Abstract
Contact interactions between different cell types play a number of important roles in development, for example in cell sorting, tissue organization, and ordered migration of cells. The nature of such heterocellular interactions, in contrast to interactions between cells of the same type, remains largely unknown. In this report, we present experimental data examining the dynamics of heterocellular interactions between epitheliocytes and fibroblasts, which express different cadherin cell adhesion molecules and possess different actin cytoskeletal organizations. Our analysis revealed two striking features of heterocellular contact. First, the active free edge of an epitheliocyte reorganizes its actin cytoskeleton after making contact with a fibroblast. Upon contact with the leading edge of a fibroblast, epitheliocytes disassemble their marginal bundle of actin filaments and reassemble actin filaments into a geometric organization more typical of a fibroblast lamella. Second, epitheliocytes and fibroblasts form cell–cell adhesion structures that have an irregular organization and are associated with components of cell adhesion complexes. The structural organization of these adhesions is more closely related to the type of contacts formed between fibroblasts rather than to those between epitheliocytes. Heterotypic epithelio-fibroblastic contacts, like homotypic contacts between fibroblasts, are transient and do not lead to formation of stable contact interactions. We suggest that heterocellular contact interactions in culture may be regarded as models of how tissue systems consisting of epithelia and mesenchyme interact and become organized in vivo.
Development of adhesive interactions between cells of the same tissue type relies upon the construction of an adhesion complex using cell-specific cadherins (1, 2). Studies examining the formation of homotypic cadherin-containing adhesive structures by cultured cells revealed that the organization of the actin cytoskeleton at sites of cell–cell contact has an essential role in the development and maintenance of the contacts (3–8). It was determined that the local architecture of the actin cytoskeleton at the leading edge of contacting cells can produce substantially different spatial organization of cell–cell adhesion complexes. Epithelial cells possessing dynamic marginal arc-like actin bundles at the edges of lamellas form cadherin-containing adhesion structures that are oriented tangentially to the leading edge of the cell (9). Fibroblasts have actin filament bundles that are oriented perpendicular to the leading edge of the cell, and the ends of the bundles protrude into the edge of the leading lamella. This organization of actin filaments results in the formation of cell–cell contacts that orient radially to the edge of the cell and are associated with the ends of the actin bundles (6). Similar to the response of fibroblasts, rows of point-like adhesions form at the ends of filopodia between contacting keratinocytes (8, 10). Furthermore, the spatial organization of cell–cell adhesions can be modified, as evidenced by the dramatic changes in structure observed after perturbation of myosin-dependent contraction and epithelio-mesenchymal transformation induced by phorbol ester (7). Thus, the structural organization of cell–cell contacts must be coordinately regulated by the dynamic activity of the cytoskeleton, the architecture of which is regulated, in turn, by multiple elements of signaling cascades (11).
During in vivo developmental processes such as organ and tissue morphogenesis there occur numerous heterocellular contact interactions that are probably as important to development as cell–cell interactions between homotypic cells. Unfortunately, our understanding of the dynamics of cadherin-associated interactions between cells of different tissue types remains largely unknown and unexplored relative to our understanding of homotypic interactions. The only description of heterotypic cadherin-containing adhesions known to the authors of this paper is a study examining melanoma cells migrating through a monolayer of endothelial cells (12).
In this paper, we present the results of experiments showing that cultured cells of two main tissue types, fibroblasts and epitheliocytes, can form heterotypic adhesion complexes upon cell–cell contact, despite having different actin cytoskeletal organization and different cadherins. It was observed that when the edge of an epithelial cell comes into contact with the surface of a fibroblast, the epithelial cell reorganizes its actin cytoskeleton and forms transient cadherin-associated contacts. Interestingly, the local organization of the actin cytoskeleton in the epithelial cell results in a small fibroblast-like lamella that protrudes over the top surface of the fibroblast. We discuss possible mechanisms that could be directing this reorganization and the physiological significance of this type of cell–cell interaction.
Materials and Methods
Cell Cultures.
Epithelial cell lines used in this study were rat IAR-2 (13) and Madin–Darby canine kidney (MDCK) cells (14). Fibroblast cell lines were rat RAT-1 (15) and human M19 cells (16). All cells were cultured in DMEM supplemented with 5–10% FCS (Harlan Bioproducts, Indianapolis) at 37°C and 5% CO2.
Heterocellular Collisions.
Culture chambers were prepared by placing a glass coverslip (22-mm square no. 1; Corning) into a 35-mm Petri culture dish and dividing the coverslip into two halves by placing a parafilm-covered plastic bar on the coverslip. Suspensions of RAT-1 fibroblasts and IAR-2 epitheliocytes were added to opposite chambers of the coverslip and incubated at 37°C in 5% CO2 . After 2–4 h, the bar was removed, and the coverslip was rinsed with warm fresh medium to remove unattached cells, placed into a new culture dish, and incubated. After 3–4 days, cells from opposite sides of the coverslip would begin making cell–cell contacts, at which time the coverslips were removed and mounted in sealed observation chambers, and cell–cell contacts were recorded by time-lapse video differential interference contract microscopy (9). Alternatively, cells of two different cell lines were simultaneously seeded on glass coverslips, producing intermixed populations of cells. After 24 h of incubation, the coverslips were removed and mounted on slides for time-lapse observations.
Immunofluorescence Localization.
To localize actin filaments, cells were fixed and labeled with rhodamine-phalloidin as described in ref. 6. Indirect immunofluorescence localization with the use of anti-E-cadherin and anti-N-cadherin antibodies (Transduction Laboratories, Lexington, KY) was carried out on cells fixed in a 1:1 mixture of acetone/methanol for 10 min at −20°C and permeabilized for 3 min with 0.1–1% Triton X-100 in PBS. Primary antibodies were localized by using FITC- (Sigma) or Alexa Fluor 488 (Molecular Probes)-conjugated anti-mouse IgG antibodies at dilutions of 1:100 or 1:200. For double staining of actin and cadherins, cells were immunolabeled with rabbit anti-actin F7 antibodies and the appropriate mouse anti-cadherin antibodies followed by incubation with goat anti-rabbit Texas Red and anti-mouse Alexa Fluor 488 secondary antibodies at 1:200 dilutions. Cells were fixed and labeled with anti-β-catenin antibodies as described (6). Microtubule staining was performed by using anti-tubulin antibody clone DM1α IgG1 (Sigma) after methanol fixation for 10 min at −20°C. Fluorescence images were collected with the use of a Bio-Rad MRC 1024 laser scanning confocal microscope equipped with Nikon optics.
Results
Morphology of Cultured Epitheliocytes and Fibroblasts.
Live epitheliocytes and fibroblasts in mixed cultures were easily distinguished from one another by their characteristic shapes when viewed by differential interference contrast microscopy and by their distinct distribution of actin bundles when examined by confocal microscopy. Epitheliocytes had rounded contours, and they often formed small, coherent islands and sheets, even in sparse cultures (Fig. 1). Fibroblasts were typically polygonal, with active lamellas at one or two poles. Fibroblasts in sparse cultures tended to remain isolated from one another or exhibited only small areas of contact.
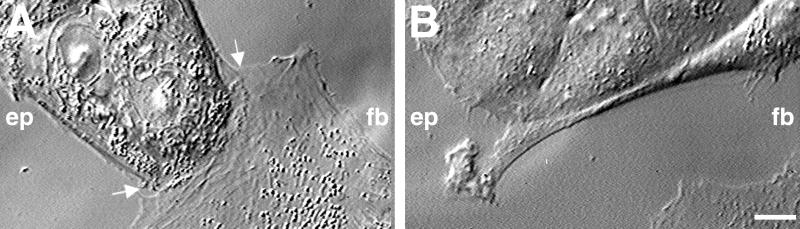
Figure 1.
Heterocellular collisions between IAR-2 epithelial cells and RAT-1 fibroblasts. (A) During the early stage of collision, the lamella of the fibroblast (fb) burrows under the free edge (see arrows) of an epithelial cell within a small island. In this example, the epithelial cell forms a wide lamellar extension at the site of contact. (B) At later stages of collision, fibroblasts tended to turn laterally and migrate along the edge of the epithelial island. Often short lamellar extensions reached out and would overlap the side of the fibroblast (also see Fig. 2 C and D). (Bar = 10 μm.)
Epithelial cells within small islands possessed characteristic marginal bundles of actin microfilaments (Fig. 2A, see arrowhead). Furthermore, epitheliocytes contained short, straight bundles of actin filaments running along the basal surface of the cells. As previously described (9), staining IAR-2 epithelial cells with anti-E-cadherin antibodies identified prominent linear contacts that ran tangentially along the edge of contact between adjacent cells (17).
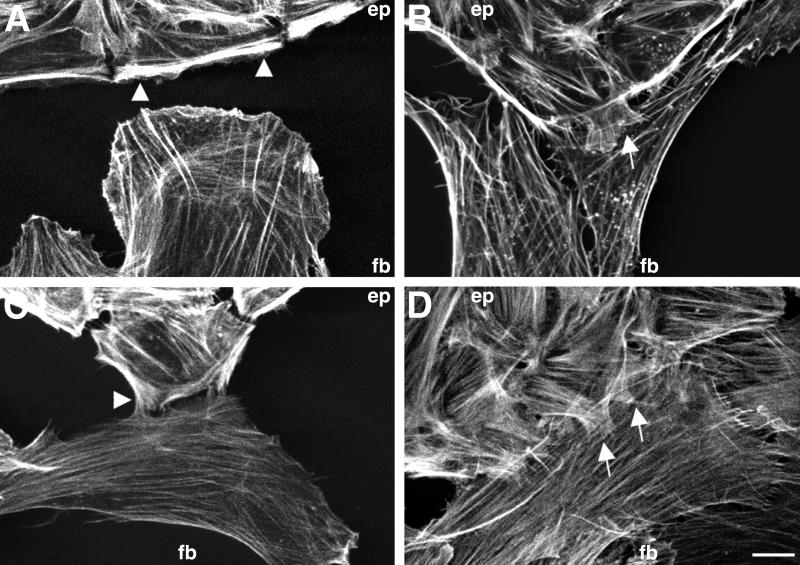
Figure 2.
Localization of actin filaments at different stages of heterocellular collisions between IAR-2 epitheliocytes and RAT-1 fibroblasts. (A) Before contact, the epithelial cells (ep) within an island exhibit typical marginal bundles (arrowheads) at their free edge, whereas fibroblasts have numerous bundles of filaments extending radially into a broad lamella. (B) Early stage of heterocellular collision, where the fibroblast has migrated beneath the edge of an epithelial island. Notice the localized disassembly of the marginal bundle in the area of collision and coincident formation of radially oriented small bundles of actin filaments within a small lamellipodial extension at the edge of the epithelial cell (see arrow). (C) At later stages of collision fibroblasts retracted the underlapped lamella and turned away from the epithelial edge. The marginal bundles within epithelial cells remain disassembled, and small, actin-rich lamellipodia (arrow) continue to retain contact with the surface of the turned fibroblast. (D) When grown in dense cultures numerous fibroblasts align themselves so that their side edge runs along the edge of epithelial islands. Epithelial cells form numerous lamellipodial extensions out onto the surface of the fibroblast (arrows), and the characteristic marginal bundles are completely disassembled. (Bar = 10 μm.)
The leading edge of fibroblasts never formed marginal bundles; instead there was a meshwork of actin filaments along with bundles of filaments that protruded into the edge of the lamella (Fig. 2A). RAT-1 fibroblasts also contained numerous straight bundles of actin filaments running parallel to the body axis. RAT-1 fibroblasts did not exhibit any staining with anti-E-cadherin antibodies; however, they did form contacts containing β-catenin that were radially oriented and associated with the ends of actin filament bundles located at the periphery of the cell (6).
Analysis of Heterocellular Collisions.
Because fibroblasts were quite motile, contacts between RAT-1 fibroblasts and IAR-2 epithelial cells typically occurred when the fibroblast moved forward toward the edge of an epithelial island. After initial contact with the epithelial edge, the lamella of the fibroblast continued moving forward and beneath the edge of the epithelial lamella (Fig. 1A, see arrow). Simultaneous with the “underlapping” of the fibroblast lamella, the edge of the epithelial cell in contact with the fibroblast became more active and appeared to extend small pseudopodial projections. Over the course of 5–20 min the fibroblast would continue moving forward, producing a 5- to 10-μm-wide zone of overlapping lamellas followed by a variable period (10–40 min) with no forward movement. After the period of immobility, the fibroblast started to extend a new active lamella from a location adjacent to the site of overlap. The new lamella would gradually widen and the fibroblast would change its orientation and begin migrating along the border of the epithelial cell island (Fig. 1B). Data were principally presented for IAR-2/RAT-1 interactions; however, collisions between MDCK epitheliocytes and M19 fibroblasts exhibited morphological changes that were similar, if not identical, to those reported for heterocellular contact between IAR-2 and RAT-1 cells (data not shown).
Reorganization of Cytoskeleton.
Cultures undergoing heterotypic cell–cell contacts were fixed and prepared for immunofluorescence microscopy to examine the organization of the actin and microtubule cytoskeletons. Formation of an underlapping lamella by fibroblasts induced a spatially and temporally correlated change in the organization of the actin cytoskeleton in epithelial cells. The two most striking changes were the disassembly of the marginal bundle at the sites of contact and the formation of actin filament-rich small lamellar extensions (Fig. 2B). Interestingly, the bundles of actin filaments in the small lamellar extension were oriented perpendicular to the edge of the epithelial lamella, an orientation that is typically seen at the edge of fibroblasts (Fig. 2 C and D). The actin-rich small lamella continued to persist at the edge of the epithelial cell, even after the fibroblast turned and started to crawl along the border of the island (Fig. 2 B–D). Epitheliocytes contained dense networks of microtubules that often ran approximately parallel to the edge of the cell. After collision, the microtubules became reoriented, with numerous microtubules penetrating into lamellar extensions (data not shown). There were no apparent changes in the overall organization of either the actin or the microtubule cytoskeleton in fibroblasts during heterotypic contact. The lack of structural reorganization of the cytoskeleton in fibroblasts during heterotypic contact is similar to our prior observation of homotypic contact interactions between fibroblasts (6).
Formation of Heterotypic Adhesions.
To determine whether epithelial cells and fibroblasts (IAR-2/RAT-1 and MDCK/M19 pairs) formed cell–cell junctional complexes, cultures undergoing collisions were fixed and probed with antibodies to E-cadherin, N-cadherin, and β-catenin. Laser scanning confocal microscopy, after staining with anti-E-cadherin antibodies, identified the presence of brightly stained tangential contacts along the edges of homotypic contacts between epithelial cells but not between adjacent fibroblasts (Fig. 3). Conversely, probing with anti-N-cadherin antibodies resulted in staining of the homotypic contacts between adjacent fibroblasts but not between epitheliocytes (Fig. 4). Both anti-E-cadherin and anti-N-cadherin antibodies identified the presence of these adhesion-specific molecules at 60–70% of the heterotypic contact sites. Generally the intensity of staining was greatest at the sites of homotypic contacts rather than heterotypic contacts (compare Fig. 3 B and C). Heterotypic contacts were also observed when colliding cells were stained with anti-β-catenin antibodies (Fig. 5).
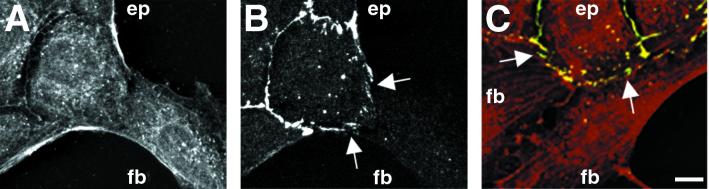
Figure 3.
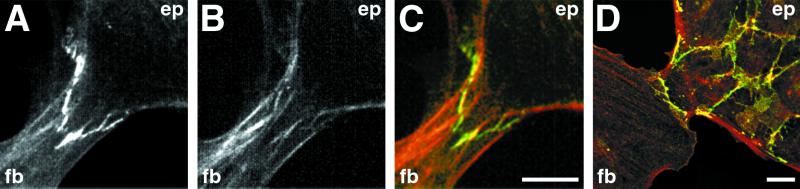
Localization of E-cadherin in heterotypic contacts. (A and B) Cells were fixed and double-stained with anti-actin (red, A) and anti-E-cadherin (green, B) antibodies. After a head-on collision between a single fibroblast (fb) and an island of epithelial cells (ep), the marginal bundle of the epithelial cell is disassembled at the site of contact, and there is no distinguishing difference between the staining patterns of the two cells at the site of contact. Staining with E-cadherin revealed the presence of fragmented clusters of E-cadherin along the zone of overlap between the epitheliocyte and the fibroblast. Note the well-defined continuous staining for E-cadherin along the edges of neighboring epithelial cells. (C) Cells were double labeled with anti-actin (red) and anti-E-cadherin (green) antibodies. Punctate yellow spots along the epithelial cell–fibroblast border (arrows) indicate the colocalization of E-cadherin and actin. Note the absence of E-cadherin (green) staining along the edge between two IAR-2 fibroblasts. (Bar = 10 μm.)
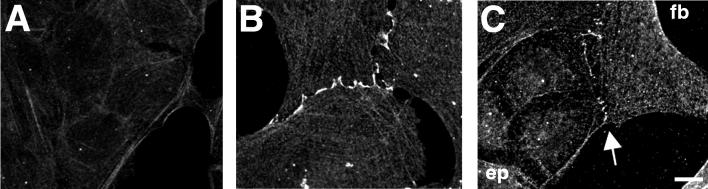
Figure 4.
Distribution of N-cadherin in IAR-2 epitheliocytes and RAT-1 fibroblasts. Cells were fixed and immunolabeled with anti-N-cadherin antibodies. (A) Anti-N-cadherin antibodies did not stain homotypic cell–cell contacts between adjacent cells in epithelial islands. (B) Homotypic contacts between fibroblasts exhibited N-cadherin staining along the edge between adjacent cells. (C) Anti-N-cadherin staining of heterotypic contacts between IAR-2 (ep) and RAT-1 (fb) cells identified the presence of discontinuous clusters of N-cadherin along the boundary between cells. (Bar = 10 μm.)
Figure 5.
Immunofluorescence labeling for β-catenin in heterotypic contact between fibroblasts and epitheliocytes. (A–C) MDCK (ep) and M19 (fb) cells were double-stained with anti-β-catenin (green) and anti-actin (red) antibodies while undergoing heterocellular contact. In this example, the MDCK cell formed a triangular extension over the M19 fibroblast. Note the presence of strong positive staining for β-catenin (A) and, in some locations, coincident positioning of actin filament bundles (B). (C) The merged image of A and B, with yellow designating areas of colocalization of β-catenin and actin. (D) Heterocellular contact between IAR-2 epithelial cells and RAT-1 fibroblasts stained with anti-β-catenin antibodies. (Bar = 10 μm.)
The shape and organization of heterotypic adhesive structures were extremely variable. Although small dots about 2 μm in diameter were predominant, it was possible to identify contact structures that were short straight “dashes” about 5–6 μm long. Occasionally, the dot and dash structures appeared to fuse into nearly straight lines or arcs that had different orientations (parallel, oblique, tangential) to each other and to the edge of the cell (Figs. 3–5). For example, a series of dashes would be oriented parallel to each other and perpendicular to the edge of the contacting cells (Fig. 4C, see arrow). Furthermore, linear adhesive structures were often, but not always, localized with actin filament bundles that were present in the zones of overlapping contact (Fig. 5 A–C).
Discussion
Dynamic Response of Fibroblasts to Heterocellular Collisions.
The sequence of changes in fibroblasts in response to contact with epithelial cells is similar to the sequence observed during the course of homotypic fibroblast–fibroblast collisions (6). In both cases there is an initial overlapping of lamellar edges, followed by a subsequent inhibition of forward lamellar movement, and finally the formation of a new expanding lamella that will pull the cell away from the site of contact. Attachment of a cell to the surface of a fibroblast appears to induce an adhesion-dependent signaling event that leads to contact inhibition of movement. This inhibition of movement must access a signaling cascade that locally inhibits actin filament polymerization, leading to the inhibition of extension of the burrowing lamellipodia. It is tempting to speculate that local contact inhibition is initiated by the same cadherin–cadherin interactions that are involved in the formation of cell–cell adhesions. For instance, this inhibition may be due to local accumulation and inactivation of p120 protein, which is associated with the cytoplasmic domain of cadherin molecules at the site of adhesions (18). When p120 protein detaches from the cytoplasmic domain of cadherin, it participates in the induction of lamellipodial extensions (19, 20). Another possible mechanism may involve signal processing induced by molecules not associated with cadherin adhesions, e.g., ephrin–ephrin receptor interactions (21, 22).
Inhibition of lamellipodial extensions in the zone of collision was associated with the activation of lamellar extensions at nearby cellular edges not involved in making cell–cell contact. This repositioning of an actively protruding lamella is responsible for the eventual change of orientation and direction of fibroblast motility. Similar reorientation of fibroblasts occurs in many different situations, for example, when cells turn away from nonadhesive areas of the substrate (see review in ref. 23) or change their direction of movement on metallic grids (24). Mechanisms at play in these types of changes in orientation of motility remain unclear.
Dynamic Reorganization of Epitheliocyte Cytoskeleton Induced by Collision.
The structural reorganization of the actin cytoskeleton observed upon heterocellular epitheliocyte–fibroblast contact is distinct from the reorganization observed in homocellular collisions between epithelial cells. When epithelial cells collide, marginal bundles at the site of contact are rapidly disassembled at multiple sites along the bundle, followed by the assembly of a meshwork of filaments and no observable forward protrusion of lamellipodia (9, 17). Upon contact with fibroblasts, the epithelial cell develops a local discontinuity within the marginal bundle, followed by assembly of thin, radially aligned bundles that project into a newly created lamellipodial projection. The lamellipodial projection was found along the upper surface of the fibroblast lamella that has burrowed beneath the epithelial cell. In addition, the organization of the microtubule cytoskeleton also changed in response to contact with the fibroblast (not shown).
Recent studies have revealed various interactions between epitheliocytes and fibroblasts (25, 26). One of the best examples of these interactions is the action of so-called scatter factor [hepatocyte growth factor (HGF)/SF], which is secreted by fibroblasts and binds to the c-met receptor of epitheliocytes. In response to HGF/SF binding, epithelial cells reorganize into motile fibroblast-like cells (27–30). HGF/SF transformation has obvious similarities with the observed reorganization of epitheliocytes upon contact with a fibroblast—in both cases the actin cytoskeleton of the epithelial cell takes on a fibroblast-like organization. Although similarity exists between the two responses, there is one significant difference, whereas HGF/SF induces a global change that affects the response of the entire cell, cell–cell collision only induces a targeted change at the site of contact. How this differential response is modulated remains a matter of speculation. Possibly during collision some receptor population on the epithelial surface is locally activated by ligands on the surface of the fibroblast. In any case, these unknown signals apparently induce two different reactions requiring localized disassembly of the marginal bundle of actin filaments and activation of actin polymerization to extend small lamellipodia.
Formation of local extension by epithelial cell has obvious functional significance in that it may serve as a “reception reaction” that locally reorganizes the actin cytoskeleton within the epithelial lamella into a form similar to that of the fibroblast. Consequently the lamella of the epithelial cell is permitted to establish heterotypic contacts that are spatially similar to homocellular fibroblast–fibroblast contact.
Formation of Heterotypic Adhesions.
Our experiments demonstrate that colliding lamellas of epitheliocytes and fibroblasts can form adhesive structures that most probably rely on the utilization of heterotypic cadherin cell adhesion molecules. According to the immunomorphological data presented, RAT-1 and M19 fibroblasts expressed N-cadherin and not E-cadherin, whereas IAR-2 and MDCK cells expressed E- but not N-cadherin. Preliminary immunoblotting data verified that epithelial cells expressed E- but not N-cadherin, whereas fibroblasts expressed N-cadherin and RAT-1 cells expressed a greatly reduced level of E-cadherin relative to epithelial cells (data not shown). The cytoplasmic adhesion plaque protein β-catenin was also localized to discrete adhesion structures, supporting the conclusion that heterotypic contacts do indeed form between epithelial and fibroblast cells. We cannot rule out the possibility that during contact between IAR-2 and RAT-1 cells, some small percentage of homotypic E-cadherin adhesion complexes are formed. Moreover, participation of some other epithelial and/or fibroblast cadherins in the observed adhesion complexes (31–33) cannot be excluded. The ability of cells with different cadherins to form “united” heterotypic contacts indicates that specificity of individual cadherins is not absolute and that these molecules are able to form trans-linkages when heterocellular membranes collide (33).
Heterotypic adhesions between epithelial and fibroblast cells were generally similar to homotypic fibroblast–fibroblast adhesions but with greater variation in shape, orientation, and association with the actin cytoskeleton. Several factors may be responsible for this variability. First, heterotypic adhesions may be formed more slowly and their shapes may represent different stages of maturation or disassembly. For example, short rows of dots often seen in these adhesions may represent the formation of adhesion molecule clusters or “puncta” associated with the ends of newly assembled actin filament bundles in the area of contact (5, 8). Second, the morphology of heteroypic adhesion is likely to change over time because the underlying lamella is eventually retracted as the fibroblast changes direction of migration and moves away from the point of contact.
Heterocellular Contacts May Be Essential for Tissue Interactions in Vivo.
Formation of “head-on” heterotypic contacts is a transient process since the advancing fibroblast will withdraw its active edge, form and reorient a new lamella, and then either become laterally associated with the edge of the epithelial island or migrate away. However, in dense culture, fibroblasts may partially or completely border on an epithelium for an indefinite time.
The physiological significance of prolonged heterocellular contacts may be to provide cues for directional migrations of cells of one type with regard to those of another type as well as to stabilize cell positions at the borders between the territories of two cell types. In other words, heterocellular interactions may be fundamentally important for the sorting of cells of different types into separate territories.
The dynamics of fibroblast–epitheliocyte collisions in cell cultures may be regarded as a simplified prototype of short-range interactions of the cells of these two main tissue types in vivo. These interactions may lead to ordered distribution of epithelial and mesenchymal cells in many tissues such as skin, gut, different glands, etc. This initial ordered distribution may be later stabilized and modified by extracellular matrix structures elaborated by the cells, e.g., by the basal laminas.
Acknowledgments
This work was supported by a Russian Foundation of Basic Investigations grant (to J.M.V., E.F., and O.I.), a gracious gift from Mrs. Harold Kaplan to the Research Exchange Program at Rutgers–Newark, and a Johnson & Johnson Discovery Award (to E.M.B.).
Abbreviations
- MDCK cells
Madin–Darby canine kidney cells
- SF
scatter factor
References
- 1.Yap A S, Brieher W M, Gumbiner B M. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 2.Troyanovsky S M. Curr Opin Cell Biol. 1999;11:561–566. doi: 10.1016/s0955-0674(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 3.Adams C L, Nelson W J, Smith S J. J Cell Biol. 1996;135:1899–1911. doi: 10.1083/jcb.135.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gumbiner B M. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 5.Adams C L, Nelson W J. Curr Opin Cell Biol. 1998;10:572–577. doi: 10.1016/s0955-0674(98)80031-8. [DOI] [PubMed] [Google Scholar]
- 6.Gloushankova N A, Krendel M F, Alieva N O, Bonder E M, Feder H H, Vasiliev J M, Gelfand I M. Proc Natl Acad Sci USA. 1998;95:4362–4367. doi: 10.1073/pnas.95.8.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krendel M, Gloushankova N A, Bonder E M, Feder H H, Vasiliev J M, Gelfand I M. Proc Natl Acad Sci USA. 1999;96:9666–9670. doi: 10.1073/pnas.96.17.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasioukhin V, Bauer C, Yin M, Fuchs E. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 9.Gloushankova N A, Alieva N A, Krendel M F, Bonder E M, Feder H H, Vasiliev J M, Gelfand I M. Proc Natl Acad Sci USA. 1997;94:879–883. doi: 10.1073/pnas.94.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasioukhin V, Fuchs E. Curr Opin Cell Biol. 2001;13:76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 11.Hall A. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 12.Voura E B, Sandig M, Siu C H. Microsc Res Tech. 1998;43:265–275. doi: 10.1002/(SICI)1097-0029(19981101)43:3<265::AID-JEMT9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Montesano R, Saint Vincent L, Drevon C, Tomatis L. Int J Cancer. 1975;16:550–558. doi: 10.1002/ijc.2910160405. [DOI] [PubMed] [Google Scholar]
- 14.Cereijido M, Robbins E S, Dolan W J, Rotunno C A, Sabatini D D. J Cell Biol. 1978;77:853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg B, Pollack R, Topp W, Botchan M. Cell. 1978;13:19–32. doi: 10.1016/0092-8674(78)90134-4. [DOI] [PubMed] [Google Scholar]
- 16.Mironov L L, Stobetskii V I, Kriuchkova G P, Kudinova S I, Karmysheva V I. Vopr Virusol. 1987;32:626–629. [PubMed] [Google Scholar]
- 17.Krendel M F, Bonder E M. Cell Motil Cytoskeleton. 1999;43:296–309. doi: 10.1002/(SICI)1097-0169(1999)43:4<296::AID-CM3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 18.Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Miyazawa K, Kitamura N, Johnson K R, Wheelock M J, Matsuyoshi N, Takeichi M, et al. J Cell Biol. 1995;128:949–957. doi: 10.1083/jcb.128.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noren N K, Liu B P, Burridge K, Kreft B. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosheva I, Shtutman M, Elbaum M, Bershadsky A D. J Cell Sci. 2001;114:695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- 21.Bruckner K, Klein R. Curr Opin Neurobiol. 1998;8:375–382. doi: 10.1016/s0959-4388(98)80064-0. [DOI] [PubMed] [Google Scholar]
- 22.Dodelet V C, Pasquale E B. Oncogene. 2000;19:5614–5619. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- 23.Vasiliev J M, Gelfand I M. Int Rev Cytol. 1977;50:159–274. doi: 10.1016/s0074-7696(08)60099-6. [DOI] [PubMed] [Google Scholar]
- 24.Rovensky Y A, Domnina L V, Ivanova O Y, Vasiliev J M. J Cell Sci. 1999;112:1273–1282. doi: 10.1242/jcs.112.8.1273. [DOI] [PubMed] [Google Scholar]
- 25.Adamson I Y, Young L, King G M. Exp Lung Res. 1991;17:821–835. doi: 10.3109/01902149109062880. [DOI] [PubMed] [Google Scholar]
- 26.Li D Q, Tseng S C. J Cell Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- 27.Birchmeier C, Gherardi E. Trends Cell Biol. 1998;8:404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- 28.Thiery J P, Chopin D. Cancer Metastasis Rev. 1999;18:31–42. doi: 10.1023/a:1006256219004. [DOI] [PubMed] [Google Scholar]
- 29.Stella M C, Comoglio P M. Int J Biochem Cell Biol. 1999;31:1357–1362. doi: 10.1016/s1357-2725(99)00089-8. [DOI] [PubMed] [Google Scholar]
- 30.Stuart K A, Riordan S M, Lidder S, Crostella L, Williams R, Skouteris G G. Int J Exp Pathol. 2000;81:17–30. doi: 10.1046/j.1365-2613.2000.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho E A, Patterson L T, Brookhiser W T, Mah S, Kintner C, Dressler G R. Development (Cambridge, UK) 1998;125:803–812. doi: 10.1242/dev.125.5.803. [DOI] [PubMed] [Google Scholar]
- 32.Mah S P, Saueressig H, Goulding M, Kintner C, Dressler G R. Dev Biol. 2000;223:38–53. doi: 10.1006/dbio.2000.9738. [DOI] [PubMed] [Google Scholar]
- 33.Steinberg M S, McNutt P M. Curr Opin Cell Biol. 1999;11:554–560. doi: 10.1016/s0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]