartilage, acute epiphyseal trabecular loss, and no change in metaphyseal trabecular bone. Whereas, growing mice (10-weeks old) can increase metaphyseal trabecular bone after 1 week of tibial compression and, by 6 weeks, incur less articular damage and similar epiphyseal trabecular bone loss than 6-months-old mice [25]. It is possible that the anabolism of WashU loading for trabecular bone was confounded by differences in the degree of knee trauma between experiments, contributing to different trabecular outcomes between studies. Similarly, the Cornell/HSS protocol here was a potent anabolic stimulus for trabecular bone of young-adult mice (4-months old) at 6 weeks, but peri-articular damage may still have affected the anabolic potential of the Cornell/HSS protocol. Overall, reduced loading levels that would minimize knee injuries but still stimulate bone formation should be investigated.
In support of previous studies [12,14], axial tibial compression using both protocols increased cortical bone volume in both mouse strains by periosteal expansion despite a lack of endocortical accrual, normal with aging. However, Cornell/HSS loaded tibiae had a greater change of bone volume at the middle time point (3 weeks) than WashU loaded tibiae, but the disparity in bone volume accrual between protocols was normalized by 6 weeks. Clearly, tibial compression is anabolic to cortical bone [2,3,11,12], yet mechanical properties of ‘adapted’ tibiae have not been previously reported. Overall, in the current study, both protocols increased the ultimate displacement and energy-to-fracture of tibiae loaded for 6 weeks. Despite accrual of cortical bone, WashU loading did not significantly increase tibial strength as would be expected [26], although non-significant trends were noted. Our study may have been underpowered to detect these modest changes (mean 8% for ultimate force of WashU groups). By contrast and similar to jumping exercise [27,28], Cornell/HSS loading led to significantly greater tibial strength (mean 17%) in both mouse strains. On the other hand, both loading protocols led to unexpected declines in stiffness, which reached significance for the Cornell/HSS loading in C57BL/6. The basis for the decrease in stiffness with loading is unclear, especially given the increase in cortical bone volume and strength, but the decrease in stiffness suggests that the loading protocol may have damaged the pre-existing bone. Further investigation is needed to evaluate the quality of the preexisting and new bone.
Our study had limitations. One limitation was that we did not include additional control groups that could discern the effects of each loading parameter. Nonetheless, we hypothesize that the two-fold higher mechanical strain rate/frequency played a key role in the greater anabolic response to the Cornell/HSS protocol. In previous work [5], doubling the strain rate from 72- to 144- N/s of rat tibiae under four-point bending, while maintaining the same frequency, increases the relative bone formation rate in the endocortical surface over three-fold and tripling the strain rate increases it five-fold. Similarly in mice, doubling the frequency of ulnar loading from 5- to 10-Hz increases the relative bone formation rate in the periosteal surface by 70% [6]. The difference in cycle number between protocols was less likely to be important, as the osteogenic response to high-magnitude strain (comparable to the current study) is reported to saturate above 36 cycles/day [7]. The greater rest-insertion between load cycles for the WashU protocol should have been to its advantage [4,8], but it is possible that any relative benefits were offset by the lower strain rate and fewer cycles. We note that the “rules” for how loading parameters influence bone formation are based on experiments focused on cortical bone, and may be different for trabecular bone. Indeed, the greatest difference between protocols was in trabecular bone, while differences in cortical bone were modest or transient. Additional work is needed to determine if loading parameters have differential effects on trabecular and cortical bone. A second limitation of our study is that the estimated peak (mechanical) strains differed by ~20% between C57BL/6 and BALB/c mice (−2800 vs. −2350 µε; both loaded at −10 N). Our main objective was to compare the two loading protocols but this difference makes it difficult to make decisive conclusions about the relative differences between mouse strains. With only a few exceptions, the relative loading effects were similar in the two mouse strains.
In conclusion, we sought to compare the structural and mechanical adaptation of tibiae in two common inbred mouse strains to two unique loading regimes over 6 weeks. Both loading protocols induced greater cortical bone, but the Cornell/HSS protocol induced peak cortical bone volume earlier and a greater accrual of trabecular bone compared to the WashU protocol. The minimal difference in relative bone accrual and strengthening between C57BL/6 and BALB/c mice suggests that tibial loading targeted similar osteogenic mechanisms innate to both mouse strains. Further, the lack of impact of animal handling, sedation and transport on tibial morphology indicates that any bone changes were imparted by localized loading. Ultimately, while the mechanism(s) underlying the osteogenesis observed here requires further study, the anabolic response from Cornell/HSS loading indicated that it is well suited for studies seeking a robust structural response in cortical and trabecular bone.
Acknowledgements
This work was supported by National Institute of Health (R01 AR047867, P30 AR057235)
M Silva has consultant/advisory role to and has received funding from Merck that is unrelated to this manuscript.
Footnotes
All other authors have stated that they have no conflict of interest.
REFERENCES
- 1.Robling AG, Burr DB, Turner CH. Skeletal loading in animals. J Musculoskelet Neuronal Interact. 2001;1:249–262. [PubMed] [Google Scholar]
- 2.De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37:810–818. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36:1030–1038. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 5.Turner CH, Owan I, Takano Y. Mechanotransduction in bone: role of strain rate. Am J Physiol. 1995;269:E438–E442. doi: 10.1152/ajpendo.1995.269.3.E438. [DOI] [PubMed] [Google Scholar]
- 6.Warden SJ, Turner CH. Mechanotransduction in the cortical bone is most efficient at loading frequencies of 5–10 Hz. Bone. 2004;34:261–270. doi: 10.1016/j.bone.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66:397–402. [PubMed] [Google Scholar]
- 8.Srinivasan S, Ausk BJ, Poliachik SL, Warner SE, Richardson TS, Gross TS. Rest-inserted loading rapidly amplifies the response of bone to small increases in strain and load cycles. J Appl Physiol. 2007;102:1945–1952. doi: 10.1152/japplphysiol.00507.2006. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res. 2002;17:1613–1620. doi: 10.1359/jbmr.2002.17.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaMothe JM, Zernicke RF. Rest insertion combined with high-frequency loading enhances osteogenesis. J Appl Physiol. 2004;96:1788–1793. doi: 10.1152/japplphysiol.01145.2003. [DOI] [PubMed] [Google Scholar]
- 11.Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res. 2010;25:2006–2015. doi: 10.1002/jbmr.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva MJ, Brodt MD, Lynch MA, Stephens AL, Wood DJ, Civitelli R. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS One. 2012;7:e34980. doi: 10.1371/journal.pone.0034980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch ME, Main RP, Xu Q, Walsh DJ, Schaffler MB, Wright TM, et al. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J Appl Physiol. 2010;109:685–691. doi: 10.1152/japplphysiol.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch ME, Main RP, Xu Q, Schmicker TL, Schaffler MB, Wright TM, et al. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone. 2011;49:439–446. doi: 10.1016/j.bone.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J. 2002;16:1280–1282. doi: 10.1096/fj.01-0913fje. [DOI] [PubMed] [Google Scholar]
- 16.Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR. Bone response to in vivo mechanical loading in two breeds of mice. Calcif Tissue Int. 1998;63:442–449. doi: 10.1007/s002239900554. [DOI] [PubMed] [Google Scholar]
- 17.Poliachik SL, Threet D, Srinivasan S, Gross TS. 32 wk old C3H/HeJ mice actively respond to mechanical loading. Bone. 2008;42:653–659. doi: 10.1016/j.bone.2007.12.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christiansen BA, Kotiya AA, Silva MJ. Constrained tibial vibration does not produce an anabolic bone response in adult mice. Bone. 2009;45:750–759. doi: 10.1016/j.bone.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41:505–515. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 21.Buie HR, Moore CP, Boyd SK. Postpubertal architectural developmental patterns differ between the L3 vertebra and proximal tibia in three inbred strains of mice. J Bone Miner Res. 2008;23:2048–2059. doi: 10.1359/jbmr.080808. [DOI] [PubMed] [Google Scholar]
- 22.Mori T, Okimoto N, Sakai A, Okazaki Y, Nakura N, Notomi T, et al. Climbing exercise increases bone mass and trabecular bone turnover through transient regulation of marrow osteogenic and osteoclastogenic potentials in mice. J Bone Miner Res. 2003;18:2002–2009. doi: 10.1359/jbmr.2003.18.11.2002. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto J, Yeh JK, Aloia JF. Differential effect of treadmill exercise on three cancellous bone sites in the young growing rat. Bone. 1999;24:163–169. doi: 10.1016/s8756-3282(98)00189-6. [DOI] [PubMed] [Google Scholar]
- 24.Robling AG, Burr DB, Turner CH. Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading. J Bone Miner Res. 2000;15:1596–1602. doi: 10.1359/jbmr.2000.15.8.1596. [DOI] [PubMed] [Google Scholar]
- 25.Ko FC, Dragomir C, Plumb DA, Goldring SR, Wright TM, Goldring MB, et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 2013 doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stadelmann VA, Bonnet N, Pioletti DP. Combined effects of zoledronate and mechanical stimulation on bone adaptation in an axially loaded mouse tibia. Clin Biomech (Bristol, Avon) 2011;26:101–105. doi: 10.1016/j.clinbiomech.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Kodama Y, Umemura Y, Nagasawa S, Beamer WG, Donahue LR, Rosen CR, et al. Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6J mice but not in C3H/Hej mice. Calcif Tissue Int. 2000;66:298–306. doi: 10.1007/s002230010060. [DOI] [PubMed] [Google Scholar]
- 28.Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S. Five jumps per day increase bone mass and breaking force in rats. J Bone Miner Res. 1997;12:1480–1485. doi: 10.1359/jbmr.1997.12.9.1480. [DOI] [PubMed] [Google Scholar]