Abstract
Background:
CD4+(TH1, and TH2) cell groups in the point of view of chemokine receptor expression were considered in blood of stomach cancer patients.
Materials and Methods:
The percentage of blood CD4+ T cells expressing chemokine receptors (before and after gastrectomy) was determined by flow cytometry (Becton Dickinson, USA) using the following chemokine receptor antibodies: anti-CCR5, anti-CXCR3, anti-CCR3 and anti-CCR4.
Results:
The means of CD4+ CCR5+ expressing cells was 1.23% ± 0.90, 0.83% ± 0.34 and 1.34% ± 0.74 in control, pre- and post-operation groups, respectively. CD4+ CXCR3+ expressing cells were 19.09% ± 8.4, 16.95% ± 5.71 and 25.08% ± 9.31, respectively. Similar pattern was seen for CD4+ CCR3+ and CD4+ CCR4+ expressing cells. Pearson correlation analysis shows no relationship between CCR3 and CCR4 expressions on TCD4 cells (r = 0.211, P = 0.126). The complex expression TH1 (CD4+ CXCR3+ CCR5+) receptors determined 1.14% ± 0.54 for control group, 0.86% ± 0.49 for pre-T and 1.57% ± 0.67 for post-T group. Moreover, the TH2 (CD4+ CCR3+ CCR4+) expression was 1.60% ± 1.05 for control group, 1.57% ± 0.83 for pre-T and 1.27% ± 0.66 for post-treatment group. Pearson correlation analysis shows that only the CCR3 and CCR5 expression was statistically correlated (r = 0.321, P = 0.018).
Conclusion:
Due to low expression of CCR5 in TH1 and CCR3 in TH2 cells, it seems that utility of these is extremely limited for clinical evaluation, but not scientific purpose. Moreover, considering the CXCR3 for TH1 cells and CCR4 expression for TH2 cells, due to considerable expression, may be practical.
Keywords: CCR3, CCR4, CCR5, chemokine receptor, CXCR3, gastric cancer
INTRODUCTION
Acquired immune system, through dendritic cells, lymphocytes, and secretion of cytokines and chemokines in human body, creates a homeostasis balance and applies appropriate responses in dealing with exogenous or endogenous antigens.[1] TCD4 lymphocytes whose most secreted cytokines are IL-2 and IFNγ are called TH1 and those whose most secreted cytokines are IL-4, IL-5, and IL-10 are called lymphocytes type II or TH2. Cytokines secreted from TH1 lymphocytes facilitate the creation of cytotoxic lymphocytes and TH2 lymphocytes help B lymphocytes (B cells) to make antibodies (humoral immunity) and suppress the activities of cytotoxic T lymphocytes.[2,3] The balance between the numbers of lymphocytes and the amount of cytokines secreted by TH1 and TH2 cells in healthy state and also in dealing with certain antigens has established indices for evaluating and following the immune system activity in healthy state and illness. For instance, according to the study of Tabata et al. on patients with gastrointestinal cancer, the ratio of TH1:TH2 showed the increase of TCD4+ secreting IL-4, IL-6, and IL-10 significantly in proportion to that of asymptomatic people. The study showed that TH2 decreased 1 month after removing tumors.[4]
A similar result was reported by Reinartz et al., although the immune system response was TH1 increasing after anti-idiotype vaccination and the increase of cytokines IL-2 and IFNγ.[5] Lue et al. obtained successful results from immunotherapy of bladder cancer with a combination of BCG and IFNα in order to create the dominance of TH1 responses and secreting related cytokines.[6] The immunotherapy of advanced prostate cancer indicated decreasing the IL-4 secretion and increasing IL-2 in peripheral blood, showed desirable results in reduction of prostate specific antigens (PSA) in patients.[7] Other studies are being conducted to evaluate the ratio of TH1:TH2 in cancers in human body and try to change the ratio in a way that TH1 cells can overcome cancers.[8]
The evaluation of TH1 and TH2 in blood needs PBMC separation process and subsequently measuring cytokines, either extracellular or intracellular proteins, and/or examining mRNA by molecular RT-PCR method. These methods require a relatively long time and a significant cost for each cytokine assesment.[9,10]
TH1 and TH2 cells are derived by TH0. TH0 cells after being stimulated by immune dendritic cells (DC) and induction by peripheral cytokines with their quantitative, e.g. IL-12, PGE2, IL-10, and IL-4, shift toward the lymphocytes types I, II or other cellular subsets.[9] Once stimulated T lymphocytes secrete numerous cytokines, they reach the final stages of differentiation in cellular subsets and are named under the types I and II following the differentiation stage and secreting related cytokines.[11] Therefore, conducting TH0 due to the initial stimulation and peripheral cytokine environment is possible experimentally.[8] No “specific” cellular surface marker, which presents lymphocyte cells in TH1 and TH2 groups, is still identified; thus, the study of cytokines produced out of lymphocyte activities is considered as a performance index and cellular activity whose measurement after laboratorial steps involves ELISA test, or cellular mRNA using RT-PCR method, or Western blot test, or lymphocytes cultivation and mediators measurement, which require a long time and high cost.[10]
Chemokines are soluble protein molecules with molecular weight of 8-14 kDa, which are produced similar to the cytokines by various cells of the body, especially immune cells in response to stimulations. Twenty receptors have been identified for the 50 types of known chemokines. The interaction between receptors and ligands on the cells has an important role in differentiation, evolution, cells homeostasis and inflammation.[12] Generally, immune cells, macrophages, fibroblasts, epithelial, endothelial and tumor cells interact with chemokine and cytokine secretion, which alters the expression of their receptors.[12,13] There are cells besides tumor cells in tumor tissues like fibroblasts, epithelial, and endothelial cells, immune cells such as DC, macrophage, and a variety of lymphocytes, which are assessable.[12,14] The amount of cytokines and chemokines produced by varieties of existing cells in tumor tissue has been found to be effective in tumor tissue to reinforce responses resulting in reduction of the volume or growth of tumor cells.[1,8,15] Also, distribution and diversity of chemokine receptors expression on various cells are different, e.g., immune dendritic cells in their maturity stage express CCR7 and CXCR4 chemokine receptors, which do not exist in their immature state. Naïve T cells have the quality of expressing CXCR4 and CCR7; however, they express CXCR3 and CXCR5 when they are activated. In this respect, TH2 cells express CCR4 and CCR8.[8,12,13,16] Studies have shown that the expression of chemokine receptors on TH1 cells differs from that of TH2, e.g., chemokine receptors on TH1 lymphocytes express CXCR3, CCR1, and CCR5, whereas TH2 lymphocytes express CCR8, CXCR4, CCR3, and CCR4.[16]
Another aspect which should be considered regarding micronutrients environment of tumor cells and the tissue in which tumor cells grow is that the expression of chemokine and chemokine receptors in various tissues of the body are different, e.g., CCL20 and CCL27 are expressed in epidermal keratinocytes, while CCL20, CCL25, and CX3CL1 are expressed in intestinal tissue, CXCL12 in bone marrow, CXCL8, CCL21, and CXCL12 in lymph nodes, and CXCL8 and CXCL12 in lungs.[12,14] The expression of these receptors is effective in lymphocyte replacement in tissues and causes interaction and the consequent immune response. Changes in the composition of TCD4+ cells and other lymphocyte subsets of acquired immune system in suppressing tumors may arise from the type of tumoral tissue, the type and amount of cytokines and chemokines, and lymphocytes of the tissue and consequently their reflection in peripheral blood.[17] In this respect, study of phenotypic changes based on the expression of chemokine receptors of blood circulation lymphocytes regarding the presence or absence of gastrointestinal malignant tumors and comparison of these indices with non-affected people was considered for a research.[18] This study was developed considering the phenotypic examination of peripheral blood lymphocytes with a higher speed using flow cytometry technique than the other techniques and judgments made on the domination of TH1 or TH2 in an immunopathological process.
MATERIALS AND METHODS
This study was conducted on 27 patients with gastric cancer who had medical records in Alzahra Hospital of Isfahan University of Medical Sciences and Shariati Hospital in Tehran. To do the tests, 3 mL blood was taken from the patients before and 2 or 3 months after surgery, and PBMC were separated for flow cytometry. Furthermore, blood samples were taken from 27 healthy people with similar age and sex to those of patients, and prepared in the same method. Blood cells in tubes containing EDTA anticoagulant were counted according to the standard method using automated hematology analyzer. The samples that were diagnosed to have gastric adenocarcinoma were included in this study and the others were excluded. The samples were mixed with the same volume of cold isotonic phosphate buffer (PBS), then they were poured in 50 cc Falcon tubes at Ficoll-Hypac density gradient of 1.077 g/ml. Once the samples were centrifuged in refrigerated centrifuges without using brake, the cellular layer was separated using Pasteur pipette and was rinsed twice with Hank's buffer in sterile tubes. After separation of PBMC, cells were divided into tubes containing 50μL of phosphate buffer for each used antibody and negative control (without antibody) and mouse isotype control for negative control. Then, 10μL of monoclonal antibody was added to each tube.[18,19]
The monoclonal antibodies used in this study were anti–human CCR4- phycoerythrin-conjugated mouse monoclonal (isotype, IgG2B clone#205410), anti-human-CCR3-carboxyfluorescein-conjugated monoclonal (isotype, rat IgG2a, colne#61828.111), Anti-human CXCR3- carboxyfluorescein-conjugated monoclonal (isotype, mouse IgG1, colne#49801), Anti-human CCR5-phycoerythrin-conjugated monoclonal (mouse IgG2B, clone#45531), and anti-human CCR3- fluorescein monoclonal antibody. (All the antibodies were secured from R&D system, USA).
Moreover, the monoclonal antibodies of PE/Cy5anti-human-CD4 (isotype mouse IgG2b, clone#okT4) with specification of mouse lgG2b (Purchased from Bio Legend Inc.) were used for measurement of CD4 receptor expression. The monoclonal antibody of Carboxyfluoresciein (CFS) conjugated mouse IgG1 antibody isotype control (clone 11711) (manufactured by R and D system, USA.) was used for measuring receptors in negative control sample to be compared with positive stained samples based on the standards and for avoiding stain interference. Once the antibody was added to all the tubes containing cellular samples, they were incubated in dark at 2–8°C for 30–45 minutes. Then, the additional antibody was rinsed with PBS buffer again and the samples of 300–400∞L of buffer were used for flow cytometry analysis.
Flow cytometry
Fluorescein Isothiocyanate (FITC) fluorochrome connected with Ab, which can absorb optical spectrum of 488 nm and reflect higher wavelengths (530 nm), as well as phicoeritrin (PE) fluorochrome, with a different absorption spectrum (570 nm) in wavelength reflection, were used to distinguish staining markers. In flow cytometer system, FL1 optical detector was designed in order to identify reflected lights at wavelength of 530 nm and FL2 was designed to absorb, identify, and distinguish reflected lights at wavelength of 575 nm. Side scatter (SSC) detector was designed to absorb, identify and assemble the lights with wavelength of 488 nm, and the value of each absorbed light is shown by software graphs using computer, which is used as statistical data. The tubes containing cells stained by monoclonal antibodies were read using flow cytometery (Becton Dickinson, USA). After CD4 cells gating and determination of the values, the calculated values for marked receptors were recorded and the results were collected as histogram or dot blot assessment for supplementary studies.[20] The representative dot blot sheets are shown in Figure 1. The data resulting from flow cytometry were analyzed using Cell Quest software and the data obtained from reading 1×104 cells were analyzed using SPSS software.
Figure 1.
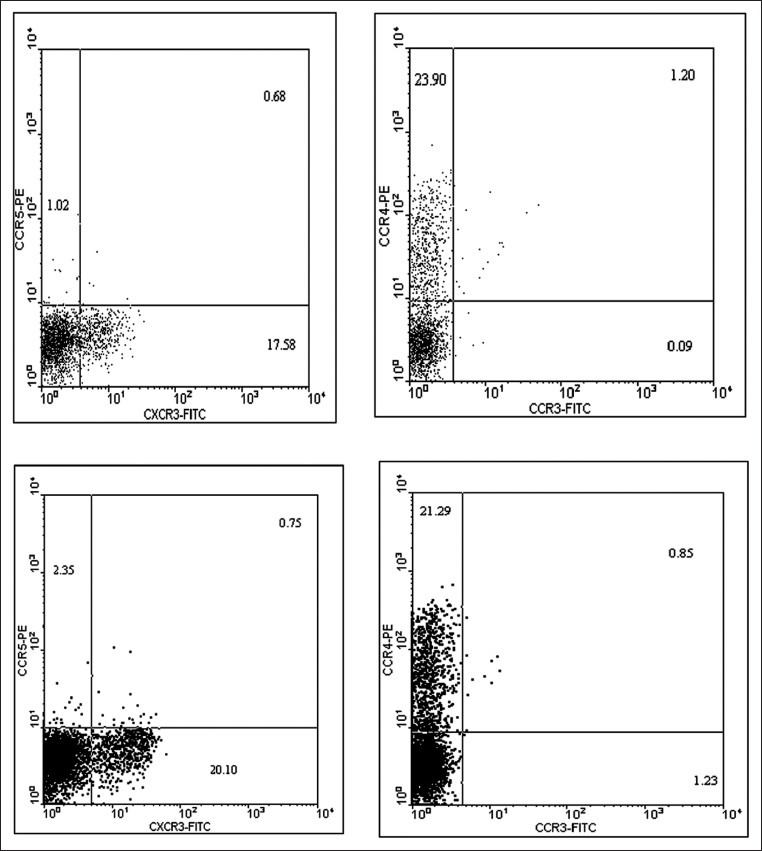
Dot plot obtained from flow cytometry software analysis of PBMC sample of the patients with gastric cancer. Every dot in such figures represents a read cell (in some occasions, similar parameters of the same cells causes the dots to coincide on the plane). Each dot plot plane is divided into four quadrants by two perpendicular lines. The dots in the bottom left quadrant represent cellular control in non-stained samples as a standard for the studied cell population or in stained samples as the cell population without studied parameters. The upper left quadrant next to Y axis shows the percentage of cells stained with the marker connected to phicoeritrin (PE) and also shows negative Fluorescein Isothiocyanate (FITC). The bottom right quadrant indicates the percentage of cells stained by the marker connected to FITC and also shows negative PE. The upper right quadrant represents the percentage of cells possessing both markers connected to FITC and PE (or double positive). The provided plots are shown as representatives for the analysis of the studied chemokine receptors expressed on CD4 cells and the percentage of stained cells was considered in statistical calculations and is shown in Tables
Statistical analysis
The results are shown by mean and standard deviation (Mean ± SD) for each group of samples. The mean comparison of independent sample test in SPSS software was used to compare the control group with patients, and the mean comparison of paired sample test was used to compare the mean before and after the surgery. Pearson correlation test was used to compare the correlation between the receptor expressions the samples. The statistical significance level was considered P-value ≤0.05.
RESULTS
Mean white blood cells (WBC) in patients with gastric cancer and control group were 7406 ± 1916/ml and 6401 ± 1783/ml, respectively. Comparison of the two groups’ mean showed a significant increase in patients’ WBC (P = 0.051). However, lymphocyte counting in the two groups showed no significant difference in the amount of lymphocytes in patients group and control group as those were 35.03% ± 5.61% (2588 ± 734) and 36.77% ± 5.43% (2343 ± 840), respectively (P = 0.261). The percentage of TCD4 lymphocytes in patients group and control group was 45.40% ± 7.03% and 45.86% ± 6.87%, respectively, which did not show a significant difference between these two groups (P = 0.810) [Tables 1 and 2].
Table 1.
WBC and lymphocytes of peripheral blood (mL) in patient and control groups

Table 2.
CD4 lymphocytes counting and percentage in peripheral blood of patient and control groups

The expression of TH1 chemokine receptors
The mean of the cells expressing CCR5 in TCD4+ cell population of control group, patients group before surgery, and patients group after surgery was calculated as 1.23% ± 0.9%, 0.83% ± 0.34%, and 1.34% ± 0.74%, respectively. Comparing the mean of control group and patients before surgery at P = 0.034 with patients after surgery at P = 0.0651 showed a meaningful decrease in CCR5 in patients before surgery. Comparing the mean for expression of CCR5 in patients before and after surgery showed a significant difference regarding the increase in expression of CCR5 after surgery (P = 0.001). As the expression of CCR5 in TCD4 cells was not quantitatively considerable, the application of them in clinical purpose (not research area) seems not practical [Table 3a].
Table 3a.
The TH1 chemokine receptor cells in lymphocytes of patient and control groups
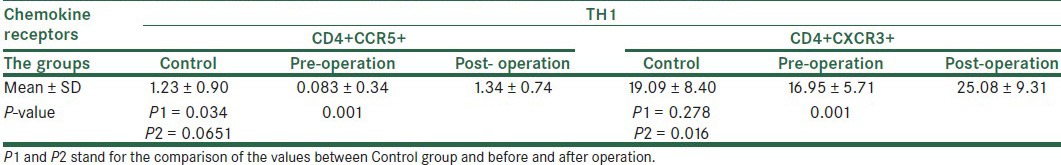
The mean of the cells expressing CXCR3 in TCD4+ cell population of control group, patient group before surgery, and patient group after surgery was calculated as 19.09% ± 8.4%, 16.95% ± 5.71%, and 25.08% ± 9.31%, respectively. The statistical analysis between the mean for expression of CXCR3 in control group and patients before surgery showed no statistical significance at P = 0.278 in control group and patients after surgery showed changes in the expression with a meaningful difference at P = 0.016. Moreover, Comparing the mean for expression of CXCR3 in patients before and after surgery showed a difference in expression of CXCR3 before and after surgery (P = 0.001). Thus, it can be said that gastric cancer is effective in the reduction of clones expressing CXCR3 in peripheral blood. Furthermore, the analysis by Pearson correlation coefficient for the expression of CCR5 and CXCR3 on TCD4 cells showed that r = 0.0267 and P = 0.0177 after surgery and r = −0.091 and P = 0.513 before surgery indicate the independent expression of these two receptors on TCD4+ cells.
The expression of TH2 chemokine receptors
The mean of the cells expressing CCR3 in TCD4+ cell population of control group, patient group before surgery, and patient group after surgery was calculated as 0.75% ± 0.93%, 0.57% ± 0.46%, and 0.62% ± 0.42%, respectively. There was no statistical difference in the mean for the expression of CCR3 of control group and patients group (P≥0.05) [Table 3b].
Table 3b.
The TH2 chemokine receptor cells in lymphocytes of patient and control groups

The mean of the cells expressing CCR4 in TCD4+ cell population as a receptor of chemokines type II in control group, patients group before surgery, and patients group after surgery was measured as 26.8% ± 10.36%, 32.29% ± 9%, and 31.69% ± 8.76%, respectively. The statistical analysis showed the increase of CCR4 expression in patients in proportion to control group before surgery at P = 0.042 and after surgery at P = 0.067. No difference in the expression of this molecule was observed in patients before and after surgery (P = 0.513). Pearson correlation coefficient between values of CCR3 and CCR4 expression in TCD4+ cell population indicated the independent expression of these two receptors in the patient lymphocytes (r = 0.255 and P = 0.259). The results showed that due to the insignificant expression of CCR3 in cell population, it cannot be used in clinical settings; however, the expression of CCR4 is considerable and can be applied in more clinical assessments.
Simultaneous examination of the expression of chemokines TH1 and TH2 receptors
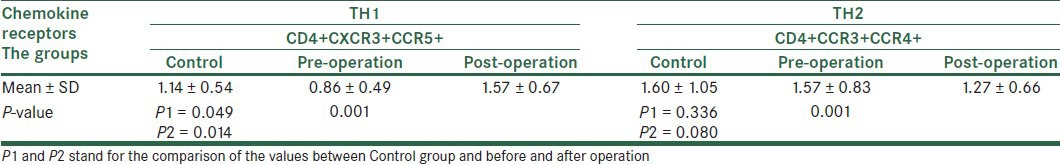
Examining the expression of CD4+CXCR3+CCR5 (TH1) and CD4+CCR3+CCR4 (TH2) was among the primary objectives of this study, however, due to the insignificant expression of CCR5 and CCR3 in each group, and simultaneous staining receptors of chemokines by the relevant antibodies and reading them using a flow cytometer, As shown in Table 4, although all the markers measured simultaneously could be calculated statistically, this measurement cannot be applied clinically due to percentage of markers. The analysis of correlation coefficient between chemokine receptor groups indicated a positive correlation only for CCR3 and CCR5 (r = 0.321 and P = 0.018). Moreover, Pearson correlation analysis in patients after surgery showed a significant statistical correlation between the expression of CCR4 and CCR5 in the cells (r = 0.401 and P = 0.038).
Table 4.
The values for chemokine receptors in TH1 and TH2 lymphocytes together
DISCUSSION
T lymphocytes are a part of acquired immune system whose clones can be activated against tumors after identifying tumor antigens and are recognizable either in blood circulation or in secondary lymphoid tissues as a variety of lymphocyte subsets. The tumor antigens are processed in lymph nodes and tissues by DC cells. DC cells induce the naive lymphocytes as effector and memory cells. The cell population, diversity and composition of cytokine and chemokine and their receptor role in interaction with tumor cells are effective in tumor's fate by providing tumor with micronutrient environment through alteration chemokine, cytokine and their receptors.[21,22,23]
In the present study, the blood lymphocyte population did not show statistical difference between patients with gastric cancer and the control group (P = 0.259). However, WBC population of the two groups differed significantly from each other (P = 0.051) showing the increase of blood leukocyte population apart from lymphocyte population in peripheral blood. The TCD4 lymphocyte population did not show a significant difference in patients and healthy people although it was higher in patients (P = 0.115). These results clearly show that the body homeostasis system moves toward a balance in proportion to the intense changes of lymphocyte population and because a small percentage (2%) of body lymphocyte population exists in blood circulation and 40% in lymph nodes, the remaining are in other tissues.[24] Therefore, phenotypic study of lymphocyte population is a way to ignore usual judgments about absolute cell counting in the blood samples in order to find how they function and the presence of activated clones in their circulation between blood and tissues. Asano et al. studied CXCR3 and CXCR4 in TH1, and TH2 classification as a model for considering malignancy indexes.[25]
In the present study, the mean for the expression of chemokine receptors (TH2; CD4, CCR3, CCR4) and (TH1; CD4, CXCR3+, CCR5) on TH1 and TH2 showed a significant difference between patients before and after gastrectomy (P = 0.001) [Table 4] indicating phenotypic changes in clones of TCD4 cells in blood circulation, which could not affect the absolute cell population significantly. Nevertheless, the phenotypic study can show cellular changes in blood circulation (in terms of clones) in people with gastric cancer.
The examination of the expression of TH1 chemokine receptors showed the reduction of clones possessing TH1 in blood in patients with gastric cancer compared to the control group (P = 0.049). Results obtained after gastrectomy showed a phenotypic increase in TH1 lymphocyte clones in peripheral blood compared with the control group and patients before surgery, which are presented in Table 4 (P = 0.014). The increase of TH1 potential by choosing CCR3 phenotype in antitumor promotion was also presented by Yoon et al.'s study.[26] These results explained phenotypic or cytokine changes in TH1 cells in other tumors, which were observed in measurement of TH1 in tumor tissues, in blood circulation and during recovery after immunotherapy. Numerous studies even using genetic methods of cytokines secreted by TH1 and TH2 and Western blot method have shown the dominance of TH2's clones in gastric cancers,[10] which are consistent with the method of the present study regarding the phenotype of measured chemokine receptors.
The phenotypic study of chemokine receptors in TH2 lymphocytes (CD4+CCR3+CCR4) in patients’ blood circulation indicated the reduction of mean percentage of TH2 after the surgery of gastric cancer (P = 0.001). Similar results were found by Ishikaw et al.'s study.[27]
The phenotypic study of TH2 in blood did not show a significant difference between patients before and after surgery with the control group (P = 0.080 and P = 0.336) [Table 4]. Thus, the probable difference in other reports may be due to the presence of TH2 cells in tumor tissue (microenvironment) and its reflection in abundant population of blood cells is not unexpected. Such a change in TH2 balance in microenvironment and also after surgery has been shown in studies by Tabate, Punoen and Pellegrini.[4,28,29]
Examining the clones of T lymphocytes affecting with gastrointestinal cancer by Berghellal et al.[30] showed that the level of serum sIL-2R in patients with colon cancer was higher than that of healthy people, which had a positive correlation with tumor stage and serum levels of IL-4, IL-6, and IL-10 and a negative correlation with IL-2 and IFNγ, showing clearly the reinforcement of immune arm of TH2 in such tumors. Similar studies by others have indicated the shift of TH1 toward TH2 in the environment for tumor growth.[19]
In the present study, comparing the changes in chemokine receptors of CCR5 and CXCR3 in TH1 group individually in patients before and after surgery showed that the expression of CXCR3 in lymphocyte population is remarkably higher than the expression of CCR5 on TCD4+ cells. Therefore, the application of CXCR3 in clinical approach is more feasible than CCR5. As shown by the results, CCR5 phenotypic changes after surgery had an increase toward the control group and there was a statistical difference in the mean for the expression of CCR5 before and after surgery (P = 0.001). The increase in the expression of CXCR3 in peripheral blood lymphocytes showed a significant difference between patients after and before surgery (P = 0.001) and with the control (P = 0.016) group showed a meaningful increase of TH1's clones in WBC population after operation.
Although phenotypic changes of CCR3 in TH2 group are not considerable quantitatively and there are changes in its expression on TCD4 cells, the reduction of cell population possessing CCR3 may not be applied in clinical studies. However, it is considered in research methods in spite of no statistical difference in its expression before and after surgery (P = 0.707). The expression of CCR4 in TH2 group on TCD4+ was quantitatively considerable. Although it was not different in patients before and after surgery (P = 0.513), it was significantly different from that of control group (P = 0.042). The independent expression of each chemokine receptor on TCD4 cells showed another aspect of the present study. The analysis of Pearson correlation coefficient showed that only chemokine receptors of CCR3 and CCR5 had a positive correlation (P = 0.024, r = 0.434, and R2 = 0.18) and CCR4 and CCR5 had a negative correlation (P = 0.038, r = −0.401, and R2 = 0.16) [Figures 2 and 3]. The expression of other chemokine receptors on TCD4 cells takes place independently, so their phenotypic changes in TH1 and TH2 lymphocytes happen individually.
Figure 2.
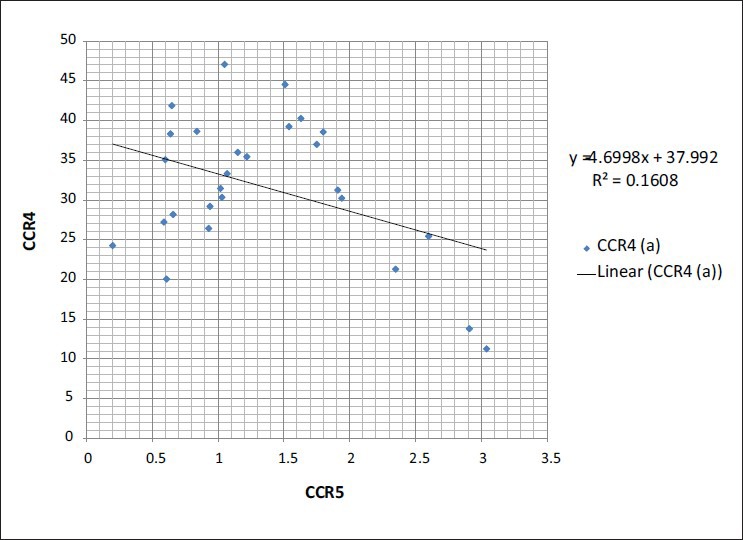
Represent the correlation of chemokine receptors in the CD4 T cells in the groups
Figure 3.
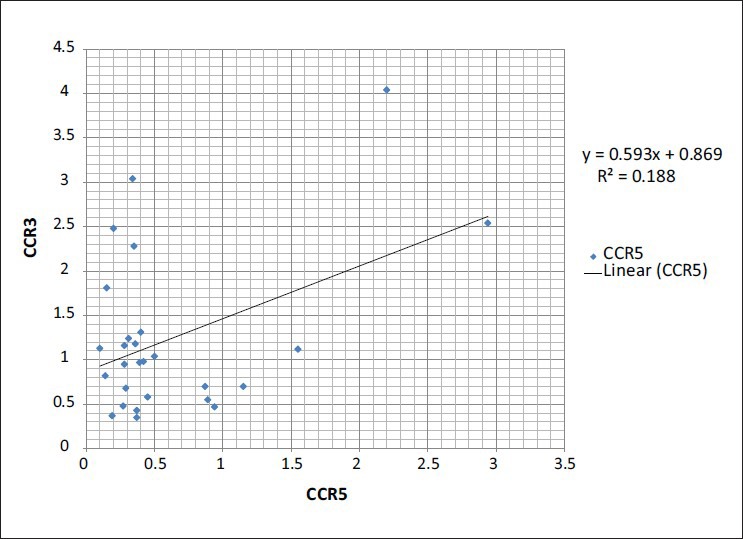
Represent the correlation of chemokine receptors in the CD4 T cells in the groups
ACKNOWLEDGMENT
This study was conducted by financial support of Deputy Research of Isfahan University of Medical Sciences (Grant 186098) during 2007-2009. We would like to acknowledge Mojgan Mokhtari (pathologist) and Shadi Babazadeh (Oncologist) for their consultation on clinical cancer.
Footnotes
Source of Support: Deputy Research of Isfahan University of Medical Sciences (Grant 186098) during 2007-2009.
Conflict of Interest: None declared
REFERENCES
- 1.Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, et al. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies? Cancer Metastasis Rev. 2006;25:333–56. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 2.Viola A, Contento RL, Molon B. T cells and their partners: The chemokine dating agency. Trends Immunol. 2006;27:421–7. doi: 10.1016/j.it.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 4.Tabata T, Hazama S, Yoshino S, Oka M. Th2 subset dominance among peripheral blood T lymphocytes in patients with digestive cancers. Am J Surg. 1999;177:203–8. doi: 10.1016/s0002-9610(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 5.Reinartz S, Boerner H, Koehler S, Von RA, Schlebusch H, Wagner U. Evaluation of immunological responses in patients with ovarian cancer treated with the anti-idiotype vaccine ACA125 by determination of intracellular cytokines--a preliminary report. Hybridoma. 1999;18:41–5. doi: 10.1089/hyb.1999.18.41. [DOI] [PubMed] [Google Scholar]
- 6.Luo Y, Chen X, Downs TM, DeWolf WC, O’Donnell MA. IFN-alpha 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette-Guerin immunotherapy. J Immunol. 1999;162:2399–405. [PubMed] [Google Scholar]
- 7.Hrouda D, Baban B, Dunsmuir WD, Kirby RS, Dalgleish AG. Immunotherapy of advanced prostate cancer: A phase I/II trial using Mycobacterium vaccae (SRL172) Br J Urol. 1998;82:568–73. doi: 10.1046/j.1464-410x.1998.00803.x. [DOI] [PubMed] [Google Scholar]
- 8.Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol. 1999;21:339–59. doi: 10.1007/BF00812261. [DOI] [PubMed] [Google Scholar]
- 9.De ST, Van MM, De BG, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–35. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 10.Yang P, Qiu G, Wang S, Su Z, Chen J, Wang S, et al. The mutations of Th1 cell-specific T-box transcription factor may be associated with a predominant Th2 phenotype in gastric cancers. Int J Immunogenet. 2010;37:111–5. doi: 10.1111/j.1744-313X.2010.00899.x. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 12.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–65. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen SJ, Crown SE, Handel TM. Chemokine: Receptor structure, interactions,and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 14.Ruffini PA, Morandi P, Cabioglu N, Altundag K, Cristofanilli M. Manipulating the chemokine-chemokine receptor network to treat cancer. Cancer. 2007;109:2392–404. doi: 10.1002/cncr.22706. [DOI] [PubMed] [Google Scholar]
- 15.Sugasawa H, Ichikura T, Kinoshita M, Ono S, Majima T, Tsujimoto H, et al. Gastric cancer cells exploit CD4+ cell-derived CCL5 for their growth and prevention of CD8+ cell-involved tumor elimination. Int J Cancer. 2008;122:2535–41. doi: 10.1002/ijc.23401. [DOI] [PubMed] [Google Scholar]
- 16.Janeway CA, Travers P, Walport M, Shlomchik MJ. 7th ed. Philadelphia: Churchill Livingstone; 2007. Immunobiology. [Google Scholar]
- 17.Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190:617–27. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii K, Sonoda K, Izumi K, Shiraishi N, Adachi Y, Kitano S. T lymphocyte subsets and Th1/Th2 balance after laparoscopy-assisted distal gastrectomy. Surg Endosc. 2003;17:1440–4. doi: 10.1007/s00464-002-9149-3. [DOI] [PubMed] [Google Scholar]
- 19.Liu XL, Gao J, Han CZ, Qiao LJ. [Pre- and post-chemotherapy expressions of Th1 and Th2 type cytokines and their clinical significance in gastric cancer patients] Zhonghua Zhong Liu Za Zhi. 2008;30:844–7. [PubMed] [Google Scholar]
- 20.Macey M.G, editor. Totowa, NJ 15: Humana Press; 2007. Flow Cytometry: Principles and Applications. [Google Scholar]
- 21.Cavallo F, Curcio C, Forni G. Immunotherapy and immunoprevention of cancer: Where do we stand? Expert Opin Biol Ther. 2005;5:717–26. doi: 10.1517/14712598.5.5.717. [DOI] [PubMed] [Google Scholar]
- 22.Negus RP, Stamp GW, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol. 1997;150:1723–34. [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Paul WE. CD4 T cells: Fates, functions, and faults. Blood. 2008;112:1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul WE. USA: Lippincott williams and wilkins; 2008. Fundamental Immunology. [Google Scholar]
- 25.Asano N, Suzuki R, Ohshima K, Kagami Y, Ishida F, Yoshino T, et al. Linkage of expression of chemokine receptors (CXCR3 and CCR4) and cytotoxic molecules in peripheral T cell lymphoma, not otherwise specified and ALK-negative anaplastic large cell lymphoma. Int J Hematol. 2010;91:426–35. doi: 10.1007/s12185-010-0513-0. [DOI] [PubMed] [Google Scholar]
- 26.Yoon SH, Yun SO, Park JY, Won HY, Kim EK, Sohn HJ, et al. Selective addition of CXCR3(+) CCR4(-) CD4(+) Th1 cells enhances generation of cytotoxic T cells by dendritic cells in vitro. Exp Mol Med. 2009;41:161–70. doi: 10.3858/emm.2009.41.3.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa M, Nishioka M, Hanaki N, Miyauchi T, Kashiwagi Y, Ioki H, et al. Perioperative immune responses in cancer patients undergoing digestive surgeries. World J Surg Oncol. 2009;7:7. doi: 10.1186/1477-7819-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrini P, Berghella AM, Del BT, Cicia S, Adorno D, Casciani CU. Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother. 1996;42:1–8. doi: 10.1007/s002620050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellegrini P, Contasta I, Berghella AM, Del BT, Casciani CU, Adorno D. The TH1 and TH2 cytokine network in healthy subjects: suggestions for experimental studies to create prognostic and diagnostic indices for biotherapeutic treatments. Cancer Biother Radiopharm. 2000;15:267–78. doi: 10.1089/108497800414365. [DOI] [PubMed] [Google Scholar]
- 30.Berghella AM, Pellegrini P, Del BT, Marini M, Tomei E, Adorno D, et al. The significance of an increase in soluble interleukin-2 receptor level in colorectal cancer and its biological regulating role in the physiological switching of the immune response cytokine network from TH1 to TH2 and back. Cancer Immunol Immunother. 1998;45:241–9. doi: 10.1007/s002620050439. [DOI] [PMC free article] [PubMed] [Google Scholar]


