Summary
Tissues such as the genital tract, skin, and lung act as barriers against invading pathogens. To protect the host, incoming microbes must be quickly and efficiently controlled by the immune system at the portal of entry. Memory is a hallmark of the adaptive immune system, which confers long-term protection and is the basis for efficacious vaccines. While the majority of existing vaccines rely on circulating antibody for protection, struggles to develop antibody-based vaccines against infections such as herpes simplex virus (HSV) and human immunodeficiency virus (HIV) have underscored the need to generate memory T cells for robust antiviral control. The circulating memory T-cell population is generally divided into two subsets: effector memory (TEM) and central memory (TCM). These two subsets can be distinguished by their localization, as TCM home to secondary lymphoid organs and TEM circulate through non-lymphoid tissues. More recently, studies have identified a third subset, called tissue-resident memory (TRM) cells, based on its migratory properties. This subset is found in peripheral tissues that require expression of specific chemoattractants and homing receptors for T-cell recruitment and retention, including barrier sites such as the skin and genital tract. In this review, we categorize different tissues in the body based on patterns of memory T-cell migration and tissue residency. This review also describes the rules for TRM generation and the properties that distinguish them from circulating TEM and TCM cells. Finally, based on the failure of recent T-cell-based vaccines to provide optimal protection, we also discuss the potential role of TRM cells in vaccine design against microbes that invade through the peripheral tissues and highlight new vaccination strategies that take advantage of this newly described memory T-cell subset.
Introduction
The development of vaccines is one of the most significant achievements of modern medicine. The use of vaccines has eliminated the threat of many debilitating and deadly diseases around the world. The lasting protection that vaccines provide depends on the ability of the immune system to generate memory against a given pathogen. While all successful vaccines thus far have relied almost solely on production of circulating antibody for protection, focus has recently shifted to T-cell-based vaccines in the face of global health threats such as human immunodeficiency virus (HIV). HIV and other sexually transmitted infections (STIs) such as herpes simplex virus (HSV) pose unique challenges in the design of an efficacious vaccine, due to both the nature of the pathogen as well as primary site of transmission.
The T-cell response to almost any immunogen occurs in three major steps: priming, expansion, and contraction. Naive T cells are largely quiescent, and they circulate through secondary lymphoid tissues at very low precursor frequencies (1). After engagement of T cell by an antigen-presenting cell via the peptide and major histocompatibility complex (MHC) and costimulatory molecules, the T cell becomes activated, or primed. Primed T cells begin to divide, thus initiating the expansion phase, during which the naive T cell differentiates into a heterogeneous population of effector T cells and acquires properties such as cytokine production and cytolytic capacity for CD8+ T cells (2). After the expansion phase, the effector T-cell population begins to contract. During this contraction phase, 90–95% of the activated T-cell pool dies, while the remaining 5–10% go on to differentiate into different types of memory T cells (2). This model of memory T-cell differentiation generally occurs after acute infection, when antigen is cleared from the host (3).
Tissues such as the skin or mucosal lining of the respiratory tract, gut, and genital tract stand as barriers against pathogen invasion. Many infectious diseases with the highest rates of morbidity and mortality begin primarily as local infections at one of these barrier sites. For example, HIV is often contracted through the genital mucosa, where infection starts with replication of a single founder virus (4, 5) in a local pool of CD4+ T cells before becoming systemic (6–8). While these tissues possess intrinsic defense mechanisms such as the production of defensins and other antimicrobial peptides (9), the immune system is critical for optimal control and elimination of invading microbes at these barriers. While systemic immunity, particularly circulating antibody, may be sufficient in protecting these peripheral sites against certain pathogens, the establishment of tissue-resident memory T cells (TRM) may be required for optimal control of pathogens such as HSV. Further understanding of how tissue-resident memory T-cell populations are generated and maintained in peripheral tissues such as skin and mucosa will aid in the design of not only vaccines but also immunotherapies for a wide variety of infections and diseases.
Education of T cells for tissue homing
Upon infection at a mucosal surface, antigen is carried into the draining lymph node (dLN) by dendritic cells (DCs) or drained directly into the dLN via the lymphatics (10). Upon reaching the lymph node, the DCs present antigen to naive T cells to induce their proliferation and differentiation. Activated T cells then leave the dLN and enter systemic circulation and are recruited to peripheral tissues through the engagement of chemokine receptors and adhesion molecules (11). T cells in circulation enter the tissue through three stages of activation: rolling and tethering to endothelial cells mediated by selectins, followed by chemokine-mediated activation of integrins, which promote firm adhesion and diapedesis (12). Trafficking of activated T cells to peripheral sites such as the skin and gut is mediated by pathways specific for each tissue. Skin-homing T cells are positive for P-selectin ligands and E-selectin ligands, including PSGL-1, cutaneous lymphocyte-associated antigen (CLA), CD43, and CD44 (13–16). Binding of these ligands to the selectins expressed on inflamed skin induces rolling on the vascular endothelium. T cells expressing the chemokine receptors CCR4 (17, 18) and CCR10 (19, 20) are stimulated via their respective chemokine ligands, CCL17 and CCL27, to activate integrins, which enable adhesion and extravasation into the tissue. Both chemokines are present in cutaneous blood vessels but not intestinal vessels (17, 20) and play essential roles in recruiting T cells to the skin during homeostasis and inflammation. T cells expressing CCR6 (21) or CCR8 (22) respond to CCL20 and CCL8, respectively, expressed in inflamed skin. Gut-homing T cells, on the other hand, express the integrin α4β7 (23), which mediates T-cell binding to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) at steady state (24, 25). MAdCAM-1 is expressed by intestinal but not cutaneous vascular endothelium (26). Although α4β7 is important for migration of T cells to the gut at homeostasis, during rotavirus infection, CD8+ T cells migrate to the gut independently of α4β7 (27), suggesting that migration to the gut is not entirely dependent on this integrin. Furthermore, a subset of α4β7 T cells is positive for CCR9, which binds to the chemokine CCL25 (28). Almost all small intestinal intraepithelial (IEL) and lamina propria (LP) T cells in mice and humans express CCR9, suggesting that this chemokine receptor is important for localization (28–30).
The expression of a particular set of homing receptors can help to determine the eventual destination of an activated T cell. Immunization through mucosal or cutaneous routes leads to the upregulation of gut- and skin-homing receptors, respectively (31). More specifically, it has been shown that tissue-associated DCs appear to be capable of imprinting the tropism of a T cell during the priming phase. Indeed, in vitro activation of T cells with DCs harvested from the peripheral lymph nodes (pLNs) can induce expression of E- and P-selectin ligands as well as responsiveness to CCR4 ligands (32). DCs isolated from Peyer’s patches (PPs) or the mesenteric lymph node (mLN) can induce expression of α4β7 as well as expression of CCR9 (33). The ability of these skin- or gut-associated DCs to promote a T-cell migration program to their respective tissues has been linked to the availability of certain metabolites that are specific for the skin or gut. For example, the expression of α4β7 and CCR9 is enhanced by the production of retinoic acid (RA), which is derived from dietary vitamin A (34–36). DCs from the mLNs and PPs, but not from pLNs, express essential enzymes required to convert retinal to RA, and these DCs produce RA during T-cell activation, which then acts on the T cell via the retinoic acid receptor (RAR) (34). In the skin, DCs convert vitamin D3, which is highly expressed in the epidermis upon exposure to UV radiation, into its active form, 1,25(OH)D3 (37). Along with IL-12, 1,25(OH)D3 induces expression of CCR10 on T cells (37). Thus, local DCs utilize unique factors that are present in peripheral tissues to imprint T cells with a tropism for that particular tissue.
Beyond the role of DCs, in vivo studies have shown that lymph node stromal cells and cells from the peripheral tissue can also influence the migratory program of activated T cells. Replacement of excised mLNs with transplanted pLNs demonstrated that T cells primed after oral administration of antigen were not able to sustain a gut-homing phenotype, despite RA production by migrating DCs that carried antigen from the tissue into the lymph node (35). In vivo, CD8+ T cells that enter the mesenteric lymph node after skin infection with vaccinia virus (VV) can briefly acquire a gut-homing phenotype (38). Furthermore, in a model of DC adoptive transfer, expression of homing receptors on T cells appears to depend more on the route by which the DCs are injected rather than the tissue origin of the DCs themselves (39, 40). As the DCs from the gut are able to suppress the expression of homing receptors to the skin on activated T cells in vitro (32), collectively these studies suggest that while tissue-derived DCs can initiate the programming of tissue tropism of T cells, additional signals from other cell types may be required to sustain the imprint. Indeed, stromal cells in the mLN express retinaldehyde dehydrogenase (RALDH) and may produce enough RA to induce upregulation of CCR9 on activated T cells (36).
For entry into tissues such as the brain, T cells may be programmed at sites that are entirely independent from the central nervous system. In a rat model of experimental autoimmune encephalitis (EAE), activated antigen-specific T cell blasts that were adoptively transferred into new hosts gained the capacity to enter the CNS only after a brief residence in the lung (41). Priming in CNS-associated lymphoid organs was proven unnecessary, as the T cells were activated via subcutaneous (s.c.) immunization and then expanded in vitro (41). While the mechanism by which target tissue-independent programming occurs is unclear, both peripheral tissue and tissue-associated DCs appear to be important for both the imprinting and maintenance of a particular homing program.
Promiscuous migration of effector T cells
As discussed above, local activation of T cells within the dLN of a peripheral organ such as the skin or gut can induce strong tropism for that particular tissue through high expression of homing receptors. However, T-cell migration appears to be more promiscuous during the effector phase of an immune response. T cells activated at any site, whether through local or systemic priming, can gain the capacity to circulate through almost all tissues in the body. Systemic priming of T cells after infection with lymphocytic choriomeningitis virus (LCMV) leads to the migration of activated antigen-specific T cells in many peripheral sites including the gut (42). Local immunizations such intranasal (i.n.) immunization with Sendai virus also leads to the migration of antigen-specific T cells to the gut as well as other tissues (42). Further examination reveals that activation of T cells in any secondary lymphoid organs (SLOs) can lead to the upregulation of homing receptors required to migrate to tissues such as the gut and skin, perhaps through the dissemination of early effector T cells to distal LNs (38). This suggests that programming T cell trafficking to a particular tissue is not necessarily dependent on priming in lymph nodes draining that site. The programming is also plastic, as T cells that have been primed in LNs associated with the gut can be ‘reprogrammed’ upon a second antigen encounter, thus changing the tropism of the cell (43). Similarly, Sendai-virus specific CD8+ T cells that have been primed intraperitoneally are incapable of migrating to the lung airway but can be recruited to that site after a secondary intranasal challenge (44). Thus, the entry of a T cell into a peripheral tissue may be established regardless of priming site and immunization route. However, priming within the dLN of a particular tissue leads to the greatest expression of receptors required to home to the associated tissue (29), indicating that ‘seeding’ of the tissue may be greatest when they are primed in SLOs draining the target site.
Migration of memory T cells at steady state
While effector T cells are capable of trafficking to almost any tissue due to their broad expression of homing receptors, memory T cells have a more restricted pattern of migration. Heterogeneity within the memory T-cell pool was originally defined in humans by expression of the chemokine receptor, CCR7 (45). CCR7+ memory T cells, termed central memory T cells (TCM) were found to express other lymphoid homing markers such as CD62L, while CCR7− memory T cells, named effector memory T cells (TEM), were found to express other chemokine receptors and integrins that would allow for recruitment to peripheral tissues at steady state (45). Indeed, these two subsets localize to distinct anatomical sites. TCM localize primarily in SLOs, while TEM are generally distributed in nonlymphoid tissues (45, 46). However, circulation of TEM through peripheral sites is not equal for all organs. Parabiosis studies and CD8+ T-cell transfer experiments have shown that while memory CD8+ T cells can rapidly equilibrate in tissues such as SLOs, lung parenchyma, and liver, entry of resting memory T cells into tissues such as the brain, intestinal mucosa, skin, and genital tract is very limited (47–49). Expression of the homing receptors that regulate migration of antigen-experienced T cells are upregulated during the priming phase. Expression of these receptors are then rapidly downregulated during the effector stage, regardless of immunization route (42). After systemic LCMV infection, for example, CCR9, α4β7, and PSGL-1, a P-selectin ligand, are upregulated on early effector CD8+ T cells. If the activated T cells do not migrate into their target tissue, expression of the homing receptors begins to decline during the peak of the effector phase. By the memory phase, skin-homing receptors are greatly downregulated, and gut-homing receptors are absent on CD8+ T cells (29). Similarly, skin scarification with VV leads to expression of both skin- and gut-homing receptors on CD8+ T cells during the effector phase, while only skin-homing receptors are expressed on memory CD8+ T cells (38). Thus, the loss of homing capability to tissues such as the gut and skin may explain the limited migration of circulating memory CD8+ T cells into these tissues. However, if an activated T cell successfully gains entry into a peripheral site during the effector phase, expression of receptors such as CCR9 and PSGL-1 are retained (29). Transfer experiments have demonstrated that memory T cells that reside in a peripheral organ, such as the lung, retain their ability to home back to the tissue or origin when placed back in circulation, likely due to continued expression of tissue-specific homing receptors (50).
Migration of memory T cells during inflammation
Under inflammatory conditions, the rules that govern the trafficking of T cells during homeostasis become altered. Along with upregulation of tissue-homing receptors on T cells, infection or immunization in vivo leads to the expression of adhesion molecules on vascular endothelial cells. Together, these conditions enable the entry of T cells into tissues that may be inaccessible during homeostasis. For example, adhesion molecules such as E-selectin and P-selectin, which are normally limited in their expression, can be upregulated on endothelial cells at sites of inflammation (51). Also, the amount of chemokine that is basally produced under non-inflammatory conditions in tissues such as the skin can be increased by inflammation, which in turn enable enhanced T-cell recruitment. The chemokine CCL27 is normally produced by keratinocytes in skin. When patients with nickel allergy are exposed to nickel, expression of CCL27 is upregulated by the pro-inflammatory cytokines IL-1β and TNFα (20). The increase in CCL27 is accompanied by an increase in the number of CCR10+ T cells in the skin (20).
Aside from the upregulation of tissue-specific chemokines, cytokines such as interferon-γ (IFNγ) and type I IFNs can induce inflammatory chemokines. In particular, the inflammatory chemokines, monokine-induced by γ IFN (MIG) (CXCL9) and IFNγ-induced protein 10 (IP-10) (CXCL10), play important roles in the recruitment of both effector CD4+ and CD8+ T cells to a wide variety of tissues during inflammation. These chemokines, along with IFN-inducible T-cell α chemoattractant (I-TAC) (CXCL11), ligate the receptor CXCR3, which is upregulated on both T-helper 1 (Th1) and CD8+ T cells upon activation (52). CXCR3 is maintained on these T-cell subsets throughout the effector and memory phase, with expression steadily increasing as memory CD8+ T cells continue to mature (53). The production of CXCR3 ligands has been shown to regulate recruitment of effector T cells into several tissues that are otherwise restricted from T-cell entry, including the central nervous system and the female genital tract (48, 54, 55). In the genital tract, the expression of CXCL9 and CXCL10 are absolutely required for the recruitment of antigen-specific CD8+ T cells. After genital infection with HSV-2, effector CD4+ T cells first enter the vaginal mucosa via type I IFN-dependent mechanisms and secrete IFNγ at the site of infection. CD4+ T-cell secretion of IFNγ, along type I IFNs secreted by other cell types, leads to the production of CXCL9 and CXCL10 in the tissue. These inflammatory chemokines then mediate the entry of effector CD8+ T cells into the genital mucosa (48). In the lung, memory CD8+ T cells are recruited into the airways via CCR5, which is ligated by multiple inflammatory chemokines, including regulated on activation, normal T-cell expressed or secreted (RANTES) (CCL5), macrophage inflammatory protein 1α (MIP-1α)(CCL2), and MIP-1β (CCL3) (56). Thus, inflammation can lead to the production of inducible chemokines such as CXCR3 and CCR5 ligands, and these chemokines can then alter the rules that regulate T-cell migration through peripheral tissues during homeostasis. While production of tissue-specific chemokines and inflammatory chemokines are both important for recruitment of T cells to sites of inflammation, it is unclear whether any sort of relationship or hierarchy exists between migration mediated by tissue-tropic homing receptors and inflammatory chemokine receptors such as CXCR3. As discussed below, some tissues lack ‘tissue-tropic’ chemokine expression at baseline and rely solely on inflammatory chemokines to establish tissue-resident memory T cells.
Access codes for effector and memory CD8+ T cell entry and retention
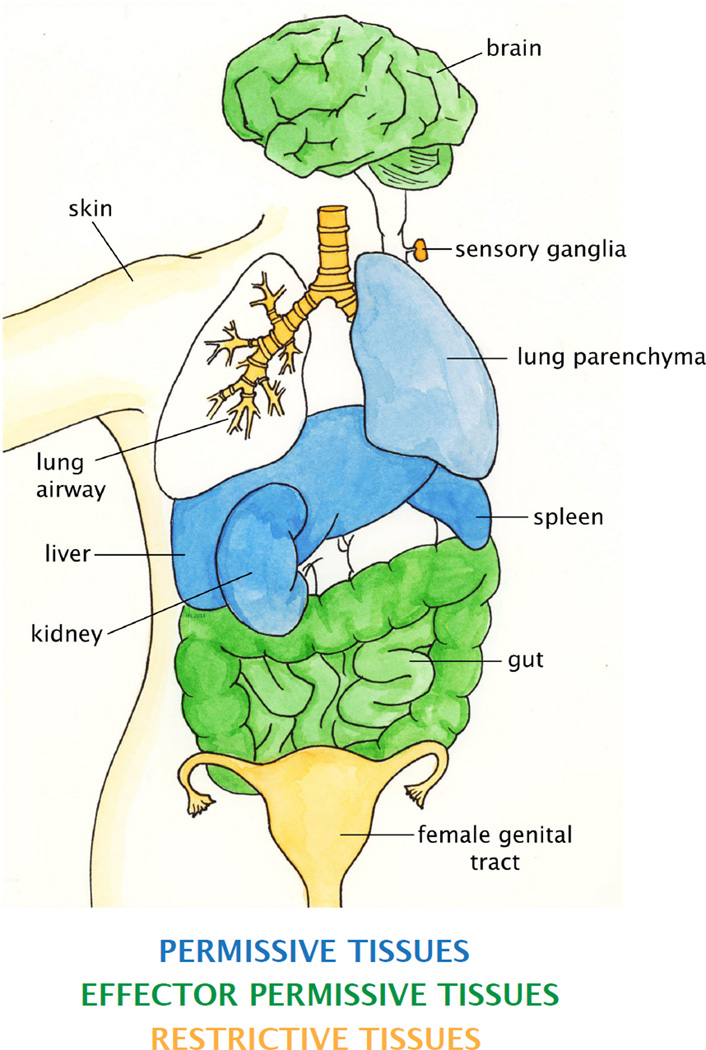
While effector CD8+ T cells can access the majority of tissues, some peripheral sites remain inaccessible by effector CD8+ T cells except under inflammatory conditions. Tissues such as the female genital mucosa do not express adhesion molecules during homeostasis and produce very little chemokines (48, 57, 58). Thus, despite high expression of CXCR3, which is the key chemokine receptor that mediates recruitment of CD8+ T cells into the vagina, neither effector nor memory CD8+ T cells circulate through the genital tract under steady state conditions (48, 59). Thus, based on these data, we characterize tissues of the body into three classes based on T cell circulation (Fig. 1, Table 1). First, ‘permissive tissues’, including organs such as the spleen, lung parenchyma, liver, kidney, and adipose tissue, are readily accessibly by both effector and CD8+ TEM without the need for any local inflammation or antigen (42, 47). Second, ‘effector permissive tissues’ are accessible by effector CD8+ T cells but not by memory CD8+ TEM and TCM. These tissues, including the gut, brain, and peritoneal cavity, are generally seeded by CD8+ T cells early during the effector phase, when homing receptors are broadly expressed on T cells (29, 47, 60). Effector CD8+ T-cell migration to these tissues does not appear to require direct infection or immunization of the tissue itself, although the presence of antigen or inflammation may enhance recruitment. After the effector phase, effector permissive tissues become inaccessible to circulating memory CD8+ T cells. A third group of organs are inaccessibly by either effector or memory CD8+ T cells at steady state. These ‘restrictive tissues’ lack tissue-tropic chemokines or adhesion molecules and are only accessible by effector CD8+ T cells when there is local inflammation leading to production of inflammatory chemokines. This third group of tissues includes skin epidermis, vaginal epithelial layer, salivary glands, lung airways, and ganglia (48, 61–64). However, both systemic and local infection can result in soluble inflammatory mediators that may affect the microenvironment of distal tissues (65). Thus, systemic and even local immunization can often result in a very low level of access for effector T cells into restrictive tissues.
Fig. 1. Categorization of tissues by T-cell migration properties.
Examples of organs that are defined as permissive tissues are shown in blue. Examples of effector permissive tissues are show in green, and restrictive tissues are shown in yellow. Not all organs that fall under each category are shown.
Table 1.
Categorization of tissues based on T cell accessibility
| CATEGORY | ACCESSIBILITY | ORGANS | TRM? |
|---|---|---|---|
| Permissive | Steady state or inflammation: effector and memory CD4 and CD8 | Secondary lymphoid organs, liver, bone marrow, | No |
| Effector permissive | Steady state: effector CD4 and CD8 Inflammation: effector and memory CD4 and CD8 | Gut, brain, peritoneal cavity | Yes |
| Restrictive | Steady state: no trafficking Inflammation: effector and memory CD4 and CD8 | Skin, vagina, lung airways, salivary glands, ganglia | Yes |
First column describes three types of tissues. Second column shows the requirements for effector and memory T cell trafficking into each of those types of tissues. Third column lists examples of the organs that fall under each category described in column 1. Fourth column indicates whether tissue-resident memory T cells are established in the tissue category.
Evidence for tissue-resident memory T cells
While the delineation of TEM and TCM remains a useful way of describing heterogeneity within the memory T-cell pool, it is becoming evident that the composition of the memory T-cell pool is more complex and dynamic. As described above, parabiosis and transplant experiments used to study memory CD8+ T-cell trafficking make it clear that resting memory CD8+ T cells cannot enter restrictive tissues. However, they also demonstrate that memory CD8+ T cells already present in tissues such as the gut, brain, and skin do not re-enter circulation and are maintained independently of the circulating memory population (29, 47, 60, 61). Experiments in which the dorsal root ganglia (DRG) of herpes simplex virus-1 (HSV-1)-infected mice were excised and placed under the kidney capsule of recipient mice also showed that the virus-specific memory CD8+ T cells within the DRG do not equilibrate with the circulating population, suggesting that the peripheral nervous system can also harbor this resident population of memory T cells (61). Multiple strategies have been used to show that the skin also contains this unique population of memory T cells. Transplantation and parabiosis studies have shown that memory CD8+ T cells that reside in the skin after infection do not recirculate (49, 61). Furthermore, when male T cells are adoptively transferred into female recipients, only male CD8+ T cells that migrate into the skin after infection survive, suggesting that the sequestration of T cells within the skin protected the skin-resident CD8+ T cells from rejection by the host immune system (66). Finally, work from our lab using HSV-2-infected parabiotic pairs also reveals that memory T-cell populations established in the genital tract do not circulate (authors’ unpublished observations). Aside from localization differences, studies examining the molecular signature of CD8+ memory T cells in the brain show that the transcriptional profile of these resident cells is different from that of circulating memory CD8+ T cells (67), which raises the intriguing question as to whether these profiles differ from tissue to tissue. Collectively, these studies make the argument that these non-circulating tissue-specific memory CD8+ T cells should be considered a separate subset called tissue-resident memory T cells (TRM). In conjunction with the rules of effector and memory CD8+ T-cell migration into tissues, permissive tissues generally do not harbor TRM. However, effector CD8+ T cells that seed the effector permissive tissues or restrictive tissues become resident memory cells if there are signals to retain them locally (Table 1). The retention signal may be in the form of antigen in the lung airways (63) or a transient burst of local chemokines in the skin and vagina (59, 68).
Although both CD4+ and CD8+ T cells can express homing receptors such as CCR4 and α4β7 that guide trafficking into tissues such as the skin and gut respectively, recent reports have highlighted differences in the pattern of CD4+ and CD8+ T-cell migration through peripheral tissues. Classic studies have shown that when cells are collected from the gut draining lymph of sheep, the majority of the population is composed of activated CD4+ T cells (69). Furthermore, when transferred back into the host, the CD4+ T cells migrate mainly to the lymph node, while the CD8+ T cells draining from the gut return to the gut, suggesting that these subsets had different trafficking patterns (69). Similarly, human afferent lymph is also mainly composed of memory-phenotype CD4 T cells (70). Indeed, more recent work with a skin model of HSV infection has shown that antigen-specific memory CD4+ and CD8+ T cells do engage in disparate migratory behavior and occupy different anatomical niches within the same tissue. After HSV infection, effector CD8+ T cells localized primarily to the epidermis, while effector CD4+ T cells were found in both the dermis and the epidermis (66). Effector CD4+ T cells within the dermis are highly motile, while both CD4+ and CD8+ T cells in the epidermis were far less active. These differences are accentuated during the memory phase, with memory CD8+ T cells within the dermis taking on a dendritic morphology. This CD8+ T-cell population forms a compartment within the epidermal layer that was maintained independently of circulating memory T cells, while memory CD4+ T cells, which localized primarily to the dermis, re-entered circulation. However, CD4+ T cells are not necessarily excluded from forming tissue-resident populations. Although not as commonly described as CD8+ TRM, parabiosis of mice after influenza infection have identified a pool of non-circulating memory CD4+ T cells in the lung (50), and CD4+ T cells that exhibit TRM characteristics have also been described in the intestine and sensory ganglia (71). Furthermore, healthy human lung also contains both CD4+ and CD8+ memory T-cell populations that are phenotypically distinguishable from circulating memory T-cell populations, demonstrating that CD4+ T cells can establish tissue residency in humans (72).
The origins of the TRM subset and how this subset relates to TCM and TEM remains unclear. Even the relationship between TEM and TCM, an area of study that has been the subject of much debate, still remains unsettled (2). Between TEM and TCM, much evidence has been presented in support of two different models of differentiation. The first model suggests that TCM are direct descendants of the TEM subset (73, 74), while the second suggests that TEM and TCM are entirely separate lineages with no conversion between the two (75, 76). As the origin and function of the TEM and TCM subsets depends on how they were primed, a combination of both models likely explains their differentiation pathways. Indeed, the TEM subset appears to arise from at least two different sources. Some IL-7R (CD127)loKLRG1hi effector CD8+ T cells, termed short-lived effector T cells, can give rise to a terminally differentiated population of TEM, while CD127hiKLRG1lo effector CD8+ T cells, termed memory precursors, can give rise to long-lived TEM that can gradually convert into TCM (77–80). Furthermore, it appears that some TCM can arise directly from a small fraction of CD62LhiCD127hi memory precursors (2). How TRM fit into the model of memory T-cell differentiation is still unknown. Effector permissive and restrictive tissues containing TRM such as the gut need to be ‘seeded’ during the early effector phase by T cells expressing the appropriate homing receptors (29). Thus, it is possible that TRM differentiation is mediated by unique signals from the microenvironment of the peripheral tissue. This idea suggests that TRM arises directly from the effector T-cell pool rather than converting from the TEM pool, as TCM might do (73, 74). Indeed, work from our laboratory shows that while effector CD8+ T cells can be recruited into the genital tract via intravaginal (ivag) treatment with the chemokines CXCL9 and CXCL10, memory CD8+ T cells, despite high expression of CXCR3, are not recruited or retained in the tissue (59), suggesting that neither TEM nor TCM are capable of becoming TRM. Furthermore, the small percentage of systemic memory CD8+ T cells that express skin-homing receptors are CD62Lhi, which suggests that any memory CD8+ TCM cells in circulation are unlikely to enter peripheral tissues (66). However, the question remains as to whether TEM could acquire tissue-residency if recruited by a strong homing signal or if transplanted into a peripheral tissue. This may become an important issue to address when designing tissue-targeted vaccines or modifying the localization of T cells in patients that were previously immunized via a systemic route.
Phenotype of TRM
Along with their localization, tissue-resident memory T cells can also be identified by the expression of a common set of surface receptors. Integrin αE (CD103) is expressed on memory CD8+ T cells in many non-permissive tissues in mice, including the skin, gut, brain, genital tract, and lung airway, while expression is low on circulating memory T cells (29, 60, 61, 68, 81). Expression of CD103 has also been reported to delineate CD8+ TRM in human mucosal tissues such as the gut and lung (82). While knockdown of CD103 on murine CD8+ T cells did not affect their entry into the CNS, it did impair their accumulation within the brain over time, signifying that CD103 plays an important role in the retention of TRM (60). Furthermore, CD8 TRM in the epidermis are shown to have a dendritic morphology (66), and it appears that CD103 may play a role in regulating the unique shape of these TRM (83). The ligand for CD103, E-cadherin, is expressed on epithelial cells. As many TRM reside in the epithelial layer of restrictive tissue, it is possible that interactions between CD103 and E-cadherin are maintaining the resident status of T cells in peripheral tissues. Indeed, CD103 facilitates the adhesion of CD8+ T cells to human keratinocytes in vitro (84). In the adult mouse brain, E-cadherin expression is limited to the cells lining the ventricles (85), while TRM clusters can be found all through the parenchyma (60), suggesting that CD103 may be binding to a unique ligand within the CNS.
The expression of CD103 on TRM appears to be mediated by different factors in different tissues. Transforming growth factor-β (TGFβ) is a key mediator of CD103 expression in the skin and the gut. Furthermore, addition of TGFβ to in vitro cultures induces the expression of CD103 on human (84) and mouse activated CD8+ T cells (86). Expression of the TGFβ dominant negative type II receptor (dnTGFβRII) prevents the upregulation of CD103 on CD8+ T cells infiltrating the gut epithelium, which in turn prevents graft rejection in a model of graft-versus-host disease (87). Similarly, dnTGFβRII that migrate to the gut after LCMV infection fail to express CD103 (86). Although it is impossible to rule out the idea that there is a small fraction of circulating CD103+ T cells that may seed peripheral tissues during entry, most evidence seem to support the idea that TRM upregulate CD103 after lodging in the tissue. In tissues such as the lung and brain, CD103 expression appears to be induced by a different mechanism involving stimulation by antigen, although the role of other factors cannot be excluded. Activated OT-I CD8+ T cells, which recognize a peptide derived from the ovalbumin protein (OVA), only upregulate CD103 expression if OVA is present in the brain where the CD8+ T cells accumulate (60). Likewise, residual antigen in the lung present after influenza infection appears to drive expression of CD103 on virus-specific CD8+ T cells (81). However, CD103 expression remains upregulated on a portion of the small TRM population that is present in the lung after all antigen has been cleared after Sendai infection, which suggests that the lung microenvironment can also mediate CD103 expression after all antigen has been cleared (88). Despite the importance of CD103 in the retention of TRM in the tissue, small T-cell populations that do not express CD103 are also present. In the brain, while the CD103− TRM population is smaller than the CD103+ population, they are still present long after immunization and localize to the same clusters that CD103+ TRM do (60). However, these CD103− CD8+ T cells appear to be functionally distinct from the CD103+ population. Thus, other molecules may be important in maintaining TRM in peripheral tissues.
Another molecule that is expressed by mouse TRM in the gut, skin, brain, lung airway, sensory ganglia, and vagina is the transmembrane C-type lectin CD69 (47, 60, 61, 63, 81, 88, 89, authors’ unpublished data). CD69 is briefly expressed on all stimulated T cells and is quickly downregulated after activation. Although the role of CD69 is yet unknown, upregulation of CD69 results in the simultaneous downregulation of the sphingosine-1-phosphate receptor 1 (S1P1) via direct protein interaction (90). S1P, a lipid mediator with chemotactic properties, is a critical factor in the egress of lymphocytes from both the thymus and the lymph nodes (91). S1P levels are higher in the blood and lymph than SLOs (92), and studies propose that CD69 is upregulated during T-cell activation to promote lymphocyte retention in the lymph node during activation (90). In peripheral tissues, while S1P expression increases with inflammation, it is unclear how this amount relates to what is found in blood and lymph (93). Thus, it is possible that TRM upregulate CD69 and consequently downregulate S1P1 in order to retain TRM within peripheral tissues, although S1P1 expression has not been directly measured on this cell population. During activation, CD69 can be transiently induced on T cells by engagement of the T-cell receptor (TCR) or by exposure to type I IFNs (93). In the gut, TRM partially depend on TGFβ for CD69 expression (86). In the lung, while antigen appears to be important for CD69 expression after influenza infection (81), expression of this molecule can be induced or maintained via antigen-independent mechanisms after Sendai virus infection (44, 88). In other restrictive tissues, however, it is unclear what induces and maintains CD69 expression on TRM, and whether CD69 plays a role in the differentiation, retention, or survival of TRM. In humans, expression of CD69 appears to be more promiscuous, as populations of CD69+ memory T cells can be found in permissive organs such as the spleen (82). In mice and humans, many TRM populations are found in tissues that are in continuous contact with the outside environment while humans are also persistently infected with many viruses (94). Thus, it is possible that upregulation of CD69 may signify the detection of low levels of inflammation by TRM in their tissue of residence.
Maintenance of TRM
One hallmark of circulating memory T cells is their ability to undergo self-renewal using the γ chain cytokines IL-7 and IL-15. Accordingly, both circulating TEM and TCM express high levels of CD127 and IL-15Rβ (CD122), which confer responsiveness to IL-7 and IL-15, respectively (95–97). Both cytokines are required for the homeostatic maintenance of memory T cells, although TCM may turnover more efficiently in response to these cytokines (73, 97). It is unknown, however, whether TRM also utilize these cytokines for their survival. As TRM are maintained long-term in most tissues without any input from circulating memory T cells, they are required to be self-sustaining within the tissue. TRM in the lung and brain express lower levels of CD127 as compared to circulating memory T cells (60, 81), and TRM in the brain and skin express lower levels of CD122 (60, 61). While a lower level of receptor expression does not necessarily translate to unresponsiveness to cytokines, TRM in the skin as well as the brain appear to divide less than their splenic counterparts, indicating that homeostatic turnover, if indeed occurring, does not occur at similar rates between circulating and resident memory T cells (60, 61). Furthermore, TRM isolated from the brain are incapable of surviving outside their tissue niche (60) and do not appear to use IL-7 or IL-15 for survival (67), indicating that there may be other factors supporting the survival and turnover of TRM in restrictive tissues. Whether there are common survival factors that maintain TRM in all tissues, or whether each individual tissue provides its own unique signal, is unknown.
Antigen is another variable that may influence the maintenance of TRM in restrictive tissues. As described above, antigen may regulate the expression of CD103 and CD69 on TRM in some tissues, which in turn may determine the retention rate of memory T cells in the tissue. It is unclear whether continuous signaling by antigen or other factors such as TGFβ is necessary for the maintenance of high CD69 and CD103 levels or whether expression of these molecules can be programmed into TRM. It is also unclear whether antigen can lead to the expression of other receptors that play a role in the retention of TRM. In the brain, although VSV is cleared between 10 to 20 days post-infection (p.i.), CD103 and CD69 remain elevated up to day 30 p.i., indicating that continued presence of virus is not necessary for TRM to express these markers (60). Likewise, in the skin, while there appears to be a slight bias towards the retention of antigen-specific TRM in the skin, non-specific T cells are also retained (61). Also, effector CD8+ T cells that are recruited to the skin via inflammation alone upregulate CD103 and are maintained up to 100 days post-recruitment (68). Finally, systemic infection with LCMV, which clears within 10 days of infection, generates populations with TRM characteristics in multiple different tissues, including in the intestine, female genital tract, and the brain (98). Clearance of antigen after influenza infection at day 10 to 20 p.i. results in very few memory CD8+ T cells in the tissue, and those cells that remain in the lung are mostly CD103 and CD69lo, indicating a requirement for extended exposure to antigen or inflammation (81). Migratory DCs appear to carry viral antigen from radioresistant cells in the lung to the mediastinal lymph node, where the antigen is then presented to T cells (99). It should be noted, however, that while CD103 and CD69 may play a role in the retention of memory CD8+ T cells in the lung, maintenance of this population requires input from circulating memory CD8+ T cells, which is a departure from one of the defining characteristics of TRM (100). Clearance of antigen leads to the decline in TRM numbers within the lung airways, which correlates with decreased protection against challenge (101). After Sendai virus infection, however, small numbers of circulating memory CD8+ T cells can still be recruited to the lung and upregulate CD103 and CD69 in the absence of cognate antigen, indicating that antigen-independent mechanisms may also be important for the long-term maintenance of residual TRM populations in the airways (88). If antigen depots are required for the stable maintenance of some TRM, then it calls into question whether these TRM can truly be considered a ‘resting’ population. Memory T cells in circulation develop properly in the absence of antigen, and continued exposure to antigen and inflammation can lead to altered homeostatic and functional properties (3). Indeed, constitutive expression of OVA antigen in the gut leads to a population of donor OVA-specific CD8+ T cells (OT-I) cells in the intestinal epithelium that was CD103low, as opposed to the endogenous CD8+ intraepithelial lymphocytes (IELs) that were CD103hi (86). However, extended antigen exposure in the lung or dLN after influenza or Sendai virus infection does not appear to lead to any impairment of the functional properties of TRM. Furthermore, virus-specific TRM in DRG that are latently infected with HSV appear to engage with infected neurons by forming what appears to be an immunological synapse, indicating that they may be receiving antigenic stimulation (102). Thus, TRM in different restrictive tissues appear to have distinct requirements for antigen in their maintenance and retention. Why TRM populations in some tissues appear to require antigen while others do not is unclear.
Function of tissue-resident memory T cells
Aside from different migration patterns, circulating TCM and TEM populations have also been reported to exhibit distinct roles in protective immunity. CD8+ TCM produce a greater amount of IL-2 and have a higher proliferative potential than CD8+ TEM, but the effector and cytolytic capabilities are very similar for both subsets (73, 103). Experiments using secondary challenges show that the route of immunization as well as the type of challenge, whether local or systemic, are both important in determining which subset dominates the recall response. Using a system in which TEM and TCM are sorted and adoptively transferred to new hosts, it was shown that TCM conferred a distinct advantage in protection against systemic challenges with LCMV or listeria monocytogenes (LM) (73). Local challenges with pathogens such as Sendai virus or challenges with pathogens that propagate in peripheral organs such as vaccinia virus (VV) show that TEM are more effective in mediating a protective response (104, 105). However, the recall responses by these two subsets are highly dynamic and can change over time. At one month post immunization, TEM generate a larger recall response as compared to TCM when challenged with Sendai virus. At 15 months post immunization, however, the balance shifts, and TCM come to dominate the response (106).
In earlier studies focusing on the role of circulating TEM and TCM during challenges at peripheral tissues, it is difficult to determine whether circulating TEM are indeed conferring the bulk of protective immunity at nonlymphoid tissues or whether TRM are playing a role. TRM reside in many tissues that stand as barriers against the outside environment and thus are expected to provide a first line of defense against invading pathogens. More recent studies have elegantly shown that rapid control of infection at peripheral tissues such as the skin, lung, brain, and vagina require the presence of TRM. Skin infection with HSV-1 results in a pool of circulating, HSV-specific memory T cells as well as a population of TRM at the site of infection (61). When a skin challenge with HSV was presented in the absence of antibody, the previously inoculated skin area showed more rapid viral control than the unimmunized flank (61). This effect was dependent on T cells, as depletion of CD4+ or CD8+ T cells led to a loss of enhanced protection (61). Memory CD8+ T cells in the lung are a critical component of heterosubtypic immunity against influenza, and decline in CD8+ TRM correlates with a loss of protection (101). Furthermore, CD4+ TRM provide heightened protection against influenza challenge compared to circulating CD4+ memory T cells (107). Lung-derived CD4+ TRM show a strong tropism for lung tissue, and after adoptive transfer, lung-derived CD4+ T cells provided better control of influenza challenge than circulating spleen-derived memory CD4+ T cells (50). Brain-resident memory CD8+ T cells are also protective against an intracranial bacterial challenge as compared to circulating memory CD8+ T cells (67). Finally, TRM established in the vaginal epithelial layer via exogenous chemokine treatment provides greater protection against a lethal vaginal HSV-2 challenge as compared to circulating HSV-2 specific memory T cells (59). The presence of TRM protects mice against weight loss and clinical symptoms of disease and confers 100% survival after challenge. Protection conferred by TRM lasts at least 3 months after immunization (59). Collectively, these studies show that TRM can provide immediate and enhanced control of pathogens that invade through barrier tissues and that circulating TEM alone may not be sufficient to provide full protection against infection at peripheral sites.
Both naive and memory T cells are thought to be activated within secondary lymphoid tissues. The environment and the architecture of the lymph node and spleen are suited to bring together the many cell types and other factors that are required to initiate a T-cell response (108). Challenge of mice that lack lymph nodes, spleen, or both show that systemic secondary responses arising from TEM and TCM require secondary lymphoid tissue (109). In accordance with their expression of lymphoid homing receptors, while both TEM and TCM could respond in the absence of lymph nodes, only TCM could respond in the absence of the spleen (109). After local challenges to peripheral sites, the generation of secondary responses by TRM appears to have quite distinct requirements. Transplantation of DRG latently infected with HSV-1 to the kidney capsule of naïve recipients causes a reactivation of HSV, which in turn generates a secondary CD8+ T-cell response (71). When infected DRG that harbor either CD45.1 or CD45.2 HSV-specific CD8+ TRM are transplanted into the same kidney capsule of a naive recipient, it was shown that there is very little migratory crossover between CD45.1 and CD45.2 secondary CD8+ T-cell effector populations (71). This suggested that priming of the CD8+ TRM upon HSV reactivation does not occur in the lymph node but within the DRG itself. Furthermore, priming of CD8+ TRM within the tissue required recruitment of inflammatory monocyte-derived dendritic cells (DCs) as well the help from DRG-resident virus-specific CD4+ T cells, which supported the expansion of activated CD8+ TRM (71). Skin challenges using VV-OVA show that CD8+ TRM can control a local viral challenge even in the presence of FTY720, an S1P1 antagonist that blocks S1P signals and prevents egress of lymphocytes from SLOs (49). This indicates that skin TRM do not need to leave the epidermis and enter the draining LN to be primed. Whether this is true for other restrictive tissue such as the genital tract remains unknown.
Tissue-resident memory T cells may be uniquely suited to provide immediate responses against infection not only because of their localization but also because of their activation status. Most circulating memory T cells have a resting phenotype, with low expression of activation markers such as CD69 and CD25 (110). Memory CD8+ T cells also express low levels of effector and cytotoxic molecules (110) but can rapidly upregulate these upon TCR stimulation or γ-chain cytokine exposure (111, 112). TRM, on the other hand, have elevated levels of CD69, as described above. Furthermore, the DRG transplantation studies described above show that virus-specific CD8+ TRM stimulated can proliferate and expand in situ (71). Along with a more activated state, TRM appear to be uniquely suited to survive the harsh environment of barrier tissues. Recent studies have shown that CD103+ TRM in both the lung (113) and brain (67) have elevated IFN induced transmembrane protein-3 (IFITM3), which is an IFN-stimulated gene (ISG). In the lung, IFITM3 appears to protect CD8+ TRM against cell death after influenza challenge by preventing infection of the TRM themselves (113). TRM also have been reported to constitutively express high levels of granzyme B, a cytolytic granule that, along with perforin, is released by cytotoxic lymphocytes such as CD8+ T cells and natural killer (NK) cells to kill infected target cells. In the brain, VSV infection leads to CD103+ TRM that express high amounts of granzyme B after virus has been cleared, as opposed to CD103− memory T cells in the brain and splenic memory T cells, which express negligible levels of granzyme B (60). Similarly, in the gut, intestinal TRM that were primed by LCMV express elevated granzyme B despite the absence of antigen (86). After clearance of epithelial HSV-1 infection, TRM in the skin contain fewer perforin and granzyme transcripts as compared to the peak of the effector phase (114). HSV-specific CD8+ T cells in the ganglia, however, retain high expression of granzymes and perforin, perhaps due engagement of the TCR by latently infected or reactivating neurons (115). Indeed, it has been reported that perforin and granzyme B secretion by virus-specific CD8+ T cells towards infected neurons is critical for suppressing reactivation of HSV-1 (115). Thus, the increased expression of effector molecules such as granzyme B, may help TRM limit the spread of pathogens at the site of infection and may also help to contain or modify the infection state of latent infections such as HSV.
Use of TRM in vaccine design
Many of the diseases with the greatest burden on society are infections that begin at peripheral tissue sites. Despite yearly vaccines, respiratory infections such as influenza remain constant threats to public health, and the danger of a pandemic arises with every new strain. Other viral infections such as human immunodeficiency virus (HIV) and HSV account for a significant amount of morbidity and mortality across the world. Both of these viruses are commonly transmitted through sexual contact, thus initiating infection at the genital tract. In women, HIV infection often begins when a single virus crosses the vaginal mucosal barrier and establishes a small founder population that is composed mainly of infected CD4+ T cells in the tissue (4, 5). These infected CD4+ T cells then proliferate and produce large quantities of infectious virus, which is carried to the dLN by infected cells such as DCs (6, 7). Viral replication in the lymph node ultimately leads to the propagation and systemic dissemination of the virus (116). HSV infection also begins in the vaginal tissue, where the virus first infects and replicates in the epithelial layer. The virus then invades the nerve endings of the innervating sensory neurons and travels up the axon via active retrograde transport into the DRG (117). Once in the neuronal soma, the virus establishes latency (118). Periodically, for reasons yet unknown, the virus will reactivate and travel back down to the original tissue of infection, where it causes the painful symptoms that are associated with genital herpes. Designing a vaccine for these infections has been particularly challenging, not only because of the tropism and mutable nature of the pathogen but also because the type of immune response required to control the infection is unknown. Most successful vaccines used today rely on high titers of circulating antibody to provide long-lasting protection. However, antibody responses that confer protective immunity against these diseases have not been achieved by vaccines thus far (119, 120). Furthermore, because HIV and HSV are very rarely naturally controlled, it has been difficult to determine the factors that would be required to eliminate these viruses upon exposure. For both HIV and HSV, the role of functional T cells has been highlighted in the control of these viruses in mice, non-human primates, and humans (9, 120–128). However, the immune response that is initiated after exposure in unimmunized hosts occurs too late to be effective in controlling infection (48, 129, 130). Thus, it appears that inducing a robust T-cell response or a combination of T and B-cell responses will be key in designing an efficacious prophylactic vaccine.
As both HIV and HSV begin as local infections in a limited population of cells, in order to efficiently prevent the spread of virus, control of infection may need to occur at the portal of entry. Thus, it is possible that the T cells that can provide protection may not be established in the correct tissue, or the established population may not be substantial enough to control the infection. Indeed, a study using in situ hybridization for simian immunodeficiency virus (SIV) RNA and in situ tetramer staining of for Gag-specific CD8+ T cells in cervical and lymphoid tissues shows that viral control correlates strongly with a high effector to target ratio and that these effectors are in close proximity to RNA+ cells (131). Furthermore, mathematical models demonstrate that early control of HIV-infected cells during the non-productive stage of infection by CD8+ T cells can have a dramatic impact on the viral load set point (132). Similarly, the density of CD8+ T cells in the genital mucosa after HSV reactivation inversely correlated with severity of disease and amount of virus in the tissue, suggesting that prevention of HSV infection may also benefit from a large and rapid T-cell response (124). Thus, establishment of TRM at the portal of pathogen entry such as the genital tract by vaccines may be key in providing the swift immune responses necessary for optimal protective immunity.
One way to manipulate the localization of antigen-specific T cells generated by vaccines is by selection of the appropriate priming route. The live attenuated smallpox vaccine is one example of how establishment of TRM can provide optimal immunity against local infections. Immunization of mice with either VV or the modified vaccinia Ankara virus (MVA) vaccine via skin scarification (s.s) or various other local and systemic routes shows that delivery of antigens via epidermal disruption induces the most robust immune responses as well as the most effective protection against skin challenge (133). Furthermore, the enhanced protection was mediated by TRM, as treatment of mice with FTY720 still led to protection against challenge (133). Vaccines against HSV that have been tested at clinical trial have generated strong antibody responses as well as some Th1 responses (134, 135). Both the Merck STEP trial vaccine and the RV144 vaccine elicited antibody as well as robust T-cell responses against HIV-1 (136–138). However, none of these vaccines have been shown to be more than modestly protective (120). While the factors that contributed to the failure of these vaccines are still being investigated, one potential reason is that these vaccines were administered parenterally. Many vaccines, including the ones that have gone to clinical trial for HSV and HIV, are administered intramuscularly (i.m.). As described earlier, effector CD8+ T cells that are generated via systemic immunization are capable of trafficking indiscriminately to many tissues, including permissive and effector permissive sites. In support of this, i.m. immunization of mice with recombinant adenovirus vectors expressing SIV epitopes leads to the circulation of antigen-specific CD8+ T cells to mucosal surfaces, including the genital tract (139). Despite this, the efficacy of human vaccines administered in this fashion are not particularly robust, suggesting that while effector CD8+ T cells can migrate through peripheral tissues after systemic or distal immunization, the size, localization, or composition of the population may not be sufficient to immediately control incoming pathogens. In support of this, i.m. or s.c. immunization of mice with a replication-deficient strain of HSV-2 leads to a similar number of systemic virus-specific CD8+ T cells as ivag immunization with an attenuated strain of HSV-2. However, while distal immunization does induce limited recruitment of these activated virus-specific CD8+ T cells to the genital tract, the number of these cells in the tissue is not as large as what is observed after ivag immunization. Furthermore, mice that are systemically immunized succumb to lethal ivag challenge with wildtype (WT) HSV-2, while ivag immunized mice are fully protected (authors’ unpublished data). Thus, while systemic immunization is capable of establishing TRM at peripheral sites, additional steps may be required to augment protection.
To try and boost mobilization of T cells to mucosal surfaces, efforts have been made to design a vaccine that uses mucosal immunization. The ‘common mucosal system’ is an idea that has gathered support from studies that show immunization at a mucosal site such as the lung can generate mucosa-associated responses, such as production of IgA, and can provide protection at distal mucosal sites (140, 141). For example, intranasal immunization with attenuated HSV-2 can lead to protection against vaginal challenge with WT HSV-2 (142). Furthermore, when macaques were immunized only i.m. with HIV-1 gp41 containing virosomes, which are lipid bilayer membrane vesicles that incorporate influenza-derived hemagglutinin and neuraminidase, they were less protected against vaginal simian-human immunodeficiency virus (SHIV) challenges as compared to animals that were immunized by both i.m. and i.n. routes (143). This protection was mostly attributed to mucosal antibodies, however. It is still unclear whether mucosal immunization can mobilize T-cell responses to other mucosal sites. Work with recombinant adenovirus 5 (rAd5), a common vector used for HIV vaccines, has shown that i.m. immunization leads to a greater frequency of antigen-specific T cells in mucosal tissues such as the genital tract as compared to intranasal immunization. However, there were overall differences in the magnitude of the response, suggesting that the differences in trafficking may be due to less efficient priming of the T-cell response (139).
Prime and pull: a novel vaccine strategy based on TRM
Our laboratory recently designed a vaccination protocol against genital herpes that can establish a robust population of antigen-specific CD8+ T cells in the genital tract after parenteral immunization. We developed this strategy with two goals in mind: (i) to try and mimic the type of optimal immunity that is found after ivag immunization with attenuated HSV-2 without having to directly infect the genital tract itself and (ii) to make it flexible enough that it could be applied to any barrier site and recruit any immune cell population. Our strategy, which we call ‘Prime and Pull’, takes advantage of the knowledge that the chemokines CXCL9 and CXCL10 are the key chemoattractants for activated CD8+ T cells for entry into the vagina (48). Subcutaneous immunization (prime) with attenuated HSV-2 generates a robust systemic virus-specific CD8+ T-cell response that is comparable to ivag immunization. However, as expected, there is very little CD8+ T-cell migration into the genital tract after s.c. infection. When the CXCL9 and CXCL10 are applied topically to the vagina (pull) during the effector phase, we observed robust recruitment of not only virus-specific CD8+ T cells but also of CD4+ T cells. Importantly, treatment with these chemokines induces very little inflammation, as indicated by the minimal influx of inflammatory cells into the tissue (59). Furthermore, the HSV-specific CD8+ T cells recruited into the genital tract by the pull are capable of establishing a resident population in the epithelial layer (unpublished data) that could be detected up to 12 weeks after the pull. Similarly to what is observed after skin infection with HSV-1, CD4+ T cells are not maintained in the vagina, indicating that they may leave the tissue to pursue an immunosurveillance role (59, 66). In correlation with the establishment of CD8+ TRM, prime and pull mice are significantly protected up to 10 weeks post-pull against a lethal vaginal challenge of WT HSV-2 compared to primed mice that were treated only with the vehicle control (59). Thus, our work suggests that TRM can be artificially established long-term in restrictive tissues such as the genital tract using the appropriate signals and that these TRM can provide protective immunity against local infections that cannot be controlled by systemic memory T cells. The surprising aspect of the success of Prime and Pull vaccine approach is that no antigen was required for entry or retention of the TRM, and a very transient presence of chemokines was sufficient to establish the TRM pool in the vagina.
Another surprising finding from the Prime and Pull study is that despite the high level of protection against clinical symptoms and the 100% survival rate of mice treated with prime and pull, mucosal viral titers were not significantly different between the prime only and prime and pull cohorts (59). We speculated that the CD8+ TRM may be playing a neuroprotective role in the tissue, as the severity of the clinical symptoms of HSV-2 infection in mice is associated with spread to the nervous system. To control mucosal viral replication, other TRM populations may be required. For example, immediate IFNγ production from Th1 cells are required for the protection observed after ivag immunization with attenuated HSV-2 (122). As described earlier, CD4+ T cells form relatively unstructured lymphoid clusters in the vaginal lamina propria of ivag immunized mice. The signals required to form and retain these clusters is a subject of ongoing study in the laboratory, but once they are elucidated, they could potentially be used to retain CD4+ T cells in the tissue after Prime and Pull. Neutralizing and other types of antibodies also play an important role in providing immunity against viral infections such as HSV and HIV. While less is known about the tissue residency of memory B cells and plasma cells in nonlymphoid tissues, B cells and antibody-secreting cells (ASCs) are capable of migrating to peripheral tissues. A model of viral encephalomyelitis in mice shows that impaired migration of virus-specific ASCs, but not T cells, into the CNS leads to decreased viral control (144). Furthermore, it has been shown in the genital tract that delivery of antibody in the vaginal lumen by the Fc-neonatal receptor (FcRn) can protect against a WT HSV-2 challenge (145). Local antibody production also appears to be an important immune correlate for control of SIV. As described above, i.n. immunization with gp41-containing virosomes leads to the production of mucosal and circulating IgA and IgG antibodies, while i.m. immunization generates only serum antibodies. The study shows that mucosal antibodies alone have virus neutralizing and transcytosis blocking antibody, and subsequently, protection is mediated by mucosal and not serum antibody (143). Collectively, these observations suggest that circulating antibody may not be sufficient to help control infection at peripheral sites and that tissue localization of B cells or antibody is required for full effectiveness. As the ideal vaccine against infections such as HSV and HIV may require cooperation between multiple cell populations such as T cells and B cells, Prime and Pull may be an effective strategy for targeting and establishing tissue residency for different arms of the immune system.
Concluding remarks and future perspective
In this review, we discuss recent evidence for a third subset of memory T cells that reside in restrictive peripheral tissues, the TRM. Tissues that are not under surveillance by circulating TEM or TCM support the establishment of resident memory T cells, which are stably maintained independently of their circulating counterparts. Due to their localization, TRM are uniquely suited to provide frontline defense against pathogens that invade peripheral barrier tissues such as the skin and the genital tract. Our recent study shows that artificially established TRM by Prime and Pull can provide superior protection against genital herpes infection compared to circulating memory T cells. In the future, it will be important to incorporate strategies to establish TRM within the target tissue when designing vaccines against infections that begin at barrier sites.
While great strides have been made in understanding the properties of TRM in recent years, many questions still remain about their generation, maintenance, and function. For example, it is unclear whether TRM arise from the same pool of memory precursors as circulating TEM and TCM, or whether TRM precursors can be distinguished by distinct receptors and allow them to establish tissue residency. Furthermore, while TEM and TCM are generally excluded from the effector permissive and restrictive tissues in which TRM are present, it is unknown whether TEM or TCM can convert to TRM once recruited into the appropriate tissue. Another unknown in this regard is the mechanism of TRM maintenance. While CD103 is upregulated on nearly all TRM, it is unknown whether there are other tissue-specific receptors that can aid in the retention of TRM in peripheral tissues. Aside from retention, it is unclear what signals mediate the survival of TRM as they express less IL-7Rα and IL-15Rβ, two receptors that are critical for the survival and turnover of circulating memory T cells. TRM do not fare well when removed from their tissue of origin, which suggests that they may adapt to unique, unknown signals within their microenvironment. Finally, while tissue residency has been described for T cells, it is unclear whether other adaptive immune cell populations such as B cells, can also establish a resident memory population. Concentration of locally produced antibody by tissue-resident memory B cells may boost the efficacy of vaccines that rely on humoral immunity to protect against peripheral infection. Thus, in future studies, it will be essential to gain a deeper understanding of tissue-resident lymphocyte biology to design vaccines and immunotherapies that provide immediate control of invading pathogens.
Acknowledgement
H. S. is supported by Ruth L. Kirschstein National Research Service Awards (NRSA) for Individual Postdoctoral Fellows (F32AI091024). This work is supported by NIH grants to A. I. (AI054359, AI062428).
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Jenkins MK, Chu HH, McLachlan JB, Moon JJ. On the composition of the preimmune repertoire of T cells specific for peptide-major histocompatibility complex ligands. Annu Rev Immunol. 2010;28:275–294. doi: 10.1146/annurev-immunol-030409-101253. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell Differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Keele BF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA 2008. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrahams M-R, et al. Quantitating the Multiplicity of Infection with Human Immunodeficiency Virus Type 1 Subtype C Reveals a Non-Poisson Distribution of Transmitted Variants. J Virol. 2009;83:3556–3567. doi: 10.1128/JVI.02132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z-Q, et al. Sexual Transmission and Propagation of SIV and HIV in Resting and Activated CD4+ T Cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 7.Miller CJ, et al. Propagation and Dissemination of Infection after Vaginal Transmission of Simian Immunodeficiency Virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douek DC, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–888. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 11.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 12.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto M, et al. CD43 functions as a ligand for E-selectin on activated T cells. J Immunol. 2005;175:8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- 14.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 15.Berg EL, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baaten B, Tinoco R, Chen A, Bradley L. Regulation of antigen-experienced T cells: lessons from the quintessential memory marker CD44. Front Immunol. 2012;3:23. doi: 10.3389/fimmu.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell JJ, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 18.Campbell JJ, O'Connell DJ, Wurbel M-A. Cutting edge: chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J Immunol. 2007;178:3358–3362. doi: 10.4049/jimmunol.178.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homey B, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 21.Homey B, et al. Up-regulation of macrophage inflammatory protein-3a/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621–6632. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 22.Islam SA, Chang DS, Colvin RA, Byrne MH, McCully ML, Moser B, et al. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ TH2 cells. Nat Immunol. 2011;12:167–177. doi: 10.1038/ni.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 24.Berlin C, et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 25.Park EJ, et al. Aberrant activation of integrin a4b7 suppresses lymphocyte migration to the gut. J Clin Invest. 2007;117:2526–2538. doi: 10.1172/JCI31570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streeter PR, Berg EL, Rouse BTN, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 27.Kuklin NA, et al. a4b7 independent pathway for CD8+ T cell-mediated intestinal immunity to rotavirus. J Clin Invest. 2000;106:1541–1552. doi: 10.1172/JCI10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svensson M, et al. CCL25 mediates the localization of recently activated CD8ab+ lymphocytes to the small-intestinal mucosa. J Clin Invest. 2002;110:1113–1121. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunkel EJ, et al. Lymphocyte CC Chemokine Receptor 9 and Epithelial Thymus-Expressed Chemokine (Teck) Expression Distinguish the Small Intestinal Immune Compartment: Epithelial Expression of Tissue-Specific Chemokines as an Organizing Principle in Regional Immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 34.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S-Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Hammerschmidt SI, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edele F, et al. Cutting Edge: Instructive Role of Peripheral Tissue Cells in the Imprinting of T Cell Homing Receptor Patterns. J Immunol. 2008;181:3745–3749. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- 37.Sigmundsdottir H, et al. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programing of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Dudda J, et al. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur J Immunol. 2005;35:1056–1065. doi: 10.1002/eji.200425817. [DOI] [PubMed] [Google Scholar]
- 40.Dudda JC, Simon JC, Martin S. Dendritic cell immunization route determines CD8+ T cell trafficking to inflamed skin: role for tissue microenvironment and dendritic cells in establishment of T cell-homing subsets. J Immunol. 2004;172:857–863. doi: 10.4049/jimmunol.172.2.857. [DOI] [PubMed] [Google Scholar]
- 41.Odoardi F, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 42.Masopust D, et al. Activated Primary and Memory CD8 T Cells Migrate to Nonlymphoid Tissues Regardless of Site of Activation or Tissue of Origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 43.Oyoshi MK, et al. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J Clin Invest. 2011;121:2210–2220. doi: 10.1172/JCI43586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takamura S, Roberts AD, Jelley-Gibbs DM, Wittmer ST, Kohlmeier JE, Woodland DL. The route of priming influences the ability of respiratory virus-specific memory CD8+ T cells to be activated by residual antigen. J Exp Med. 2010;207:1153–1160. doi: 10.1084/jem.20090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 46.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 47.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 48.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–336. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 52.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsieh M-F, et al. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J Immunol. 2006;177:1855–1863. doi: 10.4049/jimmunol.177.3.1855. [DOI] [PubMed] [Google Scholar]
- 55.Campanella GSV, et al. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci USA. 2008;105:4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohlmeier JE, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29:101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parr MB, Parr EL. Interferon-γ up-regulates intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and recruits lymphocytes into the vagina of immune mice challenged with herpes simplex virus-2. Immunology. 2000;99:540–545. doi: 10.1046/j.1365-2567.2000.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansson EL, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999;96:272–277. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 62.Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci USA. 2011;108:16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Himmelein S, St Leger A, Knickelbein J, Rowe A, Freeman M, Hendricks R. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae. 2011;2:5. doi: 10.1186/2042-4280-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hermesh T, Moltedo B, Moran TM, Lopez CB. Antiviral Instruction of Bone Marrow Leukocytes during Respiratory Viral Infections. Cell Host Microbe. 2010;7:343–353. doi: 10.1016/j.chom.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 67.Wakim LM, et al. The Molecular Signature of Tissue Resident Memory CD8 T Cells Isolated from the Brain. J Immunol. 2012;189:3462–3471. doi: 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mackay LK, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mackay CR, Marston WL, Dudler L, Spertini O, Tedder TF, Hein WR. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992;22:887–895. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- 70.Yawalkar N, Hunger RE, Pichler WJ, Braathen LR, Brand CU. Human afferent lymph from normal skin contains an increased number of mainly memory / effector CD4+ T cells expressing activation, adhesion and co-stimulatory molecules. Eur J Immunol. 2000;30:491–497. doi: 10.1002/1521-4141(200002)30:2<491::AID-IMMU491>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 71.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 72.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (TRM) are abundant in human lung: diversity, function, and antigen specificity. PLoS ONE. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 74.Bouneaud Cc, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manjunath N, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 78.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubinstein MP, et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee Y-T, et al. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol. 2011;85:4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schlickum S, et al. Integrin aE(CD103)b7 influences cellular shape and motility in a ligand-dependent fashion. Blood. 2008;112:619–625. doi: 10.1182/blood-2008-01-134833. [DOI] [PubMed] [Google Scholar]
- 84.Pauls K, et al. Role of Integrin aE(CD103)b7 for Tissue-Specific Epidermal Localization of CD8+ T Lymphocytes. J Invest Dermatol. 2001;117:569–575. doi: 10.1046/j.0022-202x.2001.01481.x. [DOI] [PubMed] [Google Scholar]
- 85.Karpowicz P, Willaime-Morawek S, Balenci L, DeVeale B, Inoue T, van der Kooy D. E-cadherin regulates neural stem cell self-renewal. J Neurosci. 2009;29:3885–3896. doi: 10.1523/JNEUROSCI.0037-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Casey KA, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.El-Asady R, et al. TGFbeta-dependent CD103 expression by CD8+ T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kohlmeier JE, Miller SC, Woodland DL. Cutting edge: Antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J Immunol. 2007;178:4721–4725. doi: 10.4049/jimmunol.178.8.4721. [DOI] [PubMed] [Google Scholar]
- 89.Al-Sadeq A, Hamad M, Abu-Elteen K. Patterns of Eexpression of vaginal T-cell activation markers during estrogen-maintained vaginal candidiasis. Allergy Asthma Clin Immunol. 2008;4:157–163. doi: 10.1186/1710-1492-4-4-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shiow LR, et al. CD69 acts downstream of interferon-a/b to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 91.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 92.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 93.Ledgerwood LG, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 94.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 95.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 96.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 97.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 98.Suvas PK, Dech HM, Sambira F, Zeng J, Onami TM. Systemic and mucosal infection program protective memory CD8 T cells in the vaginal mucosa. J Immunol. 2007;179:8122–8127. doi: 10.4049/jimmunol.179.12.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim TS, Hufford MM, Sun J, Fu Y-X, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207:1161–1172. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- 101.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- 102.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor α and CD62L. J Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 104.Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol. 2005;175:4677–4685. doi: 10.4049/jimmunol.175.7.4677. [DOI] [PubMed] [Google Scholar]
- 105.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor α CD62L. J Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 106.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen- specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mueller SN, Ahmed R. Lymphoid stroma in the initiation and control of immune responses. Immunol Rev. 2008;224:284–294. doi: 10.1111/j.1600-065X.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 109.Klonowski KD, Marzo AL, Williams KJ, Lee S-J, Pham Q-M, Lefrancois L. CD8 T Cell Recall Responses Are Regulated by the Tissue Tropism of the Memory Cell and Pathogen. J Immunol. 2006;177:6738–6746. doi: 10.4049/jimmunol.177.10.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yajima T, Nishimura H, Sad S, Shen H, Kuwano H, Yoshikai Y. A novel role of IL-15 in early activation of memory CD8+ CTL after reinfection. J Immunol. 200;174:3590–3597. doi: 10.4049/jimmunol.174.6.3590. [DOI] [PubMed] [Google Scholar]
- 112.Jenkins MR, Griffiths GM. The synapse and cytolytic machinery of cytotoxic T cells. Curr Opin Immunol. 2010;22:308–313. doi: 10.1016/j.coi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wakim LM, Gupta N, Mintern JD, Villadangos JA. Enhanced survival of lung tissue-resident memory CD8+ T cells during infection with influenza virus due to selective expression of IFITM3. Nat Immunol. 2013;14:238–245. doi: 10.1038/ni.2525. [DOI] [PubMed] [Google Scholar]
- 114.Mintern JD, Guillonneau C, Carbone FR, Doherty PC, Turner SJ. Cutting edge: tissue-resident memory CTL down-regulate cytolytic molecule expression following virus clearance. TJ Immunol. 2007;179:7220–7224. doi: 10.4049/jimmunol.179.11.7220. [DOI] [PubMed] [Google Scholar]
- 115.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 117.Cunningham AL, et al. The Cycle of Human Herpes Simplex Virus Infection: Virus Transport and Immune Control. J Infect Dis. 2006;194(Suppl):S11–S18. doi: 10.1086/505359. [DOI] [PubMed] [Google Scholar]
- 118.Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Micro. 2008;6:211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 119.Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest. 2011;121:4600–4609. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33:542–554. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- 122.Iijima N, et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schiffer JT, et al. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc Natl Acad Sci USA. 2010;107:18973–18978. doi: 10.1073/pnas.1006614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath MJ. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 126.Liu J, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Migueles SA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-{{gamma}}-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001;82:845–853. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- 129.Reynolds MR, et al. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parr MB, Parr EL. Intravaginal administration of herpes simplex virus type 2 to mice leads to infection of several neural and extraneural sites. J Neurovirol. 2003;9:594–602. doi: 10.1080/13550280390246499. [DOI] [PubMed] [Google Scholar]
- 131.Li Q, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–1729. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Althaus CL, De Boer RJ. Implications of CTL-mediated killing of HIV-infected cells during the non-productive stage of infection. PLoS ONE. 2011;6:e16468. doi: 10.1371/journal.pone.0016468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stanberry LR, et al. Glycoprotein-D,ÄìAdjuvant Vaccine to Prevent Genital Herpes. N Engl J Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 135.Corey L, et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: Two randomized controlled trials. JAMA. 1999;282:331–340. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 136.McElrath MJ, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 139.Kaufman DR, Bivas-Benita M, Simmons NL, Miller D, Barouch DH. Route of adenovirus-based HIV-1 vaccine delivery impacts the phenotype and trafficking of vaccine-elicited CD8+ T lymphocytes. J Virol. 2010;84:5986–5996. doi: 10.1128/JVI.02563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rosenthal KL, Gallichan WS. Challenges for vaccination against sexually-transmitted diseases: induction and long-term maintenance of mucosal immune responses in the female genital tract. Semin Immunol. 1997;9:303–314. doi: 10.1006/smim.1997.0086. [DOI] [PubMed] [Google Scholar]
- 141.Brandtzaeg P. Mucosal immunity in the female genital tract. J Repro Immunol. 1997;36:23–50. doi: 10.1016/s0165-0378(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 142.Morrison LA, Da Costa XJ, Knipe DM. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- 143.Bomsel M, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 144.Marques CP, et al. CXCR3-dependent plasma blast migration to the central nervous system during viral encephalomyelitis. J Virol. 2011;85:6136–6147. doi: 10.1128/JVI.00202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Z, Palaniyandi S, Zeng R, Roopenian DC, Tuo W, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci USA. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]