Summary
Influenza virus infection induces robust and highly protective B-cell responses. Knowledge gained from the analysis of such protective humoral responses can provide important clues for the design of successful vaccines and vaccination approaches and also provides a window into the regulation of fundamental aspects of B-cell responses that may not be at play when responses to non-replicating agents are studied. Here I review features of the B-cell response to viruses, with emphasis on influenza virus infection, a highly localized infection of respiratory tract epithelial cells, and a response that is directed against a virus that continuously undergoes genetic changes to its surface spike protein, a major target of neutralizing antibodies. Two aspects of the B-cell response to influenza are discussed here, namely polyreactive natural antibodies and the role and function of germinal center responses. Both these features of the B-cell response raise the question of how important antibody fine-specificity is for long-term protection from infection. As outlined, the pathogenesis of influenza virus and the nature of the antiviral B-cell response seem to emphasize repertoire diversity over affinity maturation as driving forces behind the influenza-specific B-cell immunity.
Keywords: antiviral immunity, B-1 cells, extrafollicular foci, germinal centers, plasma cells, antibody repertoire
Introduction
The influenza virus ‘lifecycle’ is intricately linked to the induction and presence of highly protective antibodies in an otherwise susceptible human population. Influenza infections of mice provide a highly relevant model to study the dynamics and the mechanisms underlying successful influenza-induced B-cell responses following virus-induced pneumonia, a response that is shaped significantly by the influenza virus replication cycle. Influenza virus employs a ‘hit-and-run’ strategy, allowing it to evade much of the immune responses mounted against it. Influenza virus infects and rapidly replicates in the epithelial cells of the respiratory tract, with peak replication occurring within 2-3 days of infection. At that time, the infected human host is highly infectious but often does not show overt signs of disease, and the virus is aerosolized and infects other susceptible individuals. Once peak viral loads are reached in the respiratory tract, the virus is usually relatively quickly cleared by a large number of innate and adaptive immune mechanisms that are triggered by the infection, including the activation of antigen-specific B cells, CD4+ and CD8+ T cells, but also innate responses such as type I interferon (IFN), natural killer (NK) cells, and B-1 cells, to name but a few.
Given the rapid dissemination to other susceptible humans, clearance of the virus from an infected host at that point thus does little to impact viral transmission. Instead, what does greatly impact the viral transmission efficiency is the existence of neutralizing antibodies on mucosal surfaces or in the serum, which can prevent reinfections. It is thought that the increased presence of neutralizing antibodies in a population of previously exposed individuals effectively interrupts the yearly influenza epidemics when susceptible hosts are no longer available and the virus is stopped in its cycle of transmission and replication.
The failure of the immune system to prevent future infections of an individual with ‘the flu’ is due to ongoing point mutations in the genes encoding the virus's surface receptors, hemagglutinin (HA) and neuraminidase (N), a process termed ‘antigenic drift’; larger exchanges of entire gene segments made possible by the fact that this virus contains its genetic information in 8 gene segments that can reassort in cells infected with two different types of influenza viruses simultaneously, a process termed ‘antigenic shift’. These shifts seem to occur roughly every 10–50 years (1). The most recent shift occurred in 2009 and led to the Swine flu pandemic of 2009/2010.
These processes emphasize the effectiveness of antibodies in preventing repeat infections with the same homotypic influenza strain but also the apparent shortcomings of the adaptive immune system in anticipating the virus’ changing antigenic face. Here we review the current literature regarding the induction and maintenance of the highly effective B-cell response to homotypic influenza virus and raise the possibility that the B-cell responses to this virus may indicate an evolutionary strategy, albeit imperfect, for protection from pathogens whose antigenic composition changes over time.
IgM and polyreactive B-cell responses to influenza
Polyreactive IgM protects from influenza infection
Many of the circulating IgM antibodies in the serum are directed against self-antigens. These antibodies form the pool of ‘natural IgM’, i.e. antibodies that are generated constitutively in the absence of antigenic challenge. In mice their source was shown to be a small subset of B cells termed B-1 cells. A poorly understood mechanism ensures the continued generation of natural poly-reactive IgM antibodies, independent of antigen-challenge (2-4). B-1 cells (CD5+ B-1a and CD5– B-1b) are distinct in development, phenotype, and tissue location from conventional (B-2) B cells (5-8), indicating fundamental differences in their biology that might be at the heart of their antigen-independent regulation. However, the origins of natural IgM in human and non-human primates are less well understood (8, 9).
Initial work on these cells and natural antibodies had focused on their self-reactivity and potential to induce autoimmunity (10-12). More recent studies now provide evidence that these antibodies bind also to pathogen-associated antigens (reviewed in 13). This is due to their overall polyreactivity, i.e. the ability of an individual IgM molecule to bind to multiple antigens (14), including the recognition of all tested influenza virus strains (15).
The airways contain significant amounts of natural IgM, which can neutralize influenza virus (16) and are likely transported to mucosal surfaces via the poly-Ig receptor (17). Influenza virus neutralization by natural IgM can also be achieved via destruction after complement activation (18). Natural IgM-mediated virus inactivation is a surprisingly powerful immune mechanism, as in the selective absence of natural IgM survival from infection influenza infection is compromised (19). This effect of polyreactive natural IgM is not restricted to protection from influenza infection. Infections of mice selectively lacking secreted (s)IgM with numerous other pathogens have shown a similar role for natural IgM in immune protection (13). Thus, production of natural IgM serves as a crucial barrier against unchecked pathogen replication prior to the establishment of specific immune responses.
One caveat about these studies comes from early findings in mice lacking secreted IgM (sIgM–). These mice showed some abnormalities on B-cell development, such as increased peritoneal cavity B-1 cell numbers (20-22), increased marginal zone B cells, and reduced populations of follicular B cells (23). In support, recent reports have linked expression of a recently identified FcμR to normal B-cell development (24). Our own recent studies confirmed such a non-redundant role for sIgM in normal B-cell development (T. Nguyen, R.A. Elsner and N. Baumgarth, unpublished data). Since numerous studies have based their conclusions on a significant role for sIgM exclusively on data obtained from comparing results in infected wildtype and sIgM–/– mice, those studies may have to be re-evaluated in light of the data that demonstrate a more pervasive effect of sIgM on the functionality of the entire B-cell compartment. Nonetheless, studies with influenza (19), LCVM, and VSV (25) showed a protective effect of short-term transfer of sIgM on viral loads and survival, thus clearly identifying IgM as capable and important in ameliorating viral infections.
Properties and functions of antiviral IgM
The importance of natural and antigen-induced IgM in immunity to the various infections is somewhat surprising, given that B cells switch towards production of IgG and/or IgA following a brief period of IgM secretion. IgM production occurs very early during an immune response, possibly preceding the development of class-switched IgG/A responses following infection or vaccination (reviewed in 26). In addition, long-term IgM secreting plasma cells are not induced. Given the relatively short half-live of IgM, about 7 days, IgM secretion peaks early and then disappears within a few weeks of infection, while IgG responses do not usually return to baseline levels. For that reason, measurements of IgM responses are not usually made when determining correlates of immune protection in vaccine trials.
All jawed vertebrates produce IgM, and IgM is the first immunoglobulin isotype produced in ontogeny, including following infections, where IgM secretion occurs as early as the second trimester of development of a human fetus, despite the fact that pathogen-specific IgG can enter the fetus via maternal blood flow (27) and provide protection. These findings indicate unique contributions of IgM to immunity and to the host's interactions with its environment. As discussed above, one such function seems to be linked to ensuring normal B-cell development, but other causes are likely. The presence of at least two FcR for IgM on B cells (FcμR and Fcμ/αR) is intriguing and could suggest regulatory feedback properties of IgM (28-30).
Because IgM is generated prior to the establishment of germinal centers, most antigen-induced IgM antibodies carry few if any mutations, and much, but not all, of the natural antibody repertoire appears germ-line encoded (31). Natural antibodies bind antigens with overall low affinities (10−3 – 10−5 M−1). However, due to the pentameric structure and its 10-binding sites, its high valency increases the avidity of natural and non-mutated IgM antibodies to a wide range (10−3 to 10−11 M−1). Average values range between 10−6 and 10−7 M−1, about the same values above which increased in vivo protection from a virus infection was no longer observed (32). Yet, the overall binding properties and the kinetics of production have led to IgM– antibodies and IgM-secreting cells being mainly ignored as inferior cousins of the more heavily mutated IgG (and IgA)-secreting B cells that are the product of germinal center responses. Given the more recent data and the high degree of evolutionary conservation, it seems clear that we do not yet have all the answers regarding the non-redundant functions of IgM and IgM-secreting B cells.
Polyreactive antiviral B cells
As indicated above, B-1 cells contribute to protection from influenza virus infection even prior to any encounter with the virus by generating influenza-binding and neutralizing natural IgM (15, 33). In BALB/c and C57BL/6 mice, about 10% of spontaneous IgM-secreting B-1 cells will generate antibodies that can bind to influenza antigen (16). Given that mice are not natural hosts for influenza, these antibodies must have arisen either by expansion of mature B-1 cell populations following exposure to a cross-reactive self- or foreign- antigens or enriched by positively selection during B-1 development. The latter is supported by data demonstrating the lack of certain specificities in the B-1 cell repertoire if a particular self-antigen is missing (34). Influenza-binding natural antibodies might be generated against conformational epitopes on the carbohydrate-rich influenza spike-proteins. This is supported by the fact that natural antibodies have hemagglutinin-inhibiting qualities (16), i.e. it is likely that many of the antibodies bind to HA. Western blot analysis with wildtype mouse serum supports these findings (YS Choi, N Baumgarth, unpublished data).
Obviously these findings contradict the current paradigm of lymphocyte development and selection, whereby self-reactive clones are deleted to avoid autoimmunity. However, given that B-1 cell-derived antibodies cross-react with pathogens, it is possible that the self-antigens that shape the repertoire of B-1 cells serve as templates for the selection of evolutionary ‘useful’ specificities (35). Such pre-existing template-selected ‘useful’ B-cell pools ensure the early generation and continuous presence of protective antibodies, independent of previous antigen encounter and make B-1 cells unique contributors to immune defense. It also emphasizes the innate-like qualities of B-1 cells. Although no B-1 cell clones have been identified that are as predominant as the invariant CD1d-restricted NK T cells among that cell population (36), they can be viewed as quite similar to this and other innate-like lymphocytes that express relatively invariant antigen receptors that arose by gene rearrangement. Given that this process induces the presence of a pool of potentially auto-reactive B cells, this highlights the need for special regulatory mechanisms to control their activation.
Polyreactivity, however, does not appear to be restricted to the pool of innate-like B cells. Recent efforts have focused on identifying the origins of broadly neutralizing antibodies against influenza virus as well as human immunodeficiency virus (HIV). Both viruses thwart ongoing vaccine efforts, by rapidly and frequently mutating and thus by evading antibody-mediated neutralization. A small subset of humans can generate broadly neutralizing antibodies to HIV, i.e. antibodies that can neutralize most HIV isolates and these individuals can control the virus without the need for therapeutic interventions (37). Sera and clones of broadly neutralizing antibody-producing cells have been studied extensively to determine why most individuals are unable to mount such a response and also what confers the breadth of the binding capacity of these antibodies. Initial studies indicated that these antibodies were not only able to bind to many HIV isolates, mostly within the CD4-binding region, they also appeared to recognize self-antigens, such as cardiolipin among others, i.e. they appeared polyreactive (38, 39). Self-reactive B cells are usually selected against during B-cell development. It is estimated that only 5% of the circulating B-cell pool in humans is self and/or-polyreactive (40). Thus polyreactive anti-HIV B cells with that specificity may usually be eliminated from the circulating B-cell pool, providing a potential explanation for the scarcity with which such antibodies develop.
A recent elegant study by Nussenzweig and colleagues (41) expanded on those findings, making the observation that in contrast to the 5% of polyreactivity observed in the normal antibody pools, 75% of monoclonal antibodies with broadly neutralizing activity against HIV are polyreactive, i.e. demonstrating the heavy selection of polyreactive clones among this potent anti-HIV response. Moreover, the study concluded that the development of polyreactivity helped to overcome one of the limitations of dimeric IgG molecules, namely their much lower avidity when the antibody binds with only one of the two potential antigen-binding sites. Given that the target of the polyreactive and broadly neutralizing antibodies is the gp140 spike-protein of HIV, of which only 10–15 molecules are expressed per virion, such homotypic, dimeric binding would be rare. The authors proposed that the polyreactivity of the antibodies allows for one of the two binding-sites to engage with gp140 with high affinity, while the other binds, with low affinity, to an unrelated protein. This heteroligation was shown to greatly increase antibody-binding avidity to gp140 in Biacore avidity measurements (41). Thus, consistent with a previous report (42), the data suggest that polyreactivity and reactivity to auto-antigens can be the result of antigen-driven clonal expansion and somatic hypermutations of conventional B cells during infections/exposure to antigens during germinal center reactions.
How widespread such development of polyreactive antibodies is in response to infections, however, remains to be studied. With regard to influenza infection, the neutralizing influenza spike proteins are expressed at relatively high densities on the virus particles and no such heteroligation has to be invoked for the development of broadly neutralizing antibodies. Broadly neutralizing antibodies to influenza, so-called ‘heterosubtypic’ or cross-reactive antibodies, which are generated by previous encounters with differing strains or substrains of influenza can be induced (reviewed in 43). The most potent seem to bind epitopes on the highly conserved helical region of the membrane-proximal stalk of HA1 and HA2 (44, 45), a promising potential target for new vaccine efforts (46).
In summary, polyreactive antibodies may arise during development by subsets of B cells that are selected on the basis of relatively strong BCR-stimulation and self-antigen recognition into subsets of natural antibody-producing cells, while such specificities among conventional B cells would lead mainly to their deletion. Other populations of polyreactive B cells may emerge during infection-induced germinal center-reactions through the process of somatic hypermutation of conventional B cells. Survival and selection of these cells seems to require the presence of T cell help (47), reducing but not eliminating the risk of infection-induced autoimmune disease. Given the example of HIV, this post-germinal center tolerance window seems to broaden the repertoire of B-cell-specificities for induction of specificities that are not part of the natural repertoire. A conclusion from the analyses of HIV broadly neutralizing antibodies seems to be that the successful outcome of germinal center-driven somatic hypermutation has to do less with increases in antibody-affinity per se but rather with increases in the breadth and diversity of antigen recognition.
Innate-like B-cell responses to influenza virus infection
Given the potential for polyreactive antibodies as contributors to protective antiviral B-cell responses and the demonstrated role of IgM-secreting B-1 cells in protection from death following influenza virus infection (19), it is important to better understand how this unusual, innate-like B-cell subset is regulated. The purposeful activation of polyreactive B cells could support early and broad immune protection, either from a primary influenza virus infection, or from associated secondary bacterial infections, which are frequent causes of death (48).
While steady-state natural serum IgM antibodies, mostly produced by B-1 cells provide passive immune protection from influenza infection (18, 19), B-1 cells also actively contribute to the influenza virus infection-induced response with increased local IgM production, measurable in the regional mediastinal lymph nodes of experimentally-infected mice, as well as in the bronchoalveolar lavage fluid (16). B-1 and B-2 cells contribute about equal amounts of IgM to this local response. Much, but not all of the influenza-specific conventional IgM response is induced via antigen-specific and T-dependent mechanisms, as virus-specific IgM secretion is greatly reduced in CD40–/– or B cell MHCII–/– mice (49, 50).
In contrast, only about 10% of the antibody-secreting B-1 cells accumulating in the regional lymph nodes after influenza infection will secrete IgM that binds to the virus. That frequency is thus not different from that found in any other tissue in which B-1 cell produce natural antibodies, mainly the spleen and bone marrow (51). This observation raises the question of whether virus neutralization via secretion of IgM is the only protective mechanism of B-1 cells in response to influenza infection. Given that 90% of the accumulating B-1 cells secrete IgM that is not directly binding to influenza, it is tempting to suggest additional, unrelated mechanisms of their action. In addition, recent studies in bacterial systems have suggested that the ability of B-1 cells to secrete GM-CSF is linked to their function (52) and earlier studies had identified B-1 cells as major producers of IL-10 (53). This together with the fact that B-1 cells migrate to secondary lymphoid tissues could indicate their involvement in the regulation of the local immune responses that go beyond their role as antibody-secreting cells.
The presence of IgM secretion that is not different than that of the repertoire of natural antibody secreting B-1 cells also points to a lack of antigen-driven clonal B-1 cell expansion in response to influenza infection. Indeed, BrdU labeling studies failed to show any evidence of clonal expansion of B-1 cells that accumulated in increased numbers in the regional lymph nodes. Thus, suggesting that infection-induced changes in B-1 cell redistribution are a major driver of the B-1 cell response to influenza. This is consistent with numerous other studies that showed that body cavity B-1 cells respond to an insult by rapidly redistributing to secondary lymphoid tissues, particularly the spleen, following their activation. For example, B-1 cells were shown to rapidly migrate from the body cavities to the gastrointestinal tract and the spleen following injection of IL-5 and IL-10 (54), mitogenic and non-mitogenic LPS (55, 56), and bacteria (57). The latter was dependent at least in part on the adaptor molecule MyD88 (57). This rapid antigen non-specific and in some cases pattern-recognition receptor-dependent activation of B-1 cells further highlights the innate-like qualities of B-1 cells. It also identifies B-1 cell populations in the body cavities as reservoirs of B-1 cells that are rapidly activated.
To study whether B-1 cells in the pleural and/or peritoneal cavity are exposed to infection-induced innate signals we recently conducted a comprehensive gene-expression microarray study, isolating B-1 cells from these sites as well as the spleen. The results were unexpected in that we found literally thousands gene-expression alterations in the pleural cavity within 2 days of influenza infection (E. E. Waffarn and N. Baumgarth, unpublished data). Qualitative differences were similar between changes observed in the pleural and peritoneal cavity B-1 cell populations, but the magnitude of those changes was greatly smaller in the peritoneal cavity, which is more distant from the site of infection.
The most obvious change in gene expression was related to a classical type I IFN-signature, which was very similar to changes we had seen previously in conventional B cells within the regional lymph nodes 48h after influenza infection (58, 59) and is in agreement also with studies by others which had shown influenza infection-induced type I IFN effects on leukocytes at sites distant from the respiratory tract (60). The data highlight the tremendous effects that even an infection as localized as influenza virus has on the physiology of the entire immune system. Our understanding of the regulation of adaptive immune responses is likely to miss important regulatory mechanisms unless these effects are taken into consideration.
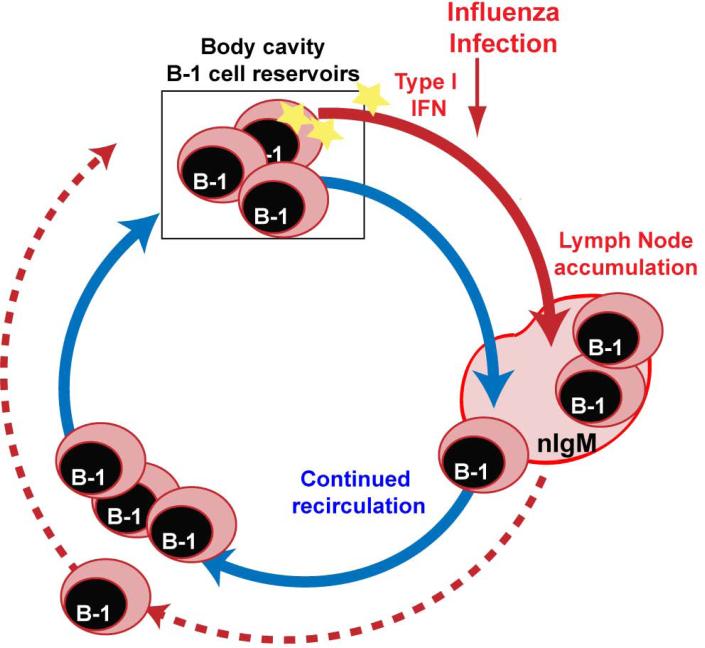
Identifying some of these signals provides potential leads to help us understand why viral infections are in general so much better than inactivated viral vaccines at inducing robust and long-lived immunity. With regard to B-1 cells, the data reveal that by being positioned within the body cavities, B-1 cells are in the vicinity of all mucosal organs and therefore positioned to act effectively as cellular reservoirs that can rapidly respond to insults by migrating to effector sites (Fig. 1).
Fig. 1. B-1 cell redistribution following influenza infection.
Published reports and our recent studies (Waffarn et al., manuscript submitted) highlight the importance of the body cavity B-1 cells as reservoirs of innate-like lymphocytes. B-1 cells appear to continuously recirculate in and out of the body cavities. After influenza virus infection, pleural cavity B-1 cells receive infection-induced signals that alter their gene expression. Type I IFN direct signaling enables B-1 cells to respond to the infection with increased accumulation in the inflamed lymph nodes at the site of infection. This accumulation may reduce the number of B-1 cells that can circulate back into the body cavities.
We are currently studying the effects of type I IFN on the responses of B-1 cells. Our initial studies suggest that this cytokine provides a non-redundant stimulus to B-1 cells by affecting their trafficking. In the absence of IFN-mediated direct signaling, B-1 cell accumulation in the lymph nodes was greatly reduced, while those cells that did accumulate produced normal levels of IgM. Further studies have linked IFN-signaling to effects on integrin-mediated B-1 cell migration (E. E. Waffarn, C. J. Hastey, N. Dixit, Y. S. Choi, S. Cherry, U. Kalinke, S. Simon and N. Baumgarth, manuscript submitted).
B-1 cells respond to influenza infection with redistribution to and differentiation at the site of infection, while the levels of natural serum antibodies to influenza remain completely unaffected (15, 16, 19). This strongly indicates that the populations of B-1 cells that respond to influenza virus infection with redistribution and those B-1 cells that generate natural antibodies are distinct. Early studies by Benner and colleagues (61, 62) followed the development of spontaneous antibody production in gnotobiotic and SPF-housed mice and demonstrated the largely antigen and T- cell-independent development of spontaneous IgM-secreting cells in two tissues: the spleen and the bone marrow. More recently studies showed that B-1 cell antibody producing cells are present in the steady-state spleen (55, 63). B-1 cell BCR-transgenic mice specific against erythrocyte antigens showed that those mice that were healthy lacked splenic B-1 cells in contrast to those generating autoantibodies that caused hemolytic anemia (54, 64). Our studies, using mice in which B-1 and B-2 cells were distinguished by the Ig-allotype they express, are consistent with those earlier studies, demonstrating the presence of IgM-secreting B-1 cells in both the spleen, and for the first time also in the bone marrow (51). While their frequencies in the bone marrow are very low, roughly 0.3% of B cells, removal of these cells completely blocked the development of serum IgM in RAG-mice for at least 6 weeks after bone marrow transfer, in contrast to mice that received complete bone marrow transfers (51). As spleen and bone marrow are also sites of long-lived antibody production by B-2 cell-derived plasma cells following vaccination or infection (65, 66), similar mechanisms ensuring their long-term survival might be at play. While some had suggested that the B-1 cell reservoirs are also the source of the natural antibodies measurable in the serum (6, 67, 68), numerous other studies including our own indicate that body cavity B-1 cells do not spontaneously produce significant amounts of natural IgM in vivo or ex vivo but are induced to do so rapidly after activation (51, 55, 63, 69). The discrepancies might have to do with the ability of body cavity B-1 cells to rapidly respond to activation with differentiation and the difficulties in obtaining pure populations of B-1 cells without stimulating them during the isolation procedures. It is also possible that the removal of B-1 cells from the body cavity environment is sufficient to induce their activation and IgM secretion.
The collective data suggest that the prevalence of B-1 cells in the body cavities is linked to the strategic positioning in response to mucosal insults, while B-1 cells in bone marrow and spleen support the production natural IgM by B-1 cells. While body cavity B-1 cells do not generate steady-state natural IgM, they can nonetheless be rapidly activated to migrate to the regional lymph nodes, as seen after influenza infection, where they contribute significantly to local IgM production. The strong effects of natural IgM on protection from influenza infection demonstrate that even low-affinity antibodies can have dramatic effects on the course of influenza virus infection.
T-dependent B-cell responses to influenza infection
Antibodies against most of the structural viral proteins are induced in response to influenza infection, although at greatly differing levels and kinetics (70). Best understood are the strong and often neutralizing responses against HA (71-73). These antibody responses are overall very strong and long-lasting and will protect from reinfection with the same influenza isolate both in humans (74) and in mice (75), while B-cell-deficient mice are vulnerable to re-infection (reviewed in 75), thus demonstrating the effectiveness of antibodies in immune protection and indicating that it is the changing nature of the virus's antigenic structures that renders antibody responses insufficient, not that the antibody response itself is insufficient or insufficiently induced.
Yet, following the influenza pandemic of 2009, multiple studies also suggested that just having been exposed to an influenza infection previously, and thus having antiviral humoral immunity, even with non or poorly matching antibodies, conferred some level of immune protection. Up to 96% of people born between 1909 and 1919 in Finland had cross-protective antibodies to the 2009 H1N1 pandemic strain, likely due to its relationship to the Spanish flu pandemic strain that circulated in the first part of the 20th century (12). These pre-existing cross-reactive antibodies might be the reason for the unexpectedly low numbers of elderly adversely affected by the 2009 pandemic compared to seasonal influenza virus strains (reviewed in (76)). Experimental studies have confirmed that specific IgG and IgA antibody production is maintained long-term after influenza virus infection of mice (77, 78).
The residual, heterosubtypic influenza immunity is thought to be comprised of antibodies against conserved regionals of the influenza virus, such as the above-mentioned stalk-region antibodies (46), and antibodies that cross-react because of similarities in the conformation of the viral structures targeted by the antibodies. The clinical and epidemiological observations highlight the importance the presence of antibodies, even those that might bind with low affinity, has for survival from influenza infection.
The identification of highly conserved structures within HIV and influenza virus have garnered much excitement and renewed interest in creating vaccines that can induce broadly neutralizing antibodies. Given the ‘experiments in nature’ in the long-term non-progressors among HIV-infected humans, it appears difficult for the virus to mutate away from the effect of the neutralizing antibodies directed against such highly conserved epitopes, supporting such approaches. However, developing a vaccine with only one or a small handful of epitopes is obviously quite dangerous.
An additional or alternative approach therefore seems worth pursuing by considering that increasing the breadth of the antibody repertoire against these viruses might provide a level of protection that, while not inducing sterilizing immunity, may nonetheless prevent high morbidity or mortality among infected individuals. As we outline below, we have come to view the development of germinal center responses following influenza infection as a means of the immune system to broaden the repertoire of B-cell responses and thereby to generate a more protective response against heterosubtypic influenza virus strains.
Extrafollicular B-cell responses to influenza infection
The local draining lymph nodes not only support the accumulation of natural antibody-secreting B-1 cells, they act also as major sites of B-cell response induction following influenza infection (50, 70). Initial B-cell stimulation occurs following their antigen encounter, which can occur through multiple routes. Dendritic cells in the lymph node medulla can capture lymph-borne virus via SIGN-R1 for presentation to B cells (79). B cells might also directly capture and/or express viral antigens in the lymph nodes or at site of infection for transport to the lymph nodes (80).
Following their antigen-induced activation, B cells are responding with the induction of either extrafollicular responses or germinal centers (Fig. 2). Extrafollicular B-cell responses will generate much of the early-induced antiviral antibody responses that can be measured by ELISPOT in draining lymph nodes or in the sera and bronchoalveolar lavage fluid by ELISA. In both mice (16, 59, 70) and ferrets (81), antibody-secreting cells are identified as early as day 3 in the cervical and mediastinal lymph nodes. This very rapid response is due to the establishment of extrafollicular foci responses. They are visible as clusters of antibody-secreting plasma blasts/cells in the T-B border regions of secondary lymphoid tissues by immunohistochemistry (82). The plasma blasts/cells that constitute the extrafollicular response appear short-lived and memory B cells are not generated. Thus, this response provides the earliest antigen-induced antibodies but does not seem to contribute to the long-term response to the virus.
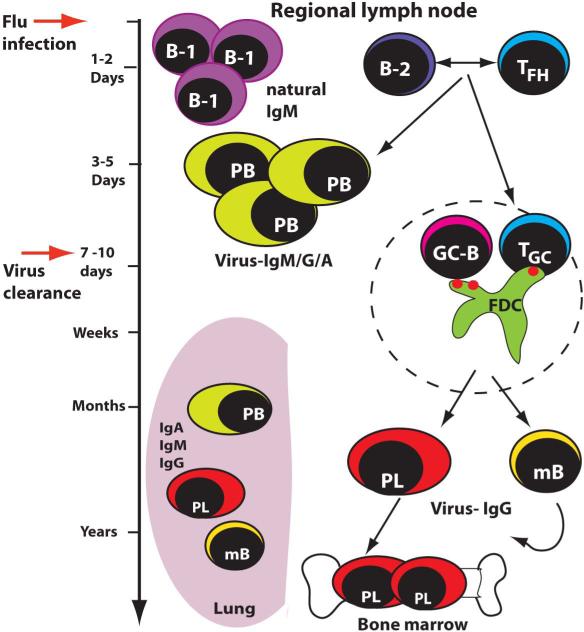
Fig. 2. Timeline of B-cell responses during influenza infection.
Within 24-48 h following influenza infection B-1a cells will accumulate in the regional lymph nodes and secrete both virus-specific and virus non-specific natural IgM. By days 3 or 4, the first IgM, IgG or IgA-producing plasmablasts are measurable. They are generated in extrafollicular foci and are mainly, albeit not exclusively, T-dependent. Germinal centers do not develop until about day 7. By that time most of the infectious virus has been cleared from the respiratory tract. Germinal centers can be found up to 5 months after influenza infection. Germinal center responses generate both long-lived plasma cells in the bone marrow as well as circulating memory B cells. Plasma cells and memory B cells are also found in the lung, were they persist for many months and even years. Their origins have not been resolved.
It is not fully resolved what drives B cells towards either an extra- or intra- follicular response. One pre-requisite for the development of extrafollicular responses appears to be the presence of B cells with high affinity for the target antigen. This was elegantly demonstrated in studies using hen-egg lysozyme (HEL) BCR transgenic mice and immunizing them with HEL or closely related antigens, which nonetheless bound to the BCR with reduced affinity (83). Not all studies are consistent with the affinity selection model, however, as some found stochastic selection of B cells into one versus the other response (84). The differences in those studies may well be due to the specific antigen used and the naïve B-cell repertoire present in the mice studied. Furthermore, it is unclear whether strong BCR-signaling alone is sufficient to drive extrafollicular B-cell responses or whether other signals are necessary. In contrast to infection with influenza virus, immunization with the same virus in adjuvant induces only very weak extrafollicular response (J. Dieter and N. Baumgarth, unpublished data). Thus, other signals are likely required to drive extrafollicular responses, signals that may not be provided with commonly used vaccines.
Serum antibodies arising from extrafollicular B-cell responses after influenza infection are not easily distinguished from their counterparts arising from germinal center responses. However, we have exploited the extensive work that was conducted in the late 1980s and early 1990s to sequence the BCR repertoire against influenza virus A/Puerto Rico/8/34 (A/PR8) in BALB/c mice. Those studies demonstrated that distinct waves of B cells, differing in their Ig-repertoire, arise during the course of vaccination with this virus (72, 73, 85). Consistent with the extrafollicular nature of early-arising antibodies, those studies demonstrated that the earliest-induced virus-specific antibodies appeared relatively short-lived and could not be boosted, while antibodies of late primary responses also contributed the secondary responses. Furthermore, these studies identified one particular germline-encoded idiotype (C12Id) as contributing nearly 25% of the earliest antibodies to immunization with A/PR8-HA and that this antibody was highly protective after passive transfer (72). Given that the extrafollicular response contributes so heavily to early immune protection, the ability to resist or to rapidly overcome influenza infection thus seems to depend considerably on the preexisting B-cell repertoire and the frequency of relatively high-affinity B cells that will form rapid, extrafollicular foci to influenza virus strains not previously experienced.
Our studies confirmed and expanded those early studies by demonstrating that C12Id-encoded B cells were follicular B cells that participated nearly exclusively in the extrafollicular foci response and were largely excluded from germinal centers (86). Consistent with the observed lack of a C12Id-contribution to a secondary response following immunization (72), the C12Id+ B cells did not give rise to memory B cells after infection, further suggesting that they do not participate in germinal center responses. We were therefore surprised to learn that despite this, C12Id+ virus-specific antibodies were produced for life in the influenza-infected mice (Rothaeusler K, Elsner RA, Nguyen T, Baumgarth N, manuscript submitted). Further studies are required to confirm the germinal center-independence of the long-term C12Id+ antibody responses. Nonetheless, the data raise questions about the role and function of germinal centers, if long-term protection from reinfection with a homotypic strain of influenza virus can be achieved in their absence.
Germinal center responses in viral infections
Following influenza infection antibody responses are induced which provide homotypic immune protection for life. The long-term maintenance of humoral immunity is provided by a combination of long-lived antibody secreting cells and memory B cells. Bone marrow and lung microenvironments foster the long-term maintenance of antibody-secreting cells (77, 78), while memory B cells appear to distribute widely, with predilections for lungs, MedLN, and the diffuse-NALT environments (87, 88). Numbers of influenza specific memory B cells in the lung far exceed those in the bone marrow (87). Whether the lung always harbors specific niches for these cells or whether they are infection-induced and how they are maintained long-term are important unresolved questions.
Memory B cells and the bone marrow resident plasma cells arise from the establishment of equally robust germinal center reactions that first appear around day 7 of infection in regional lymph nodes. Interestingly, despite the clearance of infectious virus around that same time, these germinal centers persist for many months (86, 89) (Fig. 3). These germinal centers are regarded as birthplaces of the high affinity IgG and IgA responses, as somatic hypermutation and affinity maturation of individual B cells occur only here. Yet, as outlined above, the early extrafollicular foci might also generate high-affinity antibodies. These arise from the activation of the high-affinity BCR-carrying cells among the pre-infection repertoire of germline-encoded B cells. Based on the affinity selection model the overall antibody-affinity to an influenza virus substrain might not increase over time, as the early high-affinity extrafollicular antibody response will be largely replaced by a later affinity-matured germinal center-derived antibody response. Existing evidence in the literature strongly supports this idea.
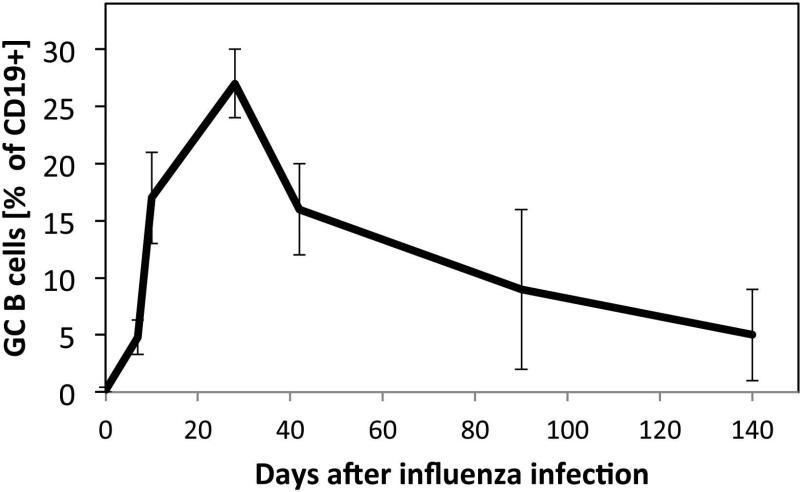
Fig. 3. Germinal center persistence after influenza infection.
Shown are the mean frequencies ± SD (n = 4-6 mice/group) of germinal center B cells among all CD19+ B cells in the regional lymph nodes at indicated times following influenza A/Puerto Rico/8/34 infection of BALB/c mice. Note the long persistence of the germinal centers, despite viral clearance around day 7. The data were as reported previously (86).
Systematic affinity measurements of early and later induced antibody responses to influenza that could validate such a model are missing. However, infections of mice with VSV demonstrated a lack of overall increases in antibody affinities over time due to the presence of high-affinity antibodies early after infection (90). Similar to the earlier studies conducted on influenza virus antibody repertoires (72, 73, 85), repertoire studies on B-cell responses after VSV infection showed a switch in V-gene usage among the antiviral B-cell clones but no overall increase in antibody-affinity over time since infection (91), which would be consistent with a switch from extrafollicular to germinal center responses. Recent studies in humans also found that plasmablasts with relatively high affinity appeared in the blood within 7 days after infection or vaccination with influenza (74). However, the latter data might be due to the reactivation of memory B cells or be the result of a truly primary response. Moreover, and surprisingly, the ability of existing serum antibodies to rapidly neutralize a virus seemed to be dependent on a certain concentration threshold, but were little influenced once avidities of antibodies exceeded 106- 107 M-1, affinities that are achieved already with pentameric polyreactive IgM antibodies (32).
Despite the undisputed fact that somatic hypermutations occur in germinal centers and can strongly increase affinity against hapten antigens, the existing literature on B-cell responses to virus infections does not support a major role for this pathway in the development of protective antibody responses to viruses (92, 93), because during these responses germinal center-derived antibodies simply replace germline-encoded antibodies and thus the germinal center response is only one B-cell response type that leads to the production of high-affinity antibodies.
Emergence of a different view of germinal center response function
If the enhancement of antibody-affinity does not drive the germinal center responses during viral infections, then what are the true benefits of this response? I would argue that the outcome of the germinal center response might be focused nearly exclusively on the potential next challenge infection and the broadening of the B-cell repertoire to counteract the ever-changing face of viruses, such as the influenza virus. The true benefit that comes from broadening the B-cell receptor repertoire might have to do less with the more rapid response that will ensue after reinfection with the same, i.e. a homotypic influenza virus, although that clearly can happen. Instead, it might provide a larger pool of B cells that have experienced a similar antigen and can respond to a challenge infection with a similar, but distinct virus, such as a heterosubtypic influenza virus. In the absence of germinal center reactions, the protective capacity of the humoral immune system would have to rely only on polyreactive B-1 cells and germline-encoded B-2 cells. While those are abundant in our empirically selected experimental models, the above-discussed germline encoded C12Id response of BALB/c mice provides one such example, they might be rare or nonexistent in outbred populations of humans or animals (Fig. 4).
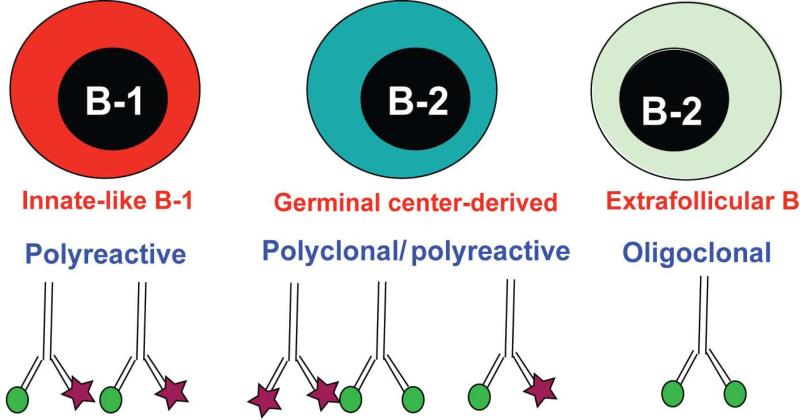
Fig. 4. B-cell responses to influenza infection differ in the diversity of their BCR repertoire.
There are at least three distinct B-cell response types that contribute to immune protection from influenza infection: B-1 cells respond to infection with the secretion of polyreactive, natural IgM, some but not all of which binds to influenza virus. These antibodies are overall of low affinity, but they can bind to many different types of antigens and their presence is required from survival from infection. Second, germinal center-derived B-2 cell responses become polyclonal over time, facilitated in part through somatic hypermutation in the antigen-binding CDR3-Ig region. While these responses generate high-affinity antibodies against the infection at hand, continued germinal center reaction can also result in the generation of polyreactive antibodies. Moreover, the repertoire of B cell clones that participate in the immune response is expanded over time. Finally, the extrafollicular foci response, which can be of high affinity, but is likely highly oligoclonal during a primary infection, as it relies on the presence of high-affinity germline-encoded antigen-specific B cells. However, these responses are supplemented by memory B cells, which can participate in a recall response by rapidly forming extrafollicular foci.
This interpretation is further supported by considering the generation of memory B cells in germinal centers. In contrast to plasma cells, which continuously produce antibodies, these cells do not secrete antibodies and thus cannot prevent a reinfection. They can, however, be activated rapidly in response to a challenge infection, where they will differentiate and produce antibodies. If the outcome of a germinal center reaction simply is to fight the same infection again, then development of plasma cells should be sufficient, as they can induce ‘sterilizing’ immunity and prevent an infection. The development of memory B cells, on the other hand, does not make much sense. However, if germinal center responses are mainly about being ready for an infection with a mutated form of a previously encountered pathogen, then the development of memory B cells is highly appropriate. It provides a broadened repertoire of B cells ready to respond and selected to fit a pathogen that is at least similar to a pathogen that it had encountered previously. These memory B cells can then respond to re-infection with rapid formation of extrafollicular foci, thereby broadening the oligoclonal repertoire of germ-line encoded B cells that participate in a truly primary, i.e. non-cross-reactive response.
While this discussion on the virtues of the germinal center response might be viewed as largely semantics, it could be of importance when considering the development of antiviral vaccines. Broadly neutralizing antibodies are an obvious and excellent target for new vaccine development efforts against highly mutating pathogens. Nonetheless, it is likely that we must also prepare for changes in these highly conserved epitopes that could be induced unintentionally when the broad presence of epitope-specific antibodies apply strong immune pressure on a handful of such viral epitopes. Causing a robust broadening of the antiviral B-cell repertoires by introducing antigens in such a way that germinal center responses that generate memory B cells for extended periods of time ensue might be an important additional design feature of a next generation vaccine that is gleaned from the highly beneficial antibody response to influenza virus.
Concluding remarks
Influenza infection triggers a robust B-cell response in the lymphoid tissues of the respiratory tract that provides immune protection from both primary and secondary infections. The regulation of this B-cell response highlights the intricate connection between the multiple B-cell clones that participate in distinct ways to the acute primary antiviral response. Waves of B cells with distinct Ig-repertoires generate a multifaceted humoral response that provides protective antibodies before, during and after infection both locally and systemically. This change in V-gene usage and the reported lack of affinity maturation of the entire B-cell repertoire in response to a viral infection can be explained by the distinct activation of early extrafollicular and later germinal center responses. The former will rapidly expand already high-affinity responses, while the latter response eventually generates high-affinity antibodies, but does so in a manner that broadens the repertoire that is now available to respond to repeat infections with viruses that likely have undergone mutations in their major neutralizing epitopes. By expanding the B-cell repertoire in germinal center reactions, based on pathogens that a host is experiencing, the immune system may modulate and expand its repertoire against specificities it is likely to encounter again. Overall this process is quite reminiscent of the pre-immune gene-conversion processes that occur in other species, such as the rabbit, where exposure to gut microbes leads to diversification of the B-cell repertoire after their emergence from the bone marrow (94).
Acknowledgement
I thank all current and past members of my laboratory for their hard work and dedication to their projects and Dr. Andy Fell for editorial comments. Current work relevant to this review was supported by grants from the US National Institutes of Health/National Institute of Allergy and Infectious Diseases (AI051354 and AI085568).
Footnotes
The author has no conflict of interest to declare.
References
- 1.Potter CW. A history of influenza. J Appl Microbiol. 2001;91:572–579. doi: 10.1046/j.1365-2672.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- 2.Bos NA, et al. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol. 1989;19:2335–2339. doi: 10.1002/eji.1830191223. [DOI] [PubMed] [Google Scholar]
- 3.Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, Nobrega A. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol. 1997;27:1557–1563. doi: 10.1002/eji.1830270635. [DOI] [PubMed] [Google Scholar]
- 4.Hooijkaas H, Benner R, Pleasants JR, Wostmann BS. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered “antigen-free” diet. Eur J Immunol. 1984;14:1127–1130. doi: 10.1002/eji.1830141212. [DOI] [PubMed] [Google Scholar]
- 5.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 6.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 7.Herzenberg LA. B-1 cells: the lineage question revisited. Immunol Rev. 2000;175:9–22. [PubMed] [Google Scholar]
- 8.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy RR, Hayakawa K, Shimizu M, Yamasaki K, Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987;236:81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa K, Hardy RR, Honda M, Herzenberg LA, Steinberg AD. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci USA. 1984;81:2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayakawa K, Hardy RR, Herzenberg LA. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986f;16:450–456. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- 12.Kasaian MT, Casali P. Autoimmunity-prone B-1 (CD5 B) cells, natural antibodies and self recognition. Autoimmunity. 1993;15:315–329. doi: 10.3109/08916939309115755. [DOI] [PubMed] [Google Scholar]
- 13.Racine R, Winslow GM. IgM in microbial infections: taken for granted? Immunol Lett. 2009;125:79–85. doi: 10.1016/j.imlet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou ZH, Zhang Y, Hu YF, Wahl LM, Cisar JO, Notkins AL. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1:51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci USA. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norderhaug IN, Johansen FE, Schjerven H, Brandtzaeg P. Regulation of the formation and external transport of secretory immunoglobulins. Crit Rev Immunol. 1999;19:481–508. [PubMed] [Google Scholar]
- 18.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 21.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci USA. 2000;97:1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker N, Ehrenstein MR. Cutting edge: selection of B lymphocyte subsets is regulated by natural IgM. J Immunol. 2002;169:6686–6690. doi: 10.4049/jimmunol.169.12.6686. [DOI] [PubMed] [Google Scholar]
- 24.Choi SC, et al. Mouse IgM Fc receptor, FCMR, promotes B cell development and modulates antigen-driven immune responses. J Immunol. 2013;190:987–996. doi: 10.4049/jimmunol.1202227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochsenbein AF, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 26.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 27.De Silva NS, Simonetti G, Heise N, Klein U. The diverse roles of IRF4 in late germinal center B-cell differentiation. Immunol Rev. 2012;247:73–92. doi: 10.1111/j.1600-065X.2012.01113.x. [DOI] [PubMed] [Google Scholar]
- 28.Honda S, et al. Enhanced humoral immune responses against T-independent antigens in Fc alpha/muR-deficient mice. Proc Natl Acad Sci USA. 2009;106:11230–11235. doi: 10.1073/pnas.0809917106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto N, et al. A novel Fc receptor for IgA and IgM is expressed on both hematopoietic and non-hematopoietic tissues. Eur J Immunol. 2001;31:1310–1316. doi: 10.1002/1521-4141(200105)31:5<1310::AID-IMMU1310>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 30.Shima H, et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. 2010;22:149–156. doi: 10.1093/intimm/dxp121. [DOI] [PubMed] [Google Scholar]
- 31.Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J Immunol. 1997;158:1175–1186. [PubMed] [Google Scholar]
- 32.Bachmann MF, et al. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 33.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 34.Hayakawa K, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 35.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 36.Elewaut D, Kronenberg M. Molecular biology of NK T cell specificity and development. Semin Immunol. 2000;12:561–568. doi: 10.1006/smim.2000.0275. [DOI] [PubMed] [Google Scholar]
- 37.Julien JP, Lee PS, Wilson IA. Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol Rev. 2012;250:180–198. doi: 10.1111/imr.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray ES, et al. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J Virol. 2009;83:8925–8937. doi: 10.1128/JVI.00758-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haynes BF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 40.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 41.Mouquet H, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grebe KM, Yewdell JW, Bennink JR. Heterosubtypic immunity to influenza A virus: where do we stand? Microbes Infect. 2008;10:1024–1029. doi: 10.1016/j.micinf.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ekiert DC, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wrammert J, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krammer F, Pica N, Hai R, Margine I, Palese P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly-protective stalk-specific antibodies. J Virol. 2013 doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zinkernagel RM, Cooper S, Chambers J, Lazzarini RA, Hengartner H, Arnheiter H. Virus-induced autoantibody response to a transgenic viral antigen. Nature. 1990;345:68–71. doi: 10.1038/345068a0. [DOI] [PubMed] [Google Scholar]
- 48.Memoli MJ, Morens DM, Taubenberger JK. Pandemic and seasonal influenza: therapeutic challenges. Drug Discov Today. 2008;13:590–595. doi: 10.1016/j.drudis.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee BO, et al. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol. 2005;175:5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- 50.Sangster MY, Riberdy JM, Gonzalez M, Topham DJ, Baumgarth N, Doherty PC. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. J Exp Med. 2003;198:1011–1021. doi: 10.1084/jem.20021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol. 2012;42:120–129. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rauch PJ, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 54.Nisitani S, Tsubata T, Murakami M, Honjo T. Administration of interleukin-5 or -10 activates peritoneal B-1 cells and induces autoimmune hemolytic anemia in anti-erythrocyte autoantibody-transgenic mice. Eur J Immunol. 1995;25:3047–3052. doi: 10.1002/eji.1830251110. [DOI] [PubMed] [Google Scholar]
- 55.Kawahara T, Ohdan H, Zhao G, Yang YG, Sykes M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol. 2003;171:5406–5414. doi: 10.4049/jimmunol.171.10.5406. [DOI] [PubMed] [Google Scholar]
- 56.Cole LE, et al. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc Natl Acad Sci USA. 2009;106:4343–4348. doi: 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ha SA, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang WL, Coro ES, Rau FC, Xiao Y, Erle DJ, Baumgarth N. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J Immunol. 2007;178:1457–1467. doi: 10.4049/jimmunol.178.3.1457. [DOI] [PubMed] [Google Scholar]
- 59.Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006;176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 60.Hermesh T, Moltedo B, Moran TM, Lopez CB. Antiviral instruction of bone marrow leukocytes during respiratory viral infections. Cell Host Microbe. 2010;7:343–353. doi: 10.1016/j.chom.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hooijkaas H, Bos N, Benner R, Pleasants JR, Wostmann BS. Immunoglobulin isotypes and antibody specificity repertoire of “spontaneously” occurring (“background”) immunoglobulin-secreting cells in germfree mice fed chemically defined ultrafiltered “antigen-free” diet. Adv Exp Med Biol. 1985;186:131–138. doi: 10.1007/978-1-4613-2463-8_16. [DOI] [PubMed] [Google Scholar]
- 62.Van Oudenaren A, Haaijman JJ, Benner R. Frequencies of background cytoplasmic Ig-containing cells in various lymphoid organs of athymic and euthymic mice as a function of age and immune status. Immunology. 1984;51:735–742. [PMC free article] [PubMed] [Google Scholar]
- 63.Ohdan H, et al. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Gal alpha 1,3Gal epitopes in alpha 1,3-galactosyltransferase-deficient mice. J Immunol. 2000;165:5518–5529. doi: 10.4049/jimmunol.165.10.5518. [DOI] [PubMed] [Google Scholar]
- 64.Murakami M, et al. Oral administration of lipopolysaccharides activates B-1 cells in the peritoneal cavity and lamina propria of the gut and induces autoimmune symptoms in an autoantibody transgenic mouse. J Exp Med. 1994;180:111–121. doi: 10.1084/jem.180.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 66.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunigy. Nature. 2002;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 67.Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, Cazenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- 68.Tumang JR, Frances R, Yeo SG, Rothstein TL. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol. 2005;174:3173–3177. doi: 10.4049/jimmunol.174.6.3173. [DOI] [PubMed] [Google Scholar]
- 69.Fairfax KA, et al. Different kinetics of Blimp-1 induction in B cell subsets revealed by reporter gene. J Immunol. 2007;178:4104–4111. doi: 10.4049/jimmunol.178.7.4104. [DOI] [PubMed] [Google Scholar]
- 70.Sealy R, Surman S, Hurwitz JL, Coleclough C. Antibody response to influenza infection of mice: different patterns for glycoprotein and nucleocapsid antigens. Immunology. 2003;108:431–439. doi: 10.1046/j.1365-2567.2003.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clarke S, Rickert R, Wloch MK, Staudt L, Gerhard W, Weigert M. The BALB/c secondary response to the Sb site of influenza virus hemagglutinin. Nonrandom silent mutation and unequal numbers of VH and Vk mutations. J Immunol. 1990;145:2286–2296. [PubMed] [Google Scholar]
- 72.Kavaler J, Caton AJ, Staudt LM, Gerhard W. A B cell population that dominates the primary response to influenza virus hemagglutinin does not participate in the memory response. Eur J Immunol. 1991;21:2687–2695. doi: 10.1002/eji.1830211107. [DOI] [PubMed] [Google Scholar]
- 73.McKean D, Huppi K, Bell M, Staudt L, Gerhard W, Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci USA. 1984;81:3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wrammert J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recover o mice from primary infleunza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 76.Monsalvo AC, et al. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med. 2011;17:195–199. doi: 10.1038/nm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hyland L, Sangster M, Sealy R, Coleclough C. Respiratory virus infection of mice provokes a permanent humoral immune response. J Virol. 1994;68:6083–6086. doi: 10.1128/jvi.68.9.6083-6086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones PD, Ada GL. Persistence of influenza virus-specific antibody-secreting cells and B-cell memory after primary murine influenza virus infection. Cell Immunol. 1987;109:53–64. doi: 10.1016/0008-8749(87)90291-7. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez SF, et al. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat Immunol. 11:427–434. doi: 10.1038/ni.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, Garcia-Sastre A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci USA. 2010;107:11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McLaren C, Butchko GM. Regional T- and B-cell responses in influenza-infected ferrets. Infect Immun. 1978;22:189–194. doi: 10.1128/iai.22.1.189-194.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MacLennan IC, et al. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 83.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jacob J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med. 1992;176:679–687. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clarke SH, Staudt LM, Kavaler J, Schwartz D, Gerhard WU, Weigert MG. V region gene usage and somatic mutation in the primary and secondary responses to influenza virus hemagglutinin. J Immunol. 1990;144:2795–2801. [PubMed] [Google Scholar]
- 86.Rothaeusler K, Baumgarth N. B-cell fate decisions following influenza virus infection. Eur J Immunol. 2010;40:366–377. doi: 10.1002/eji.200939798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joo HM, He Y, Sangster MY. Broad dispersion and lung localization of virus-specific memory B cells induced by influenza pneumonia. Proc Natl Acad Sci USA. 2008;105:3485–3490. doi: 10.1073/pnas.0800003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takahashi Y. Memory B cells in systemic and mucosal immune response: implications for successful vaccination. Biosci Biotechnol Biochem. 2007;71:2358–2366. doi: 10.1271/bbb.70142. [DOI] [PubMed] [Google Scholar]
- 89.Moyron-Quiroz JE, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 90.Roost HP, et al. Early high-affinity neutralizing anti-viral IgG responses without further overall improvements of affinity. Proc Natl Acad Sci USA. 1995;92:1257–1261. doi: 10.1073/pnas.92.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalinke U, et al. The role of somatic mutation in the generation of the protective humoral immune response against vesicular stomatitis virus. Immunity. 1996;5:639–652. doi: 10.1016/s1074-7613(00)80277-0. [DOI] [PubMed] [Google Scholar]
- 92.Kalinke U, Oxenius A, Lopez-Macias C, Zinkernagel RM, Hengartner H. Virus neutralization by germ-line vs. hypermutated antibodies. Proc Natl Acad Sci USA. 2000;97:10126–10131. doi: 10.1073/pnas.97.18.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 94.Lanning D, Zhu X, Zhai SK, Knight KL. Development of the antibody repertoire in rabbit: gut-associated lymphoid tissue, microbes, and selection. Immunol Rev. 2000;175:214–228. [PubMed] [Google Scholar]