Abstract
Resistance to infection is the ability of the host to evoke a strong immune response sufficient to eliminate the infectious agent. In contrast, maternal tolerance to the fetus necessitates careful regulation of immune responses. Successful pregnancy requires the maternal host to effectively balance the opposing processes of maternal immune reactivity and tolerance to the fetus. However, this balance can be perturbed by infections which are recognized as the major cause of adverse pregnancy outcome including pre-term labor. Select pathogens also pose a serious threat of severe maternal illness. These include intracellular and chronic pathogens that have evolved immune evasive strategies. Murine models of intracellular bacteria and parasites that mimic pathogenesis of infection in humans have been developed. While human epidemiological studies provide insight into maternal immunity to infection, experimental infection in pregnant mice is a vital tool to unravel the complex molecular mechanisms of placental infection, congenital transmission and maternal illness. We will provide a comprehensive review of the pathogenesis of several infection models in pregnant mice and their clinical relevance. These models have revealed the immunological function of the placenta in responding to, and resisting infection. Murine feto-placental infection provides an effective way to evaluate new intervention strategies for managing infections during pregnancy, adverse fetal outcome and long-term effects on the offspring and mother.
Keywords: Pregnancy, Infection, Immune response, Inflammation, Mouse, Abortion, Pre-term labor
1. Introduction
Mammalian pregnancy poses a unique challenge for the maternal immune system; tolerance needs to be maintained to the semi-allogenic fetus while effectively retaining immune reactivity to protect the mother and fetus from deleterious effects of infections. Infectious agents can reach the feto-placental compartment either through the hematogenous route or ascend the genital tract. Interestingly, the amniotic fluid and fetal-maternal membranes are often not sterile even in healthy term pregnancies (Witkin et al., 2011). Therefore, clearly mechanisms must be operative to prevent and/or limit microbial proliferation and/or its pathological consequences at the feto-maternal interface. Indeed, the placenta has been suggested to act as fortress against infections (Robbins and Bakardjiev, 2012). Nevertheless, some pathogens breach the placental barrier or intrude the amniotic cavity leading to excessive inflammation recognized as a major cause of pre-term birth (Redline, 2004). A strong maternal cell-mediated immune response to infection can also compromise fetal health even in the absence of pathogens in the reproductive mucosa (Krishnan et al., 1996b). Much of our knowledge on immunity to infection during human pregnancy is gained from retrospective epidemiological studies or in vitro infection of placental cultures. Experimental infection evoked in pregnant mice is an excellent tool to elucidate in vivo mechanisms of maternal resistance to infection and fetal tolerance to pathogen-associated danger signals. This review commences with a commentary on the suitability of mice as a model to study human gestation and immunity to infection. It then focuses on the various murine models of infections in pregnancy (Table 1 ) and their clinical relevance concluding with potential future avenues of research.
Table 1.
Murine feto-placental infection models.
| Infectious agent | Mouse strain and infection route | Placental infection | Pregnancy outcome | Maternal illness (altered relative to non-pregnant) | Immune phenotype |
|---|---|---|---|---|---|
| Bacteria | |||||
| Listeria monoytogenes | BALB/c, C57BL/6 (i.p., i.v., p.o.) | YES | Resorptions | NO | Th1, neutrophil activation at feto-maternal interface |
| Salmonella enterica Typhimurium | 129X1/SvJ C57129F1 (i.v., i.p., p.o.) | YES | Resorptions | YES (severe and fatal) | Placental inflammation, increased serum IL-6, reduced IL-12 and splenic NK response |
| Fusobacterium nucleatum | C57BL/6 (i.v.) | YES | Resorptions, still birth | NO | Induces strong IL-8 response |
| Chlamydia muridarum | C3H/HeJ | NA | NA | NA | Th1 response |
| Chalmydophila abortus | C57BL/6 (i.p. and i.v., i.n., i.g.) | YES | Resorption | MILD (resolved) | IFN-γ and neutrophil and NK cell activation |
| Coxiella burnetti | BALB/c | YES | Still birth | Endocarditis | Chronic |
| Brucella abortus | BALB/c | YES | VARIABLE | NO | Placental IFN-γ |
| Parasites | |||||
| Leishmania major | C57BL/6 | NO | Resorptions | YES | Classic Th1-Th2 imbalance |
| Plasmodium chabaudi and Plasmodium berghei | C57BL/6 | YES | Resorptions, Intrauterine growth retardation prematurity, still birth, post-natal mortality | MILD | Placental inflammation (increased MIP-1α, CCL3, IFN-γ) |
| Toxoplasma gondii | BALB/c, C57BL/6 p.o., i.p. | YES (in acute infection) | Resorption (in acute infection) | YES | Classic Th1-Th2 imbalance |
| Trypanosoma cruzi | BALB/c (i.p.) | NO | Resorptions | No | Th1 response |
| Schistosoma mansonii | C57BL/6 | NO | Vertical transmission | MILD | Th2 response |
| Viruses | |||||
| Influenza | BALB/c (i.v., i.n.) | NO | No adverse effect | YES (severe) | Elevated pulmonary inflammation (MIP-1α, IL-6, TNF-α and neutrophil infiltration) |
| Cytomegalovirus | BALB/c (intra-placental) | YES | Hearing impairment in neonate | NO | Unknown |
Abbreviations: p.o., per oral; i.n., intranasal; i.v., intravenous; i.p., intraperitoneal; NA, not available.
2. Mouse model of gestation
The use of mouse as a model to understand regulation of immunity to infection during gestation is based on a degree of similarity in the key events of gestation: implantation, placentation and parturition. Furthermore, similarity exists in the nature of immune cells that traverse the feto-placental interface and regulatory networks that protect the fetal allograft.
2.1. Implantation
Gestation in mice is 20 days when counted from the day 1 of vaginal plug formation. Similar to humans, the harmonized action of progesterone and estrogen secreted from the corpus lutea prepares the uterus for implantation. Uterine circulation is provided through blood vessels bundled at one side, named the “mesometrial side” (Lim and Wang, 2010). Attachment of the blastocyts to the luminal epithelium on the opposite side (anti-mesometrial side) occurs between days 4.0 and 4.5 and coincides with increased vascular permeability. The luminal epithelium undergoes apoptosis allowing the blastocyt to attach to the uterine lining and trophoblastic cells burrow the stroma inducing cell proliferation (decidualization). The maternal decidua lining encases the fetus and placenta initially but upon placentation is reduced to a thin anterior cap of cells. Intriguingly, even though human gestation is 280 days, the window of implantation is early, 5–9 days after ovulation and the processes are similar to those described for mice above (Lim and Wang, 2010).
2.2. Placentation
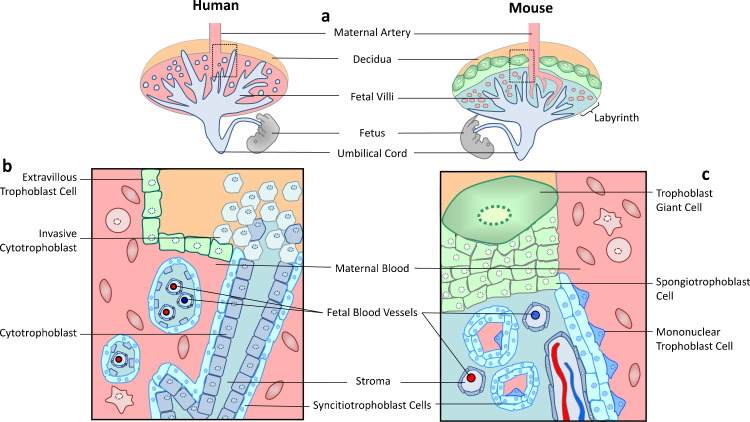
The placenta is a vital organ with physiological, endocrine and immunological functions. Both mice and humans have an invasive hemochorial placenta resulting in close opposition of fetal and maternal blood and cells (Fig. 1 ). The trophoblast layer of the placenta arises from the outer trophectoderm of the blastocyst, and trophoblast differentiation can be discerned by day 4.5 (Rossant and Cross, 2001). The distinct trophoblast layers that comprise the mouse placenta include; the labyrinth (comprising the multinucleated syncytiotrophoblast, villous chorionic trophoblast, blood vessels and stroma) which is supported by a compact layer of non-syncytial spongiotrophoblast and an outermost layer of giant trophoblast cells. The maternal blood supply passes the sphongiotrophoblast via a large central spiral artery which is remodeled post-implantation when trophoblast cells erode the endothelial cells. The maternal blood enters into tortuous spaces in the labyrinth where it bathes directly with the fetal villi, ensuring easy material exchange between the maternal and fetal blood systems. The fetal vasculature of the placenta is connected to the developing fetus by the umbilical cord (Fig. 1). In humans the extensive spiral artery remodeling culminates in the chorionic villi structure comprised of the syncytiotrophoblast and villous cytotrophoblast that is bathed in maternal blood (Fig. 1) and is analogous in function to the labyrinth of the mouse placenta. The outer column of the cytotrophoblast and extra villous trophoblast (akin to giant trophoblast cells in mouse) comprise the invasive interface with the uterus (Rossant and Cross, 2001).
Fig. 1.
Schematic diagram of mouse and human placenta. Top panel is a cartoon image of the human and mouse placenta respectively indicating orientation of the maternal decidua, spiral artery and trophoblast layers. A transverse section of the area indicated by the inset box in each image is shown in the bottom panel to highlight the cellular details. (a) List of cells and structures that are similar between human and mouse placenta. (b) Cells and structures distinctive to the human placenta. (c) Comparative cells and structures seen in the mouse placenta.
2.3. Immune cells at the feto-maternal interface
A number of maternal immune cells traverse the feto-maternal interface and have variable roles to play through the course of gestation. Murine gestation has served as a convenient model to map the localization and movement of immune cells at the feto-maternal interface using techniques such as flow cytometry, histochemistry and most recently whole mount staining microscopy (Croy et al., 2012). The emerging concept is that immune cells at the feto-maternal environment are predisposed to tolerance in the absence of sufficient “danger” signals (Mor, 2008). Below, we provide a brief description of the immune cells and their temporal regulation in response to the changing needs of the fetus.
The maternal decidua comprises as much as 30–40% leukocytes in early pregnancy and the major subsets include uterine NK (uNK) cells, dendritic cells, macrophages and T cells (Warning et al., 2011). Decidualization is tightly regulated inflammatory process which prepares the uterine bed for blastocyst implantation. Firstly, dendritic cells and uNK cells regulate stromal cell differentiation and the vascular response associated with spiral artery remodeling (Blois et al., 2011). In mice, the number of DCs and uNK cells increase at the feto-placental interface coinciding with implantation (Blois et al., 2011). The numbers of uNK cells increases to 50–70% of the leukocyte population by day 7, declining substantially in numbers by gestational day 9 (Croy et al., 2012). Depletion of DCs precipitates implantation failure (Krey et al., 2008). In the human endometrium, uNK cells emerge immediately post-ovulation (Blois et al., 2011). In mice depletion of uNK cells results in a hypo-cellular and necrotic decidua, but more importantly an undilated spiral artery and abnormal placental architecture (Croy et al., 2003, Guimond et al., 1997). Peripheral NK cells are cytotoxic, whereas uNK cells have distinct phenotype and function (Croy et al., 2003, Moffett-King et al., 2002). Firstly, uNK cells are CD56bright/CD16 (Moffett-King et al., 2002) in contrast to peripheral NK cells that are CD56dim/CD16+ (Campbell et al., 2001). CD16 is directly involved in triggering lysis of target cells, thus uNK cells lacking CD16 expression exhibit reduced cytotoxicity and switch function to IFNγ production (Croy et al., 2003, Moffett-King et al., 2002). The uNK cells invade the decidua basalis, which in both humans and mice triggers production of factors and cytokines such as leukemia inhibitory factor (LIF), IL-1, IL-6, IL-11, IFNγ, vascular endothelial growth factor (VEGF), TGF-β and IL-15 that facilitate successful implantation by modulating uNK cell maturation, angiogenesis and trophoblast differentiation (Blois et al., 2011, Croy et al., 2003). Importantly, uNK cells and DCs cross-regulate the function of the other during decidualization to balance angiogenesis with overt inflammation (Gonzalez et al., 2012).
Macrophages are present within the decidual compartment and have been implicated with pro-angiogenic functions during implantation (Nagamatsu and Schust, 2010). However, as professional antigen presenting cells macrophages may recognize allogeneic fetal antigens, and also mediate defense against infectious agents. The functional maturation of macrophages has been recently categorized into M1 and M2 analogous to T helper 1 and 2 (Th1/Th2) polarization of CD4 T cells (Sica and Mantovani, 2012). Macrophages (M1) activated under the influence of proinflammatory cytokines and danger signals secrete inflammatory cytokines such as TNF and IL-12, whereas M2 macrophages are polarized by exposure to Th2 cytokines and are characterized by enhanced scavenger and mannose receptors, reduced nitric oxide and IL-12 production (Sica and Mantovani, 2012). The polarization of decidual macrophages to the M2 phenotype has been seen in both murine and human pregnancy (Nagamatsu and Schust, 2010) and may be a mechanism of regulating immune tolerance. However, a recent report of human decidual macrophages suggests a greater plasticity among CD15+ decidual macrophages which may be either CD11chi or CD11clow capable of inflammatory and anti-inflammatory responses respectively (Houser et al., 2011). Indeed, decidual macrophage functions are important to control response to infections, as depletion of macrophages was shown to increase susceptibility to Listeria monocytogenes infection in mice (Guleria and Pollard, 2001).
Adaptive T cell immunity is necessarily restrained during pregnancy to protect the fetal allograft from potential cytotoxic effects of allo-specific CD8+ T cells and CD4+ Th1/Th17 cells (Saito et al., 2010). Indeed, a number of murine models have demonstrated that induction of allo-specific T cells can evoke abortion whereas T cell tolerance to allogeneic antigens is a hall-mark of successful pregnancy (Erlebacher, 2010). Furthermore, a recent study demonstrated that epigenetic silencing of chemokine receptors on the decidua prevents the accumulation of activated effector T cells (Nancy et al., 2012). However, even allo-antigen non-specific T cells may produce inflammatory cytokines that may have by-stander damaging effects on the fetus. An immunoregulatory role for the placenta in modulating a Th2 cytokine environment in successful pregnancy was first proposed by Wegmann et al. (1993). However, human and mouse trophoblast cells produce and/or respond to both Th1 and Th2 cytokines (Lin et al., 1993, Murphy et al., 2009). Furthermore, IL-4, IL-5, IL-9 and IL-13 gene-deficient mice exhibit normal pregnancy, suggesting that successful pregnancy can occur in the absence of a predominant Th2-biased response (Saito et al., 2010). In contrast, IFNγ, TNF and stimulation of TLRs that augments a Th1 response result in resorptions in mice (Chaouat et al., 1990) indicating a need to dampen this response. Thus, regulatory mechanisms operate at the feto-maternal interface to down-modulate both allo-specific T cell responses and damaging inflammation that may ensue from activation of T cells (D’Addio et al., 2011, Guleria et al., 2005).
T regulatory cells (Tregs) are a major class of CD4+ T cells that express the transcription factor Foxp3 and suppress immune responses against “self” and foreign antigens in a variety of physiological and pathological settings (Allan et al., 2008). Two subsets of Tregs have been recognized; thymic or peripheral origin; the latter differentiating from CD4+ T cells when binding their cognate antigen in the presence of TGFβ and absence of IL-6 (Allan et al., 2008). In humans augmented Th1-type immunity and decreased Tregs are observed following implantation failure in assisted reproduction (Saito et al., 2010). Unexplained infertility and recurrent spontaneous abortion are also associated with reduced expression of Tregs (Saito et al., 2010). Maternal Tregs are already increased in the lymph node of pregnant mice by days 2–3 of mating (Aluvihare et al., 2004, Zenclussen et al., 2006), and are evoked by the seminal plasma suggesting early induction of anti-paternal tolerance (Guerin et al., 2011). However, the expansion of Tregs occurs in both allogenic and syngenic murine mating (Aluvihare et al., 2004). Nevertheless, depletion of Tregs results in pregnancy loss in allogeneic but not syngenic mating (Aluvihare et al., 2004). Furthermore, adoptive transfer of Tregs from normal pregnant mice rescues fetal rejection in abortion-prone matings (Zenclussen et al., 2006). A pivotal recent study has shown that peripheral Foxp3 enhancer conserved sequence 1 (CNS1) that is essential for peripheral Treg differentiation is present only in placental mammals, and CNS1-deficient mice exhibit high levels of inflammation, abnormal spiral artery remodeling and fetal resorptions (Samstein et al., 2012). Thus, it has been suggested that the extrathymic differentiation of Tregs has been specifically gained during evolution of placental mammals to reinforce tolerance to paternal alloantigens presented by the fetus during prolonged gestation (Samstein et al., 2012).
Healthy human pregnancy is also associated with a decrease in Th17 cells, whereas, recurrent spontaneous abortion is associated with increased IL-17 pro-inflammatory cytokine production and increased Th17 cell infiltration into the deciduas (Lee et al., 2012). Furthermore, a role for IL-6 trans-signaling to drive the conversion of Tregs to Th17 cells in recurrent spontaneous abortion has been suggested (Saito et al., 2010). Similarly, a bias toward Th1/Th17 and away from Treg/Th2 cells and cytokines are reported in pre-mature labor and pre-elampsia in women (Saito et al., 2010). In mice as Th1 cytokines, TLR stimulation or depletion of Treg cells can evoke resorptions (Aluvihare et al., 2004, Chaouat, 2003), mechanistically this may relate to a Treg/Th17 imbalance. Indeed, many intracellular infections that are detrimental to pregnancy induce IL1β and IL-6 cytokine production that can trigger a conversion of Tregs to Th17 cells (Chaudhry et al., 2009). Furthermore, other regulatory molecules such as indoleamine-2,3-dioxygenase (IDO) expressed by the trophoblasts suppress Th17 differentiation (Baban et al., 2004, Baban et al., 2009). In conclusion, we surmise that the adaptive immunity at the feto-maternal interface is delicately balanced between a Th2/Treg and Th1/Th17 phenotype to protect the fetus from allo-recognition while providing sufficient plasticity to counter infectious threats.
Overall, the similarities between physiological processes, placentation and immune regulation in mice and human make it a convenient model to study holistically vivo responses to concurrent infection.
3. Pregnant women and infectious disease threat
Changes in physiology and immunity through the course of gestation make pregnant women react differentially to infections relative to the non-pregnant state. For decades, it has been appreciated that cell-mediated inflammatory disorders such as rheumatoid arthritis is ameliorated (Klipple and Cecere, 1989) whereas antibody mediated disorders such as systemic lupus erythematosis (Hayslett, 1991), is exacerbated during pregnancy. The Th2 response during pregnancy normally results in vigorous antibody responses and can effectively eliminate most extracellular pathogens (Jamieson et al., 2006). We first demonstrated using a murine model, the bi-directional cross-talk between pregnancy and Th1 immune response to infections; Leishmania major increased adverse pregnancy outcome in infected mice even in the absence of placental infection (Krishnan et al., 1996b). Conversely, the Th2 bias in pregnancy increased maternal susceptibility to L. major (Krishnan et al., 1996a). Infections that are threat to the pregnant mother may be extra-uterine or colonize the reproductive mucosa. The effects of infection in human pregnancy are revealed during epidemiological analysis of outbreaks. However, it is challenging to unravel the mechanism and dynamics of feto-maternal cellular interactions that influence in vivo infection by utilizing in vitro human placental cultures. Finally, select group of viruses, bacteria, parasites all pose increased threat during pregnancy, yet there is little commonality in their mechanisms of pathogenesis. Murine feto-placental infection models have increased our knowledge of modulation of immunity to infection in pregnancy.
4. Murine feto-placental infections
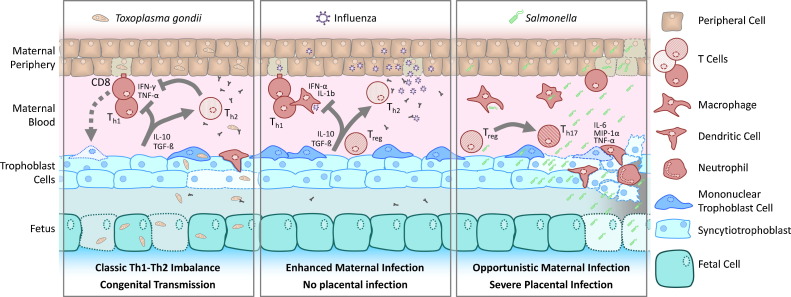
Experimental infections initiated during gestation in animal models can have three potential impacts; placental/fetal infections, acute maternal systemic illness and long-term effects on the mother or fetus (Fig. 2 ). These impacts may occur independently without consequence on the other and can differ with dose and route of infection. In the following sections, we will describe the modulation of immunity to exemplar infections that have been studied in murine pregnancy (Table 1) and discuss their clinical relevance.
Fig. 2.
Modulation of immunity to infection in pregnancy. A schematic diagram showing the different ways in which infectious agents may interact with the feto-maternal interface cells and trophoblast. The left panel shows that parasites such as T. gondii are controlled by a strong Th1 and cytotoxic CD8 T cell response. The Th1 cytokines in an infected host may inflict damage to the trophoblast leading to fetal loss. The trophoblast inturn produces IL-10 and anti-inflammatory cytokines that inhibit the Th1 response against the parasite. Macrophages and dendritic cells infected with the parasite may reach the feto-maternal interface leading to trophoblast infection and congenital transmission. Prior antibody immunity in chronically infected mothers can prevent congenital transmission. The middle panel shows that viruses such as influenza require a strong type I interferon response for clearance. However, this response may be dampened in the pregnant host due to enhanced T regulatory cell numbers and Th2 cytokine bias. High viral titers in peripheral tissue and severe maternal illness can occur despite lack of placental infection. The right panel shows that facultative intracellular bacteria such as Salmonella can reach the feto-maternal interface by escaping the maternal blood or may be carried by infected macrophages and dendritic cells. Once in the feto-placental milieu they efficiently infect trophoblast cells and profound pathogen proliferation ensues. This evokes massive inflammation and loss of placental tissue integrity. The inflammatory cytokines evoked by placental infection may convert resident T regulatory cells into Th17 cells, culminating in fetal loss and severe maternal illness.
4.1. Bacterial infections
Experimental infection of mice with a number of intracellular bacteria; L. monocytogenes, Salmonella enterica Typhimurium, Fusobacterium nucleatum, Campylobacter rectus, Porphyromonas gingivalis, Coxiella burnetti, Brucella abortus and Chlamydophilus abortus (Arce et al., 2009, Caro et al., 2009, Kim et al., 2005, Lin et al., 2003, Stein et al., 2000) is deleterious during pregnancy (Table 1). Below, we describe a few murine models of bacterial infection that have been studied depth to delineate the immune mechanisms involved.
4.1.1. Listeriosis
Listeria monocytogenes is a gram-positive, facultative intracellular food-borne pathogen that causes severe systemic disease mainly in immunocompromised individuals. In pregnant women, L. monocytogenes infects the placenta and causes miscarriage, stillbirth or neonatal meningocephalitis (Benshushan et al., 2002). However, serious maternal systemic listeriosis is rare.
The murine model of L. monocytogenes infection has been widely studied to elucidate the pathogenesis of infection and mechanisms of host immunity. The F6214-1 (4nonb) strain of L. monocytogenes is more virulent than the 10403S (1/2a) strain following intra-gastric inoculation in both pregnant and non-pregnant mice (Hamrick et al., 2003). It is usually difficult to reproduce placental infection after oro-gastric L. monocytogenes infection because of the low numbers of bacteria that cross the intestinal barrier in an immunocompetent mouse. However, intravenous inoculation of L. monocytogenes (dose range of 103 to 5 × 105) in BALB/c mice at mid-gestation (days 12–14) produces consistent placental infection with rapid fetal loss (Abram et al., 2003). As early as 6 h post infection, bacteria were detected in the placenta, followed by ∼2-log units increase per day (Le Monnier et al., 2006). On day 1 bacteria were mainly visualized along the central arterial canal adhering extraneously to the vascular wall or internalized within the invasive trophoblastic cells and were absent from the decidua basalis (Le Monnier et al., 2006). By day 2, the bacteria were visualized in both the proximal and distal central arterial canal and had spread to the decidua, spongiotrophoblast and trophoblastic giant cells. By day 3 bacteria pervaded all layers of the placenta including the labyrinth. The profound placental proliferation of L. monocytogenes occurs even in pre-immunized mice that are protected from infection in other organs (Abram et al., 2003). However, not all placentas are infected within the same uterus.
Human trophoblast cells (akin to intestinal epithelial cells) express E-cadherin receptor that binds L. monocytogenes surface protein InlA (Lecuit et al., 2004). However, mice lack E-cadherin receptor, and L. monocytogenes strains lacking InlA and InlB can infect the murine placenta, indicating other mechanisms contribute to feto-placental infection (Seveau et al., 2007). In contrast, L. monocytogenes-ActA mutant is unable to infect murine fetuses suggesting an involvement of this gene in cell-to-cell spread of bacteria through trophoblast layers (Le Monnier et al., 2007). A key observation was that trophoblasts of infected mice augmented a profound Th1 cytokine response, which led to neutrophil recruitment and limited spread of bacteria to the fetal side (Guleria and Pollard, 2000). Augmentation of innate immunity using CpG oligonucleotide pre-treatment of mice also decreased placental L. monocytogenes infection (Ito et al., 2004). Similarly, human invasive extra villous trophoblast and syncytiotrophoblast cells have been shown to restrict intracellular spread of L. monocytogenes (Zeldovich et al., 2011).
Interestingly, pregnancy does not increase systemic susceptibility to L. monocytogenes infection. Pregnant mice infected with L. monocytogenes augmented reduced IFNγ and increased IL-10 serum cytokine response suggesting a weaker systemic Th1 response to infection (Abram et al., 2003). However, the spleen and liver of pregnant L. monocytogenes infected mice exhibited lower bacterial burden and increased serum IL-6 relative to virgin infected mice (Krishnan et al., 2010, Le Monnier et al., 2006). We also demonstrated that pregnant mice infected with L. monocytogenes induce a potent systemic CD8+ T cell response, long-term memory and resistance to secondary infection (Krishnan et al., 2010). In general, trophoblast cells provide a safe niche for Listeria monocytogenes proliferation but innate immunity curtails spread of infection at the feto-maternal interface.
4.1.2. Salmonellosis
Salmonella enterica species are food-borne gram-negative facultative intracellular bacteria. Clinically, typhoid fever is caused by Salmonella typhi and Salmonella paratyphi, whereas enteric fever is caused by a large number of non-typhoidal Salmonellae (NTS). Host immunodeficiency does not increase susceptibility to typhoid fever, although placental infection and vertical transmission may occur (Hedriana et al., 1995). In contrast, NTS Salmonellosis is emerging as a major invasive disease in immunocompromised individuals, particularly in HIV-comorbidity in sub-Saharan Africa (Feasey et al., 2012). Salmonella infection during human pregnancy can cause spontaneous abortion, neonatal death and vertical transmission to the neonate besides maternal morbidity due to septic shock, osteomyelitis, polynehpritis and endocarditis (Gyang and Saunders, 2008, Ozer et al., 2009, Schloesser et al., 2004, Scialli and Rarick, 1992, van der Klooster and Roelofs, 1997).
S. enterica serotype Typhimurium (ST) is a natural pathogen for rodents and as early as 1893 was identified as the causative agent of murine typhoid (Santos et al., 2001). Genetically susceptible strains of mice infected with ST develop fever between 4 and 8 days post oral infection without diarrhea. Gross intestinal pathology includes diffused enteritis and localized inflammation in the ileal region (Santos et al., 2001). Furthermore, susceptible mice (C57BL/6) develop rapid increase in bacterial burden in spleen, liver and ceaca, and pro-inflammatory cytokine production leading to fatality within 10–15 days. In contrast, resistant strains of mice develop a chronic systemic infection without fatality (Luu et al., 2006).
We first observed that ST infection initiated intravenously in resistant strains of mice (129X1Sv/J and C57129F1) during pregnancy resulted in a rapid and fatal systemic infection, characterized by 1000-fold greater splenic bacterial burden relative to non-pregnant mice (Pejcic-Karapetrovic et al., 2007). Three sequential events appear to contribute to ST-induced feto-maternal pathology; profound bacterial growth in the placenta, induction of massive placental inflammation, and weak splenic innate immune response (Fig. 2). Firstly, following hematogenous infection with 103 bacteria, few (<50) reached the feto-placental tissue. Astoundingly, the placental bacterial burden increased to 105–107 by 14–30 h indicative of a bacterial doubling time of <2 h. In contrast the doubling time of the bacteria in the systemic organs was >10 h. ST infection evoked profound placental production of pro-inflammatory cytokines, TNFα, IL-6, G-CSF, IFNγ and IL-18 (Chattopadhyay et al., 2010). The avirulent ST ΔaroA strain did not evoke this inflammatory response despite placental proliferation. Furthermore, infection with the virulent wild-type strain led to massive GR-1 positive neutrophil infiltration throughout the labyrinth trophoblast (Chattopadhyay et al., 2010). Infected placentas rapidly lost tissue integrity exhibiting substantially reduced decidua and highly necrotic labyrinth. Furthermore, wild-type ST bacteria were found dispersed throughout the necrotic labyrinth trophoblast within 48 h post-infection. In contrast, avirulent ST ΔaroA bacteria were localized to the decidua, and did not evoke placental inflammation and fetal loss. We speculate that inflammation is triggered when virulent Salmonella infects the labyrinth trophoblast. Indeed, Salmonella utilize a specialized type III secretion system to invade non-phagocytic cells (Valdez et al., 2009). Furthermore, Salmonella secrete ∼30 different effector proteins into infected cells via their type III secretion apparatus which aids intracellular survival. Therefore, Salmonella can rapidly and easily spread across placental layers, even in the absence of tissue infarction. Bacteria may gain access to maternal decidua either directly or via infection of macrophages or reach the labyrinth trophoblast by escaping the blood sinusoids.
Innate immunity to ST plays a critical role in controlling infection as adaptive immunity to this pathogen is substantially delayed (Luu et al., 2006). Thus, ST infection in non-pregnant mice is characterized by increase in numbers of macrophages, dendritic cells, neutrophils and conspicuous increase in NK cells by 48 h, whereas this response is abrogated in pregnant-infected mice (Pejcic-Karapetrovic et al., 2007). Furthermore, the splenic NK cell cytotoxic function and serum IL-12 response was dampened in pregnant ST-infected hosts. However, the levels of certain inflammatory chemokines/cytokines such as IL-6, BLC (B lymphocyte chemoattractant)/CXCL13, granulocyte colony-stimulating factor (G-CSF), IL-1R antagonist (IL-Ra), I-309/CCL1, KC, CCL12 and MIP-2 were up regulated 5–30-fold in the serum of pregnant ST infected mice (Chattopadhyay et al., 2010). Interestingly, serum IL-10 was also increased in pregnant-infected mice, suggesting a counter host response to overcome inflammation. Crucially, depletion of systemic IL-6 in vivo by antibody treatment significantly decreased splenic burden of virulent bacteria relative to untreated pregnant-infected mice (Chattopadhyay et al., 2010). Thus, the mouse model suggests that Salmonella have evolved mechanisms for systemic dissemination in the pregnant host and cause a cytokine-storm mediated immunopathology. With the emergence of invasive NTS disease in immunocompromised hosts, susceptibility of pregnant women needs to be investigated carefully. A murine gut colonization model of Salmonella infection, after treatment of mice with streptomycin closely mimics gastroenteric fever (Santos et al., 2001) and may provide insight into the dissemination mechanism of NTS in immunocompromised humans.
4.1.3. Bacteria causing periodontal infections
Maternal periodontitis has emerged as a putative risk factor for pre-term births in humans wherein the bacteria associated with dental biofilm hamatogenously disseminate and infect the placenta (Cetin et al., 2012). Thus, there has been interest generated in studying the immune response to infection in pregnant mice infected with bacteria that can induce periodontal infections. F. nucleatum is one such anaerobic pathogen implicated in periodontal infections and placental chorioamnionitis (Gauthier et al., 2011). Systemic infection initiated on day 14.5 of murine pregnancy resulted in only transient maternal systemic colonization (Han et al., 2004). Similar to Listeria and Salmonella, localization of a small numbers of bacteria to the uterine decidua resulted in overwhelming placental infection, with premature delivery and/or stillbirth. However, organisms were suggested to enter the labyrinth only after ischemic necrosis and tissue infarction due to decidual vascular thrombosis (Han et al., 2004). Interestingly, infection of Toll-like receptor 4 (TLR4) but not TLR2 knockout mice prevented necrosis and inflammation and reduced fetal loss but did not affect bacterial titers in the placenta (Liu et al., 2007), implicating inflammation rather than bacterial burden as a cause of adverse pregnancy outcome. In another model, infection of mice with C. rectus or P. gingivalis increased expression of TLR4 occurred in infected labyrinth trophoblasts (Arce et al., 2009) suggesting that infection-induced danger signal recognition may trigger focal necrosis, neutrophils infiltration of the labyrinth and fetal loss.
Thus, murine models have revealed that following vivo infection, infected trophoblasts cells may augment an inflammatory cascade that triggers fetal loss, perhaps to preserve the mother for future successful pregnancy.
4.2. Parasitic infections
Parasitic infections in pregnancy pose multiple challenges due to cyclical parasitic life stages, differential tissue tropism at specific stages, acute or chronic phases of infection. Several parasitic infections such as malaria, toxoplasmosis, trypanosomiasis and schistosomiasis are either exacerbated in the pregnant host or lead to vertical dissemination of infection (Cardoni and Antunez, 2004, Dauby et al., 2012, Diaz-Lujan et al., 2012, Friedman et al., 2007, Hviid et al., 2010). Below are two murine models of parasitic infections that have been extensively studied in the context of the pregnant host.
4.2.1. Malaria
An estimated 300–500 million cases of malaria infection occur worldwide and women and children are at high risk of contracting severe illness (Hviid et al., 2010). A seminal study revealed that binding of Plasmodium falciparum infected erythrocytes to placental chondriotin sulphate A (CSA) results sequestration of the parasite to the feto-maternal interface (Fried et al., 2006). A number of African rodent malaria species were identified between 1948 and 1960 and two main strains have been extensively studied; Plasmodium chabaudi and Plasmodium berghei (Hviid et al., 2010, Stephens et al., 2012). Human malaria is characterized by cyclical fever and schizogony wherein the parasites invade particular erythrocyte populations. In contrast, in mice fever is not often noted, yet other syndromes such as sequestration of infected erythrocytes in organs, anemia and malaise are analogous to human infection (Hviid et al., 2010).
P. berghei infections are generally fatal for non-immune mice although pregnancy accelerates the clinical course of infection and causes resorptions, intrauterine growth retardation, pre-maturity and vertical infection. Murine placental infection is characterized by basal zone necrosis, syncytiotrophoblast hyperplasia, mononuclear infiltration, sinusoid constriction and accumulation of infected erythrocytes (Hviid et al., 2010). Most importantly, binding of P. berghei infected erythrocytes to placental tissue was inhibited by soluble CSA, indicating a similar mechanism of sequestration of infected erythrocytes as in humans (Neres et al., 2008). P. berghei infection also contributes to placental inflammation marked by increase in chemokines such as MIP-1α, CCL2, and consequent tissue damage. A model of recrudescent infection involves exposure to P. berghei prior to mating (Marinho et al., 2009). Infection then remains latent, and recurs during the second week of gestation, when parasitaemia suddenly rises, leading to infected erythrocyte sequestration in the placenta, inflammation-induced pathology and poor fetal outcome. This model mimics P. falciparum infection in pregnant women from endemic regions, including the influence of parity, as the clinical consequences were more pronounced in first pregnancy in pre-immune mice. This model revealed that multiparity decreased susceptibility to malaria due to increased IgG specific for the variant surface antigens expressed by the parasite during pregnancy (Megnekou et al., 2009).
P. chabaudi parasitemia is also initially accelerated in non-immune C57BL/6J mice infected in early pregnancy. However, the more pronounced effect was placental sequestration of P. chabaudi infected erythrocytes and higher levels of IL-1β and IFNγ particularly at the time of resorptions (Su and Stevenson, 2000). Interestingly, treatment with neutralizing TNFα antibody was reported to reduce fetal loss (Poovassery et al., 2009). Thus, murine models of placental malaria have shed light on innate mechanisms of immunity to placental malaria and its correlation to fetal health although role of adaptive immunity needs to be elucidated.
4.2.2. Toxoplasmosis
Toxoplasma gondii is an obligate intracellular protozoan parasite that usually causes primary asymptomatic or subclinical infection but can lead to chronic latent disease that may be reactivated in immunocompromised hosts (Pappas et al., 2009). The main threat of T. gondii infection during pregnancy is congenital infection of the fetus, particularly when primary infection occurs in women during gestation (Montoya and Remington, 2008). In women the frequency of vertical transmission increases when infection is acquired at later gestational age, whereas infection early in pregnancy may result in severe fetal abnormalities and abortion (Menzies et al., 2008, Montoya and Remington, 2008). The murine model of T. gondii infection mimics many aspects of human congenital infection (Menzies et al., 2008).
BALB/c mice infected with various strains of T. gondii for the first time during pregnancy vertically transmit the parasite to the fetus (Roberts and Alexander, 1992). However, chronically infected BALB/c mice do not transmit disease to the fetus, even if re-exposed to T. gondii during pregnancy which resembles chronic infection in healthy women who do not transmit the disease to the fetus (Menzies et al., 2008). The innate immune response to T. gondii infection is orchestrated through recognition of pathogen specific molecular patterns, which in turn shape effective adaptive immunity. T. gondii GPI-anchors serve as effective ligands for TLR2 and TLR4 whereas HSP70 ligates TLR4 and stimulates macrophage maturation (Debierre-Grockiego et al., 2007, Mun et al., 1999). Furthermore, T. gondii profilin interacts with TLR11 in mice, and cyclophilin binds the CCR5 receptor (Aliberti et al., 2003, Menzies et al., 2008). Thus, the recognition of T. gondii danger signals stimulates a cascade of innate cellular and soluble responses; robust NK cell activation, dendritic cell maturation, macrophage activation and production of IFNγ, IL-12, TNFα and iNOS which together limit parasite tachyzoite replication (Coutinho et al., 2012, Miller et al., 2009). Indeed dendritic cell activation following T. gondii infection in mice is rapid; within a few hours most splenic CD11c+ cells have migrated from the red pulp to the T cell areas producing IL-12 (Miller et al., 2009). This in turn triggers a strong Th1 and CD8+ T cell response against the pathogen and sustained IFNγ production (Menzies et al., 2008). Importantly, the pathogenesis of T. gondii infection in mice reveals the critical balance of Th1 and Th2 response for control of parasitemia while minimizing overt inflammation. Thus, IL-10 diminishes the ability of macrophages to kill T. gondii, but mice deficient in IL-10 die following T. gondii infection due to cytokine shock (Gazzinelli et al., 1996).
As successful pregnancy is associated with a regulated innate and Th1/Th2 response at the feto-maternal interface, the immune response to T. gondii infection may favor abortion in pregnant mice. Alternatively, pregnancy induced down-modulation of the Th1 response may interfere with the mechanisms that effectively control parasite multiplication and promote congenital transmission (Fig. 2). Both these aspects have been examined in various strains of mice (Coutinho et al., 2012). C57BL/6 mice experience higher rates of resorptions than the BALB/c strain following T. gondii infection and this correlates to higher levels of decidual necrosis and systemic TNF-α levels (Coutinho et al., 2012). However, T. gondii infection in pregnant mice results in minimal parasites in the uterus and decidua suggesting that pregnancy loss is directly attributable to the strong Th1 inflammatory response rather than high parasite burden at the feto-maternal interface (Coutinho et al., 2012). Conversely, pregnancy-induced modulation of Th1/Th2 response against T. gondii increases maternal susceptibility and congenital transmission of the parasite in murine models. For example, pregnant mice exhibit increased mortality following T. gondii infection relative to non-pregnant mice and this correlates to a decreased production of IFNγ (Luft and Remington, 1982). Hence, survival of T. gondii-infected pregnant mice can be increased by administration of IFNγ or IL-2 (Shirahata et al., 1992, Shirahata et al., 1993). Furthermore, anti-IFNγ treatment or depletion of CD8+ T cells producing IFNγ increases maternal parasitemia and enhances congenital transmission in T. gondii-infected mice (Abou-Bacar et al., 2004, Shirahata et al., 1994). This correlates to increased T. gondii parasites seen it the uterus and placentas of mice deficient in IFNγ (Shiono et al., 2007). Similarly, depletion of NK cells increases the congenital transmission of T. gondii in RAG2 −/− but not wild-type pregnant mice, suggesting a compensatory role of T and B cells as well (Combe et al., 2005). Maternal vaccination against T. gondii in mice has been successful in preventing congenital transmission to the fetus (Menzies et al., 2008). Thus, mouse models have revealed the critical balance of Th1 and Th2 immunity to T. gondii during pregnancy (Fig. 2).
4.3. Viruses
Pregnancy poses an increased risk of severe maternal illness due to a number of acute and chronic viral infections; the common rhinovirus, influenza virus, SARS coronavirus and Varicella zoster, Hepatitis E/B, HIV and cytomegalovirus (Harger et al., 2002, Jamieson et al., 2009, McDonagh et al., 2004, Wong et al., 2003). Viral infections can also lead to pre-term birth and other pregnancy complications (Jamieson et al., 2006). However, few murine models are available to study pathogenesis of viral infections.
4.3.1. Influenza
The increased risk of severe illness following influenza infection during pregnancy has been observed during all the major pandemics (Jamieson et al., 2009, Pazos et al., 2012a). Systemic infection with mouse adapted influenza virus strains results in severe morbidity and maternal mortality in pregnant mice (Marcelin et al., 2011, Pazos et al., 2012b). A model that closely resembles human respiratory illness, is infection by the intranasal route with aerosolized lethal PR8 or non-lethal X31 influenza virus at gestational day 10.5 (Pazos et al., 2012b), leading to pronounced increase in pulmonary viral titers in pregnant mice. The fetuses also exhibited significant intrauterine growth retardation and were delivered pre-term. Interestingly, treatment of mice with estrogen pellets increased their susceptibility to influenza virus infection challenge (Pazos et al., 2012b). Type I interferons play a key role in immunity to viral infections. Pregnant mice infected with influenza virus exhibited substantially reduced IFNβ mRNA and interferon stimulating genes in the lung and also systemic organs not directly infected with the virus (Pazos et al., 2012b). Similarly, infected pregnant mice show reduced lung expression of inflammatory cytokines such as IL-1β and TNFα on day 3 post-infection although number of innate immune cells (NK cells and DCs) were not reduced. In contrast, the splenic CD8+ T cell cytotoxic response to influenza infection was reduced in both pregnant and estrogen-treated animals relative to the non-pregnant state (Pazos et al., 2012b). In another study, aerosol inhalation of the pandemic A (H1N1) influenza strain by pregnant BALB/c mice evoked host fatality that correlated to increased infiltration of macrophages and neutrophils into the respiratory mucosa although viral titers in various organs were similar in pregnant and non-pregnant animals (Marcelin et al., 2011). Thus, immunity to influenza is compromised during pregnancy despite lack of placental infection (Fig. 2).
5. Conclusions and quo vadis
Murine models have long been used to unravel independently host-pathogen interactions or immune tolerance during pregnancy. However, immunity to only a few pathogens has been closely examined in pregnant mice. The knowledge of placental defenses against infection is often derived from vitro models of human placental cultures. Murine models can complement vitro studies to elucidate the manner in which placental barrier is breached. The two potential sites of transmission of infection to the placenta are (1) the blood-syncytiotrophoblast in human and labyrinth trophoblast in mouse interface where the placenta is bathed in maternal blood and (2) the uterus–trophoblast interface wherein the extra villous trophoblast in humans or giant trophoblast in mouse invade the uterine epithelium and come in contact with maternal immune cells. In vitro studies indicate that the human extra villous and syncytiotrophoblast cultures can be resistant to intracellular infections (Robbins and Bakardjiev, 2012). In vivo infection in mice can complementarily elucidate the sequence of key events during pathogenesis; primary site of infection, inflammation, tissue infarction and vertical transmission which may be variably modulated by pathogens.
Murine models are also convenient for examining the concurrent effect of multiple danger signals during gestation which may occur in endemic regions during secondary heterologous infections in chronically infected hosts, or recognition of multiple molecular patterns on the same pathogen by the immune cells. Indeed, in an elegant recent study, pregnant mice infected with murid herpes virus (MHV) demonstrated increased sensitivity to subsequent exposure to even low doses of bacterial lipopolysaccharide (Cardenas et al., 2010). Similarly, a two-hit mechanism of exposure to TLR2 and TLR4 ligands synergistically triggered pre-mature labor in mice (Ilievski and Hirsch, 2010). Another emerging concept is the role of the gut microbiome and stress-induced disequilibrium of danger signals in triggering adverse pregnancy outcome (Friebe and Arck, 2008). The interaction of endogenous danger signals with exogenous infection can be easily addressed in pregnant mice.
A recent revelation is that immunotolerance mechanisms such as naturally occurring T regulatory cells in pregnancy create holes in host defense to pathogens such as Listeria and Salmonella (Rowe et al., 2011). Many infections augment IL-6 production and IL-6 trans-signaling can convert the Treg cells to Th17 cells (Chaudhry et al., 2009). In humans, Th1/Th17-type immunity and decreased Treg cells are observed following implantation failure in assisted reproduction, unexplained infertility, recurrent spontaneous abortion (RSA), pre-eclampsia and pre-mature labor (Saito et al., 2010). These adverse pregnancy events may also have an infection etiology. Murine models may unravel the link between Tregs, Th17 and IL-6 in modulating response to infection during pregnancy.
Chronic maternal infections during pregnancy may affect the fetal immune system even without vertical transmission (Dauby et al., 2012). Deleterious effects such as modified neonatal immunity to vaccination and neurological disease in the offspring of infected mothers may occur (Elovitz et al., 2011). Alternatively, beneficial transmission of immune memory or reduction in allergy to the neonate may ensue (Stern et al., 2007). Experimental infection in murine pregnancy can provide a controlled setting to delineate the mechanism of such effects. Prophylaxis and treatment of infectious disease in endemic regions may also need differential approach during pregnancy for maximum effectiveness. Overall, only through the study of in vivo models can we unravel the complex bi-directional cross-talk between host immunity to infection and pregnancy.
Acknowledgements
Research in the laboratory of L. Krishnan is funded by the National Research Council, Canadian Institutes for Health Research and National Institutes of Allergy and Infectious Diseases.
References
- Abou-Bacar A., Pfaff A.W., Letscher-Bru V., Filisetti D., Rajapakse R., Antoni E., Villard O., Klein J.P., Candolfi E. Role of gamma interferon and T cells in congenital Toxoplasma transmission. Parasite Immunol. 2004;26:315–318. doi: 10.1111/j.0141-9838.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Abram M., Schluter D., Vuckovic D., Wraber B., Doric M., Deckert M. Murine model of pregnancy-associated Listeria monocytogenes infection. FEMS Immunol. Med. Microbiol. 2003;35:177–182. doi: 10.1016/S0928-8244(02)00449-2. [DOI] [PubMed] [Google Scholar]
- Aliberti J., Valenzuela J.G., Carruthers V.B., Hieny S., Andersen J., Charest H., Reis e Sousa, Fairlamb A., Ribeiro J.M., Sher A. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 2003;4:485–490. doi: 10.1038/ni915. [DOI] [PubMed] [Google Scholar]
- Allan S.E., Broady R., Gregori S., Himmel M.E., Locke N., Roncarolo M.G., Bacchetta R., Levings M.K. CD4+ T-regulatory cells: toward therapy for human diseases. Immunol. Rev. 2008;223:391–421. doi: 10.1111/j.1600-065X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Aluvihare V.R., Kallikourdis M., Betz A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- Arce R.M., Barros S.P., Wacker B., Peters B., Moss K., Offenbacher S. Increased TLR4 expression in murine placentas after oral infection with periodontal pathogens. Placenta. 2009;30:156–162. doi: 10.1016/j.placenta.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baban B., Chandler P., McCool D., Marshall B., Munn D.H., Mellor A.L. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J. Reprod. Immunol. 2004;61:67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Baban B., Chandler P.R., Sharma M.D., Pihkala J., Koni P.A., Munn D.H., Mellor A.L. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J. Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benshushan A., Tsafrir A., Arbel R., Rahav G., Ariel I., Rojansky N. Listeria infection during pregnancy: a 10 year experience. Israel Med. Assoc. J. 2002;4:776–780. [PubMed] [Google Scholar]
- Blois S.M., Klapp B.F., Barrientos G. Decidualization and angiogenesis in early pregnancy: unravelling the functions of DC and NK cells. J. Reprod. Immunol. 2011;88:86–92. doi: 10.1016/j.jri.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Campbell J.J., Qin S., Unutmaz D., Soler D., Murphy K.E., Hodge M.R., Wu L., Butcher E.C. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J. Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- Cardenas I., Means R.E., Aldo P., Koga K., Lang S.M., Booth C., Manzur A., Oyarzun E., Romero R., Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J. Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoni R.L., Antunez M.I. Outcome of Trypanosoma cruzi infection in pregnant BALB/c mice. Ann. Trop. Med. Parasitol. 2004;98:883–887. doi: 10.1179/00034980X3234. [DOI] [PubMed] [Google Scholar]
- Caro M.R., Buendia A.J., del R.L., Ortega N., Gallego M.C., Cuello F., Navarro J.A., Sanchez J., Salinas J. Chlamydophila abortus infection in the mouse: a useful model of the ovine disease. Vet. Microbiol. 2009;135:103–111. doi: 10.1016/j.vetmic.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Cetin I., Pileri P., Villa A., Calabrese S., Ottolenghi L., Abati S. Pathogenic mechanisms linking periodontal diseases with adverse pregnancy outcomes. Reprod. Sci. 2012;19:633–641. doi: 10.1177/1933719111432871. [DOI] [PubMed] [Google Scholar]
- Chaouat G. Innately moving away from the Th1/Th2 paradigm in pregnancy. Clin. Exp. Immunol. 2003;131:393–395. doi: 10.1046/j.1365-2249.2003.02100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouat G., Menu E., Clark D.A., Dy M., Minkowski M., Wegmann T.G. Control of fetal survival in CBA × DBA/2 mice by lymphokine therapy. J. Reprod. Fertil. 1990;89:447–458. doi: 10.1530/jrf.0.0890447. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A., Robinson N., Sandhu J.K., Finlay B.B., Sad S., Krishnan L. Salmonella enterica serovar Typhimurium-induced placental inflammation and not bacterial burden correlates with pathology and fatal maternal disease. Infect. Immun. 2010;78:2292–2301. doi: 10.1128/IAI.01186-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A., Rudra D., Treuting P., Samstein R.M., Liang Y., Kas A., Rudensky A.Y. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combe C.L., Curiel T.J., Moretto M.M., Khan I.A. NK cells help to induce CD8(+)-T-cell immunity against Toxoplasma gondii in the absence of CD4(+) T cells. Infect. Immun. 2005;73:4913–4921. doi: 10.1128/IAI.73.8.4913-4921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho L.B., Gomes A.O., Araujo E.C., Barenco P.V., Santos J.L., Caixeta D.R., Silva D.A., Cunha-Junior J.P., Ferro E.A., Silva N.M. The impaired pregnancy outcome in murine congenital toxoplasmosis is associated with a pro-inflammatory immune response, but not correlated with decidual inducible nitric oxide synthase expression. Int. J. Parasitol. 2012;42:341–352. doi: 10.1016/j.ijpara.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Croy B.A., Chen Z., Hofmann A.P., Lord E.M., Sedlacek A.L., Gerber S.A. Imaging of vascular development in early mouse decidua and its association with leukocytes and trophoblasts. Biol. Reprod. 2012 doi: 10.1095/biolreprod.112.102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy B.A., He H., Esadeg S., Wei Q., McCartney D., Zhang J., Borzychowski A., Ashkar A.A., Black G.P., Evans S.S., Chantakru S., van den H.M., Paffaro V.A., Jr., Yamada A.T. Uterine natural killer cells: insights into their cellular and molecular biology from mouse modelling. Reproduction. 2003;126:149–160. doi: 10.1530/rep.0.1260149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addio F., Riella L.V., Mfarrej B.G., Chabtini L., Adams L.T., Yeung M., Yagita H., Azuma M., Sayegh M.H., Guleria I. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J. Immunol. 2011;187:4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauby N., Goetghebuer T., Kollmann T.R., Levy J., Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect. Dis. 2012;12:330–340. doi: 10.1016/S1473-3099(11)70341-3. [DOI] [PubMed] [Google Scholar]
- Debierre-Grockiego F., Campos M.A., Azzouz N., Schmidt J., Bieker U., Resende M.G., Mansur D.S., Weingart R., Schmidt R.R., Golenbock D.T., Gazzinelli R.T., Schwarz R.T. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- Diaz-Lujan C., Triquell M.F., Schijman A., Paglini P., Fretes R.E. Differential susceptibility of isolated human trophoblasts to infection by Trypanosoma cruzi. Placenta. 2012;33:264–270. doi: 10.1016/j.placenta.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Elovitz M.A., Brown A.G., Breen K., Anton L., Maubert M., Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int. J. Dev. Neurosci. 2011;29:663–671. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A. Immune surveillance of the maternal/fetal interface: controversies and implications. Trends Endocrinol. Metab. 2010;21:428–434. doi: 10.1016/j.tem.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feasey N.A., Dougan G., Kingsley R.A., Heyderman R.S., Gordon M.A. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A., Arck P. Causes for spontaneous abortion: what the bugs ‘gut’ to do with it? Int. J. Biochem. Cell Biol. 2008;40:2348–2352. doi: 10.1016/j.biocel.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Fried M., Domingo G.J., Gowda C.D., Mutabingwa T.K., Duffy P.E. Plasmodium falciparum: chondroitin sulfate A is the major receptor for adhesion of parasitized erythrocytes in the placenta. Exp. Parasitol. 2006;113:36–42. doi: 10.1016/j.exppara.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Friedman J.F., Mital P., Kanzaria H.K., Olds G.R., Kurtis J.D. Schistosomiasis and pregnancy. Trends Parasitol. 2007;23:159–164. doi: 10.1016/j.pt.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Gauthier S., Tetu A., Himaya E., Morand M., Chandad F., Rallu F., Bujold E. The origin of Fusobacterium nucleatum involved in intra-amniotic infection and preterm birth. J. Matern. Fetal Neonatal Med. 2011;24:1329–1332. doi: 10.3109/14767058.2010.550977. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R.T., Wysocka M., Hieny S., Scharton-Kersten T., Cheever A., Kuhn R., Muller W., Trinchieri G., Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFNgamma and TNFalpha. J. Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- Gonzalez I.T., Barrientos G., Freitag N., Otto T., Thijssen V.L., Moschansky P., von K.P., Klapp B.F., Winterhager E., Bauersachs S., Blois S.M. Uterine NK cells are critical in shaping DC immunogenic functions compatible with pregnancy progression. PLoS. ONE. 2012;7:e46755. doi: 10.1371/journal.pone.0046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin L.R., Moldenhauer L.M., Prins J.R., Bromfield J.J., Hayball J.D., Robertson S.A. Seminal fluid regulates accumulation of Foxp3+ regulatory T cells in the preimplantation mouse uterus through expanding the Foxp3+ cell pool and CCL19-mediated recruitment. Biol. Reprod. 2011;85:397–408. doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- Guimond M.J., Luross J.A., Wang B., Terhorst C., Danial S., Croy B.A. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol. Reprod. 1997;56:169–179. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- Guleria I., Khosroshahi A., Ansari M.J., Habicht A., Azuma M., Yagita H., Noelle R.J., Coyle A., Mellor A.L., Khoury S.J., Sayegh M.H. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria I., Pollard J.W. The trophoblast is a component of the innate immune system during pregnancy. Nat. Med. 2000;6:589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- Guleria I., Pollard J.W. Aberrant macrophage and neutrophil population dynamics and impaired Th1 response to Listeria monocytogenes in colony-stimulating factor 1-deficient mice. Infect. Immun. 2001;69:1795–1807. doi: 10.1128/IAI.69.3.1795-1807.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyang A., Saunders M. Salmonella Mississippi: a rare cause of second trimester miscarriage. Arch. Gynecol. Obstet. 2008;277:437–438. doi: 10.1007/s00404-007-0506-2. [DOI] [PubMed] [Google Scholar]
- Hamrick T.S., Horton J.R., Spears P.A., Havell E.A., Smoak I.W., Orndorff P.E. Influence of pregnancy on the pathogenesis of listeriosis in mice inoculated intragastrically. Infect. Immun. 2003;71:5202–5209. doi: 10.1128/IAI.71.9.5202-5209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.W., Redline R.W., Li M., Yin L., Hill G.B., McCormick T.S. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect. Immun. 2004;72:2272–2279. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harger J.H., Ernest J.M., Thurnau G.R., Moawad A., Momirova V., Landon M.B., Paul R., Miodovnik M., Dombrowski M., Sibai B., Van D.P. Risk factors and outcome of varicella-zoster virus pneumonia in pregnant women. J. Infect. Dis. 2002;185:422–427. doi: 10.1086/338832. [DOI] [PubMed] [Google Scholar]
- Hayslett J.P. Maternal and fetal complications in pregnant women with systemic lupus erythematosus. Am. J. Kidney Dis. 1991;17:123–126. doi: 10.1016/s0272-6386(12)81115-2. [DOI] [PubMed] [Google Scholar]
- Hedriana H.L., Mitchell J.L., Williams S.B. Salmonella typhi chorioamnionitis in a human immunodeficiency virus-infected pregnant woman. A case report. J. Reprod. Med. 1995;40:157–159. [PubMed] [Google Scholar]
- Houser B.L., Tilburgs T., Hill J., Nicotra M.L., Strominger J.L. Two unique human decidual macrophage populations. J. Immunol. 2011;186:2633–2642. doi: 10.4049/jimmunol.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid L., Marinho C.R., Staalsoe T., Penha-Goncalves C. Of mice and women: rodent models of placental malaria. Trends Parasitol. 2010;26:412–419. doi: 10.1016/j.pt.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Ilievski V., Hirsch E. Synergy between viral and bacterial Toll-like receptors leads to amplification of inflammatory responses and preterm labor in the mouse. Biol. Reprod. 2010;83:767–773. doi: 10.1095/biolreprod.110.085464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Ishii K.J., Shirota H., Klinman D.M. CpG oligodeoxynucleotides improve the survival of pregnant and fetal mice following Listeria monocytogenes infection. Infect. Immun. 2004;72:3543–3548. doi: 10.1128/IAI.72.6.3543-3548.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D.J., Honein M.A., Rasmussen S.A., Williams J.L., Swerdlow D.L., Biggerstaff M.S., Lindstrom S., Louie J.K., Christ C.M., Bohm S.R., Fonseca V.P., Ritger K.A., Kuhles D.J., Eggers P., Bruce H., Davidson H.A., Lutterloh E., Harris M.L., Burke C., Cocoros N., Finelli L., MacFarlane K.F., Shu B., Olsen S.J. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- Jamieson D.J., Theiler R.N., Rasmussen S.A. Emerging infections and pregnancy. Emerg. Infect. Dis. 2006;12:1638–1643. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Lee D.S., Watanabe K., Furuoka H., Suzuki H., Watarai M. Interferon-gamma promotes abortion due to Brucella infection in pregnant mice. BMC Microbiol. 2005;5:22. doi: 10.1186/1471-2180-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipple G.L., Cecere F.A. Rheumatoid arthritis and pregnancy. Rheum. Dis. Clin. North Am. 1989;15:213–239. [PubMed] [Google Scholar]
- Krey G., Frank P., Shaikly V., Barrientos G., Cordo-Russo R., Ringel F., Moschansky P., Chernukhin I.V., Metodiev M., Fernandez N., Klapp B.F., Arck P.C., Blois S.M. In vivo dendritic cell depletion reduces breeding efficiency, affecting implantation and early placental development in mice. J. Mol. Med. (Berl.) 2008;86:999–1011. doi: 10.1007/s00109-008-0379-2. [DOI] [PubMed] [Google Scholar]
- Krishnan L., Guilbert L.J., Russell A.S., Wegmann T.G., Mosmann T.R., Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFNgamma response and increased production of T helper 2 cytokines. J. Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- Krishnan L., Guilbert L.J., Wegmann T.G., Belosevic M., Mosmann T.R. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions, Correlation with increased IFNgamma and TNF and reduced IL-10 production by placental cells. J. Immunol. 1996;156:653–662. [PubMed] [Google Scholar]
- Krishnan L., Pejcic-Karapetrovic B., Gurnani K., Zafer A., Sad S. Pregnancy does not deter the development of a potent maternal protective CD8+ T-cell acquired immune response against Listeria monocytogenes despite preferential placental colonization. Am. J. Reprod. Immunol. 2010;63:54–65. doi: 10.1111/j.1600-0897.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- Le Monnier A., Autret N., Join-Lambert O.F., Jaubert F., Charbit A., Berche P., Kayal S. ActA is required for crossing of the fetoplacental barrier by Listeria monocytogenes. Infect. Immun. 2007;75:950–957. doi: 10.1128/IAI.01570-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Monnier A., Join-Lambert O.F., Jaubert F., Berche P., Kayal S. Invasion of the placenta during murine listeriosis. Infect. Immun. 2006;74:663–672. doi: 10.1128/IAI.74.1.663-672.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M., Nelson D.M., Smith S.D., Khun H., Huerre M., Vacher-Lavenu M.C., Gordon J.I., Cossart P. Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes: role of internalin interaction with trophoblast E-cadherin. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6152–6157. doi: 10.1073/pnas.0401434101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.K., Kim J.Y., Lee M., Gilman-Sachs A., Kwak-Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am. J. Reprod. Immunol. 2012;67:311–318. doi: 10.1111/j.1600-0897.2012.01116.x. [DOI] [PubMed] [Google Scholar]
- Lim H.J., Wang H. Uterine disorders and pregnancy complications: insights from mouse models. J. Clin. Invest. 2010;120:1004–1015. doi: 10.1172/JCI41210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Smith M.A., Champagne C., Elter J., Beck J., Offenbacher S. Porphyromonas gingivalis infection during pregnancy increases maternal tumor necrosis factor alpha, suppresses maternal interleukin-10, and enhances fetal growth restriction and resorption in mice. Infect. Immun. 2003;71:5156–5162. doi: 10.1128/IAI.71.9.5156-5162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Mosmann T.R., Guilbert L., Tuntipopipat S., Wegmann T.G. Synthesis of T helper 2-type cytokines at the maternal–fetal interface. J. Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- Liu H., Redline R.W., Han Y.W. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J. Immunol. 2007;179:2501–2508. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- Luft B.J., Remington J.S. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infections in mice. Infect. Immun. 1982;38:1164–1171. doi: 10.1128/iai.38.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu R.A., Gurnani K., Dudani R., Kammara R., van Faassen H., Sirard J.C., Krishnan L., Sad S. Delayed expansion and contraction of CD8+ T Cell response during infection with virulent Salmonella typhimurium. J. Immunol. 2006;177:1516–1525. doi: 10.4049/jimmunol.177.3.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin G., Aldridge J.R., Duan S., Ghoneim H.E., Rehg J., Marjuki H., Boon A.C., McCullers J.A., Webby R.J. Fatal outcome of pandemic H1N1 2009 influenza virus infection is associated with immunopathology and impaired lung repair, not enhanced viral burden, in pregnant mice. J. Virol. 2011;85:11208–11219. doi: 10.1128/JVI.00654-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho C.R., Neres R., Epiphanio S., Goncalves L.A., Catarino M.B., Penha-Goncalves C. Recrudescent Plasmodium berghei from pregnant mice displays enhanced binding to the placenta and induces protection in multigravida. PLoS ONE. 2009;4:e5630. doi: 10.1371/journal.pone.0005630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh S., Maidji E., Ma W., Chang H.T., Fisher S., Pereira L. Viral and bacterial pathogens at the maternal–fetal interface. J. Infect. Dis. 2004;190:826–834. doi: 10.1086/422330. [DOI] [PubMed] [Google Scholar]
- Megnekou R., Hviid L., Staalsoe T. Variant-specific immunity to Plasmodium berghei in pregnant mice. Infect. Immun. 2009;77:1827–1834. doi: 10.1128/IAI.01321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies F.M., Henriquez F.L., Roberts C.W. Immunological control of congenital toxoplasmosis in the murine model. Immunol. Lett. 2008;115:83–89. doi: 10.1016/j.imlet.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Miller C.M., Boulter N.R., Ikin R.J., Smith N.C. The immunobiology of the innate response to Toxoplasma gondii. Int. J. Parasitol. 2009;39:23–39. doi: 10.1016/j.ijpara.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Moffett-King A., Entrican G., Ellis S., Hutchinson J., Bainbridge D. Natural killer cells and reproduction. Trends Immunol. 2002;23:332–333. doi: 10.1016/s1471-4906(02)02261-5. [DOI] [PubMed] [Google Scholar]
- Montoya J.G., Remington J.S. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 2008;47:554–566. doi: 10.1086/590149. [DOI] [PubMed] [Google Scholar]
- Mor G. Inflammation and pregnancy: the role of Toll-like receptors in trophoblast-immune interaction. Ann. N. Y. Acad. Sci. 2008;1127:121–128. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- Mun H.S., Aosai F., Yano A. Role of Toxoplasma gondii HSP70 and Toxoplasma gondii HSP30/bag1 in antibody formation and prophylactic immunity in mice experimentally infected with Toxoplasma gondii. Microbiol. Immunol. 1999;43:471–479. doi: 10.1111/j.1348-0421.1999.tb02430.x. [DOI] [PubMed] [Google Scholar]
- Murphy S.P., Tayade C., Ashkar A.A., Hatta K., Zhang J., Croy B.A. Interferon gamma in successful pregnancies. Biol. Reprod. 2009;80:848–859. doi: 10.1095/biolreprod.108.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu T., Schust D.J. The contribution of macrophages to normal and pathological pregnancies. Am. J. Reprod. Immunol. 2010;63:460–471. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- Nancy P., Tagliani E., Tay C.S., Asp P., Levy D.E., Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal–fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neres R., Marinho C.R., Goncalves L.A., Catarino M.B., Penha-Goncalves C. Pregnancy outcome and placenta pathology in Plasmodium berghei ANKA infected mice reproduce the pathogenesis of severe malaria in pregnant women. PLoS ONE. 2008;3:e1608. doi: 10.1371/journal.pone.0001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer O., Cebesoy F.B., Sari I., Davutoglu V. A case of Salmonella typhi endocarditis in pregnancy. Am. J. Med. Sci. 2009;337:210–211. doi: 10.1097/MAJ.0b013e31818128a3. [DOI] [PubMed] [Google Scholar]
- Pappas G., Roussos N., Falagas M.E. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 2009;39:1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Pazos M., Sperling R.S., Moran T.M., Kraus T.A. The influence of pregnancy on systemic immunity. Immunol. Res. 2012 doi: 10.1007/s12026-012-8303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M.A., Kraus T.A., Munoz-Fontela C., Moran T.M. Estrogen mediates innate and adaptive immune alterations to influenza infection in pregnant mice. PLoS ONE. 2012;7:e40502. doi: 10.1371/journal.pone.0040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejcic-Karapetrovic B., Gurnani K., Russell M.S., Finlay B.B., Sad S., Krishnan L. Pregnancy impairs the innate immune resistance to Salmonella typhimurium leading to rapid fatal infection. J. Immunol. 2007;179:6088–6096. doi: 10.4049/jimmunol.179.9.6088. [DOI] [PubMed] [Google Scholar]
- Poovassery J.S., Sarr D., Smith G., Nagy T., Moore J.M. Malaria-induced murine pregnancy failure: distinct roles for IFNgamma and TNF. J. Immunol. 2009;183:5342–5349. doi: 10.4049/jimmunol.0901669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline R.W. Placental inflammation. Semin. Neonatol. 2004;9:265–274. doi: 10.1016/j.siny.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Robbins J.R., Bakardjiev A.I. Pathogens and the placental fortress. Curr. Opin. Microbiol. 2012;15:36–43. doi: 10.1016/j.mib.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C.W., Alexander J. Studies on a murine model of congenital toxoplasmosis: vertical disease transmission only occurs in BALB/c mice infected for the first time during pregnancy. Parasitology. 1992;104(Pt 1):19–23. doi: 10.1017/s0031182000060753. [DOI] [PubMed] [Google Scholar]
- Rossant J., Cross J.C. Placental development: lessons from mouse mutants. Nat. Rev. Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- Rowe J.H., Ertelt J.M., Aguilera M.N., Farrar M.A., Way S.S. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10:54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S., Nakashima A., Shima T., Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- Samstein R.M., Josefowicz S.Z., Arvey A., Treuting P.M., Rudensky A.Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal–fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R.L., Zhang S., Tsolis R.M., Kingsley R.A., Adams L.G., Baumler A.J. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 2001;3:1335–1344. doi: 10.1016/s1286-4579(01)01495-2. [DOI] [PubMed] [Google Scholar]
- Schloesser R.L., Schaefer V., Groll A.H. Fatal transplacental infection with non-typhoidal Salmonella. Scand. J. Infect. Dis. 2004;36:773–774. doi: 10.1080/00365540410020802. [DOI] [PubMed] [Google Scholar]
- Scialli A.R., Rarick T.L. Salmonella sepsis and second-trimester pregnancy loss. Obstet. Gynecol. 1992;79:820–821. [PubMed] [Google Scholar]
- Seveau S., Pizarro-Cerda J., Cossart P. Molecular mechanisms exploited by Listeria monocytogenes during host cell invasion. Microbes Infect. 2007;9:1167–1175. doi: 10.1016/j.micinf.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Shiono Y., Mun H.S., He N., Nakazaki Y., Fang H., Furuya M., Aosai F., Yano A. Maternal–fetal transmission of Toxoplasma gondii in interferon-gamma deficient pregnant mice. Parasitol. Int. 2007;56:141–148. doi: 10.1016/j.parint.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Shirahata T., Muroya N., Ohta C., Goto H., Nakane A. Correlation between increased susceptibility to primary Toxoplasma gondii infection and depressed production of gamma interferon in pregnant mice. Microbiol. Immunol. 1992;36:81–91. doi: 10.1111/j.1348-0421.1992.tb01644.x. [DOI] [PubMed] [Google Scholar]
- Shirahata T., Muroya N., Ohta C., Goto H., Nakane A. Enhancement by recombinant human interleukin 2 of host resistance to Toxoplasma gondii infection in pregnant mice. Microbiol. Immunol. 1993;37:583–590. doi: 10.1111/j.1348-0421.1993.tb01680.x. [DOI] [PubMed] [Google Scholar]
- Shirahata T., Yamashita T., Ohta C., Goto H., Nakane A. CD8+ T lymphocytes are the major cell population involved in the early gamma interferon response and resistance to acute primary Toxoplasma gondii infection in mice. Microbiol. Immunol. 1994;38:789–796. doi: 10.1111/j.1348-0421.1994.tb01858.x. [DOI] [PubMed] [Google Scholar]
- Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Lepidi H., Mege J.L., Marrie T.J., Raoult D. Repeated pregnancies in BALB/c mice infected with Coxiella burnetii cause disseminated infection, resulting in stillbirth and endocarditis. J. Infect. Dis. 2000;181:188–194. doi: 10.1086/315166. [DOI] [PubMed] [Google Scholar]
- Stephens R., Culleton R.L., Lamb T.J. The contribution of Plasmodium chabaudi to our understanding of malaria. Trends Parasitol. 2012;28:73–82. doi: 10.1016/j.pt.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D.A., Riedler J., Nowak D., Braun-Fahrlander C., Swoboda I., Balic N., Chen K.W., Vrtala S., Gronlund H., van H.M., Valenta R., Spitzauer S., von M.E., Vercelli D. Exposure to a farming environment has allergen-specific protective effects on Th2-dependent isotype switching in response to common inhalants. J. Allergy Clin. Immunol. 2007;119:351–358. doi: 10.1016/j.jaci.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Su Z., Stevenson M.M. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect. Immun. 2000;68:4399–4406. doi: 10.1128/iai.68.8.4399-4406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez Y., Ferreira R.B., Finlay B.B. Molecular mechanisms of Salmonella virulence and host resistance. Curr. Top. Microbiol. Immunol. 2009;337:93–127. doi: 10.1007/978-3-642-01846-6_4. [DOI] [PubMed] [Google Scholar]
- van der Klooster J.M., Roelofs H.J. Management of Salmonella infections during pregnancy and puerperium. Neth. J. Med. 1997;51:83–86. doi: 10.1016/s0300-2977(97)00037-5. [DOI] [PubMed] [Google Scholar]
- Warning J.C., McCracken S.A., Morris J.M. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction. 2011;141:715–724. doi: 10.1530/REP-10-0360. [DOI] [PubMed] [Google Scholar]
- Wegmann T.G., Lin H., Guilbert L., Mosmann T.R. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a Th2 phenomenon? [see comments] Immunol. Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Witkin S.S., Linhares I.M., Bongiovanni A.M., Herway C., Skupski D. Unique alterations in infection-induced immune activation during pregnancy. BJOG. 2011;118:145–153. doi: 10.1111/j.1471-0528.2010.02773.x. [DOI] [PubMed] [Google Scholar]
- Wong S.F., Chow K.M., de S.M. Severe acute respiratory syndrome and pregnancy. BJOG. 2003;110:641–642. doi: 10.1046/j.1471-0528.2003.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldovich V.B., Robbins J.R., Kapidzic M., Lauer P., Bakardjiev A.I. Invasive extravillous trophoblasts restrict intracellular growth and spread of Listeria monocytogenes. PLoS Pathog. 2011;7:e1002005. doi: 10.1371/journal.ppat.1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenclussen A.C., Gerlof K., Zenclussen M.L., Ritschel S., Zambon B.A., Fest S., Hontsu S., Ueha S., Matsushima K., Leber J., Volk H.D. Regulatory T cells induce a privileged tolerant microenvironment at the fetal–maternal interface. Eur. J. Immunol. 2006;36:82–94. doi: 10.1002/eji.200535428. [DOI] [PubMed] [Google Scholar]