Abstract
Background
The trait of mindfulness varies among meditation-naïve individuals and is associated with attentional and autonomic regulation, two neurocognitive functions that become impaired in addiction. It was hypothesized that alcohol dependent inpatients with comparatively high levels of trait mindfulness would exhibit significant autonomic recovery from stress-primed alcohol cues mediated by greater attentional disengagement from such cues.
Methods
58 alcohol dependent inpatients participated in affect-modulated psychophysiological cue-reactivity protocol and a spatial cueing task designed to assess alcohol attentional bias (AB). Associations between trait mindfulness, alcohol AB, and an index of autonomic activity, high-frequency heart rate variability (HFHRV), were examined via multivariate path analysis.
Results
Higher trait mindfulness was significantly associated with less difficulty resisting the urge to drink and greater HFHRV recovery from stress-primed alcohol cues. After statistically controlling for the correlation of mindfulness and perceived difficulty resisting drinking urges, the association between mindfulness and HFHRV recovery was partially mediated by attentional disengagement from alcohol cues (model R2 = .30).
Discussion
Alcohol dependent inpatients higher in mindfulness are better able to disengage attention from alcohol cues, which in turn predicts the degree of HFHRV recovery from such cues. Trait mindfulness may index cognitive control over appetitive responses reflected in superior attentional and autonomic regulation of stress-primed alcohol cue-reactivity.
Keywords: mindfulness, attentional bias, heart rate variability, stress, addiction
INTRODUCTION
Identification of malleable traits that can counter risk mechanisms implicated in addiction is of paramount importance to treatment development efforts. Individuals differ substantially with regard to their vulnerability to the acquisition, maintenance, and reinstatement of addictive behaviors; these individual differences have been traced to variation in neurocognitive functions that have been mapped in recent multi-systems conceptualizations (Garland, Boettiger, & Howard, 2011; George & Koob, 2010). Functions subserved by regions of prefrontal cortex appear to be central in regulating the cognitive, affective, and autonomic mechanisms underpinning addiction. Among such functions, attentional control, behavioral monitoring, interoceptive awareness, and self-regulation may modulate the impaired insight, automatic appetitive habits, and stress reactivity that promote addictive behavior (Goldstein et al., 2009).
The trait of mindfulness is characterized by this constellation of functions. Persons with high levels of trait mindfulness exhibit the propensity to attend to present moment experience as a means of becoming aware of their automatic reactions, and by doing so remain nonreactive in the face of distressing thoughts, emotions, and somatic sensations (Brown, Ryan, & Creswell, 2007; Garland, 2007; Garland, Fredrickson et al., 2010). Trait mindfulness appears to vary naturally among meditation-naive individuals and is inversely correlated with risk factors such as stress, thought suppression, and affective dysregulation (Baer, Smith, Hopkins, Krietemeyer, & Toney, 2006), which have been linked with addictive behaviors (Cheetham, Allen, Yucel, & Lubman, 2010; Garland, Boettiger, & Howard, 2011; Palfai, Monti, Colby, & Rohsenow, 1997). Moreover, this trait appears to be plastic and modifiable by training; participation in mindfulness-oriented interventions is associated with significant increases in trait mindfulness which in turn mediate the effects of mindfulness training on psychological symptom reduction (Carmody & Baer, 2008). Thus, mindfulness training interventions (e.g., Bowen et al., 2009; Garland, Gaylord, Boettiger, & Howard, 2010) may exert salutary effects on addictive processes through their promotion of trait mindfulness.
One addictive process that is likely offset by trait mindfulness is the addiction attentional bias (AB), i.e., preferential attention to substance-relevant cues that have been conferred incentive salience through mesocorticolimbic sensitization (Robinson & Berridge, 2008). Substance dependent individuals exhibit automatic attentional responses to drug-related cues (Field & Cox, 2008), as evidenced on dot probe tasks by shorter reaction times to probes replacing alcohol photos relative to probes replacing neutral photos. On tasks where substance cues are presented for 200 ms or less, addiction AB is believed to index initial orienting, because orienting, disengagement, and re-orienting to a new complex stimulus requires more than 200 ms (Duncan, Ward, & Shapiro, 1994; Theeuwes, 2005), whereas AB for longer duration stimuli (> 500 ms) is believed to index delayed attentional disengagement (Field & Cox, 2008). Among alcohol users, AB towards alcohol cues is primed by stress (Field & Powell, 2007) and associated with craving (Field, Munafo, & Franken, 2009), and alcohol consumption (Field & Eastwood, 2005). Conversely, greater attentional disengagement from alcohol cues is found among persons receiving treatment for alcohol use disorders (Townshend & Duka, 2007), and is predictive of successful treatment outcomes (Fadardi & Cox, 2009; Schoenmakers et al., 2010). Given evidence of significant correlations between trait mindfulness and self-reported attentional control (Baer et al., 2006; Herndon, 2008), decreased errors on sustained attention tasks (Schmertz, Anderson, & Robins, 2009), and improved selective attention, inhibitory control, and cognitive flexibility (Moore & Malinowski, 2009), it seems likely that persons recovering from addiction with high levels of trait mindfulness would have greater capacity to disengage attention from substance-related stimuli. In support of this hypothesis, a recent study of recovering alcohol dependent adults in residential treatment identified a significant inverse relationship between trait mindfulness and AB for alcohol-related stimuli presented for 2000 ms that remained robust even after controlling for alcohol dependence severity, craving, and perceived stress (Garland, Boettiger, Gaylord, West Channon, & Howard, 2011). It is plausible that the enhanced capacity for attentional disengagement from alcohol cues exhibited by alcohol dependent persons higher in trait mindfulness may be subserved by individual differences in prefrontal cortex (PFC) and anterior cingulate cortex (ACC) functionality, as these brain structures have been implicated in addictive AB (Ersche et al., 2011; Hester & Garavan, 2009; Luijten et al., 2011).
A second addictive process that may be associated with trait mindfulness is autonomic recovery from stress and alcohol cue-exposure. In the context of the present paper, we refer to “autonomic recovery” to indicate change in parasympathetic nervous system activation after presentation and subsequent withdrawal of an emotional stimulus. According to the neurovisceral integration model (Thayer & Lane, 2000, 2009), linkages between central (e.g., PFC and ACC) and autonomic (e.g., vagus nerve) nervous system networks coordinate the self-regulation of attention, cognition, and emotion while exerting regulatory influences over perturbations to visceral homeostasis, such as those that might be evoked in abstinent substance dependent individuals exposed to stressful and/or substance-related stimuli. According to the model, high frequency heart rate variability (HFHRV), which reflects parasympathetic control of the heart (Berntson et al., 1997), is an index of central autonomic network integrity and cognitive control over attention and emotion (Thayer, Hansen, Saus-Rose, & Johnsen, 2009). Neural activations in the PFC and ACC exert downstream influences on HFHRV during the experience of emotion (Lane et al., 2009), fine-tuning the cardiac pacemaker during the mobilization of energy resources in proportion to the perceived motivational demands of external and internal stimuli (Thayer & Lane, 2009). Thus, HFHRV should increase proportionally to the intensity of cognitive emotion regulation. Elevated HFHRV during stress exposure may indicate spontaneous regulation of negative emotional responses (Butler, Wilhelm, & Gross, 2006; Pu, Schmeichel, & Demaree, 2010), while elevated HFHRV during alcohol alcohol cue-exposure may reflect active regulation of appetitive motivational responses (i.e., cravings) elicited by alcohol cues; the latter interpretation is supported by findings of increased heart rate variability during exposure to appetitive stimuli when consumption of the desired substance is not permitted (Ingjaldsson, Laberg, & Thayer, 2003; Segerstrom & Nes, 2007). Conversely, lower HFHRV during cue exposure may index attentional fixation on such cues (Garland, Carter, Ropes, & Howard, 2011). The inability to disengage attention from alcohol cues may result in perseverative cognition coupled with heightened and prolonged autonomic nervous system reactions (Brosschot, 2010; Brosschot, Gerin, & Thayer, 2006), as indexed by reduced or delayed autonomic (i.e., HFHRV) recovery (Key, Campbell, Bacon, & Gerin, 2008; Verkuil, Brosschot, de Beurs, & Thayer, 2009).
Perseveration on internal representations of alcohol cues that are no longer present in the physical environment sustains physiological activation in the absence of such cues and lead to chronic stress states (Brosschot, Pieper, & Thayer, 2005). Maintaining internal representations of alcohol cues in working memory may modulate autonomic responses associated with substance cue-exposure such as heart-rate variability (Ingjaldsson, Thayer, & Laberg, 2003) and blood pressure (Fox, Bergquist, Hong, & Sinha, 2007). Such cue-induced autonomic responses show a high concordance with the subjective experience of craving (Sinha et al., 2003), which is characterized by a wide array of predominately aversive interoceptive responses (Bergquist, Fox, & Sinha, 2010), including increased heartbeat, tension, jitteriness, and restlessness. Among abstinent substance dependent individuals, protracted autonomic arousal induced by stress, attentional fixation, and perseveration on alcohol-related cognitions may drive relapse (Garland, Boettiger, & Howard, 2011). For such individuals, alcohol consumption may be a form of palliative coping (Olff, Langeland, & Gersons, 2005), an attempt to allay the stress, autonomic arousal, and aversive subjective states that co-occur with perseveration on alcohol-related thoughts, memories, and urges. However, mindful individuals with greater capacity for self-regulation, either innate or developed in treatment, may successfully inhibit such perseverative cognition and downregulate concomitant autonomic reactivity, manifested as greater HFHRV recovery.
To the extent that mindful alcohol dependent persons can successfully regulate stress and alcohol cue-reactivity, the ability to disengage attention from alcohol cues when they are no longer present should predict the degree of autonomic recovery from such cues. The aim of the present investigation was to explore this proposition in a sample of abstinent alcohol dependent adults in residential treatment. Specifically, it was hypothesized that alcohol dependent persons evincing higher levels of trait mindfulness would exhibit greater attentional disengagement from alcohol cues and greater HFHRV recovery from stress-primed alcohol cue exposure than alcohol dependent persons with lower levels of trait mindfulness. Furthermore, it was hypothesized that the relationship between trait mindfulness and HFHRV recovery would be mediated by attentional disengagement from alcohol cues. However, it is possible that, relative to their less mindful counterparts, alcohol dependent individuals endorsing higher levels of trait mindfulness may simply have less difficulty resisting the urge to drink and therefore evidence greater HFHRV recovery from alcohol cues. Hence, multivariate path analysis was employed to test both mediational hypotheses via simultaneous estimation of multiple linear equations.
METHODS
Subjects
Study participants were 58 alcohol-dependent adults who had resided for an average of 22.2 ± 3.6 months in a residential treatment facility. All participants met lifetime DSM-IV criteria for alcohol dependence as established by a semi-structured psychiatric interview. Most participants were male (81%) and African American (55.2%); 39.7% were Caucasian. With regard to income in the year before entering treatment, 56.9% had earned < $20,000, and 29.3% had earned $20,000–$40,000. The mean age of the sample was 39.8 (SD = 9.3). The mean number of DSM-IV alcohol dependence criteria met by participants was 6.5 (SD = 1.0), and the mean total AUDIT score for the sample was 32.4 (SD = 5.6). The mean number of standard alcoholic drinks consumed per day in the year before entering treatment was 18.9 (SD = 10.8). All participants reported having continuously abstained from use of psychoactive substances during their residence in the treatment facility. Reports of abstinence were corroborated by periodic, random urinalyses, as well as through daily observation by program staff. No participants were taking medications that might have affected cardiovascular function.
Procedure
During a single assessment session conducted on premises at the treatment facility, study participants first completed several standardized psychosocial instruments. Next, participants were engaged in a computer-based spatial cueing task as a measure of alcohol AB. Last, participants completed an affect-modulated cue-reactivity protocol (c.f., Cheetham et al., 2010), that is, one in which stress cue-exposure primed responses to subsequently presented alcohol cues. All measures were administered in this same order across study participants. This study was conducted with IRB approval from the University of North Carolina at Chapel Hill IRB board and was therefore performed in accordance with the ethical standards laid down in the Declaration of Helsinki. All participants gave informed consent prior to their inclusion in the study.
Measures
Mindfulness
The Five Facet Mindfulness Questionnaire (FFMQ, α = .81), comprised of 39 likert-type items rated on a five-point scale (1 = never or very rarely true, 5 = very often or always true), was used to measure trait mindfulness. The FFMQ yields a total score (computed by summing responses across all 39 items) and scores for five internally consistent mindfulness factors each with their own convergent and predictive validity: nonreactivity to inner experience (tapped by items such as “I watch my feelings without getting lost in them”), observing and attending to experience (“I pay attention to sensations, such as the wind in my hair or the sun on my face”), describing and discriminating emotional experiences (“I’m good at finding words to describe my feelings”), nonjudging of experience (reverse coded item: “I tell myself I shouldn’t be feeling the way that I am feeling”), and acting with awareness (reverse coded item: “I find myself doing things without paying attention”) (Baer et al., 2006).
Alcohol attentional bias
A spatial cueing task created in E-Prime 2.0 (PST Inc., Pittsburgh, PA) and presented on an IBM T60 laptop with a 15” screen was used to measure alcohol AB. In each trial, first a fixation cross was presented for 500 ms. Next, two grayscale images appeared side by side: one image was neutral in content, the other was alcohol-related. The pair of images was presented for 500 ms. Left/right position of the alcohol images and presentation duration were both randomized and counterbalanced across 20 practice trials and 160 trials. Following a 50 ms inter-stimulus interval, a target probe (two dots) replaced one of the images and a distracter probe (one dot) replaced the other image; probes appeared for 100 ms. Participants were instructed to indicate the location of the target probe by responding with a left or right button press on a keypad. Target probes randomly replaced alcohol and neutral images with equal frequency. The inter-trial interval was 500 ms.
Some parameters of the task employed here vary slightly from the visual probe tasks often used to study addiction-related AB (c.f., Field, Mogg, Zetteler, & Bradley, 2004), but accord with well-validated cognitive neuroscience methods used to probe attentional processes. In light of the large body of research that suggests attentional effects are more robust when targets appear with distracters relative to when targets are presented alone (for a review, see Carrasco, 2006), in our spatial cueing task, stimuli (one or two dots) appear in both cue locations, requiring participants to discriminate between target and distracter probes. This task design was chosen to enhance AB detection and eliminate confounding contributions of automatic, reflexive attention that are not related to the emotional salience (e.g., alcohol-relatedness) of the image cues. In particular, both sudden onsets and offsets have been found to capture attention (Hopfinger & Maxwell, 2005; Theeuwes & Chen, 2005), and if a probe appeared in only location, participants’ attention would be reflexively captured by the probe (Theeuwes, 1991) irrespective of the emotional salience of the preceding cue. Including a place marker in the opposite target probe location, requires the participant’s attention to be directed to the spatial location of the target probe and ensures that response selection cannot be based on reflexive detection of the probe through peripheral vision. In addition, use of target and distracter probes requires greater attentional resources than detection of a single probe and thus this design may have more power to resolve attentional shifts elicited by alcohol cues. Specifically, the use of two probes may lead to longer RTs when attention is originally captured to a non-target location, and greater facilitation of responding when attention is already directed to the target location, thereby increasing our ability to measure addiction-related attentional effects. While the use of two probes may add an additional mental task compared to single-probe tasks, other forms of discrimination tasks, such as those requiring participants to report the direction a target arrow, have found reliable attentional biases toward drug-related stimuli (Field et al., 2004; Field & Powell, 2007).
Alcohol stimuli included 13 photographs of alcoholic drinks (i.e., liquor, beer, etc), as well as 7 photos of persons drinking alcohol. Neutral stimuli included 13 photos of kitchen items and 7 photos of persons in kitchen scenes. Stimulus sets were analyzed with respect to their spatial frequency content to ensure that they did not differ in terms of basic visual properties, which could elicit reflexive attentional capture irrespective of image content. On measures of spectral peak (Neutral: 0.0180, Alcohol: 0.0176, t(38)=0.383, p=0.704) and spectral width (Neutral: 59.20, Alcohol: 59.29, t(38)=−0.027, p=0.979), the two stimulus sets were not significantly different.
HFHRV cue-reactivity
HFHRV responses to stress-primed alcohol cues were measured during an affect-modulated psychophysiological cue-reactivity protocol. First, disposable Ag-AgCl electrodes were attached to participants’ right and left pectoral muscles. Electrocardiogram (ECG) data was sampled at 500 Hz and recorded continuously throughout the protocol on a Biopac MP150 data acquisition system (Biopac Systems, Goleta, CA). Next, participants were instructed to remain motionless, silent, and “not think about anything in particular” for a 5-minute baseline. To ensure that participants had habituated to the experimental task situation (e.g., the experimenter, the psychophysiological sensors, etc.) during the baseline period prior to stimulus presentation, we used a visual analogue scale to assess stress and difficulty resisting alcohol urges before and after this resting baseline. Stress and craving were significantly reduced over the baseline period, t(54) = 3.35, p = .001, t(54) = 2.82, p = .007, respectively, suggesting that participants had indeed habituated to the experimental task situation. Next, 30 aversive photographs from the International Affective Picture System (IAPS) were serially presented on a 15” laptop screen for 10 seconds each (total duration: 5 min). Participants were asked to fixate on the image stream while holding as still as possible. After this presentation, participants again rated their current levels of stress, urge to drink, and difficulty resisting a drink. Next, 30 photographs of beer, wine, and distilled liquor (12 of which included individuals drinking or preparing to drink alcohol) were serially presented for 10 seconds each (total duration: 5 min), and participants were again instructed to keep still and fixate on the image stream. Last, participants were instructed to remain silent and motionless for five minutes as they recovered and returned to baseline. Hereafter we refer to this phase of the protocol as the recovery period.
Subjective cue-reactivity
After each phase of the cue-reactivity protocol (resting baseline, stress cue-exposure, alcohol cue-exposure, recovery period) participants were asked to rate current levels of stress and perceived difficulty with resisting the urge to drink on two, 10-point visual analogue scales (VAS) (0 = not at all, 9 = extreme). Participants were asked the following: “How stressed do you feel right now?” and “If your favorite alcoholic drink were in front of you right now, how hard would it be for you to resist drinking it?”
Data analysis
For AB data, trials with extreme RTs, defined as those with RTs longer than +3 SD above the individual mean (Field et al., 2004), were discarded as outliers (mean = 2.5 ± 1.5 per participant); error trials were also discarded. For each participant, AB scores were calculated by subtracting their mean RT to target probes replacing alcohol photos from their mean RT to target probes replacing neutral photos, such that positive bias scores indicate an AB toward visual alcohol cues. All data are reported as means ± SD unless otherwise noted.
R-R intervals were detected in the ECG data using automated routines in Nevrokard aHRV software (Medistar, Stegne, Ljubljana, Slovenia). The R-wave file was then visually inspected to correct misidentified or omitted R-waves. Kubios 2.0 (Biosignal Analysis and Medical Imaging Group, University of Finland) was used for spectral analysis of R-waves, applying a fast Fourier transform to extract HFHRV from a de-trended, end-tapered interbeat interval time series. HFHRV in the respiratory frequency band (0.15 – 0.40 Hz) was selected as our estimate of vagally-mediated HRV. Heart rate (HR) and HFHRV indices were averaged across the 5-minute baseline, 5-minute stress cue-exposure, 5-minute alcohol cue-exposure, and 5-minute recovery period. The present analysis focused on two particular contrasts: HFHRV reactivity, that is, changes in HFHRV between baseline and alcohol cue exposure, and HFHRV recovery, that is, changes in HFHRV between alcohol cue exposure and the recovery period. HFHRV reactivity was computed as the difference (Δ) between the 5-minute mean baseline level and the 5-minute mean during alcohol cue-exposure. HR and HFHRV recovery were computed as the difference (∆) between the 5-minute mean alcohol cue-exposure level and the 5-minute mean during the recovery period.
Bivariate correlations, t-tests, and repeated measures analyses of variance were performed with SPSS 16.0. Potential multicollinearity issues were screened by examining the variance inflation factor (VIF) of each variable. To examine whether the relation between trait mindfulness and HRV recovery was mediated by alcohol AB and/or perceived difficulty resisting the urge to drink, path analysis was conducted within a structural equation modeling framework with AMOS 17.0, which uses Full Information Maximum Likelihood (FIML) methods to estimate missing data. FIML has been shown to produce approximately unbiased estimations of regression coefficients for small samples (N ~ 60) with up to 20% of missing data (Scholmer, Baumer, & Card, 2010). The overall model fit was assessed by examining the chi-square statistic and the Comparative Fit Index (CFI; Bentler, 1990), as well as the Root Mean Squared Error of Approximation (RMSEA) Index (Hu & Bentler, 1998). CFI values approaching 1 indicate better model fit, with .90 being the conventional cut-off for a model with adequate fit. RMSEA scores closer to 0 indicate better model fit, with .05 being a commonly accepted cut-off for a well-fitting model.
RESULTS
Alcohol attentional bias
Mean accuracy on the spatial cueing task was 97.2%± 0.4%. Mean RT to target probes replacing alcohol photos presented for 500 ms was 363.0 ± 123.0 ms, whereas mean RT for neutral photos was 359.7 ± 124.5 ms. 500 ms AB data were approximately normally distributed (yielding a non-significant Kolmogorov-Smirnov test for normality). Paired t-tests revealed nonsignificant differences between RTs to alcohol and neutral photos presented for 500 ms, t(50) = 1.14, p=.25. Although the overall sample mean AB was −3.3 ms, a slight average attentional bias away from alcohol cues, this value was not significantly greater than zero. This finding is parallel to those of Noel et al. (2006), who found a nonsignificant AB in abstinent alcohol dependent patients. However, there was substantial heterogeneity in individual AB scores, such that nearly one-half of the individuals in the sample had an attentional bias towards alcohol cues (n = 22, 44%), and the other half had an attentional bias away from alcohol cues (n = 28, 56%); hence, an individual difference analysis was warranted to model the heterogeneity in AB responses.
HFHRV cue-reactivity and recovery
Mean HFHRV changed significantly over the course of the affect modulated cue-reactivity protocol, F(3,147) = 9.69, p < .001, ηρ2 = .17. Analyses of planned contrasts revealed a significant increase in HFHRV from baseline through stress cue-exposure F(1,49) = 10.64, p = .002, ηρ2 = .17, and a significant decrease in HFHRV from alcohol cue-exposure through the recovery period, F(1,49) = 8.3, p < .01, ηρ2 = .15.
HR cue-reactivity and recovery
Mean HR changed significantly over the course of the affect modulated cue-reactivity protocol, F(3,156) = 14.58, p < .001, ηρ2 = .22. Analyses of planned contrasts revealed a significant decrease in HR from baseline through stress cue-exposure F(1,52) = 22.09, p < .001, ηρ2 = .30, and a significant increase in HR from alcohol cue-exposure through the recovery period, F(1,52) = 4.11, p < .05, ηρ2 = .07.
Subjective cue-reactivity and recovery
Subjective stress changed significantly over the course of the affect modulated cue-reactivity protocol, F(3,156) = 26.60, p < .001, ηρ2 = .34. Participants reported significant increases in subjective stress from baseline through stress cue-exposure, F(1,52) = 34.13, p < .001, ηρ2 = .40, and significant decreases in stress from alcohol cue-exposure through the recovery period, F(1,52) = 17.39, p < .001, ηρ2 = .25. Perceived difficulty resisting drinking urges also changed significantly over the course of the affect modulated cue-reactivity protocol, F(3,156) = 9.06, p < .001, ηρ2 = .15. Participants reported significantly increased difficulty resisting the urge to drink from baseline through cue-exposure, F(1,52) = 4.5, p < .05, ηρ2 = .08, and significantly decreased difficulty resisting alcohol urges from cue-exposure through the recovery period, F(1,52) = 18.42, p < .001, ηρ2 = .26.
Trait mindfulness is associated with alcohol AB and HFHRV recovery but not HFHRV reactivity
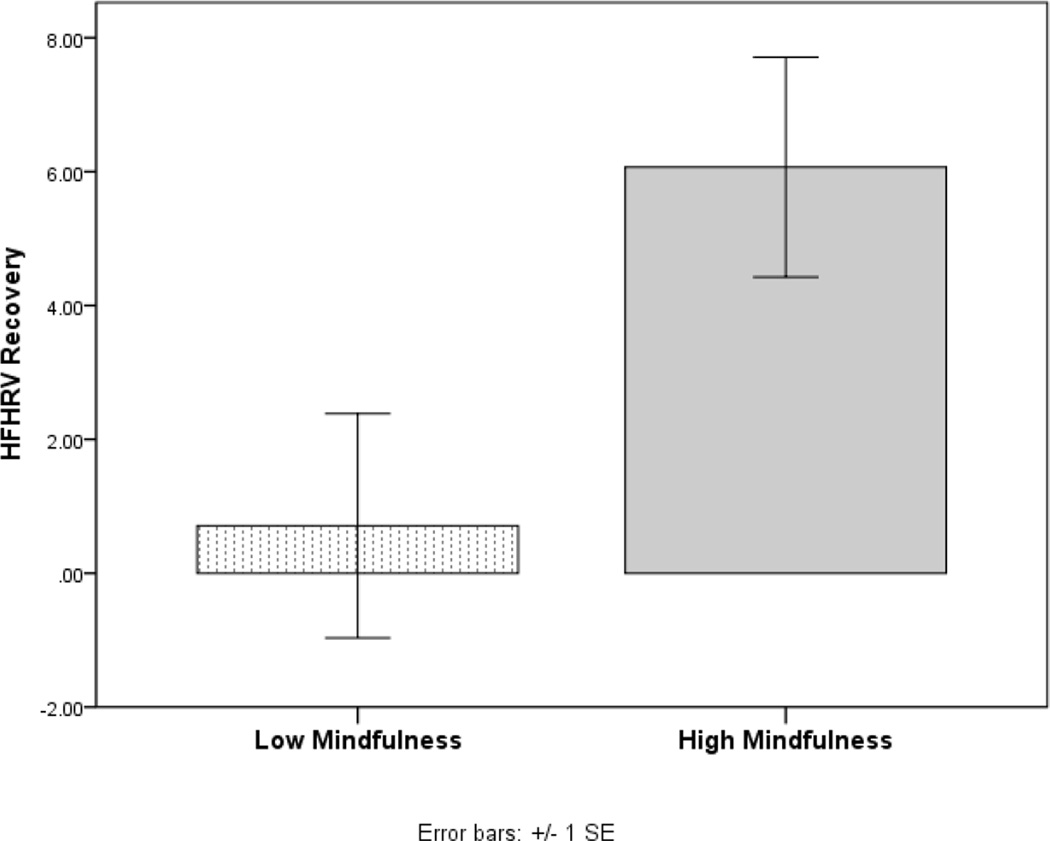
Trait mindfulness was significantly associated with HFHRV recovery from alcohol cue exposure, r = .36, p < .01. To aid in visualizing the relationship between trait mindfulness and HFHRV recovery, we used a median split on trait mindfulness to divide the sample into high and low trait mindfulness subgroups, and plotted differences in HFHRV recovery between these subgroups (see Figure 1). Similarly, trait mindfulness was significantly correlated with change in HR from alcohol cue exposure to the recovery period, r = .29, p < .05. Persons reporting higher levels of trait mindfulness experienced greater HFHRV and HR recovery from alcohol cue exposure. Furthermore, trait mindfulness was marginally associated with alcohol AB, r = −.28, p = .05, such that participants endorsing higher levels of mindfulness tended to exhibit increased attentional disengagement from alcohol cues. In contrast, trait mindfulness was not statistically predictive of HFHRV reactivity, r = −.04, p > .10 or HR reactivity, r = −.21, p > .10.
Figure 1.
Differences in HFHRV recovery from stress-primed alcohol cue-exposure between alcohol dependent patients high and low in trait mindfulness (defined by median split).
Attentional disengagement from alcohol cues is associated with HFHRV recovery but not HFHRV reactivity
Alcohol AB was significantly associated with HFHRV recovery from alcohol cue-exposure, r = −.31, p =.03, such that recovering alcohol dependents who were better able to disengage and re-orient attention from alcohol cues experienced the largest decreases in HFHRV from alcohol cue-exposure to the recovery period. Alcohol AB was statistically unrelated to HFHRV reactivity, r = −.00, p > .10.
Perceived difficulty resisting the urge to drink is associated with trait mindfulness, alcohol AB, and HFHRV recovery
Trait mindfulness was inversely associated with perceived difficulty resisting the urge to drink alcohol at baseline prior to cue exposure, r = −.36, p < .01. Baseline perceived difficulty resisting the urge to drink was inversely associated with changes in HFHRV from alcohol cue-exposure to the recovery period, r = −.27, p < .05, such that persons who reported greater difficulty resisting the urge to drink had less HFHRV recovery from alcohol cue exposure. Alcohol AB was significantly correlated with changes in perceived difficulty resisting the urge to drink alcohol, r = .31, p < .05 from alcohol cue-exposure to the recovery period (see Table 1 for correlations between selected study variables).
Table 1.
Zero-order correlations among selected study variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Trait Mindfulness | 1 | ||||||||
| 2. HFHRV Cue-Reactivity | −.04 | 1 | |||||||
| 3. HFHRV Cue-Recovery | **.36 | −.58 | 1 | ||||||
| 4. HR Cue-Reactivity | −.24 | −.21 | .06 | 1 | |||||
| 5. HR Cue-Recovery | −.06 | .18 | −.21 | **−.45 | 1 | ||||
| 6. Alcohol AB | †−.28 | −.00 | *.31 | .07 | .01 | 1 | |||
| 7. Baseline Difficulty Resisting Drinking Urges | **−.36 | .06 | *−.27 | −.24 | *.29 | .05 | 1 | ||
| 8. Change in Difficulty Resisting Urges from Baseline to Cue-Exposure | −.12 | −.16 | .02 | −.06 | *.33 | −.26 | .14 | 1 | |
| 9. Change in Difficulty Resisting Urges from Cue-Exposure to Recovery | −.08 | .18 | −.20 | −.03 | −.21 | *.31 | .17 | ***−.76 | 1 |
p = .05,
p < .05,
p < .01,
p <.001
Path analysis
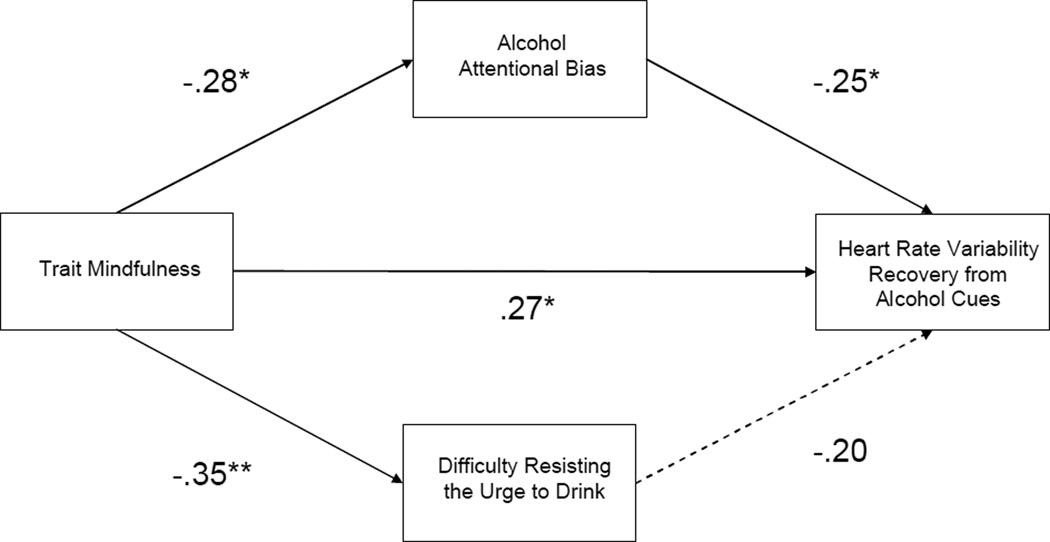
Multivariate path analysis was employed to test a model in which either alcohol AB or baseline difficulty resisting the urge to drink could mediate the relationship between trait mindfulness and HFHRV recovery, controlling for differences in age and gender (see Figure 2). This model exhibited good fit: χ2/df = 1.13, p = .34; CFI = .92, RMSEA = .05 (.00, .17). Controlling for the significant association between trait mindfulness and difficulty resisting the urge to drink, alcohol AB partially mediated the relation between trait mindfulness and HFHRV recovery. Thus, alcohol dependent persons endorsing higher levels of mindfulness experienced larger decreases (recovery) in HFHRV from stress-primed alcohol cue-exposure, and such decreases are associated with a greater ability to disengage and re-orient attention from alcohol cues. The overall model explained 30.3% of the variance in HFHRV recovery.
Figure 2.
Multivariate path model indicating that the relation between trait mindfulness and high-frequency heart rate variability recovery from alcohol cues was partially mediated by attentional disengagement from alcohol cues, after controlling for the association of mindfulness and perceived difficulty resisting alcohol urges. * p < .05 ** p < .01.
DISCUSSION
Among this sample of alcohol dependent adults undergoing an affect-modulated cue-reactivity protocol, higher trait mindfulness was associated with less subjective difficulty resisting alcohol urges and greater cardiovascular recovery from alcohol cues. Further, persons exhibiting greater capacity for attentional disengagement from alcohol cues experienced larger recovery in HFHRV from alcohol cue-exposure. After statistically controlling for the association between trait mindfulness and perceived difficulty resisting the urge to drink, the relation between trait mindfulness and HFHRV recovery could be accounted for, in part, by attentional disengagement from alcohol cues. To the extent that mindful individuals were better able to disengage their attention from alcohol cues, such reduced alcohol AB predicted the degree to which their heart rate variability recovered from alcohol cue-exposure levels.
Alcohol dependent inpatients, regardless of their level of mindfulness, exhibited an elevation in HFHRV and decrease in HR from baseline through stress cue-exposure. This increase in HFHRV in response to stress provocation likely indexes prefrontal regulation of the stress reaction via parasympathetic nervous system activation of the “vagal brake,” resulting in HR deceleration (Porges, 1995; Thayer & Lane 2009). In contrast, persons reporting higher levels of mindfulness exhibited greater HFHRV and HR recovery from stress-primed alcohol cue-exposure than those persons endorsing lower levels of mindfulness. The pattern of HFHRV recovery observed among persons with higher levels of mindfulness may be interpreted as evidence of contextually-appropriate disengagement of neurocognitive resources (Thayer et al., 2009; Thayer & Lane, 2000, 2009) during the recovery period, at which time no stressful or alcohol-related stimuli were present. In the absence of emotionally-provocative stimuli, persons endorsing high levels of mindfulness may have disengaged their attention from alcohol-related cognitions and may have ceased to maintain representations of stress- and alcohol-related cues online in working memory (indicated by deactivation of the central autonomic network reflected in decreased vagally-mediated HFHRV and concomitant increases in HR). This interpretation is consistent with conceptualizations of trait mindfulness as involving the capacity to “let go” of emotional reactions (Brown et al., 2007). Moreover, path analytic findings supported the notion that persons endorsing higher levels of trait mindfulness were better able to disengage attentional resources from processing alcohol-related cognitions, and consequently experienced significant HFHRV recovery in the absence of alcohol cues.
In contrast, alcohol dependent inpatients endorsing lower trait mindfulness evidenced less HFHRV recovery than their more mindful counterparts. Such attenuated HFHRV recovery may be indicative of a failure to disengage attentional resources from alcohol-related cognitions held online in working memory. In the present study, alcohol dependent inpatients low in mindfulness may have continued to perseverate about alcohol after alcohol cues had been removed. Indeed, rumination after stress exposure is associated with reduced HFHRV recovery (Key et al., 2008). Thayer and Friedman (2002) assert that interruption of perseverative cognition is accomplished via inhibitory processes associated with parasympathetic control of cardiovascular activity; hence, individuals with deficient inhibitory functions are less able to habituate to innocuous stimuli and consequently suffer from perseveration and anxiety. Given evidence of an inverse association between mindfulness and rumination (Borders, Earleywine, & Jajodia, 2011; Deyo, Wilson, Ong, & Koopman, 2009), it is plausible that alcohol dependent inpatients reporting lower levels of trait mindfulness, who were less able to disengage their attention from alcohol cues, continued to ruminate over thoughts of alcohol during the recovery period. This continued ruminative engagement with internal representations of alcohol cues held in working memory may have been reflected in the prolonged HFHRV responses of less mindful study participants.
Importantly, although individuals higher in mindfulness reported less difficulty resisting the urge to drink, this association was statistically independent of the mediational relationship between trait mindfulness, alcohol AB, and HFHRV recovery. Thus, it is not merely that mindful alcohol dependent inpatients have weaker appetitive responses towards alcohol and thus exhibit faster autonomic recovery. Above and beyond the subjective sense of being able to resist alcohol urges, mindful individuals exhibit greater attentional disengagement from alcohol cues which, in turn, facilitates situationally-adaptive withdrawal of parasympathetic activation after such cues are no longer present.
To be clear, the measure of cardiovascular recovery used in the present study (delta) is a measure of the extent of recovery over the 5-minute recovery period, not a measure of the time course of recovery over a prolonged period. Measures of intensity or amplitude of short-term change in cardiovascular activation may not reflect the full range of pathogenic effects caused by perseverative cognition (Brosschot, Gerin, & Thayer, 2006). Given that cardiovascular reactivity may extend from minutes after the presentation of a stressor through the rest of the day and into nocturnal sleep, ambulatory monitoring of HR and HRV is needed to ascertain “where exactly prolonged activation started and how long it exactly lasted after the stressor” (Pieper, Brosschot, van der Leeden, & Thayer, 2010). Furthermore, such methods are necessary to employ the area-under-the-curve analytic approach suggested by Brosschot, Gerin, & Thayer (2006). Future studies should employ ambulatory monitoring after stress-primed alcohol cue-exposure to determine how trait mindfulness and alcohol AB impact the total amount of stress-induced cardiovascular activation over time.
One limitation of the present study is its inability to dissociate changes in HFHRV evoked by arousal from changes in HFHRV evoked by heightened attention or emotion regulation. Indeed, the somatic signature of arousal can be confounded with that of heightened attention or cognitive processing (c.f., Lacey, 1967). Moreover, because the study was focused on stress-primed alcohol cue responses, participants were not allowed to return to baseline between the stress cue and alcohol cue exposure periods. As such, there is no way of knowing if the observed changes in HFHRV reflect recovery from stress cues, alcohol cues, or the interaction of the two, and therefore it is not possible to conclusively interpret the HFHRV responses observed in the present study. Yet, this limitation may be partially offset by triangulation of HFHRV responses with attentional and self-report data, which bolstered the present interpretations of the findings. Similarly, because the spatial cueing and cue-reactivity tasks were not counterbalanced, task order effects cannot be ascertained. Future research designs should employ counterbalanced, experimental manipulations to examine the differential effects of stress provocation, emotion regulation, and cognitive processing on HFHRV cue responses among alcohol dependent persons with varying levels of trait mindfulness.
Furthermore, in spite of reports that heavy drinkers have AB towards alcohol cues on visual probe tasks, the mean alcohol AB for our sample was not statistically significantly different than zero. Inspection of individual differences revealed that the sample was roughly split into persons with AB towards alcohol cues and those with AB away from such cues. One might expect significant heterogeneity of AB responses among alcohol dependent inpatients in long-term residential treatment, who tend to exhibit varying degrees of treatment response. Alternatively, the lack of a significant mean AB in this study may stem from our use of a spatial cueing task, which differed somewhat from the usual tasks used to assess alcohol AB. Though, a prior study established the sensitivity and construct validity of the spatial cueing task by identifying a significant positive correlation between alcohol AB as revealed by this task and level of alcohol consumption (Garland, Boettiger, Gaylord et al., 2011). Current study findings should be replicated using other means of assessing alcohol AB, such as the dot probe and addiction Stroop tasks. The lack of a significant mean alcohol AB might have also resulted from the nature of the study participants, who, after an average of 22 months residing in a treatment milieu without access or exposure to alcohol, may have exhibited attenuated appetitive responses reflected in nonsignificant mean alcohol AB. Thus, conclusions based on study findings cannot be generalized to untreated alcohol dependent individuals or patients earlier in treatment.
In addition, HFHRV findings may have been confounded because neither respiration rate nor tidal volume was controlled in the present analyses (Grossman & Taylor, 2007) although there is substantial debate in the literature regarding the importance of such corrections (e.g., Denver, Reed, & Porges, 2007). In spite of these caveats, it is notable that individual difference analysis identified a statistically significant association between alcohol AB and HFHRV recovery from alcohol cues, which to our knowledge is the first report of such a finding in the literature. This noteworthy finding is congruent with theoretical views of HFHRV as an index of attentional control mediated by central autonomic network activity. In future studies, measures of galvanic skin response could be used to confirm whether the identified effects on heart rate variability recovery are due primarily to changes in parasympathetic efflux. Further, neuroimaging technologies could be used to investigate the cortical and subcortical activations underlying functional linkages between trait mindfulness, alcohol AB, and heart rate variability.
In sum, treated alcohol dependent persons who have developed trait mindfulness may successfully regulate cue-reactivity by virtue of their ability to disengage attention and recover from stress and alcohol cues. As such, results of the current study complement those of our prior clinical trial of mindfulness training for alcohol dependent adults, which found significant effects of training on alcohol AB and heart rate variability recovery from stress-primed alcohol cue-exposure (Garland, Gaylord et al., 2010). Insofar as trait mindfulness in untrained alcohol dependent persons is associated with greater attentional disengagement and autonomic recovery from alcohol cue-exposure, mindfulness training, which exerts therapeutic effects in part through the development of trait mindfulness (Carmody & Baer, 2008), may leverage and augment similar neurovisceral mechanisms implicated in stress-precipitated alcohol dependence (Garland, Boettiger, & Howard, 2011). Future research should employ rigorous psychophysiological methods coupled with tasks drawn from cognitive neuroscience to disentangle how state and trait mindfulness interact during mindfulness training (Garland, Fredrickson et al., 2010; Lutz, Dunne, & Davidson, 2007) to promote recovery from stress and addictive impulses.
Acknowledgements
This research was supported by Grant Number T32AT003378 from the National Center for Complementary and Alternative Medicine and a Francisco J. Varela Research Grant from the Mind and Life Institute, Boulder, CO.
REFERENCES
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Bergquist KL, Fox HC, Sinha R. Self-reports of interoceptive responses during stress and drug cue-related experiences in cocaine-and alcohol-dependent individuals. Experimental and Clinical Psychopharmacology. 2010;18(3):229–237. doi: 10.1037/a0019451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufman PG, Malik M, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Borders A, Earleywine M, Jajodia A. Could mindfulness decrease anger, hostility, and aggression by decreasing rumination? Aggressive Behavior. 2011;36(1):28–44. doi: 10.1002/ab.20327. [DOI] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, et al. Mindfulness-based relapse prevention for substance use disorders: A pilot efficacy trial. Substance Abuse. 2009;30:295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF. Markers of chronic stress: prolonged physiological activation and (un)conscious perseverative cognition. Neuroscience & Biobehavioral Reviews. 2010;35(1):46–50. doi: 10.1016/j.neubiorev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60(2):113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Pieper S, Thayer JF. Expanding stress theory: prolonged activation and perseverative cognition. Psychoneuroendocrinology. 2005;30(10):1043–1049. doi: 10.1016/j.psyneuen.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM, Creswell JD. Mindfulness: Theoretical foundations and evidence for its salutary effects. Psychological Inquiry. 2007;18(4):211–237. [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43(6):612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. Journal of Behavioral Medicine. 2008;31(1):23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Yucel M, Lubman DI. The role of affective dysregulation in drug addiction. Clinical Psychology Review. 2010;30(6):621–634. doi: 10.1016/j.cpr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Denver JW, Reed SF, Porges SW. Methodological issues I the quantification of respiratory sinus arrhythmia. Biological Psychology. 2007;74(2):286–294. doi: 10.1016/j.biopsycho.2005.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo M, Wilson KA, Ong J, Koopman C. Mindfulness and rumination: does mindfulness training lead to reductions in the ruminative thinking associated with depression? Explore (NY) 2009;5(5):265–271. doi: 10.1016/j.explore.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Duncan J, Ward R, Shapiro K. Direct measurement of attentional dwell time in human vision. Nature. 1994;369(6478):313–315. doi: 10.1038/369313a0. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Muller U, et al. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Archives of General Psychiatry. 2011;67(6):632–644. doi: 10.1001/archgenpsychiatry.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadardi JS, Cox WM. Reversing the sequence: reducing alcohol consumption by overcoming alcohol attentional bias. Drug and Alcohol Dependence. 2009;101(3):137–145. doi: 10.1016/j.drugalcdep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug and Alcohol Dependence. 2008;97(1–2):1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology (Berl) 2005;183(3):350–357. doi: 10.1007/s00213-005-0202-5. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Zetteler J, Bradley BP. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology (Berl) 2004;176(1):88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychological Bulletin. 2009;135(4):589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Powell H. Stress increases attentional bias for alcohol cues in social drinkers who drink to cope. Alcohol and Alcoholism. 2007;42(6):560–566. doi: 10.1093/alcalc/agm064. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcoholism: Clinical & Experimental Research. 2007;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Garland EL. The meaning of mindfulness: A second-order cybernetics of stress, metacognition, and coping. Complementary Health Practice Review. 2007;12(1):15–30. [Google Scholar]
- Garland EL, Boettiger CA, Gaylord SA, West Channon V, Howard MO. Mindfulness is inversely associated with alcohol attentional bias among recovering alcohol dependent adults. Cognitive Therapy & Research. 2011 doi: 10.1007/s10608-011-9378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Boettiger CA, Howard MO. Targeting cognitive-affective risk mechanisms in stress-precipitated alcohol dependence: An integrated, biopsychosocial model of allostasis, automaticity, and addiction. Medical Hypotheses. 2011;76:745–754. doi: 10.1016/j.mehy.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Carter K, Ropes K, Howard MO. Thought suppression, impaired regulation of alcohol urges, and Addiction Stroop predict affect-modulated cue-reactivity among alcohol dependent adults. manuscript submitted for publication. 2011 doi: 10.1016/j.biopsycho.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Fredrickson BL, Kring AM, Johnson DP, Meyer PS, Penn DL. Upward spirals of positive emotions counter downward spirals of negativity: Insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical Psychology Review. 2010;30:849–864. doi: 10.1016/j.cpr.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: Results from a randomized controlled pilot trial. Journal of Psychoactive Drugs. 2010;42(2):177–192. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience & Biobehavioral Reviews. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution, and biobehavioral functions. Biological Psychology. 2007;74(2):263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, et al. The neurocircuitry of impaired insight in drug addiction. Trends in Cognitive Sciences. 2009;13(9):372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon F. Testing mindfulness with perceptual and cognitive factors: External vs. internal encoding, and the cognitive failures questionnaire. Personality and Individual Differences. 2008;44(1):32–41. [Google Scholar]
- Hester R, Garavan H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacology Biochemistry & Behavior. 2009;93(3):270–277. doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Maxwell JS. Appearing and disappearing stimuli trigger a reflexive modulation of visual cortical activity. Brain Research & Cognitive Brain Research. 2005;25(1):48–56. doi: 10.1016/j.cogbrainres.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54(12):1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Key BL, Campbell TS, Bacon SL, Gerin W. The influence of trait and state rumination on cardiovascular recovery from a negative emotional stressor. Journal of Behavior Medicine. 2008;31(3):237–248. doi: 10.1007/s10865-008-9152-9. [DOI] [PubMed] [Google Scholar]
- Lacey JT. Somatic response patterning and stress: Some revision of activation theory. In: Appley M, Trumbell R, editors. Psychological stress: Some issues in research. New York: Appelton Century Crofts; 1967. pp. 14–37. [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44(1):213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, van den Brink W, Hester R, Field M, Smits M, et al. Neurobiological substrate of smoking-related attentional bias. Neuroimage. 2011;54(3):2374–2381. doi: 10.1016/j.neuroimage.2010.09.064. [DOI] [PubMed] [Google Scholar]
- Lutz A, Dunne JD, Davidson RJ. Meditation and the neuroscience of consciousness: An introduction. In: Zelazo PD, Moscovitch M, Thomspson E, editors. The Cambridge Handbook of Consciousness. New York: Cambridge University Press; 2007. pp. 497–549. [Google Scholar]
- Moore A, Malinowski P. Meditation, mindfulness and cognitive flexibility. Conscious Cogn. 2009;18(1):176–186. doi: 10.1016/j.concog.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Noel X, Colmant M, Van Der Linden M, Bechara A, Bullens Q, Hanak C, et al. Time course of attention for alcohol cues in abstinent alcoholic patients: the role of initial orienting. Alcoholism: Clinical and Experimental Research. 2006;30(11):1871–1877. doi: 10.1111/j.1530-0277.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Gersons BP. Effects of appraisal and coping on the neuroendocrine response to extreme stress. Neuroscience & Biobehavioral Reviews. 2005;29(3):457–467. doi: 10.1016/j.neubiorev.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Palfai TP, Monti PM, Colby SM, Rohsenow DJ. Effects of suppressing the urge to drink on the accessibility of alcohol outcome expectancies. Behaviour Research and Therapy. 1997;35(1):59–65. doi: 10.1016/s0005-7967(96)00079-4. [DOI] [PubMed] [Google Scholar]
- Pieper S, Brosschot J, van der Leeden R, Thayer JF. Prolonged cardiac effects of momentary assessed stressful events and worry episodes. Psychosomatic Medicine. 2010;72:570–577. doi: 10.1097/PSY.0b013e3181dbc0e9. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Pu J, Schmeichel BJ, Demaree HA. Cardiac vagal control predicts spontaneous regulation of negative emotional expression and subsequent cognitive performance. Biological Psychology. 2010;84:531–540. [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmertz SK, Anderson PL, Robins DL. The relation between self-report mindfulness and performance on tasks of sustained attention. Journal of Psychopathology and Behavioral Assessment. 2009;31 [Google Scholar]
- Schoenmakers TM, de Bruin M, Lux IF, Goertz AG, Van Kerkhof DH, Wiers RW. Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug and Alcohol Dependence. 2010;109(1–3):30–36. doi: 10.1016/j.drugalcdep.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Scholmer GL, Baumer S, Card NA. Best practices for management of missing data in counseling psychology. Journal of Counseling Psychology. 2010;57(1):1–10. doi: 10.1037/a0018082. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychological Science. 2007;18(3):275–281. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170(1):62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH. Stop that! Inhibition, sensitization, and their neurovisceral concomitants. Scandinavian Journal of Psychology. 2002;43(2):123–130. doi: 10.1111/1467-9450.00277. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Exogenous and endogenous control of attention: the effect of visual onsets and offsets. Perception and Psychophysics. 1991;49(1):83–90. doi: 10.3758/bf03211619. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Irrelevant singletons capture attention. In: Itti L, Rees G, Tsotsos JK, editors. Neurobiology of attention. London: Elsevier Academic Press; 2005. pp. 418–424. [Google Scholar]
- Theeuwes J, Chen CY. Attentional capture and inhibition (of return): the effect on perceptual sensitivity. Perception and Psychophysics. 2005;67(8):1305–1312. doi: 10.3758/bf03193636. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Avoidance of alcohol-related stimuli in alcohol-dependent inpatients. Alcoholism: Clinical and Experimental Research. 2007;31(8):1349–1357. doi: 10.1111/j.1530-0277.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- Verkuil B, Brosschot JF, de Beurs DP, Thayer JF. Effects of explicit and implicit perseverative cognition on cardiac recovery after cognitive stress. International Journal of Psychophysiology. 2009;74(3):220–228. doi: 10.1016/j.ijpsycho.2009.09.003. [DOI] [PubMed] [Google Scholar]