Abstract
Background:
Acute promyelocytic leukemia (APL) is an acute leukemia diagnosed by translocation of chromosomes 15 and 17 [T (15,17)] and aggregation of neoplastic promyelocytes which are incapable of being converted into mature cells. Today, many tend to use medicinal herbs in studies and clinical applications for treatment of cancers. Cinnamon with scientific name “cinnamomumzelanicum” is a shrub of Laurales order, lauraceae family with cinnamomum genus. It is a medicinal shrub with anti-proliferation effect on tumor cells. This study was conducted to determine the effects of aqueous cinnamon extract on HL-60 cells as a model for APL.
Materials and Methods:
In this in vitro experimental study, HL-60 cell line was cultured under the influence of cinnamon extract's concentrations of 0.01, 0.1, 1, and 2 mg/ml in with intervals of 24, 48, and 72 h. Growth inhibition and toxic effects of cinnamon extract were evaluated through tetrazolium salt reduction. The effect of this herb on the cell cycle was studied by flow cytometry. The Hoechst stain was used to detect apoptotic cell nuclei.
Results:
Cinnamon extract inhibited the growth of HL-60 cells as correlated with concentration and time. After 72 h of treating HL-60 cells with 0.01 mg/l cinnamon extract, the growth of cells was inhibited by 90.1%. Cinnamon extract stopped the cell cycle in G1 phase and the Hoechst staining verified the apoptotic process in those cells.
Conclusion:
Considering the inhibitory property of cinnamon extract, we recommend it as a single drug or besides other medications for treating promyelocytic leukemia.
Keywords: Acute promyelocytic leukemia, apoptosis, cell cycle, cinnamon extract, HL-60 cells
INTRODUCTION
Acute promyelocytic leukemia (APL) is a severe type of acute leukemia which is commonly caused by chromosomal translocation of retinoic acid receptor gene on chromosome 17 and promyelocytic leukemia gene on chromosome 15. This disorder leads to fusion of PML and RARα genes.[1,2] This type of leukemia is distinguished by quick onset of symptoms, weak response to chemotherapy, disseminated intravascular coagulation disorder, and high mortality rate.[3,4] Among the treatment protocols for cancers are chemotherapy and radiotherapy.[5] As the drugs commonly used in chemotherapy have many side effects, they are usually immune suppressors[6,7] and cause problems for patients. An important step toward treatment of cancer will be taken if a suitable drug with toxic effect on cancer cells and nontoxic effect on other normal cells is discovered. One of the significant resources of such drugs may be the natural compounds especially in medicinal herbs which are identified through studying old textbooks and also screening these herbs.[8,9]
In this respect, the first step was to evaluate the toxic effect of herbal extracts on cancer cell lines. One of the prevalent strategies in cancer treatment is the prevention of DNA synthesis or mitosis through blocking the progress of cell cycle in malignant cells. There are different medicinal herbs with toxic effect that may stop the growth and proliferation of malignant and transformed cells.[10]
Cinnamon is a plant with scientific name “cinnamomumzelanicum” and general name “cinnamon”. This evergreen shrub belongs to the lauraceae family endemic to Sri Lanka and Southeast regions of India.[11] Although cinnamon with its peppery taste is mostly used as spices for cooking in the kitchen, its therapeutic applications should not be ignored. This plant is one of the oldest medicinal herbs which have been used in traditional medicine as an important drug. Different parts of this plant including its bark have many therapeutic effects such as strengthening of the heart, stomach, intestines, improvement of kidney function, and increase in libido.[12]
The significance of this drug is mainly for its volatile oil. Its major compounds are cinnamaldehyde, eugenol, and safrole with insulin-like activity that can be effective in diabetic treatment.[13,14] Furthermore, these compounds have positive effect on reduction of triglyceride, cholesterol, and lipoprotein with blood low density.[15]
Cinnamon is used against important body pathogens including escherichia coli, helicobacter pylori, and candida albicans due to its anti-fungal and anti bacterial properties.[16] Consumption of this spice inhibits the oxidation of organic matter in body and reduces free radicals due to its potent antioxidant effect.[17]
Studies have shown that cinnamon extract is effective in healing wounds made on Wistar rats.[18] Other therapeutic effects of this plant like treatment of nausea, diarrhea, and enhancement of cognitive power have been reported.[19,20]
Moreover, researchers have shown that aqueous cinnamon extract inhibits the proliferation of acute lymphoid leukemia cell line.[21]
The aim of the present study, was to evaluate the effect of aqueous cinnamon extract on viability reduction of cell lineages of APL.
MATERIALS AND METHODS
Preparation of aqueous cinnamon extract
The cinnamon bark was purchased from a well-known apothecary in Khorramabad, Iran. It was evaluated in terms of material and species by MsAhmadi, an expert in Jahad-e-Keshavarzi. The cinnamon bark was initially grinded into powder, and then was homogenized by a homogenizer. The extract was made by soaking the powder.[22] Solutions with concentrations of 0.01, 0.1, 1, and 2 mg/l were prepared in the medium (RPMI 1640) and were sterilized by using filters of millipore with size of 0.22 μm. They were kept in a refrigerator till being used in the study.
HL-60 human cell line was supplied from cell bank of Pasteur Institute of Iran. The cells were incubated in T25 flasks (NUNC Co. in Denmark) and culture medium of RPMI-1640 (Gibco Co. in Germany) containing 10% fetal calf serum (FCS), streptomycin antibiotics (100 μg/ml), and penicillin (100 IU/ml, CinnaGen, Iran) at 37°C in an incubator (Memmert, Germany) with 5% CO2 and 90% humidity of the atmosphere inside.
Incubation of cells by various concentrations of aqueous cinnamon extract
Aqueous cinnamon extract with concentrations of 0.01, 0.1, 1, and 2 mg/ml was prepared in culture medium and incubated with HL-60 cells in temporal intervals of 24, 48, and 72 h. Cell-free culture medium was used as blank control and cell culture medium without drug was used as a control of living cells.
Evaluation of the toxic effect of aqueous cinnamon extract
The toxic effect of aqueous cinnamon extract on HL-60 cells was examined using tetrazolium salt (methyl thiazoltetrazolium-MTT) reduction test. Tetrazolium salt is reduced to formazan crystals due to living cell activity. In this respect, 10,000 cells were counted by Neubauer slide and then transferred to 96-well plate. Different concentrations of aqueous cinnamon extract were added to them after 24 h. After incubation of HL-60 cells by various concentrations of aqueous cinnamon extract at intervals of 24, 48, and 72 h, 20 μl (5 mg/ml) of MTT solution was added to the plates which were incubated at 37°C and 5% CO2. After 4 h of incubation at 37°C, MTT stain was absorbed by mitochondria of cells and changed into purple crystals. At this time, the surface liquid was removed and 200 μl of acidic isopropanol solution (Merck Co., Germany) was added to the related wells.
Then, the plates were placed on the shaker for 30 min. After that, 180 μl of the solution in all wells was transferred to another plate and was read at wavelength of 570 nm by an ELISA reader. The percentage of growth inhibition was calculated by the following equation:[23]
Growth inhibition rate =![]()
Evaluation of the cell cycle
The cell cycle was evaluated using flow cytometer (Partec, Germany). In this respect, one to two million cells were incubated with cinnamon extract concentrations of 0.01 and 2 mg/ml at intervals of 72 and 24 h, respectively. The living cells without drug were used as a control sample. Having been collected and centrifuged at the speed of 800 rpm, the cells were washed with cold PBS (phosphate buffer saline) and fixed with 70% ethanol. In order to examine the cell cycle phases, the samples were mixed with 1 mg of propidium iodide (PI) dissolved in 1 ml of PBS and 25 mg of RNaseA (dissolved in 1 ml of PBS) and were evaluated using a flow cytometer.[21] The percentage of cell population of G1, S, and G2 phases was determined by the software specific to the flow cytometer.
Study of the apoptosis
The Hoechst33342 stain was applied to distinguish the apoptotic cell nucleus. This stain is exclusively used in chromatin staining and displays the apoptotic cell nuclei with the help of a fluorescent microscope. In this respect, once 2 million of HL-60 cells were counted by Neubauer slide, they were incubated with aqueous cinnamon extract concentrations of 0.01 mg/ml and 2 mg/ml for 24 and 72 h, respectively. The living cells without drug were used as a control sample. Then, 25 μl of the incubated cell suspension along with the control sample was mixed with 1 μl of 10 μg/ml Hoechst stain. After that, 10 μlit of the mixture was poured on a clean slide and at least 200 cells were counted and examined under a fluorescent microscope with lens of 10×.
The collected data were entered into SPSS18 software and analyzed using descriptive statistical methods such as tables and bar charts with error bars. The inferential statistical tests of one-way ANOVA, repeated measures ANOVA, and Tukey test were used to analyzed the data. All the tests were done at significance level of 0.05.
RESULTS
The assessment of methyl thiazoltetrazolium
As shown in the Figure 1, following the incubation of various concentrations of aqueous cinnamon extract with cancer cells for 24 h, the highest cytotoxicity effect was observed at concentrations of 0.01 mg/ml and 0.1 mg/ml. The rate of this cytotoxicity for the above concentrations was 74% and 71.4%, respectively.
Figure 1.
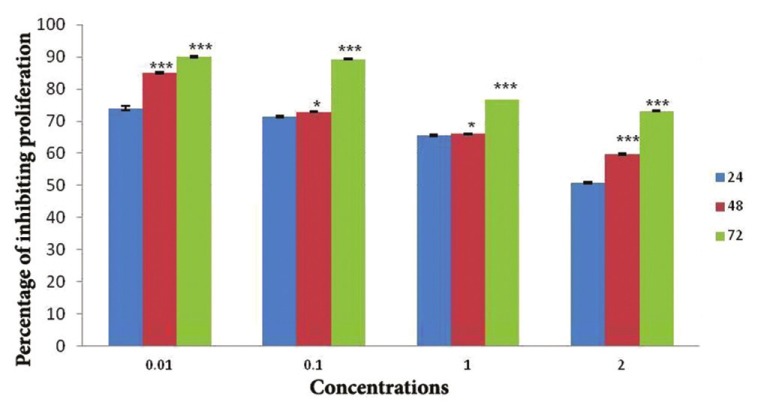
The results of analyzing variations in percentage of inhibiting proliferation of HL-60 cell line incubating with aqueous cinnamon extract concentrations of 0.01, 0.1, 1, and 2 mg/ml after 24, 48, and 72 h (*P < 0.05 and ***P < 0.001)
The results also showed that after 48-hour incubation of various concentrations of aqueous cinnamon extract with HL-60 cells, the low concentrations, i.e. 0.01 mg/ml and 0.1 mg/ml, caused 85.5% and 72.9% of cell death, respectively.
The 72-h incubation of HL-60 cells with various concentrations of aqueous cinnamon extract also indicated the correlation of cytotoxicity with the concentration of cinnamon extract, as the percentage of dead cells increased when the concentration of the extract was reduced. The rate of cytotoxicity, within 72 h, for the concentrations of 0.01 mg/ml and 0.1 mg/ml was 90.1% and 89.3%, respectively.
Cytotoxicity effect of aqueous cinnamon extract correlates to contact time and concentration of the extract. Moreover, equal concentrations at different intervals were compared with each other. There was a significant difference in the rate of cytotoxicity among different times (*P < 0.05 and ***P < 0.001).
Hoechst 33342 staining
The fastest way to detect apoptosis is the observation of variations in nucleus morphology of incubated cells using Hoechst staining. Hoechst staining is one of the most common methods for chromatin staining which is used in many studies. In the present study, apoptotic cells were observed with morphology of smaller body cell with scattered DNA fragments, sometimes peripheral. The healthy cells were observed to show large oval-shaped body with single nucleus looking littleblurred. The important point is that DNA fragments in apoptotic cells were stained and detected as blue fluorescent dye, while DNA of healthy cells were not stained and consequently they appeared and looked like spots under microscopes.
The results of apoptotic cell count showed that the number of apoptotic cells in incubated samples increased remarkably compared to that of control samples. Based on the prepared slides, HL-60 cell line incubated with 0.01 mg/ml aqueous cinnamon extract within 72 h showed the highest rate of apoptosis. The number of apoptotic cells in incubation of HL-60 cell line with 2 mg/ml aqueous cinnamon extract within 24 h showed a significant increase as compared to that of control samples.
Comparison of the Figures 2a, b, and c showed that the number of apoptotic cells in Figures 2b and c was significantly increased than as compared to that of control samples. In Figures 2a and b, apoptotic cells increased considerably compared to those cells incubated with 2 mg/ml aqueous cinnamon extract shown in Figure 2 and Figure 3.
Figure 2a.
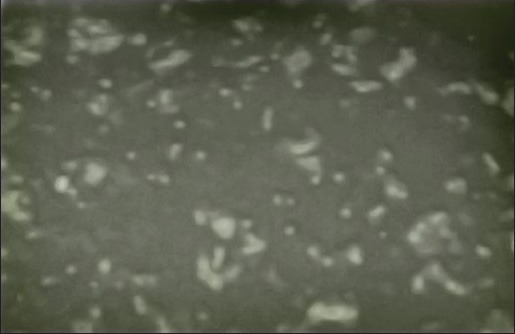
The control samples of HL-60 cell line which were not incubated at all. As seen in the figure, no apoptotic cell is observed under magnification of ×10
Figure 2b.
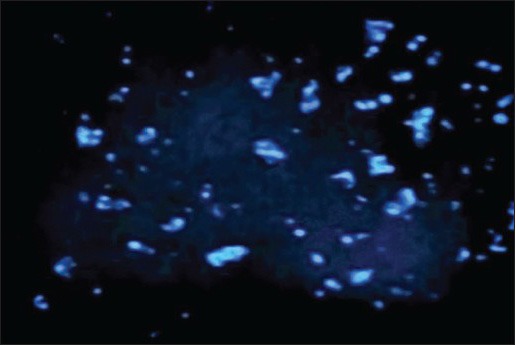
Fluorescent microscope image of HL-60 cells incubated with 2 mg/ml aqueous cinnamon extract for 24 h, then, stained with Hoechst dye. The apoptotic cells are clearly distinguished under magnification of ×10
Figure 2c.
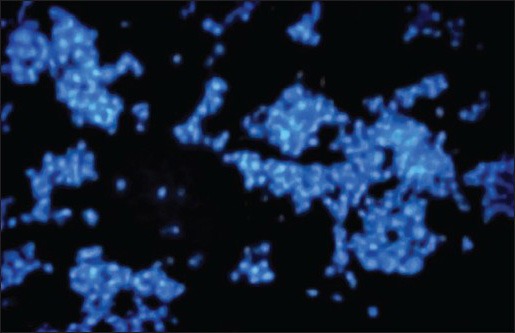
Fluorescent microscope image of HL-60 cells incubated with 0.01 mg/ml aqueous cinnamon extract for 72 h, then, stained with Hoechst dye. The number of apoptotic nuclei in this slide obviously ncreased compared to that of control samples under magnification of ×10
The analysis of cell cycle (SubG1 phase)
DNA content of a cell often provides some information about cell cycle. Therefore, measurement of DNA may be valuable in cell biology. In particular, measurement of DNA can offer some information on the function of cytotoxic drugs. There are different methods for measuring DNA and all of them use dyes which exclusively bind to the nucleic acids and their level of fluorescence increases at the time of binding. However, measurement of DNA content of a cell indicates a statistical manifestation of the cell cycle.
The charts established through flow cytometry presents four parameters as follows: M1 which shows the rate of apoptosis; M2 which shows the number of cells in G0-G1 phase; M3 which indicates the number of cells in S phase; and M4 which represents the number of cells in G2-M phase.
Comparison of three Figures 3a, b, and c showed the number of cells in SubG1 phase in the control sample as 4.06 which increased to 32/14 in HL-60 cells incubated with 2 mg/ml cinnamon extract for 24 h and then increased to 88.11 in cells incubated with 0.01 mg/ml cinnamon extract for 72 h. This increase showed the number of cells which existed in the final phase of apoptosis.
Figure 3a.
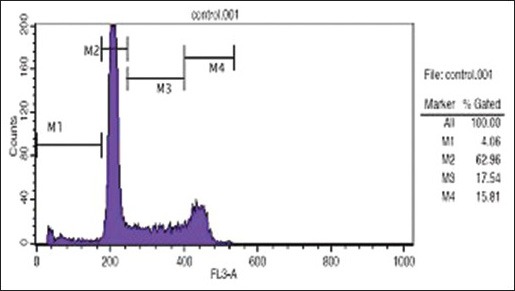
Analysis of cell cycle distribution method and DNA content in each phases of G2/M, S, G1, and SubG1 in HL-60 cell line (control sample)
Figure 3b.
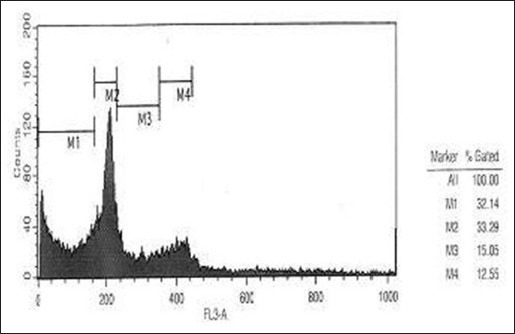
Evaluating the effect of 2 mg/ml aqueous cinnamon extract and 24 h of incubation on distribution and progression of cell cycle in HL-60 cell line
Figure 3c.
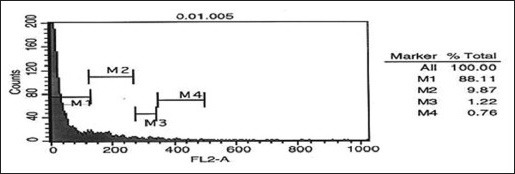
Evaluating the effect of 0.01 mg/ml aqueous cinnamon extract on distribution and progression of cell cycle in HL-60 cell line
Moreover, the number of cells in G0-G1 phase in samples incubated with cinnamon extract was reduced significantly compared with that of control sample. This reduction in the sample incubated with 0.01 mg/ml cinnamon extract for 72 h was also higher than that of other samples. The number of cells in G0-G1 phase in control sample was 62.96 which decreased to 33.29 in the experimental sample incubated with 2 mg/ml cinnamon extract for 24 h and then decreased to 9.87 in the experimental sample incubated with 0.01 mg/ml cinnamon extract for 72 h. This great reduction showed that the cells stopped in the SubG1 phase instead of entering G0-G1 phase of the cell cycle; therefore, they did not enter the cell cycle and became apoptotic.
DISCUSSIONS
Nowadays, a lot of efforts have been made to deal with cancers using herbal drugs extracted from plants or other natural resources. Among such efforts are the study of anti-cancer effect of herbal flavonoids and native medicinal herbs on the growth of cancer cells.[24,25] One of the reasons of much attention to herbal compounds, may be due to the fact that humans have been eating these materials for many years and are sure about their safety. Nature is a rich source of useful compounds whose effects have not been known yet. However, there have been known compounds such as atropine, digoxin, ergotamine, hyoscine, and many others that are among successful drugs.[26]
Among types of cancer, leukemias and lymphomas with global prevalence of 3-5% in individuals are of special importance.[27] Extensive studies are being conducted to find anti-cancer drugs with fewer side effects. These studies largely use cell lines of leukemia and lymphoma.[28] The drugs commonly used in chemotherapy have many side effects, they are also immune suppressor.[29,30] An important step for treatment of cancer will be taken if a suitable drug with toxic effect on cancer cells and non-toxic effect on normal cells is discovered. Natural compounds found in medicinal herbs are significant resources of such drugs which are identified by studying old textbooks and also by screening these herbs.[24,25]
As shown by the results of the present study, the aqueous cinnamon extract inhibits proliferation of HL-60 cell line that indicates the antiproliferative effect of this extract. This plant has been proved to have a large amount of antioxidant. It is noteworthy that in some diseases like Parkinson, cardiovascular diseases, and cancers, too many free radicals are produced and application of cinnamon extract is recommended for treatment of these diseases by the researchers due to its rich antioxidant content.[17]
The results of this study showed that the 0.01 mg/ml aqueous cinnamon extract in a 24-h culture could remove 74.6% of cancer cells. This cytotoxicity percentage was reduced when the amount of extract increased at 2 mg/ml concentration of the extract, the above percentage decreased to 50.7%. Incubation of cancer cells with 0.01 mg/ml aqueous cinnamon extract after 48 h caused 85.5% of cell death. Within 72 h of incubation, minimum and maximum concentration of 0.01 mg/ml and 2 mg/ml caused 90.1% and 73.2% of cell death, respectively.
It was shown that the cinnamon extract inhibited the growth of HL-60 cells as it correlated with concentration and time and this process is associated with apoptotic induction and cessation of cycle in G1 phase. Schoene et al. studied three cell lines of leukemia and found that aqueous cinnamon extract induced growth inhibition in the form of apoptosis in cell lines of U937, Wurzburg, and Jurkat, depending on the concentration of cinnamon extract. Moreover, Hyeon Ka, et al. indicated that cinnamaldehyde extracted from cinnamon induced apoptosis in HL-60 cell line. Therefore, the results of this study on the apoptotic induction largely conform to the results obtained from other cell lines.
These study give us the opportunity to find compounds which exclusively target cancer cells. The results suggest further studies on the effects of aqueous cinnamon extract on normal cell lines, then in animal models, and finally in humans through clinical trials in order to identify exact effect of this extract. In this respect, the effects of the aqueous cinnamon extract in human may be proved and special drugs can be formulated through further studies and by detecting effective anticancer compounds of cinnamon extract.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Wang ZY, Chen Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood. 2008;111:2505–15. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 2.Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–903. [PubMed] [Google Scholar]
- 3.Randolph TR. Acute promyelocytic Leukemia (AML-M3)-part 1: Pathophysiology, clinical diagnosis, and differentiation therapy. Clin Lab Sci. 2000;13:98–105. [PubMed] [Google Scholar]
- 4.Mchenzie SL. 2nd ed. Bultimore: Williams and Wilkins; 1996. Text book of hematology; pp. 385–7. [Google Scholar]
- 5.Jackson N, Menon BS, Zarina W, Zawiwi N, Naing NN. Why is acute leukemia more common in males? A possible gender determined risk linked to the ABO blood group genes. Ann Hematol. 1999;78:233–6. doi: 10.1007/s002770050507. [DOI] [PubMed] [Google Scholar]
- 6.Mrozek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: Are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–48. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sirard C, Lapidot T, Vormoor J, Cashman JD, Doedens M, Murdoch B, et al. Normal and leukemic SCID-repopulating cells (SRC) coexist in the bone marrow and peripheral blood from CML patients in chronic phase, whereas leukemic SRC are detected in blast crisis. Blood. 1996;87:1539–48. [PubMed] [Google Scholar]
- 8.Newman DJ, Cragg GM, Sanders KM. Natural Products as sources of new drugs over the period. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 9.Srivastara V, Negi AS, Kumar JK, Gupta MM, Khanuja SP. Plant based anticancer molecules: A chemical and biological profile of some important leads. Bioorg Med Chem. 2005;13:5892–908. doi: 10.1016/j.bmc.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 10.Hwang JS, Ji HJ, Koo KA, Lee NH, Yeo HK, Cheong SW, et al. AIP1, a water-soluble fraction from Artemisia iwayomogi, suppresses thymocyte apoptosis in vitro and down-regulates the expression of fas gene. Biol Pharm Bull. 2005;28:921–4. doi: 10.1248/bpb.28.921. [DOI] [PubMed] [Google Scholar]
- 11.Mirheidar H. Tehran: Islamic Culture Press; 2004. Plant knowledge; Application of plants in prevention and remedy of diseases; pp. 323–8. [Google Scholar]
- 12.Shah AH, AL-Shareef AH, Ageel AM, Qureshi S. Toxicity studies in mice of common spices Cinnamomumzeylanicum bark and piper lonum fruits. Plant Foods Hum Nutr. 1998;52:231–9. doi: 10.1023/a:1008088323164. [DOI] [PubMed] [Google Scholar]
- 13.Singh G, Maurya S, DeLampasona MP, Catalan CA. A comparision of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oil. Food Chem Toxicol. 2007;45:1650–61. doi: 10.1016/j.fct.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RA, Broadburst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP, et al. Isolation and characterization of polyphenol type- A polymers from cinnamon with Insulin-like biological activity. J Agric Food Chem. 2004;52:65–70. doi: 10.1021/jf034916b. [DOI] [PubMed] [Google Scholar]
- 15.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–8. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 16.Nir Y, Potasman I, Stermer E, Tabak M, Neeman I. Controlled trial of the effect of cinnamon extract on Helicobacter pylori. Helicobacter. 2000;5:94–7. doi: 10.1046/j.1523-5378.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 17.Calnan CD. Cinnamon dermatitis from an ointment. Contact Dermatitis. 1976;2:167–70. doi: 10.1111/j.1600-0536.1976.tb03018.x. [DOI] [PubMed] [Google Scholar]
- 18.Kamath JV, Rana AC, Chowdhury AR. Pro-healing effect of cinnamomumzeylanicum bark. Phytother Res. 2003;18:970–2. doi: 10.1002/ptr.1293. [DOI] [PubMed] [Google Scholar]
- 19.Skidmore-Roth L. 2nd ed. St. Louis: Mosey; 2002. Handbook of herbs and natural supplements; p. 38. [Google Scholar]
- 20.Adame J, Adame H. Parte 1. In: Adame J, dame H, editors. Curatives plants of north mexico Parte 1. Monterret Mexico: Ediciones Castillo; 2000. pp. 21–260. [Google Scholar]
- 21.Schoene NW, Kelly MA, Polansky MM, Anderson RA. Water-soluble polymeric polyphenols from cinnamon inhibit proliferation and alter cell cycle distribution patterns of hematologic tumor cell lines. Cancer Lett. 2005;230:134–40. doi: 10.1016/j.canlet.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Shariati S. Isfahan: Mani Publication; 1992. Extraction of hydroalcoholic extract of useful products of herbal drugs with methods of identifying; pp. 12–3. [Google Scholar]
- 23.Sundartan SG, Milner JA. Impact of organs compounds in garlic oncanine mammary tumor cells in cultures. Cancer lett. 1993;174:85–90. doi: 10.1016/0304-3835(93)90048-e. [DOI] [PubMed] [Google Scholar]
- 24.Spiridonov NA, Konovalov DA, Arkhipov VV. Cytotoxicity of some Russian ethnomedicinal plants and plant compounds. Phytother Res. 2005;19:428–32. doi: 10.1002/ptr.1616. [DOI] [PubMed] [Google Scholar]
- 25.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 26.Valenti J. 1st ed. Isfahan University of Medical Sciences, Isfahan Rah-e-Kamal Publication; 2002. Herbal Medicine, treatment of diseases using herbs, translated by Ahmad Emami. [Google Scholar]
- 27.Lau CB, Ho CY, Kim CF, Leung KN, Fung KP, Tse TF, et al. Cytotoxic activities of Coriolusversicolor (Yunzhi) extract on human Leukemia and lymphoma cells by induction of apoptosis. Life Sci. 2004;75:797–808. doi: 10.1016/j.lfs.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Drexler HG, Minowada J. History and classification of human leukemia-lymphoma cell lines. Leuk Lymphoma. 1998;31:305–16. doi: 10.3109/10428199809059223. [DOI] [PubMed] [Google Scholar]
- 29.Herberton R, Hancock BW. Hodgkin Lymphoma in adoleocents. Cancer Treat Rev. 2005;31:339–60. doi: 10.1016/j.ctrv.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Vazquez E, Lucaya J, Castellote A, Piqueras J, Sainz P, Olive T, et al. Neuroimaging in pediatric leukemia and lymphoma: Differential diagnosis. Radiographics. 2002;22:1411–28. doi: 10.1148/rg.226025029. [DOI] [PubMed] [Google Scholar]


