Abstract
Objective
Preclinical data suggest an important role for the sarcoma proto-oncogene tyrosine kinase (SRC) in the oncogenesis of epithelial ovarian cancer (EOC) or primary peritoneal carcinoma (PPC). The Gynecologic Oncology Group (GOG) conducted a Phase II trial to evaluate the efficacy and safety of dasatinib, an oral SRC-family inhibitor in EOC/PPC and explored biomarkers for possible association with clinical outcome.
Methods
Eligible women had measurable, recurrent or persistent EOC/PPC and had received one or two prior regimens which must have contained a platinum and a taxane. Patients were treated with 100 mg orally daily of dasatinib continuously until progression of disease or adverse effects prevented further treatment. Primary endpoints were progression-free survival (PFS) ≥6 months and response rate. Serial plasma samples were assayed for multiple biomarkers. Circulating free DNA was quantified as were circulating tumor and endothelial cells.
Results
Thirty-five (35) patients were enrolled in a two-stage sequential design. Of the 34 eligible and evaluable patients, 20.6% (90% confidence interval: 10.1%, 35.2%) had a PFS ≥6 months; there were no objective responses. Grade 3–4 toxicities were gastrointestinal (mostly nausea and emesis; n=4), pulmonary (dyspnea and/or pleural effusion; n=4) and pain (n=5), and infrequent instances of anemia, malaise, insomnia, rash, and central nervous system hemorrhage. Lack of clinical activity limited any correlation of biomarkers with outcome.
Conclusion
Dasatinib has minimal activity as a single-agent in patients with recurrent EOC/PPC.
Keywords: dasatinib, ovarian, cancer, SRC, inhibition
INTRODUCTION
Despite initially high remission rates, at least 75% of women diagnosed with advanced stage epithelial ovarian carcinoma (EOC) will relapse and ultimately die of their disease [1]. Treatment of these patients once they develop recurrent disease remains a major problem. The need for new therapeutic strategies is evident. The SRC family of kinases (SFK) is a nine member group of membrane associated non-receptor tyrosine kinases that are involved in a variety of cellular signaling pathways [2]. The SFK is involved in the oncogenesis of numerous tumors including ovarian cancer. SRC regulates many intracellular signaling pathways responsible for various important tumor cell functions such as proliferation, motility and invasion, angiogenesis, and survival. SRC has been found to be overexpressed in a majority of late stage ovarian tumors and cell lines [3].
SRC is a component of signaling pathways downstream of many growth factor receptors, including epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR), and MNNG transforming gene product (c-MET) [4]. Increased resistance to traditional chemotherapy is modulated by SRC through increased activity of RAS and AKT [5]. Inhibition of SRC enhanced the activity of cytotoxic agents, including cisplatin, gemcitabine, and paclitaxel through the activation of caspase-3 in pre-clinical models [5–7]. In addition, tumor growth was blunted when human ovarian cancer cells carrying an antisense SRC construct were implanted into mice bearing these xenografts [8].
VEGF is an important growth factor for ovarian cancer cells [9]. VEGF was significantly down-regulated by SRC inhibition and microvessel density was reduced [10]. Anti-VEGF therapy has demonstrated activity in patients with recurrent and primary disease [11,12]. VEGF stimulation of its receptor increased tyrosine phosphorylation of focal adhesion kinase, p130 CAS and paxillin [13]. Increased activity of these mediators leads to an increased epithelial-to-mesenchymal transition (EMT), a crucial step to enhancing the metastatic potential of these cancer cells [14]. SRC is a key intermediate in the EMT process [15,16]. In addition, caveolin-1 expression indirectly promotes cell-cell adhesion in ovarian cancer cells [17,18]. SRC interferes with caveolin function also promoting EMT and encouraging tumor spread.
Dasatinib is a potent oral inhibitor of breakpoint cluster region-Abelson fusion protein (BCR-ABL), c-KIT, ephrin type-A receptor 2 (EPHA2), c-FMS, and SFK [19,20]. These kinases are implicated in oncogenic process and maintaining the metastatic phenotype of many cancers. Dasatinib’s mechanism of action depends on it successfully competing for the ATP binding site contained in the kinase domain. The agent is widely approved for chronic myelogenous leukemia and Ph+ acute lymphoblastic leukemia and is now under investigation for treating various solid tumors [21].
Based on these observations, the evaluation of dasatinib in patients with recurrent EOC was undertaken by the Gynecologic Oncology Group (GOG). Translational research (TR) objectives were included to explore the association between biomarkers and patient outcome. Biomarkers included cell-free DNA (cfDNA), circulating tumor cells (CTCs), circulating endothelial cells (CECs), circulating endothelial precursors (CEPs), and seven plasma biomarkers relevant to dasatinib treatment (EGF and its soluble receptor [sEGFR], VEGF and its soluble receptors [sVEGFR1, sVEGFR2, sVEGFR3], and insulin like growth factor binding protein 2 [IGFBP2]).
PATIENTS AND METHODS
Eligibility
Eligible patients had a histologically-confirmed diagnosis of EOC or primary peritoneal carcinoma, measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST) [22], a GOG performance status of 0–2, and adequate bone marrow (absolute neutrophil count ≥1,500/μL, platelet count ≥100,000/μL), renal (serum creatinine ≤1.5 x the upper limit of normal), and hepatic function (total bilirubin ≤1.5 x the upper limit of normal, and transaminases and alkaline phosphatase ≤2.5 x the upper limit of normal). No biomarker based method was used for patient selection. Eligible patients were permitted to have up to two prior cytotoxic regimens. If a patient had only one prior regimen, she was required to have a platinum-free interval of less than 12 months or to have progressed during or have persistent disease after platinum-based therapy. Prior biological agents were permitted other than those known to inhibit SRC. Patients with prior radiation to more than 25% of marrow bearing areas, therapeutic warfarin treatment, or signs and/or symptoms of bowel obstruction were excluded. Patients provided written informed consent consistent with federal, state, and local institutional review board at each participating GOG institution in accordance with assurances filed with and approved by the Department of Health and Human Services.
Treatment Plan/Dose Modifications
Dasatinib (Bristol-Myers Squibb, New York, NY) was administered orally at an initial dose of 100 mg once daily until disease progression or adverse effects required interruption, reduction or discontinuation of therapy. Dose level −1 was 70 mg daily and dose level +1 was 70 mg twice daily. Although dosing was continuous, a cycle was defined as 28 days. Dasatinib was supplied by Bristol-Myers Squibb, Inc.
Toxicity was graded using the National Cancer Institute Common Toxicity Criteria version 3.0 (NCI-CTCAEv3.0). For first occurrence of febrile neutropenia and/or documented grade 4 neutropenia, dasatinib was held until the absolute neutrophil count (ANC) was grade ≤2 then reduced by one dose level. Patients with grade 4 thrombocytopenia had their drug held until grade ≤1 and then were reduced by one dose level. The next cycle of dasatinib did not begin until ANC was ≥1,500/μl and platelet count was ≥100,000/μl. Therapy was allowed to be delayed up to a maximum of two weeks. Patients who failed to recover adequate counts within this time were removed from study. Prophylactic use of myeloid growth factors was prohibited. Patients who experienced grade ≥2 non-hematologic toxicity had therapy held until resolution to grade 0–1 up to a maximum of 14 days. Dasatinib was then restarted at 70 mg daily. If toxicity recurred to grade 2 or worse, the patient would discontinue study drug. Exceptions to the above modifications included: liver function tests were required to be grade 3 or worse toxicity before dose modification was required. There was no dose adjustment for fatigue or alopecia. Doses were reduced for gastrointestinal toxicities only if they could not be controlled with medical management. Fluid retention (pleural effusion and/or ascites) was managed with early initiation of diuretic treatment (furosemide and/or spironolactone). Cavity drainage was performed as clinically indicated. Once a patient’s dose was reduced, no subsequent increases were permitted.
Patients with no grade ≥1 toxicities after cycle 1 were escalated one dose level (70 mg twice daily) beginning with cycle 2 of treatment. This dose escalation was included based on early data that solid tumors may require higher doses of dasatinib to achieve clinical activity compared with doses used to treat patients with CML [23,24].
Response Assessment
Patients were evaluated clinically every four weeks and radiographically every eight weeks. The same evaluation modality was used throughout for each patient on study. Response criteria used were as defined by RECIST [22].
Translational Research
Plasma and whole blood specimens were collected for TR prior to cycles 1, 2 and 3. Detailed methodology and references for isolating and phenotyping CTC and CEC/CEP can be found in the supplemental material [online only]. Methods for determining circulating levels of serum biomarkers and extraction and quantification of total plasma cell-free DNA (cfDNA) are also summarized in the supplemental online material.
Statistical Methods
We anticipated that the effect of dasatinib might be either cytotoxic or cytostatic. Therefore, the primary endpoint of this study included both objective tumor response and the proportion of patients alive and progression-free after six months PFS-6). Time on study was assessed from date of registration and included all eligible treated patients.
The null hypothesis, i.e. an “uninteresting” level of efficacy, was determined from analysis of an historical GOG dataset, based on a similar patient population from clinical trials of study drugs now considered inactive or minimally active. The null hypothesis jointly specified the probability of a patient experiencing a tumor response to be ≤10% and the probability of a patient being alive and free from PFS-6 to be ≤15%. For the purpose of study design, a 20% increase (to 25% for tumor response or to 35% for PFS-6) were considered clinically significant. The two-stage, bivariate, flexible method of Sill and Yothers [25] was used with a goal of limiting patient exposure to inactive agents while restricting the probabilities of type I and type II errors to about 10%. If the regimen were to demonstrate sufficient activity in the first stage (with 35 patients, this required ≥5 objective tumor responses or ≥8 patients with PFS-6), then the study would target a total of 53 patients (cumulatively) in stage 2. Signed-rank tests were used to test changes from baseline in biomarkers, and Wilcoxon rank sum tests were used to compare changes or ratios for patients who had PFS for at least 6 months versus those who did not. Proportional hazards models were used to compare PFS by high (≥median) versus low (<median) baseline (pre-cycle 1) levels of each parameter; both unadjusted models and models adjusted for age and performance status were examined.
RESULTS
Patients and Eligibility
Thirty-five patients were enrolled. One patient was deemed ineligible because inadequate data were available. Patient characteristics are listed in Table 1. A majority of patients (58.8%) received two prior regimens. All patients had performance status of 0 or 1.
Table 1.
Demographics (n=34)
| Characteristics | Category | No. | % |
|---|---|---|---|
| Age (years) | 40–49 | 4 | 11.8 |
| 50–59 | 9 | 26.5 | |
| 60–69 | 13 | 38.2 | |
| 70–79 | 6 | 17.6 | |
| 80–89 | 2 | 5.9 | |
| Race | Asian | 1 | 2.9 |
| African American | 1 | 2.9 | |
| American Indian | 1 | 2.9 | |
| White | 31 | 91.2 | |
| Performance Status | 0 | 27 | 79.4 |
| 1 | 7 | 20.6 | |
| Site of Disease | Ovary | 29 | 85.3 |
| Primary peritoneal | 5 | 14.7 | |
| Cell Type | Adenocarcinoma, Unsp. | 4 | 11.8 |
| Large Cell Carcinoma | 1 | 2.9 | |
| Clear Cell Carcinoma | 2 | 5.9 | |
| Mucinous Adenocarcinoma | 1 | 2.9 | |
| Mixed Epithelial Carcinoma | 1 | 2.9 | |
| Squamous Cell Carcinoma | 1 | 2.9 | |
| Serous Adenocarcinoma | 24 | 70.6 | |
| Grade | 1: Well differentiated | 3 | 8.8 |
| 2: Moderately differentiated | 4 | 11.8 | |
| 3: Poorly differentiated | 27 | 79.4 | |
| Prior Chemotherapy | 1 | 14 | 41.2 |
| Regimens | 2 | 20 | 58.8 |
Treatment and Response
Patients received a median of two cycles (range, 1–12) of protocol therapy. Of 23 patients who received two or more cycles, 15 were escalated to 140 mg of dasatinib daily (70 mg bid) and two were reduced to 70 mg daily.
There were no responders. Also, only seven patients (20.6%; 90% confidence interval: 10.1%, 35.2%) were PFS-6 (Table 2). Therefore, the protocol criteria for continuing to second stage of accrual were not met. Of the seven patients with PFS ≥6 months, five had two prior treatment regimens while two had received one prior regimen, and all had platinum-resistant disease (<6 months platinum-free interval). Median PFS was 2.1 months (first and third quartiles: 1.8 and 4.9 months). Median overall survival (OS) was 17.7 months (first quartile: 10.8 months, third quartile has not been reached; Figure 1).
Table 2.
Responses
| Characteristic | Category | No. | % |
|---|---|---|---|
| Response | In creasing disease | 18 | 52.9 |
| Stable disease | 11 | 32.4 | |
| Indeterminatea | 5 | 14.7 | |
| PFS > 6 Months | No | 27 | 79.4 |
| Yes | 7 | 20.6 | |
| Cycles of Treatment | 1 | 11 | 32.4 |
| 2 | 12 | 35.3 | |
| 3 | 5 | 14.7 | |
| 4 | 1 | 2.9 | |
| 5 | 2 | 5.9 | |
| 6 | 2 | 5.9 | |
| 12 | 1 | 2.9 |
1 patient did not have any post-baseline scans
1 patient did not have a post-baseline scan until 13 weeks after treatment started
3 patients received less than one cycle and had scans 1, 4 and 7 weeks post-treatment – insufficient to declare stable disease
Figure 1.
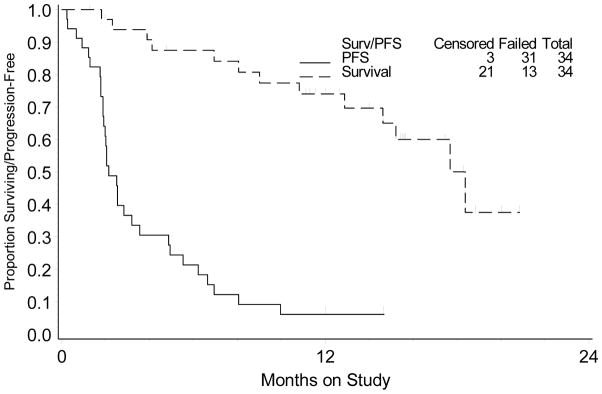
Kaplan-Meier plots for overall and progression-free survival.
Toxicity
The most commonly observed grade 3 toxicities were gastrointestinal (mostly nausea and emesis), pulmonary (dyspnea and/or pleural effusion), and pain (Table 3). There were single cases of grade 3 or 4 anemia, malaise, insomnia, rash, and central nervous system hemorrhage.
Table 3.
Toxicities (n=34)
| AE Category | Grade (max across cycles) | ||
|---|---|---|---|
| 3 | 4 | ||
| Anemia | 1 | 0 | |
| Constitutional | 1 | 0 | |
| Insomnia | 1 | 0 | |
| Malaise | 1 | 0 | |
| Dermatologic (rash) | 1 | 0 | |
|
| |||
| Gastrointestinal | 7 | 0 | |
| Nausea/emesis | 4 | 0 | |
| Diarrhea | 1 | 0 | |
| Ascites | 1 | 0 | |
| Obstruction | 2 | 0 | |
|
| |||
| CNS Hemorrhage | 0 | 1 | |
| Pain | 5 | 0 | |
| Pulmonary | 4 | 0 | |
| Dyspnea | 3 | 0 | |
| Pleural effusion | 1 | 0 | |
Translational research
Dasatinib related biomarkers
Bead-based immunoassays were used to measure circulating levels of seven dasatinib related biomarkers in plasma collected pre-cycles 1 (n=27), 2 (n=23), and 3 (n=15). Fifteen patients submitted samples at all three time points. Median biomarker levels (ng/mL) are presented (Supplemental Table S1). There was a significant increase in the levels of sVEGFR2, sVGEFR3, and IGFBP2 between baseline and pre-cycle three in these patients. There was no association between baseline levels of plasma biomarkers and outcome (PFS and OS) nor between changes in biomarker levels and six-month PFS outcome (data not shown).
Circulating cfDNA
Total cfDNA from plasma collected pre-cycles 1 (n=28), 2 (n=23), and 3 (n=15) was quantified using a real-time PCR TaqMan Assay and primers directed to β-actin, β-globin, and GAPDH. Median cfDNA totals (GE/mL) are presented (Supplemental Table S2). cfDNA increased from baseline to pre-cycle 2 and decreased between pre-cycle 2 and pre-cycle 3; these changes were not significant. There were no statistically significant associations between baseline measures of cfDNA and outcome (PFS and OS) nor between changes in biomarker levels and six-month PFS outcome (data not shown).
Circulating Tumor and Endothelial Cells
Whole blood was collected pre-cycles 1 (n=26), 2 (n=21), and 3 (n=12) for CTC and CEC enumeration; CEC VEGFR expression was examined. Twenty-six patients were evaluated for at least one time point; nine patients submitted samples at all three time points. Median biomarker values are presented (Supplemental Table 3). Sixteen patients had ≥1 CTC at one of the three timepoints, with nine patients having ≥1 at baseline. All patients had CECs (range 14–800) at each timepoint tested. There were no statistically significant associations between baseline CTCs or CECs and outcome (PFS and OS) nor between changes in biomarker levels and PFS-6 outcome (data not shown).
CEC VEGFR expression was used to examine whether dasatinib had an effect on endothelial cell activation and whether activation correlates with outcome. We anticipated the percent of VEGFR+ CEC to decrease with positive anti-angiogenic treatment effect. There was no significant association between baseline CEC VEGFR expression and outcome (PFS and OS) nor between changes in biomarker levels and six-month PFS outcome (data not shown).
DISCUSSION
Dasatinib was well tolerated but had minimal activity in patients with recurrent ovarian or primary peritoneal carcinoma. With SRC reported to be overexpressed in approximately 90% of ovarian cancers [3], no prescreening was performed to determine eligibility.
SRC occupies a strategic position in many cell signaling pathways affecting cell proliferation, growth, and survival [4]. It has an important role in mediating the epithelial-to-mesenchymal transition enhancing the metastatic potential of ovarian cancer cells [15,16]. Activated SRC is required for VEGF expression, which has already been shown to be important in ovarian cancer biology and as an important therapeutic target. SRC expression was associated with a drug resistant phenotype and SRC inhibition by transfecting SRC antisense oligonucleotides (or by small molecule inhibitors) reversed drug resistance in preclinical models [6]. Gene expression profiles of ovarian cancer tumors were able to identify with greater than 80% accuracy which tumors were likely to be resistant to primary platinum-based therapy [26,27]. These profiles identified expression signatures consistent with activation of SRC which in the future may be used to direct therapeutic strategies.
SRC protein expression usually correlated with the degree of SRC pathway deregulation. The more deregulated the pathway, the higher the activated protein expression. Platinum-resistant ovarian cancers have been shown to be more likely to have deregulation of the SRC pathway [5,26]. Kaplan-Meier survival analysis showed that SRC pathway deregulation is associated with a poor survival. Konecny and colleagues tested a panel of 34 ovarian cancer cell lines and reported that 71% were highly sensitive to dasatinib [28]. Teoh et al, evaluated the in vitro activity of dasatinib alone and in combination with paclitaxel and carboplatin in ovarian cancer cell lines [29]. Dasatinib demonstrated anti-proliferative activity alone and synergistic activity with the cytotoxic in the cell lines with either SRC pathway deregulation and/or high SRC protein expression. Dasatinib did not decrease SRC protein expression, but completely abrogated the activation of SRC in all cell lines tested. These data suggest dasatinib may help prime cells for apoptosis induced by cytotoxic chemotherapy. Investigators at Duke University have conducted a phase I trial of carboplatin, paclitaxel, and dasatinib [NCT00672295], but have yet to report these results.
It is not surprising that there was little single agent activity of dasatinib in this trial in light of what has been learned about SRC since the conception and conduct of this trial. Preclinical data support evaluating SRC inhibition in cancers with a predilection for developing metastases to bone based on SRC’s suppression of osteoclast function. A phase II trial of single agent dasatinib investigated its activity in chemotherapy-naïve men with castrate resistant prostate cancer (CRPC). A 43% stable disease rate (SDR) at 12 weeks and a 19 % SDR was observed.(30) This level of activity was very similar to the results of current trial in patients with recurrent ovarian cancer. A biochemical marker of drug activity was identified with a decline in N-telopeptide, a marker if bone resorption predictive of adverse skeletal events, in men with CRPC treated with dasatinib. Dasatinib has been similarly studied in patients with triple negative breast cancer, Her 2 positive/hormone receptor positive breast cancer with response rates of 4–5%. No or minimal activity also was observed in phase II trials of monotherapy dasatinib in patients with head and neck cancer, glioma and small cell lung cancer (21,31). None of these trials pre-selected patients based on any predictive biomarkers of dasatinib activity. While SRC may be effective (under some circumstances) in suppressing tumor growth, it is not able as monotherapy to cause regression of established tumors. There are compensatory pathways that can bypass blockade of SRC. For example, SRC silencing results in significant increase in FGR levels (another member of the SFK) [32]. Alternatively, activation of JAK may re-establish downstream STAT signaling after inhibition of SFKs [33].
The low activity of dasatinib as a single agent should not preclude evaluation as part of combination regimens, either concomitantly or in sequence prior to chemotherapy if it is borne out that dasatinib can serve a priming function in ovarian cancers with a high degree of SRC pathway deregulation. In this regard, Pathak and Godwin have recently completed in vitro high-throughput screening (HTS) using a siRNA library targeting signaling molecules related to receptor tyrosine kinases in combination with dasatinib. Such screening would identify second-site molecules that can be targeted in combination with SRC inhibition to synergistically improve dasatinib efficacy in patients and to identify a potential gene signature predictive of response to dasatinib therapy (personal communication). The clinical significance of these genes identified through the HTS as being capable of synergizing with SRC inhibition is now being evaluated in blinded tumor biopsy samples from the patients that participated in this trial.
Our correlative studies did not show any significance between the biomarkers tested and patient outcome, mainly due to no clinical response on this study. There was a significant increase in the levels of sVEGFR2, sVEGFR3, and IGFBP2 between baseline and pre-cycle 3 in the 15 patients submitting samples at all three time points similar to the data reported by Strauss and colleagues [34]. There were no statistically significant changes in the three housekeeping genes from baseline in these patients. The mechanisms of the occurrence of cfDNA in blood under normal and pathological conditions are not yet fully understood. cfDNA might be influenced by apoptosis, necrosis, decreased DNAase activity in circulating cancer cells, as well as clearance by liver/kidney, and modification status of cfDNA. In this study, 16 patients had ≥1 CTC at one of the three time points; 9 had ≥1 at baseline. Further investigation of the relationship between cfDNA and circulating CTCs and the predictive value of changes in CTC in patients with ovarian cancer is needed.
Supplementary Material
RESEARCH HIGHLIGHTS.
There were no objective responses observed in patients with recurrent ovarian cancer treated with dasatinib.
Seven of 34 patients had PFS ≥ 6 months
The agent was well tolerated with the major toxicities being grade 3 nausea/emesis, pain and pulmonary (dyspnea +/− effusion)
There were no detectable relationships between clinical outcome and biomarkers
Acknowledgments
This study was supported, in part, by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical and Data Center (CA 37517), R01 CA140323 (to AKG), and P50 CA083639 (to AKS), P50 CA093638 (AKG and RJS), and CA006927 (RJS). AKG was also supported by the Chancellors Distinguished Chair in Biomedical Sciences Professorship, and AKS is also supported by the Betty Anne Asche Murray Distinguished Professorship.. The following Gynecologic Oncology Group member institutions participated in this study: Abington Memorial Hospital, Walter Reed Army Medical Center, Colorado Gynecologic Oncology Group P.C., Milton S. Hershey Medical Center, University of North Carolina School of Medicine, Indiana University School of Medicine, Rush-Presbyterian-St. Luke’s Medical Center, State University of New York at Stony Brook, Fox Chase Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Wisconsin Hospital, Women and Infants Hospital, The Hospital of Central Connecticut, and Community Clinical Oncology Program.
The authors thank De-Yu Shen in the Department of Gynecologic Oncology and Reproductive Medicine at MD Anderson Cancer Center for performing the quantitative PCR, the GOG Tissue Bank for their assistance with the banking and distribution of specimens and Veridex for providing specimen collection kits. The authors acknowledge support from The University of Kansas Cancer Center and the Kansas Bioscience Authority Eminent Scholar Program (AKS).
Footnotes
CONFLICT OF INTEREST
The co-authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61:183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002;1602:114–30. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 3.Wiener JR, Windham C, Estrella BC, Parikh NU, Thall PF, Deavers MT, et al. Activated SRC protein tyrosine kinase is overexpressed in late-stage human ovarian cancer. Gynecol Oncol. 2003;88:73–9. doi: 10.1006/gyno.2002.6851. [DOI] [PubMed] [Google Scholar]
- 4.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 5.Pengetnze Y, Steed M, Roby KF, Terranova PF, Taylor CC. Src tyrosine kinase promotes survival and resistance to chemotherapeutics in a mouse ovarian cancer cell line. Biochem Biophys Res Commun. 2003;309:377–83. doi: 10.1016/j.bbrc.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Chen T, Pengetnze Y, Taylor CC. Src inhibition enhances paclitaxel cytotoxicity in ovarian cancer cells by caspace-9 independent activation of caspace-3. Mol Cancer Ther. 2005;4:217–24. [PubMed] [Google Scholar]
- 7.Ceppi P, Papotti M, Monica V, Lo Iacono M, Saviozzi S, Pautasso M, et al. Effects of Src kinase inhibition induced by dasatinib in non-small cell lung cancer cell lines treated with cisplatin. Mol Cancer Ther. 2009;8:3066–74. doi: 10.1158/1535-7163.MCT-09-0151. [DOI] [PubMed] [Google Scholar]
- 8.Wiener JR, Nakano K, Kruzelock RP, Bucana CD, Bast RC, Jr, Gallick GE. Decreased Src tyrosine kinase activity inhibits malignant human ovarian cancer tumor growth in a nude mouse model. Clin Cancer Res. 1999;5:2164–70. [PubMed] [Google Scholar]
- 9.Martin L, Schilder R. Novel approaches in advancing the treatment of epithelial ovarian cancer: the role of angiogenesis inhibition. J Clin Oncol. 2007;25:2894–901. doi: 10.1200/JCO.2007.11.1088. [DOI] [PubMed] [Google Scholar]
- 10.Han LY, Landen CN, Trevino JG, Halder J, Lin YG, Kamat AA, et al. Antiangiogenic and antitumor effects of SRC inhibition in ovarian cancer. Cancer Res. 2006;66:8633–9. doi: 10.1158/0008-5472.CAN-06-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 12.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 13.Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, et al. Biological significance of focal adhesion kinase in ovarian cancer: Role in migration and invasion. Am J Path. 2004;165:1087–95. doi: 10.1016/S0002-9440(10)63370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 15.Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010;223:14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 16.Boyer B, Bourgeois Y, Poupon MF. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene. 2002;21:2347–56. doi: 10.1038/sj.onc.1205298. [DOI] [PubMed] [Google Scholar]
- 17.Miotti S, Tomassetti A, Facetti I, Sanna E, Berno V, Canevari S. Simultaneous expression of caveolin-1 and E-cadherin in ovarian carcinoma cells stabilizes adherens junctions through inhibition of Src-related kinases. Am J Pathol. 2005;167:1411–27. doi: 10.1016/S0002-9440(10)61228-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson B, Nesland JM, Goldberg I, Kopolovic J, Gotlieb WH, Bryne M, et al. Caveolin-1 expression in advanced-stage ovarian carcinoma – a clinicopathologic study. Gynecol Oncol. 2001;81:166–71. doi: 10.1006/gyno.2001.6156. [DOI] [PubMed] [Google Scholar]
- 19.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 20.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-Chloro-6-methyl-phenyl-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src-Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–61. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 21.Araujo J, Logothetis C. Dasatinib: A potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. 2010;36:492–500. doi: 10.1016/j.ctrv.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer. National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Luo FR, Yang Z, Camuso A, Smykla R, McGlinchey K, Fager K, et al. Dasatinib (BMS-354825) pharmacokinetics and pharmacodynamic biomarkers in animal models predict optimal clinical exposure. Clin Cancer Res. 2006;12:7180–6. doi: 10.1158/1078-0432.CCR-06-1112. [DOI] [PubMed] [Google Scholar]
- 24.Luo FR, Barrett YC, Yang Z, Camuso A, McGlinchey K, Wen ML, et al. Identification and validation of phospho-SRC, a novel and potential pharmacodynamic biomarker for dasatinib (SPRYCEL™), a multi-targeted kinase inhibitor. Cancer Chemother Pharmcol. 2008;62:1065–74. doi: 10.1007/s00280-008-0699-5. [DOI] [PubMed] [Google Scholar]
- 25.Sill MW, Yothers GA. Technical Report 06–08. Department of Biostatistics, University of Buffalo; A method for utilizing bivariate efficacy outcome measures to screen agents for activity in the 2-stage phase II clinical trials. Website: http:/sphhp.buffalo.edu/biostat/research/techreports/index.php. [Google Scholar]
- 26.Dressman HK, Berchuck A, Chan G, Zhai J, Bild A, Sayer R, et al. An integrated genomic-based approach to individualized treatment of patients with advanced-stage ovarian cancer. J Clin Oncol. 2007;25:517–25. doi: 10.1200/JCO.2006.06.3743. [DOI] [PubMed] [Google Scholar]
- 27.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–7. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 28.Konecny GE, Glas R, Dering J, Manivong K, Qi J, Finn RS, et al. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Brit J Cancer. 2009;101:1699–708. doi: 10.1038/sj.bjc.6605381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teoh D, Yeni TA, Tubatt JM, Adams DJ, Grace L, Starr MD, et al. Dasatinib (BMS-35482) has synergistic activity with paclitaxel and carboplatin in ovarian cancer cells. Gynecol Oncol. 2011;121:187–92. doi: 10.1016/j.ygyno.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–8. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puls LN, Eadens M, Messersmith W. Current status of Src inhibitors in solid tumors. The Oncologist. 2011;16:566–78. doi: 10.1634/theoncologist.2010-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HS, Han HD, Armaiz-Pena GN, Stone RL, Nam EJ, Lee JW, et al. Functional roles of Src and Fgr in Ovarian carcinoma. Clin Cancer Res. 2011;17:1713–21. doi: 10.1158/1078-0432.CCR-10-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen B, Peng S, Woods DM, Wistuba I, Bell D, El-Naggar AK, et al. STAT-5A-mediated SOCS2 expression regulates JAK2 and STAT3 activity following c-SRC inhibition in head and neck squamous carcinoma. Clin Cancer Res. 2012;18:127–39. doi: 10.1158/1078-0432.CCR-11-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strauss L, Sy O, Fairchild J, Fu C, Rybicki A, Yoganathan S, et al. Biomarker analysis in phase 2 single-agent trials of dasatinib for breast cancer. SABCS. 2009:2034. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


