Abstract
High performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometer was used for separation and identification of phenolic and other compounds in the water extracts of Saraca asoca (Roxb.), De. Wilde. The aim of the study was to identify and evaluate the distribution of phenolic compounds in the different parts of the plant. The identity of compounds was established through the comparison with standards and characteristic base peaks as well as other daughter ions. In crude extracts, 34 catechin derivatives, 34 flavonoids, and 17 other compounds were identified. Interestingly, further analysis of compounds showed plant part specific unique pattern of metabolites; that is, regenerated bark is observed to be the best source for catechin/catechin derivative while flowers were found to be the source for wide variety of flavonoids. Moreover, these plant part specific compounds can be used as biomarkers for the identification of plant material or herbal drugs. Overall, the present study provides for the first time a comprehensive analysis of the phenolic components of this herb which may be helpful not only to understand their usage but also to contribute to quality control as well.
1. Introduction
Bark decoction of S. asoca (Roxb.), De. Wilde (Caesalpiniaceae), has been mentioned as one of the most famous Indian treatise Charaka Samhita (100 A.D.) for the treatment of various types of gynaecological disorders. Bhavprakash Nighantu, another Indian treatise, referred to it as a uterine tonic for regularizing the menstrual disorders. Bark of the plant is well reported for its stimulating effect on endometrium and ovarian tissues and being used to treat menorrhagia. S. asoca contains significant amounts of phenolic compounds that are considered to be the biologically active components. Water extracts of the plant parts are being used to prepare various Ayurvedic and herbal drugs being rich source of catechin, epicatechin, epigallocathechin, and their polymers and glucosides [1, 2]. Catechins are well reported for various kinds of biological activities and are useful for the symptomatic treatment of several gastrointestinal, respiratory, and vascular diseases. The antioxidant activity of flavonoids has been studied with regard to retarding the aging of cells and protection against cancer and coronary or cardiovascular disease [3–5].
Various techniques are in use to identify phenolic compounds such as thin layer chromatography, high performance thin layer chromatography, gas chromatography, UV detection, high performance liquid chromatography (HPLC), and mass spectrometry. These methods are useful to detect a limited number of known compounds but are not applicable for the characterization of unknown polyphenols in crude mixtures. Quadrupole time-of-flight mass spectrometry (Q-TOFMS) is excellent technique to analyze multicomponents in the complex herbal extracts due to accurate mass measurement, high resolution, and ion separation [6]. Rapid data mining procedures and aligning algorithm tools have been used to process huge raw data generated from metabolome analyses [7]. These processed data were thereafter used successfully in various pharmacophysiological studies such as disease diagnostics, human nutritional science, and drug discovery [8, 9].
In the present study, HPLC coupled with Q-TOFMS in positive mode was used to generate nontargeted MSn data from various crude extracts prepared by taking different parts of S. asoca. As on date scanty information is available from S. asoca, rather no one reported a comprehensive profile of phenolic compounds from this plant. Therefore, nontargeted MSn data was generated and processed by using Mass Hunter qualitative software for identification of phenolic compounds from the various prepared extracts of S. asoca.
2. Experimental
2.1. Reagents
Standard compounds and solvents lidocaine, D-camphor, 5-7-isoflavone, formic acid and acetic acid (HPLC grade), acetonitrile, and formic acid and water (LCMS grade) were purchased from Sigma-Aldrich (St. Louis, MO. USA). Phenolic standards protocatechuic acid, coumaric acid, and quercetin were obtained from Sigma (St. Louis, MO, USA). Epicatechin, catechin, gallic acid, ferulic acid, and caffeic acid were purchased from Fluka (Buchs, Switzerland). The purity of the standards was more than 98%, and stock solutions were prepared as at 1 mg/L in methanol. Working standard solutions were made by diluting the stock solutions with mobile phase of HPLC.
2.2. Plant Material
Bark, regenerated bark, leaves, and flowers of S. asoca were collected from Botanical Garden of National Research Institute of Basic Ayurvedic Sciences, CCRAS, (Department of AYUSH), Nehru Garden, Kothrud, Pune, in February 2012 (winter season). The collected plant materials were identified, and voucher specimens (no. 207) were kept at the medicinal plant museum of the institute.
2.3. Extraction and Sample Preparation
Fresh plant materials were extracted overnight (at 25 and 70°C) with deionized water (Direct-Q, Millipore) and methanol in sequence (1 : 1 w/v). Extraction steps were repeated three times to ensure complete recovery of metabolites. The pooled supernatant phases were filtered through 0.22 μ filters (HiMedia), concentrated under vacuum to dryness (FreeZone 4.5 Labconco, CA, USA), and stored at −80°C till further use. All the samples were given abbreviated name as: bark water, hot water, and methanol extract (B), regenerated bark water, hot water, and methanol extract (RB), leaves water and hot water extract (L), and flower water and hot water extract (F). The extracts were reconstituted in HPLC mobile phase (5.0 mg/mL) for further analytical studies. Standard compounds lidocaine, D-camphor, and 5-7-isoflavone (5.0 ppm) were mixed in the samples.
2.4. HPLC
Experiments were performed on Agilent 1290 Infinity Series HPLC interfaced with an Agilent 6538 Accurate-Mass Q-TOF. A ZORBAX 300SB reverse phase column (C18, 4.5 mm × 250 mm, and 5 μm particle size) with guard column of same diameter and pore size was used at a flow rate of 0.2 mL/min. The column temperature was maintained at 40°C. The mobile phase used for HPLC was combination of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile). The gradient was varied linearly 5–10% in 15 min, 10–45% in 22 min, 45–65% in 30 min, 65–90% in 35 min, and finally to 5% B at 45 min. Sample volume of 20 μL was injected by autosampler.
2.5. Q-TOFMS Conditions
Q-TOFMS was calibrated and tuned as recommended by the manufacturer to get accuracy less than 5 ppm. Instrument was operated in positive ion polarity mode and extended dynamic range (1700 m/z, 2 GHz) with following parameters: gas temperature 350°C, nebulizer 50 Psi, gas flow 11 L/min, capillary voltage 3500 V, nozzle 500 V, skimmer voltage 65 V, octapole RF 250 V, octapole DC1 48 V, and fragmentor voltage 175 V. MSn data was collected in total ion counting mode, and spectra were acquired in the range 100–1100 m/z with acquisition rate 3 spectra s−1. To assure the mass accuracy of recorded data, standards of lidocaine and 5, 7-isoflavone were infused with samples along with continuous internal calibration with the use of signals at a range of m/z 121.05 to m/z 922.0098 (as per instrument standards).
3. Results and Discussion
3.1. HPLC/MS/MS Conditions Optimization
The HPLC-Q-TOFMS was tested with several basic and acid ionizers, but formic acid 0.1% was found to be most suitable among the tested conditions to resolve most of the compounds present in the crude extracts. In this condition ionic strength became appropriate, and the signal-to-noise ratio increased in the positive ion mode. However, negative mode also gets refined, but positive mode showed better ionization; therefore, it was selected to study the extracts. Being the crude extracts, several gradient profiles were tested, but used gradient profile allowed maximum separation of compounds in the extracts. Mixed standard solutions were tested in order to establish the optimum MSn conditions. The fragmentation voltage was varied from 50 to 250 V and the collision energy from 5 to 45 V. The best results were obtained at fragmentation voltage 175 and ramping collision energy.
3.2. Analysis Catechins from Standards and S. asoca Extracts
Figure 1 is showing some of important and previously known compounds identified from S. asoca. Standard MSn spectra of some important compounds were obtained under positive electron spray ionization (+ESI) conditions as discussed previously. The spectra generated for catechins by +ESI gave the protonated molecule [M + H]+ and some fragments even at relatively low fragmentation and collision energy voltages. Catechin, (−)-epicatechin, and (−)-epigallocatechin yielded the protonated molecule [M + H]+ (m/z 291) along with other characteristic ions at m/z 123, 139, 161, and 207 [10]. For instance, other fragments of m/z 207, 219, and 275 were observed in the spectra. The retro-Diels-Alder fragmentation ions are reported as characteristic fingerprints for the presence of catechins in complex matrices. [M + H-galloyl + H–H2O]+ is a general fragmentation pattern observed for all catechin gallates and gallocatechin gallates [11]. Fragmentation of the predominant positive ions in nontargeted MSn mode was used to obtain information about the molecular masses of conjugates and sugar moieties bound to the aglycones. The total ion chromatograms in positive mode of the extracts in Figure 2 are showing visual changes in profiles of different parts. The positive full-scan LC/MS analysis produced peaks for derivatives of catechins which were identified by scanning the characteristics fragment ions and matching standards available in the literature (Table 1).
Figure 1.
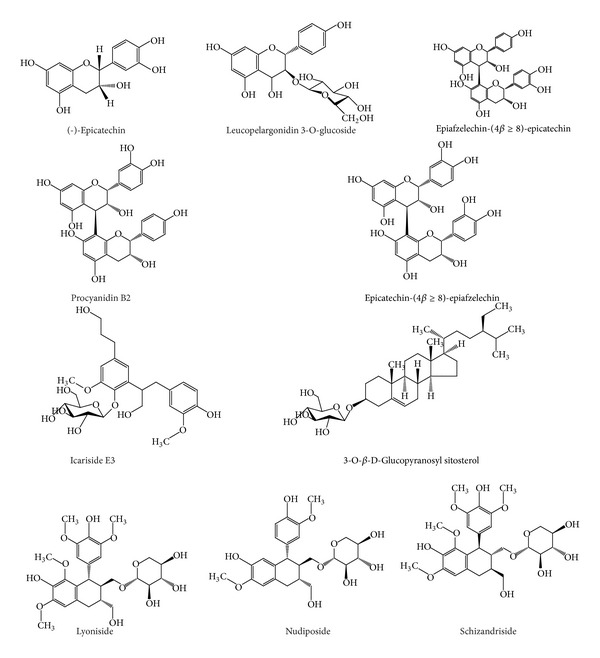
Structures of some known compounds from S. asoca.
Figure 2.

BPC scans of S. asoca regenerated bark hot water (a), bark hot water (b), regenerated bark water (c), bark water (d), flower hot water (e), flower water (f), leaves hot water (g), leaves water (h), methanol bark (i), and regenerated bark methanol (j) extracts. Peaks assignment is listed in Table 1.
Table 1.
Identified catechins and their derivatives in different parts of S. asoca.
| S. no. | RT | Name of compound | Product ions (m/z) | Calculated mass | Exact mass | Sample* |
|---|---|---|---|---|---|---|
| 1 | 6.23 | Gallocatechin 3-O-gallate | 139.02, 289.2, 361.341, and 459.59 | 458.137 | 458.08 | F |
| 2 | 7.03 | Gallic acid hexoside | 111.001, 159.234, 171.0423, 219.161, and 239.128 | 332.073 | 332.071 | F, B, RB |
| 3 | 7.30 | Gallic acid derivative | 153.249, 171.034 | 193.0352 | ALL | |
| 4 | 7.72 | Protocatechuic acid | 109.04, 127.062 | 155.051 | 154.031 | F, L |
| 5 | 8.20 | Catechol | 111.006 | 110.006 | 110.036 | ALL |
| 6 | 10.90 | Gallic acid | 109.024, 127.131, and 153.323 | 170.041 | 170.021 | F, L |
| 7 | 21.99 | Catechin derivative | 127.021, 139.0156, 165.012, 271.213, 291.213, 409.210, and 569.351 | 740.221 | — | RB |
| 8 | 22.41 | Catechin derivative | 139.237 | 351.825 | — | L |
| 9 | 23.64 | (epi)Catechin-(epi)catechin-(epi)catechin | 127.138, 151.335, 163.411, 245.710, 301.779, 409.722, 427.707, 451.665, 527.526, 578.477, and 715.239 | 866.081 | 866.211 | RB, B |
| 10 | 23.70 | Procyanidin B3 | 127.131, 275.741, 287.749, 291.742, 409.709, 417.690, and 427.690 | 578.516 | 578.14 | B |
| 11 | 23.80 | Procyanidin B2 | 127.13, 139.23, 289.163, 291.177, and 409.206 | 578.463 | 578.53 | |
| 12 | 23.83 | Catechin diglucoside | 123.102, 139.023, 165.041, 285.101, 291.179, 315.179, 383.277, and 453.202 | 598.294 | — | RB |
| 13 | 23.88 | Tannin | 127.103, 139.236, 163.23, 287.231, 301.268, 393.213, and 409.321 | 724.25 | 724.242 | RB |
| 14 | 23.95 | Procyanidin B1 | 127.131, 139.237, 163.405, 271.743, 287.749, 291.781, 301.766, 409.708, 427.686, and 543.509 | 578.463 | 578.14 | ALL |
| 15 | 24.18 | Procyanidin C1 | 127.137, 139.244, 289.775, 291.756, 409.722, 545.504, and 577.476 | 866.082 | 866.205 | RB, B |
| 16 | 24.37 | Catechin derivative | 127.013, 139.137, 289.265, 301.215, 393.243, 409.213, 427.209, 464.204, and 563.231 | 871.099 | — | RB |
| 17 | 24.80 | Epiafzelechin | 107, 139.236, 149.33, 169.44, 191.57, 233.67, and 257.74 | 274.779 | 274.084 | B, RB |
| 18 | 24.83 | Hydroxy catechin | 123.103, 139.243, 151.355, 163.412, 181.520, 207.637, and 215.668 | 302.798 | — | B |
| 19 | 24.87 | Catechin glucoside rhamnose | 139.037, 275.279 | 583.507 | — | B |
| 20 | 24.97 | Epicatechin | 123.097, 139.098, 147.104, 165.132, and 207.229 | 291.237 | 290.27 | ALL |
| 21 | 25.05 | Galloyl-isorhamnetin | 317.213 | 468.205 | — | F, L |
| 22 | 25.29 | Catechin | 123.097, 139.098, 147.104, 165.132, and 207.229 | 291.215 | 290.27 | ALL |
| 23 | 25.30 | Afzelechin-(4alpha→8)-catechin | 107.051, 139.245, 147.311, 231.702, 273.772, 287.761, 291.754, 393.751, 409.722, 411.7024, and 427.705 | 562.574 | 562.15 | B, RB |
| 24 | 25.48 | Proanthocyanidin trimer | 127.0124, 139.123, 151.133, 163.133, 247.243, 271.254, 287.261, 301.279, 397.232, 409.272, 427.2046, 449.256, 534.133, 577.173, 679.280, 695.2561, and 713.238 | 864.036 | B | |
| 25 | 25.53 | Dicatechin gallate | 287.248, 409.203, and 579.263 | 730.193 | — | B |
| 26 | 26.04 | Tricatechin gallate | 239.02, 247.134, 279.265, 518.135, and 579.238 | 1018.944 | — | F |
| 27 | 26.22 | Propelargonidin trimer | 119.009, 139.123, 151.023, 231.168, 289.262, 300.277, 325.275, 329.255, 381.242, 393.326, 409.204, 419.17, 425.175, 435.187, 451.148, 471.166, 546.119, 555.199, 577.144, 680.1736, and 699.246 | 850.055 | 850.21 | RB |
| 28 | 26.44 | Catechin-(4alpha→8)-gallocatechin-(4alpha→8)-catechin | 139.012, 153.021, 271.243, 287.246, 331.231, 417.201, 544.141, 563.153, 587.127, and 714.2103 | 881.99 | 882.22 | F |
| 29 | 26.76 | (−)-Epicatechin-3-O-gallate | 123.09, 139.237, 153.321, 165.433, 273.760, and 291.781 | 442.661 | 442.09 | F |
| 30 | 27.40 | Catechin O-glucoside | 123.102, 139.023, 165.041, 291.179, 367.234, and 411.259 | 452.202 | — | RB |
| 31 | 27.80 | Lignan | 137.012, 145.123, 151.156, 167.125, 181.174, 189.126, 285.145, 317.174, 361.267, and 465.276 | 464.136 | — | RB |
| 32 | 28.32 | Petunidin gallate | 317.782 | 485.693 | — | L |
| 33 | 29.80 | (−)-Gallocatechin | 139.243, 289.345, and 291.786 | 306.004 | 306.07 | RB |
| 34 | 37.55 | Cyanidin 3-(2G-galloylrutinoside) | 748.492 | 747.49 | 747.482 | RB |
*Abbreviations B, F, L, and RB in sample column represent bark, flower, leaves, and regenerated bark, respectively.
C- and O-glycosides were identified on the basis of previous reports. In the positive ion full-scan mass spectrum, the C-glycosides showed only the prominent [M + H]+ ion with losses of 120 and 150 u (X + [M + H–120]+ and X + [M + H–150]+). The analysis of protonated C-glycosides by ESI-Q-TOFMS has proven that the ions of X + [M + H–90]+, X + [M + H–120]+, and X + [M + H–150]+ are the characteristic product ions for polyphenol C-glycosides, and the losses of 120 and 150 u are more favourable [12], whereas in polyphenol O-glycoside X+ [M+H–162]+ was characteristic ion due to neutral loss of 162 u in the product ion spectra.
Using the standards and identification of characteristic ions, 34 catechins and their derivatives were identified from the samples. The gradient of water containing 0.1% formic acid and acetonitrile 0.1% formic acid method produced well-shaped peaks for (−)-epicatechin, catechin, and epigallocatechin at 24.447, 25.261, and 23.8 min, respectively [10]. (−)-Epicatechin and catechin were differentiated on the basis of their retention time related to spectra of standard compounds. Moreover, several new derivatives of catechin were identified, and some remain unidentified (Table 1). Catechin-O-glucoside and catechin di-O-glucoside were identified for the first time as these give characteristic peaks of catechin along with neutral loss of 162 u due to loss of glucose moiety. Six catechin derivatives were found throughout the sample. Other catechin derivatives were observed to be specific with respect to plant parts which can be used as plant part specific markers and can be helpful in standardization of herbal drugs. Regenerating bark was found to have maximum number of catechin derivatives and tannins which might be induced under stress of regeneration and to prevent infections due to damage in bark.
On the basis of inclusive analysis of phenolic compounds, pathway of flavonoids and their derivatives biosynthesis in S. asoca were explored (Figure 3). These compounds showed unique pattern of metabolites in the plant parts. In the study, S. asoca was found to be a rich source for catechins that accumulate in all the organs especially in bark. Contrary to this, epicatechin-3-O-gallate, and epigallocatechin-3-O-gallate were observed in the leaves and flowers of this herb.
Figure 3.
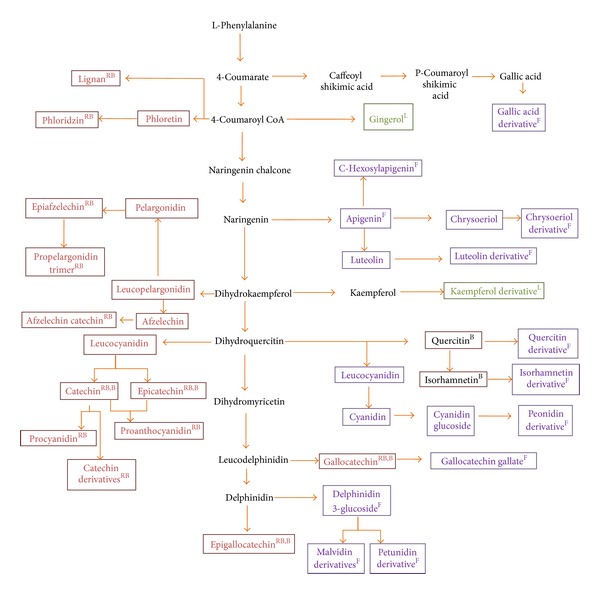
Plant part specific flavonoids biosynthesis pathway in Saraca asoca. Brown, violet, and green boxes correspond to the compound present in regenerated bark (RB), flower (F), and leaves (L) of S. asoca, respectively.
3.3. Analysis of Flavonoids from Standards and S. asoca Extracts
Samples of S. asoca were analysed for flavonoids and found to have apigenin, kaempferol, peonidin, quercetin, isorhamnetin, chrysoeriol, and their derivatives. However maximum numbers of flavonoids were observed in the flower extracts of herb. In this study, total 34 flavonoids were characterized. Most of them were unambiguously identified by comparing retention times and MS data with those of the reference standards and discussed in the literature. Concerning the presence of aglycones in S. asoca, up to now several aglycones have been described in the literature [13]. The product ion spectra of apigenin, kaempferol, peonidin, quercetin, isorhamnetin, and chrysoeriol (Figure 1) were identified by comparing the product ion spectra and retention times with those of standards provided with a useful tool for the confirmation of the presence of these six aglycones in S. asoca extracts for the first time. Aglycones were identified by product ions generated by neutral losses of CH3 group, H2O, and CO as described previously [14, 15]. Glycosides of flavonoids were identified as described previously in case of catechins counting the loss of 162, 150, 120, and 90 u which are characteristics of flavonoids O- and C-glucosides. Total ion chromatogram was screened for loss of 162, 150, and 120 u. All the fragments were assigned with an accuracy of less than 5 ppm with few exceptions. Aglycones were fixed by comparing the product ions from standards and the literature. Resulted flavonoid glycosides are given in Table 2. Peonidin, quercetin, delphinidin, isorhamnetin, petunidin, and malvidin glycoside were mainly observed in flowers as shown in biosynthesis pathway (Table 2, Figure 3).
Table 2.
Identified polyphenols and their glycosides in different parts of S. asoca.
| S. no. | RT | Name of compound | Product ions (m/z) | Calculated mass | Exact mass | Sample* |
|---|---|---|---|---|---|---|
| 1 | 11.96 | Kaempferol | 112.01, 147.03, 163.134, 211.224, and 243.232 | 286.264 | 286.240 | L |
| 2 | 15.18 | Kaempferol 3-diglucoside-7-glucoside-p-coumaloyl | 471.167 | 918.198# | 918.191 | B, F, RB |
| 3 | 15.43 | C-Hexosyl-apigenin | 283.125, 367.723 | 528.528 | 528.520 | F |
| 4 | 21.40 | Quercetin-3-rhamnoside | 129.01, 141.025, 233.177, 287.147, 303.281, and 449.214 | 448.218 | — | F, L |
| 5 | 22.10 | Petunidin-3-O-beta-glucopyranoside | 317.125 | 479.122# | 479.118 | F |
| 6 | 22.33 | Unknown gingerol type glycoside | 139.123, 181.143, 265.213, 33.242, and 351.273 | 512.251# | — | L |
| 7 | 22.59 | Pentahydroxyflavone-O-glucoside | 129.12, 137.153, 153.123, and 305.833 | 466.265# | 466.157 | F, L |
| 8 | 22.72 | C-Hexosyl-luteolin O-hexoside, O-pentoside | 299.213, 329.142, 353.125, and 383.217 | 743.286 | 742.278 | FW |
| 9 | 23.20 | Peonidin-3-O-β-galactopyranoside | 301.145 | 463.184# | 463.124 | F, L |
| 10 | 23.57 | Dihexosyl quercetin | 303.124, 465.213 | 626.134 | 626.150 | F |
| 11 | 23.78 | Quercetin | 123.10, 137.24, 151.33, and 285.77 | 302.796 | 302.04265 | B, RB |
| 12 | 24.10 | Quercetin-3′,7-di-O-glucoside | 287.249, 449.364 | 610.411 | 610.52 | F |
| 13 | 24.14 | Isorhamnetin sophorose | 317.263, 479.223 | 640.167# | 640.160 | F |
| 14 | 24.52 | 6-Hydroxykaempferol | 123.102, 139.214, 147.31, 151.3358, 165.43, 181.518, 193.57, 207.63, 215.668, 243.722, 261.67, and 285.785 | 302.796 | 302.042 | B |
| 15 | 24.52 | C-Hexosyl-chrysoeriol O-hexoside | 301.124, 463.256 | 624.204# | 624.17 | F |
| 16 | 24.70 | 3,5,7,2′,6′-Pentahydroxyflavone | 215.727, 243.82, and 289.811 | 306.864# | 304.058 | L |
| 17 | 24.77 | Malvidin-3-galactoside | 331.772 | 493.112# | 493.134 | F |
| 18 | 24.80 | Peonidin glucoside derivative | 301.767, 463.654 | 776.145# | — | F |
| 19 | 24.88 | Peonidin-3,5-O-di-β-glucopyranoside | 286.0235, 301.0235 | 625.241# | 625.176 | F |
| 20 | 24.90 | Phloridzin# | 275.421 | 437.542# | 436.136 | RB |
| 21 | 24.99 | Peonidin-3-O-alpha-arabinopyranoside | 133.023, 177.253, 301.271, and 415.123 | 433.105# | 433.113 | F |
| 22 | 25.03 | (+)-Dihydrokaempferol | 107.04, 123.09, 127.13, 139.23, 149.29, 163.407, 166.472, 179.434, 215.654, 243.715, 259.759, 271.744, and 289.763 | 288.763 | 288.063 | ALL |
| 23 | 25.08 | 3-O-Hexosyl-quercetin | 133.197, 145.295, 153.356, and 301.178 | 464.075 | 464.10 | F |
| 24 | 25.22 | Leucopelargonidin 3-O-glucoside | 137.124, 291.178, and 303.155 | 452.185 | 452.131 | ALL |
| 25 | 25.26 | Apigenin | 107.043, 119.003, 149.013, 153.123, 174.155, 215.165, 228.213, and 243.214 | 270.103 | 270.05 | ALL |
| 26 | 25.43 | Malvidin-diglucoside | 331.275, 493.213 | 655.257# | 655.187 | F, L |
| 27 | 25.46 | Isorhamnetin-3-coumaroylglucopyranoside | 317.275, 463.266 | 624.224# | — | B |
| 28 | 25.70 | Isorhamnetin | 115.043, 123.176, 147.109, 165.133, 257.177, 297.020, and 302.054 | 316.02# | 316.06 | B |
| 29 | 26.5 | Delphinidin-3-O-β-glucopyranoside | 303.213 | 465.231# | 465.103 | F |
| 30 | 27.28 | Quercetin-3-O-Arabinoside | 131.01, 137.024, 151.125, 181.123, 257.263, 285.214, 303.214, and 360.225 | 434.2 | 434.214 | B |
| 31 | 27.43 | Quercetin 3,4′-di-glucoside-3′-(6-caffeoylglucoside) | 625, 787, and 487.685 | 950.265 | 950.257 | RB |
| 32 | 30.20 | 7-Acetyloxy-2-methylisoflavone | 107.08, 111.077, 121.134, 125.068, 151.100, 161.187, 179.194, 193.170, 221.191, 237.146, 249.178, 259.176, and 277.103 | 294.102# | 294.089 | F |
| 33 | 30.47 | Peonidin | 286.0235, 301.068 | 301.067# | 301.071 | ALL |
| 34 | 34.60 | Isorhamnetin-3-O-glucoside | 302.155, 317.013 | 478.032# | 478.111 | F |
*Abbreviations B, F, L and RB in sample column represent bark, flower, leaves, and regenerated bark, respectively.
#Compound detected for the first time in S. asoca.
3.4. Analysis of Other Compounds from S. asoca Extracts
Compounds other than catechin and flavonoid derivatives were identified with help of standard mass spectral libraries from http://spectra.psc.riken.jp and http://www.massbank.jp [16, 17]. Table 3 is showing compounds and their product ions. Unidentified compounds were mentioned as unknown or derivative of known compounds.
Table 3.
Other compounds identified in different parts of S. asoca.
| S. no. | RT | Name of compound | Product ions (m/z) | Calculated mass | Exact mass | Sample* |
|---|---|---|---|---|---|---|
| 1 | 7.10 | L-Homocitrulline | 100.123, 127.061, 155.280, and 173.213 | — | 189.111 | ALL |
| 2 | 10.22 | Dehydrogenated-decarboxy-neobetanin | 341.771 | 502.598 | — | RB |
| 3 | 15.70 | Ecdysone | 123.045, 233.213, 279.253, 297.256, 313.257, 325.252, 393.383, 429.266, 447.256, 465.225, and 482.167 | 482.167 (M + H + NH3)+ | 464.122 | L |
| 4 | 20.26 | 17-Decarboxy-betanin | 345.289 | 506.217 | 506.152 | B, RB |
| 5 | 22.02 | Triterpenoid hexose | 126.98, 323.711, 429.738, and 505.594 | 666.330 | 666.40 | F, L |
| 6 | 22.35 | 11-Hydroxy-sec-O-β-D-glucosylhamaudol | 293.794 | 454.695 | — | B |
| 7 | 24.3 | D-(+)-Cellotriose | 203.201, 325.298, 343.231, and 487.241 | 504.208 | 504.169 | B, RB |
| 8 | 24.65 | Unknown diglucoside | 323.245, 485.6 | 647.343 | 646.335 | LHW |
| 9 | 24.94 | 14-Hydroxycarpesterol | 127.012, 139.123, 163.102, 257.251, 275.231, 291.215, 301.253, 337.296, 401.196, 409.203, 427.196, and 560.293 | 578.222 | 578.22 | ALL |
| 10 | 25.44 | Icariside E3 | 115.023, 145.125, and 188.156 | 524.206 | 524.225 | F, L |
| 11 | 25.50 | 3-O-beta-D-Glucopyranosyl sitosterol# | 397.213, 415.282 | 576.406 | 576.438 | B, RB |
| 12 | 26.11 | 7-Dehydrocholesterol glucoside | 120.8, 133.1, 159.2, 247.2, 259.2, 368.2, and 385.2 | 546.2 | — | RB, B |
| 13 | 26.50 | Phytolaccagenic acid 3-O-glucose (1′′→3′) galactose | 249.772, 517, and 679 | 840.329 | 840.321 | RB |
| 14 | 27.90 | Unknown | 123.09, 153.121, 271.25, 394.243, and 542.197 | 882.993 | — | F |
| 15 | 28.2 | Tyramine-betaxanthin | 163.149, 249.244, and 287.219 | 330.244 | 330.12 | B, RB |
| 16 | 34.36 | 4-Methylthio-n-butyl glucosinolate | 186.001, 286.23, 316.993, and 398.505 | 477.900 | 477.984 | B, RB |
| 17 | 37.90 | Tripalmitin type compound | 393.89, 313.9816, 239.89, 155.333, and 137.3193 | 554.672 | — | F |
*Abbreviations B, F, L, and RB in sample column represent bark, flower, leaves, and regenerated bark, respectively.
4. Conclusions
The rational use of S. asoca plant parts for declining uterine diseases is mainly due to presence of flavonoidal glycosides, catechins, oligomeric procyanidins, and steroids. The detailed identification of the phenolic composition of S. asoca provides the background necessary to evaluate the biological activity of the identified compounds and to develop an understanding of the potential benefit of the herb. A number of steroidal compounds were also observed in all plant parts but could not be identified very well due to limited fragmentations. The qualitative and comparative method showed good results in terms of identification of flavonoids. Variety of catechin derivatives were found to be elevated in regenerating bark. One possible reason for the elevation of flavonoids could be the protective effect of these compounds against plant infections. Part specific compounds as shown in Tables 1, 2, and 3 can be used as biomarkers for the identification of plant material or herbal drugs. This comprehensive analysis of the phenolic components of herb will be helpful not only in the quality control of this herb and its products but also in understanding medicinal importance of different parts of the herb. Besides this, the content of desire compound can be enhanced in specific part of the plant by using metabolic engineering where the present data will be very useful and supportive.
References
- 1.Gahlaut A, Taneja P, Shirolkar A, Nale A, Hooda V, Dabur R. Principal component and partial least square discriminant based analysis of methanol extracts of bark and regenerated bark of Saraca asoca . International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(4):331–335. [Google Scholar]
- 2.Saha J, Mitra T, Gupta K, Mukherjee S. Phytoconstituents and HPTLC analysis in Saraca asoca (Roxb.) Wilde. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(1):96–99. [Google Scholar]
- 3.Galli RL, Shukitt-Hale B, Youdim KA, Joseph JA. Fruit polyphenolics and brain aging. Annals of the New York Academy of Sciences. 2002;959:128–132. doi: 10.1111/j.1749-6632.2002.tb02089.x. [DOI] [PubMed] [Google Scholar]
- 4.Zamora-Ros R, Not C, Guinó E, et al. Association between habitual dietary flavonoid and lignan intake and colorectal cancer in a Spanish case—control study (the Bellvitge Colorectal Cancer Study) Cancer Causes & Control. 2013;24(3):549–557. doi: 10.1007/s10552-012-9992-z. [DOI] [PubMed] [Google Scholar]
- 5.Testai L, Martelli A, Cristofaro M, Breschi MC, Calderone V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. Journal of Pharmacy and Pharmacology. 2013;65(5):750–756. doi: 10.1111/jphp.12032. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhary A, Kaur P, Kumar N, Singh B, Awasthi S, Lal B. Chemical fingerprint analysis of phenolics of Albizia chinensis based on ultra-performance LC-electrospray ionization-quadrupole time of flight mass spectrometry and antioxidant activity. Natural Product Communications. 2011;6(11):1617–1620. [PubMed] [Google Scholar]
- 7.Shirolkar A, Gahlaut A, Hooda V, Dabur R. Phytochemical composition changes in untreated stem juice of Tinospora cordifolia (W) Mier during refrigerated storage. Journal of Pharmacy Research. 2013;7(1):1–6. [Google Scholar]
- 8.Cevallos-Cevallos JM, Reyes-De-Corcuera JI, Etxeberria E, Danyluk MD, Rodrick GE. Metabolomic analysis in food science: a review. Trends in Food Science & Technology. 2009;20(11-12):557–566. [Google Scholar]
- 9.Goodacre R, Vaidyanathan S, Dunn WB, Harrigan GG, Kell DB. Metabolomics by numbers: acquiring and understanding global metabolite data. Trends in Biotechnology. 2004;22(5):245–252. doi: 10.1016/j.tibtech.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Shirolkar A, Gahlaut A, Chhillar AK, Dabur R. Quantitative analysis of catechins in Saraca asoca and correlation with antimicrobial activity. Journal of Pharmaceutical Analysis. 2013 doi: 10.1016/j.jpha.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen D, Wu Q, Wang M, Yang Y, Lavoie EJ, Simon JE. Determination of the predominant catechins in Acacia catechu by liquid chromatography/electrospray ionization-mass spectrometry. Journal of Agricultural and Food Chemistry. 2006;54(9):3219–3224. doi: 10.1021/jf0531499. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Zhang X, Xue X, Zhang Y, Xiao H, Liang X. Rapid identification of polyphenol C-glycosides from Swertia franchetiana by HPLC-ESI-MS-MS. Journal of Chromatographic Science. 2009;47(3):190–196. doi: 10.1093/chromsci/47.3.190. [DOI] [PubMed] [Google Scholar]
- 13.Careri M, Elviri L, Mangia A. Validation of a liquid chromatography ion spray mass spectrometry method for the analysis of flavanones, flavones and flavonols. Rapid Communication in Mass Spectrometry. 2009;13(23):2399–2405. doi: 10.1002/(SICI)1097-0231(19991215)13:23<2399::AID-RCM805>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Madeira PJ, Borges CM, Florêncio MH. Electrospray ionization Fourier transform ion cyclotron resonance mass spectrometric and semi-empirical calculations study of five isoflavone aglycones. Rapid Communications in Mass Spectrometry. 2010;24(23):3432–3440. doi: 10.1002/rcm.4791. [DOI] [PubMed] [Google Scholar]
- 15.Justino GC, Borges C, Florêncio MH. Electrospray ionization tandem mass spectrometry fragmentation of protonated flavone and flavonol aglycones: a re-examination. Rapid Communications in Mass Spectrometry. 2009;23(2):237–248. doi: 10.1002/rcm.3869. [DOI] [PubMed] [Google Scholar]
- 16. http://spectra.psc.riken.jp.
- 17. http://www.massbank.jp.


