Abstract
Background
Inherited leukodystrophies are progressive, debilitating neurological disorders with few treatment options and high mortality rates. Our objective was to determine national variation in the costs for leukodystrophy patients, and to evaluate differences in their care.
Methods
We developed an algorithm to identify inherited leukodystrophy patients in de-identified data sets using a recursive tree model based on ICD-9 CM diagnosis and procedure charge codes. Validation of the algorithm was performed independently at two institutions, and with data from the Pediatric Health Information System (PHIS) of 43 U.S. children’s hospitals, for a seven year time period, 2004–2010.
Results
A recursive algorithm was developed and validated, based on six ICD-9 codes and one procedure code, that had a sensitivity up to 90% (range 61–90%) and a specificity up to 99% (range 53–99%) for identifying inherited leukodystrophy patients. Inherited leukodystrophy patients comprise 0.4% of admissions to children’s hospitals and 0.7% of costs. Over seven years these patients required $411 million of hospital care, or $131,000/patient. Hospital costs for leukodystrophy patients varied at different institutions, ranging from 2 to 15 times more than the average pediatric patient. There was a statistically significant correlation between higher volume and increased cost efficiency. Increased mortality rates had an inverse relationship with increased patient volume that was not statistically significant.
Conclusions
We developed and validated a code-based algorithm for identifying leukodystrophy patients in deidentified national datasets. Leukodystrophy patients account for $59 million of costs yearly at children’s hospitals. Our data highlight potential to reduce unwarranted variability and improve patient care.
Keywords: Leukodystrophy, cost analysis, hospitalization, pediatric, health services research
Introduction
Inherited leukodystrophies affect almost 1 in 7500 children with mortality greater than 30%.1 Children with leukodystrophies have serious medical complications including epilepsy, developmental regression, and intellectual impairment.2,3 Treatment for leukodystrophies is primarily supportive, with the exception of hematopoietic cell transplantation or enzymatic therapies for a minority of patients in a few specific diseaseategories.4–6
Children with chronic neurological diseases such as leukodystrophies account for almost 1/3rd of all charges at U.S. children’s hospitals,7 and it is not known what accounts for this substantial health-care utilization requirement. Care practices for children with leukodystrophies varies between institutions as there are no unifying guidelines or protocols. Creating a standard approach to therapy is limited by lack of data regarding national disease burden, overall healthcare utilization and costs, and discrepancies of in-patient care, studies, and therapies for leukodystrophy patients across treatment centers. Since inpatient admissions account for the majority of the cost associated with care of leukodystrophy patients,8 strategies to develop standardized care models require improved understanding of inpatient utilization related to complications of these disorders.
Our objective was to determine national variations in the care for leukodystrophy patients, including differences in cost, mortality, and efficiency of admissions. However, current ICD-9-CM codes do not represent the wide diversity of current leukodystrophy diagnoses. Further, use of ICD-9-CM codes without manual verification is less than 20% specific for “gold-standard” true inherited leukodystrophies.1 Thus, evaluation of any large administrative database based solely on ICD-9 codes would be inaccurate and limited. To overcome this issue we sought to develop a methodology to identify leukodystrophy patients in national administrative datasets and to query this data.
To more accurately and sensitively identify leukodystrophy patients we developed and validated a recursive tree model algorithm based on ICD-9-CM diagnosis and procedure charge codes from our defined leukodystrophy cohort.1 We then queried and analyzed the U.S. children’s hospital’s Pediatric Health Information System (PHIS) administrative database to determine costs, utilization of care, and variability of costs at different U.S. children’s hospitals. PHIS is a database of demographic information and diagnosis and procedure codes from inpatient and emergency room encounters at 40+ tertiary care children’s hospitals in the United States.9,10 Herein we discuss our approach, findings, and the implications for future studies of the clinical care of leukodystrophy patients.
Methods
This study was approved by the Institutional Review Boards at the University of Utah, Intermountain Healthcare (Intermountain), and Stanford University.
The original cohort of 122 patients with clinically confirmed inherited leukodystrophies, together with 542 patients demonstrated not to have a leukodystrophy (664 patients total)1 was used to develop the algorithm. These patients presented over a nine-year period (1999–2007) at a children’s hospital providing both primary and tertiary care (Primary Children’s Medical Center, Salt Lake City, UT). Cases were identified through a computerized search of International Classification of Disease, 9th Edition, Clinical Modification (ICD-9-CM, referred to subsequently as ICD-9) diagnosis codes, and confirmed by manual chart review and review of laboratory results and MRI images.1 Patients were identified using ICD-9 codes 330.0, 330.1, 330.2, 330.3, 330.8, 330.9, 349.89, 323.9, and 341.9 (respectively leukodystrophy, cerebral lipidoses, cerebral degeneration in generalized lipidoses, cerebral degeneration of childhood in other diseases classified elsewhere, other specified cerebral degenerations in childhood, unspecified cerebral degenerations in childhood, other unspecified disorders of the nervous system, unspecified cause of encephalitis, myelitis, and encephalomyelitis, demyelinating disease of central nervous system, unspecified). All charts were manually reviewed by one of the study pediatric neurologists to determine whether a patient met inclusion and exclusion criteria.
Patients were included in the study if they met the following criteria: 1) age less than 19 years at their initial presentation for evaluation of their symptom(s) which lead to the diagnosis of leukodystrophy; 2) brain MRI findings showing abnormalities of white matter signal consistent with the diagnosis of a leukodystrophy; and 3) MRI results obtained prior to determination of an alternative diagnosis not typically considered a leukodystrophy (such as Batten disease).
Patients were excluded from the study if they met any of the following criteria: (1) they had a known (past medical history) or likely reason for their leukodystrophy, including multiple sclerosis, optic neuritis, transverse myelitis, encephalitis, meningitis, cerebellitis, acute disseminated encephalomyelitis, prematurity, hypoxic-ischemic injury, hemolytic-uremic syndrome, chemotherapy exposure, immunodeficiency, or trauma; (2) they had hypomyelination on an initial MRI which was not present upon reimaging at a later time point and in whom there were not persistent neurological abnormalities; (3) they had a history of headaches with < 4 punctate white matter hyperintensities on brain MRI; or (4) no CNS imaging available for review by the study investigators even in cases where a diagnosis (for example, adrenoleukodystrophy) had been made based on biochemical or genetic testing.
PCMC is the sole tertiary care pediatric hospital in Utah, and receives referrals from Wyoming, Idaho, Montana, and parts of Nevada, Colorado, and Arizona. PCMC is part of Intermountain, which maintains administrative and clinical electronic records captured from multiple information systems related to admissions, emergency room, and clinic visits in an enterprise data warehouse (EDW). The EDW also captures charge codes for lab tests and radiology procedures, such as brain MRIs. The Intermountain charge codes were converted to equivalent PHIS standardized charge codes known as Clinical Transaction Classification (CTC) codes. We queried the EDW using unique identifiers assigned to each of the 664 patients and extracted all ICD-9 diagnosis codes and CTC codes for all inpatient admissions.
We used a recursive tree model to generate an algorithm based on ICD-9 and CTC codes for the 664 patient cohort. We fitted a tree model using binary recursive partitioning whereby the data were successively split along coordinate axes of the predictor variables so that at any node, the split which maximally distinguishes the response variable in the left and the right branches is selected.11 Splitting continued until the nodes were pure or data too sparse (Supplemental Figure 1). We used hundreds of ICD-9 and CTC codes to build the tree, with the goal of a minimal number of commonly used codes to achieve acceptable sensitivity and specificity.
The final algorithm protocol employed two steps: first, nine ICD-9 codes1 were used to interrogate a database to identify a pool of potential patients and generate the “preliminary” dataset (ICD-9 codes 330.0, 330.1, 330.2, 330.3, 330.8, 330.9, 349.89, 323.9, and 341.9). Next, we applied our recursive tree algorithm on the preliminary dataset to make final determinations.
Ultimately, we found that three different recursive tree algorithms consisting of three, six, or seven codes were able to identify correctly true leukodystrophy patients at different levels of sensitivity and specificity. The codes for the seven-code model were 315.8 (other specified delays in development), 330.0 (leukodystrophy), 323.9 (unspecified causes of encephalitis, myelitis, and encephalomyelitis), 348.8 (other conditions of brain), 276.2 (acidosis), and 294.10 (dementia in conditions classified elsewhere); together with CTC code 411052 (brain MRI).
The Pediatric Health Information System (PHIS) administrative database includes demographic information, diagnosis and procedure codes, from inpatient and emergency room encounters at 43 tertiary care children’s hospitals in the United States.9,10 The hospitals are affiliated with the business alliance of Child Health Corporation of America (Shawnee Mission, Kansas). Data is deidentified before entry into the PHIS database.
U.S. children’s hospital data was queried using the PHIS database with the seven-code algorithm for the time period 1/1/2004 −12/31/2010. Of the hospitals reporting information to PHIS, three were excluded because they did not report any leukodystrophy patients, and one hospital was excluded that did not report any financial data for its leukodystrophy patients. Data collected included number of patients seen; number of encounters; charges; length of stay; number of MRIs; and number of CTs. Data was normalized to the total group of patients seen in PHIS during the same time period. Data was analyzed for the entire group of PHIS hospitals, as well as on a hospital-by-hospital basis.
PHIS cost data was determined from each hospital. Costs were the “Total Adjusted Cost (RCC-based)”. This metric is determined when each PHIS hospital reports charges for their financial data, grouped by CTC codes. Each hospital provides a cost to charge ratio (ratio cost-charge: RCC), which is then adjusted by the geographical location of the hospital. Individual costs are determined by the formula of: (individual charge × RCC). Charges in the PHIS database are adjusted for the wage and price index (published annually in the Federal Register).
Results
Algorithm Development and Validation
We developed, tested, and validated different algorithms based on ICD-9 diagnosis and procedure charge codes to develop a sensitive and specific method to identify inherited leukodystrophy patients in de-identified, code-based national databases. This approach is necessary because specific codes for the different leukodystrophies are not available; and because use of ICD-9 codes without manual verification is less than 20% specific for “true” inherited leukodystrophies.1 We determined all of the ICD-9 diagnosis codes and CTC charge codes for in-patient admissions on the original cohort of 664 patients.1 This included 122 confirmed, inherited leukodystrophy patients (see Methods), and 542 “false-positive” patients which manual chart review had demonstrated were not inherited leukodystrophies.
Then, we developed tree-based algorithms using binary recursive partitioning (Supplemental Figure 1). We developed three different algorithms, based on the use of three codes, six codes, or seven codes. Composition of the different algorithms was: three code model: ICD-9 codes 315.8, 330.0, and 323.9; six code model: 315.8, 330.0, 323.9; 348.8, 276.2, and 294.10; seven code model: 315.8, 330.0, 323.9; 348.8, 276.2, 294.10, and CTC code 411052.
We performed three tests to validate our algorithms and determine their sensitivity and specificity. First, we tested the algorithms at our institution for the years 1999–2007 on the “Original Cohort” (Supplemental Table 1). The original cohort consists of the patients used to develop the algorithm. Second, we tested the algorithms using PHIS database-extracted data for PCMC only, but for the years 2008–2010 (the “PHIS Validation” cohort). These years and patients were not part of the original cohort. This second group of patients was then manually confirmed by chart review. Third, we tested our algorithms at a different institution by applying them to leukodystrophy patients presenting to the children’s hospital (LPCH) at Stanford University for the time range 1/1/2006 through 6/30/2012.
Using the three code tree model (ICD-9 codes 315.8, 330.0, and 323.9), the original cohort has a sensitivity of 84% and specificity of 83%; while the PHIS validation cohort had a sensitivity of 36% and specificity of >99%. With a six code algorithm (ICD-9 codes 315.8, 330.0, 323.9, 348.8, 276.2, and 294.10), the original cohort had a sensitivity of 90% and specificity of 85%; while the PHIS validation cohort has a sensitivity of 63% and specificity of >99%. We added one CTC code (411052; brain MRI), to create the seven-code model, and found that the original cohort had a sensitivity of 90% and specificity of 92%; with a NPV of 84% and PPV of 95%. When this seven-code model was applied to the PHIS validation cohort, a sensitivity of 71% and specificity of >99% was generated. When we tested our seven-code algorithm at LPCH, we had a sensitivity of 61% and specificity of 53%, with a positive predictive value of 13% and negative predictive value of 92%. Addition of additional codes more than used in the seven-code model did not increase specificity, and led to a decrease in sensitivity.
U.S. Children’s Hospital Data
To determine costs and query disease burden for pediatric leukodystrophy patients we used our algorithm to query the PHIS inpatient children’s hospital database for the years 2004–2010. Data for thirty-nine hospitals was analyzed; three hospitals were excluded from analysis because they had no apparent leukodystrophy patients; and one hospital was excluded that did not have financial data.
We identified 3,143 unique leukodystrophy patients with 13,554 patient admissions, or 4.3 admissions per patient (Table 1). Leukodystrophy patients accounted for 0.38% of total admissions even though they were only 0.09% of patients (38.9 admissions/ 10,000 admissions; total PHIS admissions was 3.2 million patients). This is almost three times as many admissions as the average patient.
Table 1.
Summary of leukodystrophy patient numbers and costs from PHIS database of U.S. children’s hospitals. Results are compared to the total for all PHIS patients, and to the average PHIS patient. Years 2004–2010, PHIS database total n= 2,272,591 patients; total cost $60,166,647,000.
| Category | Number (%) |
|---|---|
| Unique leukodystrophy patients | 3,143 (0.09%) |
| Leukodystrophy patients/10,000 total patients | 9.02 |
| Leukodystrophy patient admissions | 13,554 (0.38%) |
| Leukodystrophy admissions/10,000 patient admissions | 38.9 |
| Leukodystrophy patient costs | $411,374,000 (0.68%) |
| Average leukodystrophy patient cost | $130,886 |
| Average PHIS patient cost | $26,475 |
| Cost ratio (and range) of leukodystrophy:average patient | 4.9 (2.2 – 20.3) |
| Costs ratio per admission (and range) of leukodystrophy:average | 1.8 (1.0–4.9) |
| Average leukodystrophy patient admissions | 4.3 |
| Average PHIS patient admissions | 1.5 |
| Admissions ratio (and range) of leukodystrophy:average patient | 2.9 (2.0 – 5.4) |
The total cost of inpatient care for leukodystrophy patients was $411 million dollars. For the 7-year study period, the average total cost for a leukodystrophy patient was $131,000, which is five times greater than the average PHIS patient cost of $26,000. Even taking into account the greater number of admissions, each leukodystrophy patient admission was almost twice as expensive as an admission for the average PHIS patient (Table 2).
Table 2.
Comparison of variation for leukodystrophy patients across PHIS children’s hospitals, for years 2004–2010. All numbers refer to leukodystrophy patients. Results are total for the 7-year period. Mortality rate is calculated as a percent of the total leukodystrophy patient cohort at each hospital. The cost/patient is the total cost of in-patient health care costs for each leukodystrophsy patient.
| Measure | Total | Average/Hospital | Range |
|---|---|---|---|
| Number of patients | 3,143 | 81 | 14–172 |
| Number of admissions (n) | 13,554 | 348 | 81–705 |
| Admissions/patient | --- | 4.3 | 3.0–7.3 |
| Mortalities (n) | 268 | 6.9 | 1–17 |
| Mortality % | --- | 8.8 | 2.4–18.1 |
| Hospital costs ($thousands) | 411,374 | 10,548 | 1,835–27,602 |
| Cost/patient ($) | --- | 131,380 | 60,013–239,872 |
| Total hospital days | 136,776 | 3,507 | 826–7,307 |
| Average length of stay/patient (days) | --- | 10.1 | 6.7–14.1 |
| MRIs (n) | 4,755 | 122 | 15–292 |
| MRI/patient | --- | 1.5 | 0.7–2.5 |
Institution Variability in Care
We compared patient and cost information across the various children’s hospitals reporting data to PHIS to determine the variability in care for patients at different institutions (Table 2).
Leukodystrophy patients had an average of 4.3 admissions over the seven-year study period; but at different hospitals this rate varied from 3 to 7.3 admissions per patient. Average mortality was 7 leukodystrophy patient deaths per hospital or a rate of 8.8%, but this varied across institutions ranging from 1 to 17 patient deaths or 2.4% to 18.1%.
Cost on average for a leukodystrophy patient was more than five (5.31) times the average pediatric patient, but the hospitals ranged from 2.2 times to 20.3 times the average patient cost. Several potential contributors to cost were also elevated in leukodystrophy patients: average length of stay was 10 days but varied from 6 to 14 days; and each patient had on average 1.4 MRI scans, varying at different hospitals from 0.7 to 2.5 scans per patient.
To determine whether there were intrinsic differences in hospital care, we compared measures of care for leukodystrophy patients across institutions (Table 3). We compared institutions based on the percentage make-up of their total patient base by leukodystrophy patients (percent of patients); the percentage of total admissions that were leukodystrophy patients (percent of admissions); and the percentage of total costs incurred by leukodystrophy patients (percent of costs). To compare for whether institutions were admitting their patients with similar frequencies, we calculated percent of patients divided by percent of admissions.
Table 3.
Hospital efficiencies for leukodystrophy patients (see graphical representation in Figure 1).
i. Efficiency of admissions (costs efficiency). A value closer to 1 indicates that the percent of costs was equal, or nearly equal, to the percent of admissions. Calculated by dividing percent of admissions/percent of costs. ii. Volume of admissions. This calculates the relative proportion of leukodystrophy patient admissions, compared to total patient admissions. iii. Admission frequency, controls for whether certain institutions were admitting their patients with similar frequencies, calculated by percent of patients/percent of admissions. Definitions: Percent of admissions: percent of patients at each hospital that are leukodystrophy patients. Percent of costs: percent of costs at each hospital accounted for by leukodystrophy patients. Percent of patients: the percent of patients at each hospital that are leukodystrophy patients.
| Category | Average/Hospital | Range | Standard deviation |
|---|---|---|---|
| i. Efficiency (cost) | 0.59 | 0.20–0.96 | 0.14 |
| ii. Volume | 0.21 | 0.05–0.45 | 0.07 |
| iii. %Pat/%Adm | 0.36 | 0.19–0.50 | 0.07 |
We determined the relative efficiency of costs by dividing the percentage of leukodystrophy admissions by the percentage of costs accounted for by these patients. A higher efficiency score (approaching 1) indicates that the percentage of costs is proportional to the percentage of admissions. More admissions with low costs would have a high efficiency. In contrast, few admissions but with high costs for each admission would have low efficiency. We found that hospital efficiencies had a range of 0.20 to 0.96 with an average of 0.59 and standard deviation of 0.14 (Table 3). For efficiency, 27 (of 39) hospitals were within one standard deviation, while 37 were within two standard deviations.
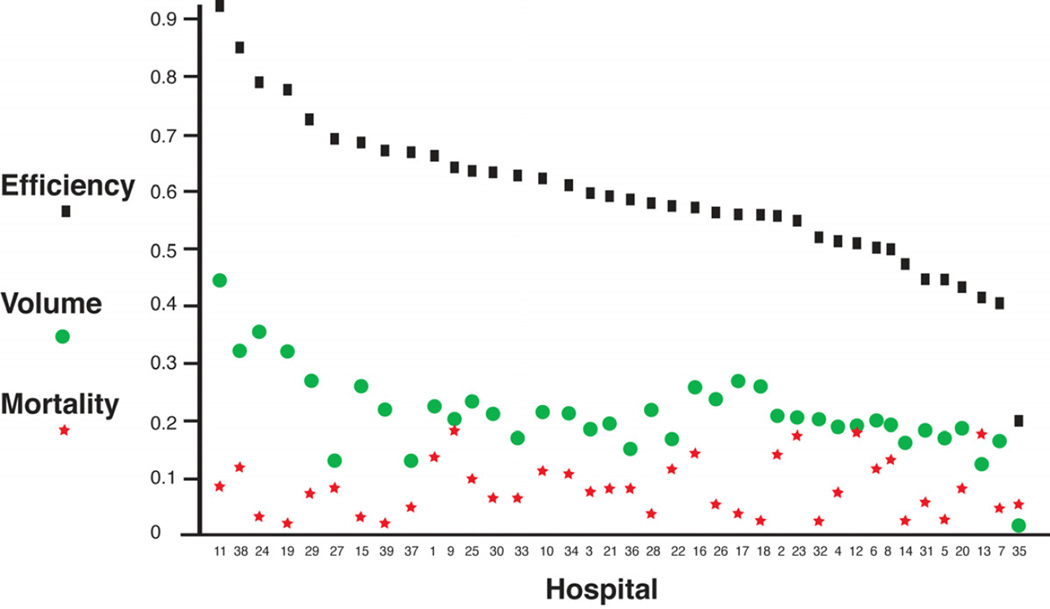
When compared as trends (Figure 1), institutions had wide variations in cost efficiency. There was a statistically significant correlation between higher volume and increased cost efficiency, with a correlation coefficient r 0.770. Increased mortality rates had an inverse relationship with increased patient volume that was not statistically significant, with a correlation coefficient r 0.076.
Figure 1.
Leukodystrophy patient cost efficiency, volume, and mortality at different PHIS children’s hospitals. Patient volume and cost efficiency are statistically significantly correlated; volume and mortality are not. Efficiency is shown as black squares; volume as green circles; and mortality as red stars. X-axis is the institution code; y-axis is percent (see text for definitions).
Discussion
We report the first analysis of U.S. healthcare costs and mortality outcomes for children hospitalized with leukodystrophies. We developed and validated an algorithm comprised of seven codes to evaluate leukodystrophy patients in national data sets. We found that leukodystrophy patients accounted for $411 million in costs at children’s hospitals over seven years. This is $59 million per year. This cost is more than five times that of the average patient. Our algorithm likely underestimates the total number of patients and their associated costs.
Prior to this study there has been no national data on healthcare utilization or costs for children with inherited leukodystrophies. Since ICD-9 diagnosis codes are not specific for inherited leukodystrophies,1 we developed a recursive code-based algorithm comprised of seven codes generated using tree-model statistical analysis. We manually validated our approach in our known data set as well as in a novel data set, and at two separate institutions. Our approach has a sensitivity between 63 and 90%, and specificity between 53 and 99%. The use of the International Classification of Diseases, revision 10 (ICD-10), starting in October 2014, could improve the ability to track and report leukodystrophy-specific outcomes. For example, adrenoleukodystrophy and metachromatic leukodystrophy will have separate codes (E71.3 and E75.2, respectively). However, other leukodystrophies including Canavan’s disease and Pelizaeus-Merzbacher disease will fall under the general leukodystrophy category (E75.29), while Alexander disease and vanishing white matter disease are not explicitly listed.
Children’s hospitals reporting data to PHIS experienced a large range of leukodystrophy patient admissions and costs. These institutions hospitalized leukodystrophy patients between 2.0 times to 5.2 times more than the average pediatric patient. Charges per encounter were up to 5 times greater than the average pediatric patient, and total charges per patient were between 2.32 times to 15.20 times the average pediatric patient. Certain leukodystrophy patients may have had higher costs because of their treatments. For example, patients who receive hematopoietic stem cell transplantation/bone marrow transplant have higher costs, although we have shown previously that these treatments are not the sole determinants of high costs in leukodystrophy patients.8 However, with our current analysis we did not dissect the costs within the leukodystrophy patients in PHIS. Future studies could examine national data to address whether certain subsets of leukodystrophy patients have higher costs, and what the reasons for these higher costs are.
Comparison of our findings across the different hospitals included in PHIS revealed several important points. First, cost efficiency varied greatly, and was directly correlated with the number of leukodystrophy patients seen. There was an almost 5-fold difference in efficiency at different institutions.
We defined cost efficiency as the efficiency of hospitalization of leukodystrophy patients, to determine if the percent of charges was proportional to the percent of admissions. A higher efficiency score, approaching 1 (although technically scores higher than 1 are also possible), indicates that the percentage of costs is proportional to the percentage of admissions. More admissions with low costs would have a high efficiency. In contrast, few admissions but with high costs for each admission would have low efficiency.
The cost efficiency is a ratio, not an absolute cost, and it is calculated at each hospital for leukodystrophy patients in comparison to the other PHIS patients. Therefore the use of efficiency ratios allows comparisons between hospitals that can have different charges and costs.12
We found that those hospitals that cared for the largest volume of leukodystrophy patients were the most cost efficient. That is, the hospitals that admitted more leukodystrophy patients charged less per admission than hospitals that admitted fewer leukodystrophy patients. We controlled for the relative frequency with which leukodystrophy patients were admitted at each PHIS hospital, to insure that certain hospitals did not have disproportionate rates of hospitalization that would affect their efficiency calculation.
Second, patient mortality did not correlate with the volume of leukodystrophy patients. Future studies should examine the clinical characteristics and reasons for admission in order to understand and improve the efficiency at hospitals. These findings could help in the design of care models for leukodystrophy patients.
Our study has several limitations. We have demonstrated that our algorithm has a sensitivity of approximately 70%, so we are likely undercounting the total number of leukodystrophy patients. Therefore, our estimation of costs is also likely an underestimation. However, the definition of inherited leukodystrophy used to define the original study cohort,1 specifically, our inclusion of genetically unconfirmed leukodystrophies, could potentially result in an overestimation of cases. However, because genetically unconfirmed leukodystrophies represent such a large portion of leukodystrophies overall- typically up to 50% of cases overall,1, 12 we consider the inclusion of this “unconfirmed” group necessary and important. For institutional comparisons we do not have data to determine how severe each patient’s disease is, so we are making an assumption that the average severity of these patients is relatively similar across all the reporting hospitals. PHIS hospitals include 43 children’s hospitals: not all children’s hospitals have data represented in PHIS; and not all children are admitted to a pediatrics hospital. The PHIS database only includes costs from in-patient hospitalizations, so we do not have comparison data on costs as outpatients. However, we have shown previously that in-patient hospitalization account for a significant majority of costs for leukodystrophy patients.8 Another limitation is that our comparison group of “average” pediatric patients in PHIS included a mix of different diagnoses, ranging from bronchiolitis to complex congenital heart disease. In addition, we do not have outcomes for patients, and we assume that each set of patients at each hospital consists of a distributed population with a random distribution of severity of diseases, variability of disease course, and treatments provided. Finally, costs data is a relatively inexact measure of resource utilization and varies across institutions; standardization methods across institutions will assist to more precisely determine variability in care and costs.13
A particular strength of our approach is that we have validated the codes used in database searches and defined statistical ranges for our data. We have been able to use the combination of national database searches with institution-specific fine detail analysis to determine very precise information concerning hospitalization features.8 We expect this approach to yield continued important insights. For example, with our approach we can define causes of infections in leukodystrophy patients, first by detailed analysis in the IH database, and with verification nationally. Future directions can include the use of improved, more detailed national databases such as the PHIS+ consortium.14,15 Our strategy of data analysis provides a general roadmap for approaching issues of costs and care in neurologically-impaired children.
We have generated a powerful methodology for identification of leukodystrophy patients in national data sets, and generation of data to improve treatment care models and standardize care. Further, we have shown previously that the number of hospital days, respiratory care such as ventilators, and infections, are significant drivers of costs for leukodystrophy patients.8 Expensive procedures, such as MRI, also add to these costs. Children with chronic neurologic diseases such as leukodystrophies are responsible for 1/3rd of total costs at children’s hospitals.7 Further, hospital-associated costs account for the single largest component of health care spending.16 Thus our approach and findings offer a roadmap for strategies to understand patient costs, and to improve and standardize care for a broad range of chronic neurological conditions.
Supplementary Material
Acknowledgements
Dr. Bonkowsky is supported by a grant from the Primary Children’s Medical Center Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES:
The authors report no conflicts of interest.
Author Contributions:
All authors assisted with data analysis, writing, and revising the manuscript for content. CJB, JL, KVH, JW, CN, EKK, and JLB were involved in data acquisition. CJB, KVH, and JLB conceived the study. CJB, KVH, XS, and JLB performed the statistical analyses.
References
- 1.Bonkowsky JL, Nelson CR, Kingston JL, Filloux FM, Mundorff MB, Srivastava R. The burden of inherited leukodystrophies in children. Neurology. 2010;75(8):718–725. doi: 10.1212/WNL.0b013e3181eee46b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raymond G, Eichler Fatemi A, Naidu S. Leukodystrophies. London: Mac Keith Press; 2011. [Google Scholar]
- 3.Perlman SJ, Mar S. Leukodystrophies. Adv Exp Med Biol. 2012;724:154–171. doi: 10.1007/978-1-4614-0653-2_13. [DOI] [PubMed] [Google Scholar]
- 4.Sevin C, Aubourg P, Cartier N. Enzyme, cell and gene-based therapies for metachromatic leukodystrophy. J Inherit Metab Dis. 2007 Apr;30(2):175–183. doi: 10.1007/s10545-007-0540-z. [DOI] [PubMed] [Google Scholar]
- 5.Orchard PJ, Tolar J. Transplant outcomes in leukodystrophies. Semin Hematol. 2010 Jan;47(1):70–78. doi: 10.1053/j.seminhematol.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Kohlschütter A, Eichler F. Childhood leukodystrophies: a clinical perspective. Expert Rev Neurother. 2011 Oct;11(10):1485–1496. doi: 10.1586/ern.11.135. [DOI] [PubMed] [Google Scholar]
- 7.Berry JG, Poduri A, Bonkowsky JL, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med. 2012 Jan;9(1):e1001158. doi: 10.1371/journal.pmed.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson C, Mundorff MB, Korgenski EK, Brimley CJ, Srivastava R, Bonkowsky JL. Determinants of Health Care Use in a Population-Based Leukodystrophy Cohort. J Pediatr. 2012 Oct 13; doi: 10.1016/j.jpeds.2012.08.046. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kittle K, Currier K, Dyk L, Newman K. Using a pediatric database to drive quality improvement. Semin Pediatr Surg. 2002 Feb;11(1):60–63. doi: 10.1053/spsu.2002.29367. [DOI] [PubMed] [Google Scholar]
- 10.Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055. doi: 10.1001/jama.299.17.2048. [DOI] [PubMed] [Google Scholar]
- 11.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Belmont CA: Wadsworth International Group; 1984. [Google Scholar]
- 12.Schiffmann R, van der Knaap MS. Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology. 2009;72(8):750–759. doi: 10.1212/01.wnl.0000343049.00540.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keren R, Luan X, Localio R, Hall M, McLeod L, Dai D, Srivastava R for the Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of Comparative Effectiveness Research Topics in Hospital Pediatrics. Arch Pediatr Adolesc Med. 2012 Oct;1:1–10. doi: 10.1001/archpediatrics.2012.1266. [DOI] [PubMed] [Google Scholar]
- 14.Narus SP, Srivastava R, Gouripeddi R, et al. Federating clinical data from six pediatric hospitals: process and initial results from the PHIS+ Consortium. AMIA Annu Symp Proc. 2011;2011:994–1003. [PMC free article] [PubMed] [Google Scholar]
- 15.Gouripeddi R, Warner PB, Mo P, et al. Federating Clinical Data from Six Pediatric Hospitals: Process and Initial Results for Microbiology from the PHIS+ Consortium. AMIA Annu Symp Proc. 2012;2012:281–290. [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser Foundation. Trends in health care costs and spending. The Henry J. Kaiser Family Foundation; [Accessed January 21, 2013]. Available at: http://www.kff.org/insurance/upload/7692_02.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.