Abstract
Over the past two decades, many magnetic resonance spectroscopy (MRS) studies reported lower N-acetylaspartate (NAA) in key brain regions of patients with schizophrenia (SZ) compared to healthy subjects. A smaller number of studies report no difference in NAA. Many sources of variance may contribute to these discordant results including heterogeneity of the SZ subject populations and methodological differences such as MRS acquisition parameters, and post-acquisition analytic methods.
The current study reviewed proton MRS literature reporting measurements of NAA in SZ with a focus on methodology.
Studies which reported lower NAA were significantly more likely to have used longer echo times (TEs), while studies with shorter TEs reported no concentration difference. This suggests that NAA quantitation using MRS was affected by the choice of TE, and that published MRS literature reporting NAA in SZ using a long TE is confounded by apparent differential T2 relaxation effects between SZ and healthy control groups.
Future MRS studies should measure T2 relaxation times. This would allow for spectral concentration measurements to be appropriately corrected for these relaxation effects. In addition, as metabolite concentration and T2 relaxation times are completely independent variables, this could offer distinct information about the metabolite of interest.
Keywords: T2 relaxation, NAA, schizophrenia, MRS
1 INTRODUCTION
Spatially resolved magnetic resonance spectroscopy (MRS) has proven to be a powerful and non-invasive tool for the investigation of the neurochemistry of the working healthy and pathological brain. MRS has been used as an investigational tool in schizophrenia (SZ) research following the development of spatially selective pulse sequences and water suppression techniques in the late 1980’s which enabled the in vivo detection of brain metabolite resonances.
One brain metabolite commonly examined in MRS studies is N-acetylaspartate (NAA). NAA is a free amino acid that is biosynthesized in neuronal mitochondria. It is found almost exclusively in neurons, including axons and dendrites, and is considered a marker for neuronal viability and integrity. Changes in NAA concentrations could be caused by changes in neuronal density or neuronal dysfunction (such as changes in glucose metabolism or mitochondrial function). A number of proton MRS (1H-MRS) studies have reported reduced NAA in the frontal and temporal lobes and other structures of patients with SZ. However, a lesser number of studies report no difference in NAA between patients with SZ and healthy controls (HCs). What are the possible origins of the disparate findings? There are many potential sources of variance which may contribute to these conflicting results including differences in clinical and demographic characteristics (such as medication status or duration of illness), and also the choice of specific MRS acquisition parameters, techniques, and analytic methods (Sanches et al., 2004).
The fundamental principle underlying proton MRS is that for each MRS visible metabolite, the fundamental frequency at which the nucleus of each hydrogen atom (proton) resonates is shifted by a small amount (measured in parts-per-million, ppm) from the basic resonant frequency of a single, isolated, proton. Chemically identical hydrogen nuclei within an MRS visible metabolite experience similar local magnetic fields and nuclear spin-spin interactions and therefore have a characteristic chemical shift along the resonance frequency axis, which results in a spectral peak that is a chemical signature of that group of protons within that metabolite. The peak intensity or area under the spectral peak is proportional to the number of nuclei contributing to that peak, which is determined by the concentration of that metabolite within a selected volume of interest (voxel) (Jansen et al., 2006).
As discussed below, several MRS acquisition parameters and subject tissue characteristics ultimately affect the measured spectral peak area. When these myriad factors are properly accounted for, or held constant, a “raw” peak integral (area under the peak) is obtained which is proportional to the concentration of the metabolite of interest. These raw spectral measurements reflect absolute metabolite concentrations which may then be further normalized into conventional units or expressed as dimensionless concentration ratios to some within-subject reference metabolite such as creatine (Cr). The use of metabolite (or water) ratios does correct for differences in excitation within a voxel of interest. However, when using this method, if a change in normalized data is observed, it is impossible to tell whether the numerator (the metabolite of interest) or the denominator (the reference metabolite, often Cr) is changing (Jansen et al., 2006). In early MRS studies, the creatine spectral peak (Cr-PCr) was commonly chosen as the reference metabolite as it was hypothesized to be constant and comparable between brain regions or participant populations; however it has been demonstrated that this assumption does not always hold, even in healthy individuals. In fact, coefficients of variation are higher in ratio studies than in absolute quantification studies (Schirmer and Auer, 2000; Li et al., 2003). The assumption of uniform concentration of a reference metabolite is even more unreliable in abnormal populations such as patients with SZ (Ongur et al., 2010b). Therefore, although the use of a reference metabolite such as Cr was commonly used in the early MRS literature, in recent years this practice has diminished in favor of absolute concentration measures with the caveat that normalization to the absolute water reference peak is still common practice as discussed below.
However, there are a number of other methodological considerations that affect spectral quantification as well, including radiofrequency coil properties, calibration procedures, spectral fitting methods, voxel corrections for fractional cerebral spinal fluid (CSF)/gray matter/white matter content, macromolecule suppression, and spectral editing techniques (Jansen et al., 2006). The acquisition of a spatially resolved spectroscopic signal for a metabolite of interest requires the selection of a significant number of spectrometer acquisition parameters. Each of these parameters has an impact on the characteristics of the spectrometer signals used to excite the specific brain region being analyzed, and in the resultant spectrum obtained from the excitation echoes. The conversion of an integrated area under a spectral peak for a specific resonance line to a metabolite concentration requires a number of approximations. A general expression for this relationship between signal intensity I and metabolite concentration [M] is:
| [1] |
where I = signal intensity, c1 = constant, N = number of equivalent atoms per molecule, [M] = metabolite concentration, V = volume, B1(r) = reception field distribution, L = function of radiofrequency coil loading, θ = RF excitation tip angle, TE = acquisition delay or echo time (depending upon method), T2* = spin-spin transverse relaxation time including static field effects, TR = pulse repetition time, and T1 = spin-lattice relaxation time.
The goal in MRS experiments is to hold values of c1, N, V, B1(r), and θ constant, to the extent possible, or, when necessary, to correct for variations. For example, L (the amount of power necessary to transmit the signal) and the signal to noise ratio (SNR) is dependent on the volume of the object near the coil (ie. the size and tissue composition of the head being examined) and by the electrical impedance of the coil when “loaded” with a subject’s head. Larger, denser objects require more transmitted power to achieve a constant flip angle θ. As the size of the participant’s head cannot be controlled, this is a source of variability, although some experimenters attempt to control for this by measuring the power received by the coil and the SNR and calculating the volume of the head (Jansen et al., 2006). T1 is assumed to be constant, and in most proton MRS experiments, T1 variability is considered to have a negligible effect especially at a longer TR. Saturation of longitudinal magnetization due to repeated pulses in standard MRS pulse sequences also tend to reduce T1 effects. Most TRs for these experiments range from 1500–3000ms, and the T1 for NAA at 1.5 and 3T is ~1300–1400 ms (Rutgers and van der Grond, 2002; Traber et al., 2004). This review does assume that T1 is not variable between groups, however this could be an interesting topic of a future study, especially one focused on phosphorus MRS findings as the variability would manifest as a TR-dependence (long vs. short) in the observed MRS signal.
Thus, after eliminating all other terms of the above equation as sources of variance, this review will focus on the possibility that differential NAA concentration measurements between experiments could be due to the selection of long versus short TEs during signal acquisition because long TE experiments are more sensitive to any differences in T2 relaxation times between HC and SZ groups. The T2 relaxation time reflects the mean decay time of the MR signal or free-induction decay (FID) for a given metabolite, and different metabolites have different T2 relaxation times. Mobile molecules will have longer T2 times (longer FIDs) than less mobile molecules. Therefore, if the local micro-environment in which the metabolite of interest resides is altered, then relaxation times (and therefore measures of metabolite concentrations) may also be affected. This is especially important when normalizing metabolites that are intracellular only (ie NAA) to molecules which are found in both the intracellular and extracellular space (ie Cr) as changes in the relaxation times of metabolites in these two compartments could be differentially affected by an abnormal environment. Studies that normalize to water rather than Cr do not avoid this problem either, as previous studies have found schizophrenia-related changes in water proton relaxation times (Andreasen et al., 1991; Williamson et al., 1992; Supprian et al., 1997; Pfefferbaum et al., 1999; Aydin et al., 2007; Ongur et al., 2010b). For instance, some studies have found that within groups of patients with SZ, T2 relaxation times of intracellular metabolites (Cr + phosphocreatine, choline containing compounds) are reduced compared to that of HC subjects (Ongur et al., 2010b). The authors suggest that this could be due to a decrease in neuronal cell volumes and/or increased macromolecule concentrations resulting in increased metabolite-macromolecule interactions and more rapid loss of transverse magnetization (decreased T2 relaxation time) (Ongur et al., 2010b).
The existence of significant discrepancies in MRS research literature examining SZ has motivated this review of these assumptions and analysis of the published results. These questions were tangentially addressed in an insightful review and meta-analysis by Steen et al., (2005) which concluded that some of the inconsistency in findings on NAA within the literature are due to many of these studies being underpowered. The present review focuses instead on published proton MRS literature reporting proton MRS measurements of NAA in SZ research with a focus on the choice of TE. These analyses reveal evidence that certain analytic assumptions may not hold in comparisons of quantitative spectroscopic data between patients with SZ and HCs.
2 METHODS
A pubmed search was performed with the keywords schizoph*, spectroscopy, “magnetic resonance”, brain, and limited to English language articles on humans with an abstract available. This search resulted in 351 articles. Of these, 239 were excluded. For exclusion details, see Table 1. In addition, three more articles were found in reference lists (Renshaw et al., 1995; Bertolino et al., 1998; Deicken et al., 1999) resulting in 115 journal articles. A table of data from all studies included in the analysis can be found in Table 2. Studies were not examined for details of patient selection such as matching to control participants by age, sex, symptom profiles, or for duration of illness. Only peer reviewed, published reports were included in the analysis. Subjects who meet diagnostic criteria for schizophrenia comprise a very heterogeneous population, and the studies included in the analysis, in the aggregate, reflect this diversity reporting results from study populations that span different age ranges, illness durations, and symptom severity.
Table 1.
Articles excluded from the final analysis
| N | Reason for exclusion |
|---|---|
| 58 | Non-MRS methodology |
| 57 | Review articles with no new data |
| 55 | Phosphorus MRS only |
| 29 | Methods only/no new statistics/correlational only |
| 25 | No separate healthy control or patient groups |
| 8 | Postmortem/cerebrospinal fluid/serum study |
| 2 | Spectral normalization to choline |
| 2 | N > 5 |
| 2 | Did not report TE |
| 1 | Not in humans |
TABLE 2.
Data from all studies included in the analysis
| Finding | TE | N | Brain Region | Normalization | Citation |
|---|---|---|---|---|---|
| = | 40ms | 7 SZ; 7 HC | BG | Cr | (Ando et al., 2002) |
| = | 35ms | 32 SZ; 17 HC | Parietal WM; Thal | Concentration | (Auer et al., 2001) |
| ↓ in FE = in chronic | 30ms (multi-TE) | 12 FE; 16 chronic; 15 HC | Genu of Corpus Callosum | Concentration | (Aydin et al., 2007) |
| ↓ | 30ms (multi-TE) | 14 SZ; 15 HC | Genu of Corpus Callosum | Concentration | (Aydin et al., 2008) |
| = | 20ms | 10 SZ; 10 HC | mPFC | Concentration | (Bartha et al., 1997) |
| = in GM | 20ms | 11 FE SZ; 11 HC | Left mesial-TL | Concentration | (Bartha et al., 1999) |
| ↓ | 171ms | 10 SZ; 10 HC | dlPFC, HP | Cr | (Bertolino et al., 1996) |
| ↓ | 272ms | 10 SZ; 10 HC | dlPFC, HP | Cr | (Bertolino et al., 1998a) |
| ↓ | 272ms | 14 SZ; 14 HC | dlPFC, HP | Cr | (Bertolino et al., 1998c) |
| ↓ in dlPFC & HP = in others | 272ms | 12 SZ; 12 HC | dlPFC, HP, Thal, superior temporal gyrus, ACC, PCC, Occip, OFC, PFC WM, centrum semiovale | Cr | (Bertolino et al., 1998b) |
| ↓ | 272ms | 13 SZ; 13 HC | Bilateral dlPFC | Cr | (Bertolino et al., 2000) |
| ↓ | 272ms | 24 SZ; 24 HC | HP, dlPFC | Cr | (Bertolino et al., 2003) |
| ↓ in HP = in others | 272ms | 17 SZ; 17 HC | dlPFC, HP, Thal, superior temporal gyrus, ACC, PCC, Occip, OFC, PFC WM, centrum semiovale, putamen, inferior temporal gyrus, superior cingulate, | Cr | (Blasi et al., 2004) |
| = | 272ms | 25 SZ; 19 HC | BG, FL | Cr | (Block et al., 2000) |
| = | 30ms | 11 SZ; 15 HC | TL | Concentration | (Bluml, 1999) |
| ↓ | 136ms | 16 SZ; 12 HC | FL | Cr | (Brooks et al., 1998) |
| = | 68ms | 28 SZ; 20 HC | TL, FL | Concentration | (Buckley et al., 1994) |
| ↓ haloperidol = clozipine | 40ms BG; 30ms FL | 38 SZ; 21 HC | BG, FL | Concentration | (Bustillo et al., 2001) |
| = | 40ms | 11 SZ; 11 HC | BG | Concentration | (Bustillo et al., 2002a) |
| ↓ FL medicated = in others | 40ms | 20 SZ; 10 HC | FL, Occip | Concentration | (Bustillo et al., 2002b) |
| = | 40ms | 32 SZ; 21 HC | FL, Occip, caudate, cerebellar | Concentration | (Bustillo et al., 2008) |
| ↓ | 20ms | 14 SZ; 10 HC | ACC | Concentration | (Bustillo et al., 2010) |
| ↓ in H = in others | 272ms | 47 SZ; 66 HC | HP, ACC, posterior cingulate, centrum semiovale, Occip, FL WM, Thal, putamen, OFC, dlPFC, superior temporal gyrus | Cr | (Callicott et al., 1998) |
| ↓ in HP & PFC = in others | 272ms | 13 SZ; 18 HC | ACC, putamen, PFC, HP, Thal | Cr | (Callicott et al., 2000b) |
| = | 272ms | 36 SZ; 73 HC | Centrum semiovale, superior temporal gyrus, OFC, ACC, posterior cingulate, Occip, FL WM | Cr | (Callicott et al., 2000a) |
| ↓ | 21ms dlPFC; 19 ms midTL | 10 SZ; 14 HC | dlPFC, midTL | Cr | (Cecil et al., 1999) |
| = Occip ↓ in others | 30ms | 23 SZ; 22 HC | FL (L and R), TL (L and R), Occip | Concentration | (Chang et al., 2007) |
| ↓ | 20ms | 23 SZ; 10 HC | FLWM | Cr | (Choe et al., 1994) |
| ↓ | 20ms | 34 SZ; 20 HC | PFC (L and R) | Cr | (Choe et al., 1996) |
| ↓ | 135ms | 24 SZ; 15 HC | FL (L) | Concentration | (Deicken et al., 1997b) |
| ↓ | 135ms | 26 SZ; 16 HC | ACC (R and L) | Concentration | (Deicken et al., 1997a) |
| ↓ | 135ms | 30 SZ; 28 HC | HP (R and L) | Concentration | (Deicken et al., 1998) |
| ↓ | 135ms | 23 SZ; 18 HC | HP (L and R) | Concentration | (Deicken et al., 1999) |
| ↓ | 135ms | 17 SZ; 10 HC | Thal (L and R) | Concentration | (Deicken et al., 2000) |
| ↓ | 135ms | 20 SZ; 15 HC | CB | Concentration | (Deicken et al., 2001) |
| ↓ with deficit syndrome = in all together | 30ms | 17 SZ; 5 deficit syndrome; 22 HC | mPFC (L and R) | Cr | (Delamillieure et al., 2000b) |
| = | 30ms | 27 SZ; 24 HC | Thal (L and R) | Cr | (Delamillieure et al., 2000a) |
| = | 30ms | 17 SZ; 14 HC | mPFC, Thal, HP | Cr | (Delamillieure et al., 2002) |
| ↓ pons = cerebellum | 30ms | 12 SZ; 8 HC | Cerebellum, pons | Cr | (Eluri et al., 1998) |
| ↓ | 135ms | 19 SZ; 16 HC | Anterior cingulate gyrus | Concentration | (Ende et al., 2000) |
| ↓ | 135ms | 15 SZ; 15 HC | Bilateral Thal | Concentration | (Ende et al., 2001) |
| ↓ Thal & HP = putamen | 135ms | 13 SZ; 15 HC | Putamen, HP, Thal | Concentration | (Ende et al., 2003) |
| = pons, dentate nucleus ↓ in others | 135ms | 14 SZ; 14 HC | Pons, CB, cerebellar cortex, dentate nucleus | Concentration | (Ende et al., 2005) |
| ↓ non-med. HP = in others | 35ms | 32 SZ; 18 HC | BG, PFC, HP | Cr | (Fannon et al., 2003) |
| = | 135ms | 13 SZ; 12 HC | BG (L and R) | Cr | (Fujimoto et al., 1996) |
| ↓ medial TL; = FL | 135ms | 15 SZ; 15 HC | Medial TL (L), FL (L) | Cr | (Fukuzako et al., 1995) |
| ↓ | 60ms | 64 SZ; 51 HC | Medial TL (L) | Cr | (Fukuzako et al., 1996) |
| ↓ | 60ms | 40 SZ; 40 HC | Medial TL (L) | Cr | (Fukuzako et al., 1999) |
| = | 35ms | 15 short prodromal; 15 long prodromal; 19 HC | FL (L), TL (L), Thal (L) | Cr | (Galinska et al., 2009) |
| = FL ↓ others | 68ms | 18 SZ; 18 HC | BG (L), frontal, parieto-Occip | Cr | (Goto et al.) |
| = | 270ms | 13 SZ; 13 HC | Frontal cortex (L and R), Thal (L and R) | Cr | (Hagino et al., 2002) |
| = | 30ms | 18 SZ; 31 HC | BG, FL, Thal (L and R), TL | Cr | (Heimberg et al., 1998) |
| ↓ | 126ms | 12 SZ; 13 HC | Frontal (L) | Cr | (Hendren et al., 1995) |
| ↓ | 135ms | 22 SZ; 22 HC | Thal (L and R) | Concentration | (Jakary et al., 2005) |
| = TL ↓ in others | 272ms | 21 SZ; 31 HC | ACC, FL, TL | Cr | (Jessen et al., 2006) |
| = | 20ms | 10 SZ; 10 HC | HP | Cr | (Kegeles et al., 2000) |
| = | 20ms | 11 high risk; 12 HC | ACC | Cr | (Keshavan et al., 1997) |
| ↓ | 30ms | 40 SZ; 46 HC | Caudate | Concentration | (Keshavan et al., 2009) |
| ↓ | 80ms | 29 SZ; 44 HC | HP (L) | Concentration | (Klar et al., 2010) |
| = GM ↓WM | 144ms | 10 SZ; 9 HC | PFC, TL, PL, Occip | Concentration | (Lim et al., 1998) |
| ↓ in left = right | 135ms | 25 SZ; 32 HC | HP (L and R) | Concentration | (Maier et al., 1995) |
| = | 135ms | 26 SZ; 38 HC | HP (L and R) | Concentration | (Maier and Ron, 1996) |
| = | 135ms | 26 SZ; 38 HC | HP (R) | Concentration | (Maier et al., 2000) |
| ↓ | 272ms | 49 SZ; 37 HC | Thal (L and R) | Cr | (Martine z-Granados et al., 2008) |
| ↓ in SZ w/GS = in SZ w/o GS | 30ms | 15 SZ w/GS; 15 SZ w/o GS; 15 HC | BG (L), CV?, HP (L) | Cr | (Miyaoka et al., 2005) |
| = in recent onset ↓ in chronic | 136ms | 16 recent onset; 19 chronic; 20 HC | dlPFC (L and R) | Cr | (Molina et al., 2005) |
| ↓ dlPFC (R) in chronic = others | 136ms | 17 FE SZ; 17 chronic SZ; 20 HC | dlPFC (L and R) | Cr | (Molina et al., 2006) |
| ↓ | 136 | 11 SZ; 10 HC | dlPFC (L and R) | Cr | (Molina et al., 2007) |
| ↓ on right | 50ms | 11 SZ; 11 HC | HP/amygdala | Concentration | (Nasrallah et al., 1994) |
| = | 40ms | 10 SZ; 10 HC | BG | Concentration | (Ohara et al., 2000) |
| ↓ | 20ms | 15 FE; 20 chronic | Left dlPFC | Concentration | (Ohrmann et al., 2007) |
| ↓ dlPFC = ACC | 32ms | 43 SZ; 37 HC | ACC, dlPFC | Concentration | (Ohrmann et al., 2008) |
| ↓ Thal = FL | 136ms | 20 SZ; 18 HC | FL, Thal | Cr | (Omori et al., 2000) |
| = | 272ms | 11 pediatric; 11 SZ; 20 HC | ACC (inferior and superior; L and R), Putamen, caudate, frontal WM (L and R), frontal cortex (L and R), Occip (L and R), parietal WM (L and R), parietal (L and R), Thal (L and R) | Concentration | (O’Neill et al., 2004) |
| = | 30ms (multi-TE) | 21 SZ; 19 HC | ACC, POC | Concentration | (Ongur et al., 2008) |
| ↓ ACC = POC | 30ms (multi-TE) | 17 SZ; 21 HC | ACC, POC | Concentration | (Ongur et al., 2010a) |
| ↓ | 20ms | 24 SZ; 20 HC | FL (L and R) | Cr | (Pae et al., 2004) |
| ↓ | 35ms | 30 SZ; 15 HC | Dorsal ACC | Concentration | (Premkumar et al., 2010) |
| = | 20ms | 15 SZ; 14 HC | Medial FL | Concentration | (Purdon et al., 2008) |
| = | 80ms | 26 SZ; 23 HC | Bilateral dorsal ACC | Cr | (Reid et al., 2010) |
| ↓ | 30ms | 13 SZ; 15 HC | TL (L and R) | Cr | (Renshaw et al., 1995) |
| = | 35ms | 10 w/deficit syndrome; 10 w/o; 11 HC | Middle PFC (L), inferior parietal (L) | Concentration | (Rowland et al., 2009) |
| = | 30ms | 29 SZ; 31 HC | dlPFC (L), HP | Concentration | (Rusch et al., 2008) |
| = | 35ms | 14 SZ; 15 HC | Cingulate gyrus | Cr | (Sarramea Crespo et al., 2008) |
| = | 28.5ms | 4 SZ; 9 HC | BG, Occip | Cr | (Sharma et al., 1992) |
| ↓PCG? = temporal | 144ms | 19 SZ; 18 HC | PCG??, TL | Cr | (Shimizu et al., 2007) |
| = | 135ms | 21 SZ; 21 HC | BG | Concentration | (Shioiri et al., 1996) |
| = | 102ms | 19 SZ; 18 HC | Medial PFC Cortex | Concentration | (Shirayama et al., 2010) |
| = | 136ms | 25 SZ; 26 HC | dlPFC (L and R) | Concentration | (Sigmundsson et al., 2003) |
| = | 20ms | 32 SZ; 24 HC | FL | Concentration | (Stanley et al., 1996) |
| ↓ early onset = others | 20ms | 8 early onset; 10 late onset; 34 HC | dlPFC | Concentration | (Stanley et al., 2007) |
| = | 145ms | 10 SZ; 10 HC | FL (L and R) | Concentration | (Steel et al., 2001) |
| ↓ Thal = others | 30ms | 27 SZ; 27 HC | HP (L), ACC, Thal (L) | Concentration | (Stone et al., 2009) |
| = | 35ms | 106 SZ (separated by med); 21 HC | FL, TL, Thal | Concentration | (Szulc et al., 2007) |
| ↓ | 30ms | 14 SZ; 13 HC | FL (L) | Concentration | (Tanaka et al., 2006) |
| ↓ medial TL = others | 30ms | 42 SZ; 40 HC | Medial TL (L and R) frontal (L and R) Occip (L and R) | Concentration | (Tang et al., 2007) |
| ↓ ACC = BG | 18ms | 31 SZ; 26 HC | ACC, BG | Concentration | (Tayoshi et al., 2009) |
| = | 5ms | 13 SZ; 3 HC | ACC | Concentration | (Terpstra et al., 2005) |
| = | 20ms | 21 SZ; 28 HC | ACC (L), Thal (L) | Concentration | (Theberge et al., 2002) |
| = | 20ms | 21 SZ; 21 HC | ACC (L), Thal (L) | Concentration | (Theberge et al., 2003) |
| ↓ FL = OC | 20 ms | 13 SZ; 12 HC | FL, OC | Cr | (Thomas et al., 1998) |
| = | 120ms | 12 SZ; 12 HC | CB | Cr | (Tibbo et al., 2000) |
| = | 30ms | 21 SZ; 33 HC | dlPFC (L), HP (L) | Concentration | (van Elst et al., 2005) |
| = | 30ms | 29 SZ; 24 HC | ACC (L and R), HP (L and R) | Concentration | (Venkatraman et al., 2006) |
| ↓ | 135ms | 15 SZ; 15 HC | HP | Concentration | (Weber-Fahr et al., 2002) |
| = | 30ms | 22 SZ; 41 HC | Frontal cortex | Cr | (Wobrock et al., 2008) |
| ↓ dlPFC = others | 135ms | 56 SZ; 21 HC | dlPFC (L), medial TL (L) | Cr | (Wood et al., 2003) |
| ↓ | 30ms | 15 SZ; 14 HC | Dorsal ACC (L and R), rostral ACC (L and R) | Concentration | (Wood et al., 2007) |
| = | 30ms | 34 SZ; 19 HC | HP (L and R) | Concentration | (Wood et al., 2008) |
| = | 35ms | 15 SZ; 14 HC | ACC | Cr | (Yamasue et al., 2002) |
| = Thal w/o GS ↓ others | 30ms | 15 w/GS; 15 SZ w/o; 20 HC | ACC (L), insular cortex, Thal | Cr | (Yasukawa et al., 2005) |
| ↓ Thal = others | 140ms | 22 SZ; 22 HC | ACC, dlPFC, Thal | Concentration | (Yoo et al., 2009) |
| ↓ | 20ms | 16 SZ; 14 HC | TL (L and R) | Cr | (Yurgelun-Todd et al., 1996) |
| ↓ dlPFC (L) = dlPFC (R) | 136ms | 8 SZ; 33 HC | dlPFC (L and R) | Concentration | (Zabala et al., 2007) |
ACC=anterior cingulate cortex; BG=basal ganglia; CB=cerebellar vermis; dlPFC=dorso-lateral prefrontal cortex; FL=frontal lobe; HP=hippocampus;Occip=occipital lobe; PCC=posterior cingulate cortex; PL=parietal lobe; Thal=thalamus; TL=temporal lobe
HC=healthy control; SZ=schizophrenic; L=left; R=right
Reported results for NAA concentration in patients with SZ and HC participants, the spectrometer acquisition parameter TE, normalization technique (the ratio of NAA to Cr versus examination of absolute concentration of NAA), and region of interest were recorded for each study. The regions of interest included: Anterior Cingulate (ACC), Basal Ganglia, Cerebellum, Frontal Lobe (including dorso-lateral Prefrontal Cortex, Orbitofrontal Cortex, and Prefrontal Cortex), Hippocampus/Temporal Lobe, Thalamus/Putamen, Occipital lobe, Parietal Lobe, and other (including Centrum, Pons, Insular Cortex, Cingulate Gyrus, Centrum Semiovale, Dentate Nucleus, gray matter (whole brain) and white matter (whole brain). When more than one region of interest was examined, each region was counted as a separate experiment. The goal of this analysis is to test for acquisition parameter dependence of published results and since the regional distribution of NAA T2 abnormalities is unknown each separate experiment was considered independent. This assumption is not required for the region specific results reported. Also, when more than one population was examined (ie. different medication groups, or participants with and without deficit syndrome), each population was counted as a separate experiment. A cut off of 40 ms was used to differentiate long TE from short TE methods. This choice of 40 ms was driven by a natural partition in the data set with the vast majority of studies having either a TE over 100 ms or ≤ 40 ms. In fact, of all 115 journal articles included in the current analyses, only 4 studies had a TE between 40 and 100 ms (Table 2).
A statistical test for TE dependence of the published NAA results was performed using the 1-sided Fisher’s Exact Test with TE (short versus long) and NAA finding (decrease versus no change) as the variables of interest in a 2×2 table. For details of breakdown of analysis groups see Table 3. All N’s refer to the number of experimental results (all regions, both normalization methods, and all studies), rather than the number of subjects. First, data from all studies (both those using normalization to Cr and those quantifying absolute concentrations) were broken down by region. As discussed in the introduction, normalization to an internal “reference metabolite” may create additional confounds due to possible reference metabolite variation between subjects, so experiments were then broken down into two groups: studies normalizing to Cr (n=200) and studies which yielded absolute concentrations of NAA (n=186). Finally, data both Cr normalization and absolute concentration studies and all regions of interest were tested (n=333). Studies which included both normalization techniques were included in each individual data set (when they were segregated into studies which normalized to Cr versus studies which yielded absolute concentrations), however only absolute concentration data for these studies were included in the “all studies, all regions” analysis).
TABLE 3.
Details of breakdown of analysis groups
| Analysis Set | Total N* | Lower NAA, TE ≥ 40 ms | Lower NAA, TE < 40ms | Unchanged NAA, TE ≥ 40 ms | Unchanged NAA, TE < 40 ms |
|---|---|---|---|---|---|
| All brain regions | |||||
| Cr normalization and NAA concentration | 333 | 56 | 54 | 99 | 124 |
| Cr normalization only | 195 | 28 | 37 | 52 | 78 |
| NAA concentration only | 185 | 35 | 25 | 52 | 73 |
| Region of interest analysis; Cr normalization and NAA concentration | |||||
| Frontal Cortex | 100 | 15 | 19 | 28 | 38 |
| Occipital Lobe | 15 | 0 | 0 | 7 | 8 |
| Parietal Lobe | 11 | 1 | 1 | 4 | 5 |
| Hippocampus and Temporal Lobe | 71 | 19 | 14 | 14 | 24 |
| Thalamus and Putamen | 41 | 10 | 4 | 10 | 17 |
| ACC | 36 | 4 | 9 | 10 | 13 |
| Basal Ganglia | 31 | 0 | 4 | 12 | 15 |
| Cerebellum | 8 | 4 | 0 | 2 | 2 |
| “Other” | 20 | 3 | 3 | 11 | 3 |
| Region of interest analysis; Cr normalization only | |||||
| Frontal Cortex | 63 | 13 | 10 | 14 | 26 |
| Occipital Lobe | 8 | 0 | 0 | 4 | 4 |
| Parietal Lobe | 3 | 1 | 1 | 0 | 1 |
| Hippocampus and Temporal Lobe | 43 | 10 | 12 | 7 | 14 |
| Thalamus and Putamen | 31 | 2 | 9 | 13 | 4 |
| ACC | 13 | 1 | 2 | 6 | 4 |
| Basal Ganglia | 18 | 0 | 2 | 4 | 12 |
| Cerebellum | 2 | 0 | 0 | 1 | 2 |
| “Other” | 14 | 1 | 3 | 7 | 3 |
| Region of interest analysis; NAA concentration | |||||
| Frontal Cortex | 53 | 5 | 10 | 14 | 24 |
| Occipital Lobe | 9 | 0 | 0 | 3 | 6 |
| Parietal Lobe | 8 | 0 | 0 | 4 | 4 |
| Hippocampus and Temporal Lobe | 39 | 12 | 4 | 7 | 16 |
| Thalamus and Putamen | 21 | 8 | 2 | 3 | 8 |
| ACC | 25 | 3 | 7 | 6 | 9 |
| Basal Ganglia | 16 | 0 | 2 | 8 | 6 |
| Cerebellum | 6 | 4 | 0 | 1 | 1 |
| “Other” | 8 | 3 | 0 | 5 | 0 |
N refers to the total number of experiments, not the total number of participants or studies. Please see the Methods section for more details.
3 RESULTS
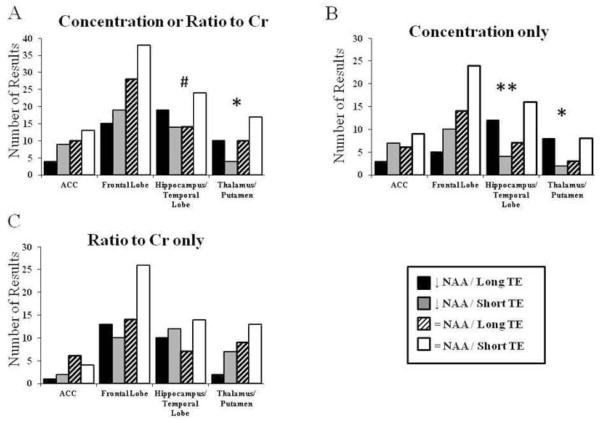
See Table 2 for a complete list of studies, study findings, regions of interest in each study, and TE used in each study. The first analysis included data normalized to Cr as well as data which quantified absolute concentrations (Figure 1A). The comparison of NAA findings between long and short TE studies was significant within the thalamus and putamen (Figure 1A; Fischer’s Exact Test p=0.039). All other comparisons were not significant, although there was a trend level change within the hippocampus and temporal cortex (Figure 1A; Fischer’s Exact Test p=0.053). The second analysis included only data from studies which quantified absolute concentrations of NAA (Figure 1B). The comparison of NAA findings between long and short TE studies was significant within the thalamus and putamen (Figure 1B; Fischer’s Exact Test p=0.023), as well as the hippocampus and temporal cortex (Figure 1B; Fischer’s Exact Test p=0.007). In a final analysis, only regional data from studies which used metabolite ratios normalized to Cr were included, and all comparisons were not significant (Figure 1C).
Figure 1.
Count of results organized by NAA concentration finding and TE methodology. This includes studies quantifying absolute NAA concentration for each region of interest from both quantification methods:-- normalizing to Cr or quantifying absolute NAA concentration (A), data from studies which only quantified absolute NAA concentration (B), and studies which only normalized to Cr (C). Data are separated by brain region. Data are expressed as a number of results. # = p < 0.10, * = p < 0.05, ** = p < 0.01
Because the field strength at which the experiment was conducted affects metabolite specific T1 and T2 relaxation rates, there may be an enhancement or minimization of the T2 effect when different field strengths are used. Therefore, we also conducted the analyses, including only studies that were conducted at 1.5T. When this analysis included data which normalized to Cr as well as data which quantified absolute concentration, the comparison of NAA findings between long and short TE studies was significant within the thalamus and putamen (Fischer’s Exact Test p=0.042. When this analysis included only data from studies which quantified absolute concentrations of NAA, the comparison of NAA findings between long and short TE studies was significant within the thalamus and putamen (Fischer’s Exact Text p=0.043), as well as the hippocampus and temporal cortex (Fischer’s Exact Test p=0.013). When this analysis included only data normalized to Cr, all comparisons were not significant.
Finally, data from all regions of interest were examined. Although there are a number of limitations associated with this analysis, such as combining data from assessments of both gray and white matter, these analysis are meant to assess findings within the literature as a whole, rather than only assessing findings from regions of interest that have attracted sufficient attention to merit several publications. In this all-inclusive analysis, there was a significant difference in comparison of NAA finding between long and short TE studies when only data from studies which quantified absolute concentration were included in the analysis (Figure 2; Fischer’s Exact Test p=0.024). Comparisons which included only data normalized to Cr, and comparisons including both Cr normalization and absolute concentrations were not significant (Figure 2). For the same rational mentioned above, we repeated this analysis including only studies that were conducted at 1.5T. When we included only data only from studies which quantified absolute concentrations of NAA, the comparison of NAA findings between long and short TE studies decreased to a trend level of significance (Fischer’s Exact Test p=0.057).
Figure 2.
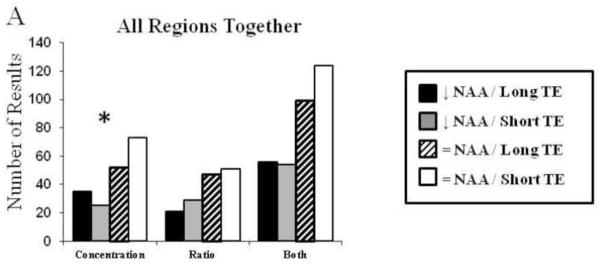
Count of results organized by NAA concentration finding and TE methodology. This includes studies quantifying absolute NAA concentration for all brain regions assessed.
4 DISCUSSION
The analyses in the current study provide evidence that the published literature to date on MRS studies of schizophrenia is partially confounded by T2 effects. Studies with one set of acquisition parameters (long TE) were much more likely to report lower concentrations of NAA in patients with SZ, while studies with different acquisition parameters (short TE) were likely to report no change. Previous publications have also noted this possibility (Olson et al., 2003; Sanches et al., 2004; Tunc-Skarka et al., 2009), however the current study uses a much larger data set to confirm this bias. Several MRS studies have now reported metabolite and water T2 relaxation differences in some brain regions of schizophrenic subjects, further supporting the assertion that T2 effects have likely been confounding measurements reported in the MRS schizophrenia literature for decades (Tunc-Skarka et al., 2009; Ongur et al., 2010b).
Even so, these studies do also confirm reduced NAA concentrations in patients with schizophrenia. Indeed, one caveat to our analysis is that our use of the χ2 technique necessitated that we treat each study with equal weight, regardless of sample size. A meta-analytic approach to the same data may yield different results, perhaps showing reduced NAA even at longer TEs. In addition, it is important to note that Tunc-Skarka et al report NAA T2 findings in relatively homogeneous white matter voxels whereas Ongur et al, and other studies reporting T2 relaxation times in NAA and other brain metabolites in gray matter voxels are subject to partial volume effects including both concentration and relaxation rate differences for NAA (and other metabolites) in white vs. gray matter. While partial volume corrections are routinely done for fractional gray vs. white voxel composition, they are rarely, if ever, done for differential relaxation rates. As discussed below, between group water proton T2 relaxation differences also contribute to the list of potential confounds.
So what does this mean for future MRS studies? It should be noted that long TE methods were originally used because early gradient systems on MRI scanners had significant difficulties with eddy currents that made short TE spectra difficult to interpret. Hardware advances, driven by explosive interest in the use of echo-planar MRI largely resolved this issue. This advance, coupled with the growing awareness of the potential confounding effects of long TE MRS acquisition (with resultant T2 effects on spectral quantification), has allowed for a shift away from longer echo times in the published MRS literature since 2004 (Figure 3). While short TE MRS studies minimize T2 weighting, the advantages of measuring metabolite T2 times should not be overlooked.
Figure 3.
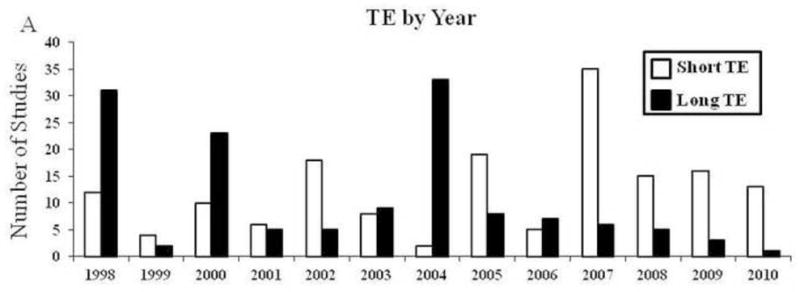
Number of studies with long versus short TE organized by year. Data are expressed as a count of studies.
For example, when metabolite T2 times are measured, absolute metabolite concentrations may be properly corrected for residual T2 weighting – even at short echo times. This would be especially important in instances when T2 effects on the MRS signal may oppose and partially or completely obscure changes due to concentration. As one hypothetical example: if NAA concentration within a voxel is increased due to increased neuronal cellular packing density, but NAA T2 is decreased (due to decreased neuronal cell volume and increased spin-spin interactions with macromolecules causing more rapid signal dephasing), these factors would tend to cancel each other out, and the (uncorrected) concentration acquired by a simple single TE MRS experiment could indicate that NAA concentration is not changing when it is actually increased. However, if the experimenter were to measure the NAA T2 relaxation time as well as the T2 corrected NAA concentration, both important changes within the microstructural environment could be identified. Choice of normalization technique also introduces complex T2 considerations. Even the preferred water normalization method, which uses the unsuppressed water signal as a normalizing factor can be affected by between group differences in tissue water concentration and water proton T2 differences. Higher water proton T2 relaxation times have been reported in schizophrenia (Tunc-Skarka et al., 2009; Ongur et al., 2010b). Using water as a reference denominator, we can predict that higher water T2 would artificially lower the normalized NAA concentration measurement in the context of unchanged or reduced NAA T2. This would have increased the likelihood of reporting lower NAA for studies at longer TE which did not correct for T2 relaxation effects.
In addition, metabolite concentration and T2 relaxation times are completely independent variables which offer distinct and complimentary information about the metabolite of interest. Changes in T2 relaxation times reflect changes in the metabolite-specific compartment of interest which is different for each metabolite. NAA T2 relaxation occurs primarily within the intra-neuronal cytosolic compartment, and changes in T2 relaxation times in individuals with SZ may correlate with specific changes within neurons. Decreased NAA T2 (reflecting more rapid NAA MRS signal dephasing due to spin-spin interactions with macromolecules in the cytosol) is consistent with smaller cell volume rather than complete loss of neurons. In this case, the suggestion that some component of decreased NAA may be due to T2 relaxation effects would be an encouraging finding as it might suggest a potentially reversible pathologic change, whereas complete neuronal loss is more problematic. Decreased NAA T2 is also consistent with the observed reduced gray matter volume, increased packing density, smaller cell size, and the relatively preserved number of neurons reported in the post-mortem literature for subjects with schizophrenia (Olson et al., 2003; Ongur et al., 2008; Ongur et al., 2010b; Ongur et al., 2010a). Similarly, T2 relaxation time measurements of other commonly studied MRS visible metabolites may yield additional insight into metabolite-specific micro-environments. For example, the portion of choline that is visible using MRS is the free component (non-bound) both within neurons, myelin, and in the extracellular compartment. Changes in choline T2 contrasted with changes in choline concentration can answer questions that concentration data alone cannot address.
In the future, experiments should be designed such that spectra are acquired at multiple TEs to provide an estimate of the individual T2 relaxation times for each metabolite. A study by Tunc-Skarka et al. (2009) took just this approach. Using a protocol including 30 ms, 80 ms, 200 ms, 300 ms, and 420 ms TEs, they scanned 23 patients with SZ and 29 HCs. They found a trend for reduced NAA concentrations at the lowest TE, which became significant in all longer TE experiments. They also found shortened NAA T2 relaxation times in the patients, and conclude that this is consistent with changes in microstructural white matter in patients with SZ. They found no changes in concentration or T2 relaxation time in any other metabolites, including glutamate, choline, and creatine. Although it does take additional time, designing experiments using this or a similar method will allow accurate metabolite concentrations to be calculated which take into account changes in metabolite T2 relaxation times in addition to metabolite concentration. This will avoid potential errors in concentration measurements as well as provide new and complimentary information about the local micro-environment of each metabolite species.
Acknowledgments
This research was supported by NIH grant T32 DA15036.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ando K, Takei N, Matsumoto H, Iyo M, Isoda H, Mori N. Neural damage in the lenticular nucleus linked with tardive dyskinesia in schizophrenia: a preliminary study using proton magnetic resonance spectroscopy. Schizophr Res. 2002;57:273–279. doi: 10.1016/s0920-9964(01)00290-0. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Ehrhardt JC, Swayze VW, 2nd, Tyrrell G, Cohen G, Ku JS, Arndt S. T1 and T2 relaxation times in schizophrenia as measured with magnetic resonance imaging. Schizophr Res. 1991;5:223–232. doi: 10.1016/0920-9964(91)90080-b. [DOI] [PubMed] [Google Scholar]
- Auer DP, Wilke M, Grabner A, Heidenreich JO, Bronisch T, Wetter TC. Reduced NAA in the thalamus and altered membrane and glial metabolism in schizophrenic patients detected by 1H-MRS and tissue segmentation. Schizophr Res. 2001;52:87–99. doi: 10.1016/s0920-9964(01)00155-4. [DOI] [PubMed] [Google Scholar]
- Aydin K, Ucok A, Cakir S. Quantitative proton MR spectroscopy findings in the corpus callosum of patients with schizophrenia suggest callosal disconnection. AJNR Am J Neuroradiol. 2007;28:1968–1974. doi: 10.3174/ajnr.A0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin K, Ucok A, Guler J. Altered metabolic integrity of corpus callosum among individuals at ultra high risk of schizophrenia and first-episode patients. Biol Psychiatry. 2008;64:750–757. doi: 10.1016/j.biopsych.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Bartha R, Williamson PC, Drost DJ, Malla A, Carr TJ, Cortese L, Canaran G, Rylett RJ, Neufeld RW. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54:959–965. doi: 10.1001/archpsyc.1997.01830220085012. [DOI] [PubMed] [Google Scholar]
- Bartha R, al-Semaan YM, Williamson PC, Drost DJ, Malla AK, Carr TJ, Densmore M, Canaran G, Neufeld RW. A short echo proton magnetic resonance spectroscopy study of the left mesial-temporal lobe in first-onset schizophrenic patients. Biol Psychiatry. 1999;45:1403–1411. doi: 10.1016/s0006-3223(99)00007-4. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Callicott JH, Nawroz S, Mattay VS, Duyn JH, Tedeschi G, Frank JA, Weinberger DR. Reproducibility of proton magnetic resonance spectroscopic imaging in patients with schizophrenia. Neuropsychopharmacology. 1998a;18:1–9. doi: 10.1016/S0893-133X(97)00090-0. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Callicott JH, Elman I, Mattay VS, Tedeschi G, Frank JA, Breier A, Weinberger DR. Regionally specific neuronal pathology in untreated patients with schizophrenia: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 1998b;43:641–648. doi: 10.1016/s0006-3223(97)00555-6. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Esposito G, Callicott JH, Mattay VS, Van Horn JD, Frank JA, Berman KF, Weinberger DR. Specific relationship between prefrontal neuronal N-acetylaspartate and activation of the working memory cortical network in schizophrenia. Am J Psychiatry. 2000;157:26–33. doi: 10.1176/ajp.157.1.26. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Nawroz S, Mattay VS, Barnett AS, Duyn JH, Moonen CT, Frank JA, Tedeschi G, Weinberger DR. Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. Am J Psychiatry. 1996;153:1554–1563. doi: 10.1176/ajp.153.12.1554. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Kumra S, Callicott JH, Mattay VS, Lestz RM, Jacobsen L, Barnett IS, Duyn JH, Frank JA, Rapoport JL, Weinberger DR. Common pattern of cortical pathology in childhood-onset and adult-onset schizophrenia as identified by proton magnetic resonance spectroscopic imaging. Am J Psychiatry. 1998c;155:1376–1383. doi: 10.1176/ajp.155.10.1376. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Sciota D, Brudaglio F, Altamura M, Blasi G, Bellomo A, Antonucci N, Callicott JH, Goldberg TE, Scarabino T, Weinberger DR, Nardini M. Working memory deficits and levels of N-acetylaspartate in patients with schizophreniform disorder. Am J Psychiatry. 2003;160:483–489. doi: 10.1176/appi.ajp.160.3.483. [DOI] [PubMed] [Google Scholar]
- Blasi G, Bertolino A, Brudaglio F, Sciota D, Altamura M, Antonucci N, Scarabino T, Weinberger DR, Nardini M. Hippocampal neurochemical pathology in patients at first episode of affective psychosis: a proton magnetic resonance spectroscopic imaging study. Psychiatry Res. 2004;131:95–105. doi: 10.1016/j.pscychresns.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Block W, Bayer TA, Tepest R, Traber F, Rietschel M, Muller DJ, Schulze TG, Honer WG, Maier W, Schild HH, Falkai P. Decreased frontal lobe ratio of N-acetyl aspartate to choline in familial schizophrenia: a proton magnetic resonance spectroscopy study. Neurosci Lett. 2000;289:147–151. doi: 10.1016/s0304-3940(00)01264-7. [DOI] [PubMed] [Google Scholar]
- Bluml S. In vivo quantitation of cerebral metabolite concentrations using natural abundance 13C MRS at 1.5 T. J Magn Reson. 1999;136:219–225. doi: 10.1006/jmre.1998.1618. [DOI] [PubMed] [Google Scholar]
- Brooks WM, Hodde-Vargas J, Vargas LA, Yeo RA, Ford CC, Hendren RL. Frontal lobe of children with schizophrenia spectrum disorders: a proton magnetic resonance spectroscopic study. Biol Psychiatry. 1998;43:263–269. doi: 10.1016/S0006-3223(97)00462-9. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Moore C, Long H, Larkin C, Thompson P, Mulvany F, Redmond O, Stack JP, Ennis JT, Waddington JL. 1H-magnetic resonance spectroscopy of the left temporal and frontal lobes in schizophrenia: clinical, neurodevelopmental, and cognitive correlates. Biol Psychiatry. 1994;36:792–800. doi: 10.1016/0006-3223(94)90591-6. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Lauriello J, Petropoulos H, Hammond R, Hart B, Brooks WM. High choline concentrations in the caudate nucleus in antipsychotic-naive patients with schizophrenia. Am J Psychiatry. 2002a;159:130–133. doi: 10.1176/appi.ajp.159.1.130. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Lauriello J, Rowland LM, Thomson LM, Petropoulos H, Hammond R, Hart B, Brooks WM. Longitudinal follow-up of neurochemical changes during the first year of antipsychotic treatment in schizophrenia patients with minimal previous medication exposure. Schizophr Res. 2002b;58:313–321. doi: 10.1016/s0920-9964(02)00210-4. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Jung R, Brooks WM, Qualls C, Hammond R, Hart B, Lauriello J. Proton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2008;33:2456–2466. doi: 10.1038/sj.npp.1301631. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Lauriello J, Rowland LM, Jung RE, Petropoulos H, Hart BL, Blanchard J, Keith SJ, Brooks WM. Effects of chronic haloperidol and clozapine treatments on frontal and caudate neurochemistry in schizophrenia. Psychiatry Res. 2001;107:135–149. doi: 10.1016/s0925-4927(01)00102-0. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C, Hammond R, Brooks WM, Lauriello J. 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psychiatry. 2010;15:629–636. doi: 10.1038/mp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Egan MF, Mattay VS, Langheim FJ, Weinberger DR. Selective relationship between prefrontal N-acetylaspartate measures and negative symptoms in schizophrenia. Am J Psychiatry. 2000a;157:1646–1651. doi: 10.1176/appi.ajp.157.10.1646. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Bertolino A, Mattay VS, Langheim FJ, Frank JA, Weinberger DR. Hippocampal N-acetyl aspartate in unaffected siblings of patients with schizophrenia: a possible intermediate neurobiological phenotype. Biol Psychiatry. 1998;44:941–950. doi: 10.1016/s0006-3223(98)00264-9. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000b;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Lenkinski RE, Gur RE, Gur RC. Proton magnetic resonance spectroscopy in the frontal and temporal lobes of neuroleptic naive patients with schizophrenia. Neuropsychopharmacology. 1999;20:131–140. doi: 10.1016/S0893-133X(98)00063-3. [DOI] [PubMed] [Google Scholar]
- Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry. 2007;62:1396–1404. doi: 10.1016/j.biopsych.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe BY, Suh TS, Shinn KS, Lee CW, Lee C, Paik IH. Observation of metabolic changes in chronic schizophrenia after neuroleptic treatment by in vivo hydrogen magnetic resonance spectroscopy. Invest Radiol. 1996;31:345–352. doi: 10.1097/00004424-199606000-00006. [DOI] [PubMed] [Google Scholar]
- Choe BY, Kim KT, Suh TS, Lee C, Paik IH, Bahk YW, Shinn KS, Lenkinski RE. 1H magnetic resonance spectroscopy characterization of neuronal dysfunction in drug-naive, chronic schizophrenia. Acad Radiol. 1994;1:211–216. doi: 10.1016/s1076-6332(05)80716-0. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Pegues M, Amend D. Reduced hippocampal N-acetylaspartate without volume loss in schizophrenia. Schizophr Res. 1999;37:217–223. doi: 10.1016/s0920-9964(98)00173-x. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Zhou L, Schuff N, Weiner MW. Proton magnetic resonance spectroscopy of the anterior cingulate region in schizophrenia. Schizophr Res. 1997a;27:65–71. doi: 10.1016/S0920-9964(97)00082-0. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Johnson C, Eliaz Y, Schuff N. Reduced concentrations of thalamic N-acetylaspartate in male patients with schizophrenia. Am J Psychiatry. 2000;157:644–647. doi: 10.1176/appi.ajp.157.4.644. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Feiwell R, Schuff N, Soher B. Evidence for altered cerebellar vermis neuronal integrity in schizophrenia. Psychiatry Res. 2001;107:125–134. doi: 10.1016/s0925-4927(01)00103-2. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Zhou L, Corwin F, Vinogradov S, Weiner MW. Decreased left frontal lobe N-acetylaspartate in schizophrenia. Am J Psychiatry. 1997b;154:688–690. doi: 10.1176/ajp.154.5.688. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Zhou L, Schuff N, Fein G, Weiner MW. Hippocampal neuronal dysfunction in schizophrenia as measured by proton magnetic resonance spectroscopy. Biol Psychiatry. 1998;43:483–488. doi: 10.1016/S0006-3223(97)00490-3. [DOI] [PubMed] [Google Scholar]
- Delamillieure P, Constans J, Fernandez J, Brazo P, Dollfus S. Proton magnetic resonance spectroscopy (1H-MRS) of the thalamus in schizophrenia. Eur Psychiatry. 2000a;15:489–491. doi: 10.1016/s0924-9338(00)00522-8. [DOI] [PubMed] [Google Scholar]
- Delamillieure P, Constans JM, Fernandez J, Brazo P, Benali K, Courtheoux P, Thibaut F, Petit M, Dollfus S. Proton magnetic resonance spectroscopy (1H MRS) in schizophrenia: investigation of the right and left hippocampus, thalamus, and prefrontal cortex. Schizophr Bull. 2002;28:329–339. doi: 10.1093/oxfordjournals.schbul.a006942. [DOI] [PubMed] [Google Scholar]
- Delamillieure P, Fernandez J, Constans JM, Brazo P, Benali K, Abadie P, Vasse T, Thibaut F, Courtheoux P, Petit M, Dollfus S. Proton magnetic resonance spectroscopy of the medial prefrontal cortex in patients with deficit schizophrenia: preliminary report. Am J Psychiatry. 2000b;157:641–643. doi: 10.1176/appi.ajp.157.4.641. [DOI] [PubMed] [Google Scholar]
- Eluri R, Paul C, Roemer R, Boyko O. Single-voxel proton magnetic resonance spectroscopy of the pons and cerebellum in patients with schizophrenia: a preliminary study. Psychiatry Res. 1998;84:17–26. doi: 10.1016/s0925-4927(98)00043-2. [DOI] [PubMed] [Google Scholar]
- Ende G, Braus DF, Walter S, Henn FA. Lower concentration of thalamic n-acetylaspartate in patients with schizophrenia: a replication study. Am J Psychiatry. 2001;158:1314–1316. doi: 10.1176/appi.ajp.158.8.1314. [DOI] [PubMed] [Google Scholar]
- Ende G, Braus DF, Walter S, Weber-Fahr W, Henn FA. Multiregional 1H-MRSI of the hippocampus, thalamus, and basal ganglia in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2003;253:9–15. doi: 10.1007/s00406-003-0398-5. [DOI] [PubMed] [Google Scholar]
- Ende G, Braus DF, Walter S, Weber-Fahr W, Soher B, Maudsley AA, Henn FA. Effects of age, medication, and illness duration on the N-acetyl aspartate signal of the anterior cingulate region in schizophrenia. Schizophr Res. 2000;41:389–395. doi: 10.1016/s0920-9964(99)00089-4. [DOI] [PubMed] [Google Scholar]
- Ende G, Hubrich P, Walter S, Weber-Fahr W, Kammerer N, Braus DF, Henn FA. Further evidence for altered cerebellar neuronal integrity in schizophrenia. Am J Psychiatry. 2005;162:790–792. doi: 10.1176/appi.ajp.162.4.790. [DOI] [PubMed] [Google Scholar]
- Fannon D, Simmons A, Tennakoon L, O’Ceallaigh S, Sumich A, Doku V, Shew C, Sharma T. Selective deficit of hippocampal N-acetylaspartate in antipsychotic-naive patients with schizophrenia. Biol Psychiatry. 2003;54:587–598. doi: 10.1016/s0006-3223(03)00185-9. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Nakano T, Takano T, Takeuchi K, Yamada K, Fukuzako T, Akimoto H. Proton magnetic resonance spectroscopy of basal ganglia in chronic schizophrenia. Biol Psychiatry. 1996;40:14–18. doi: 10.1016/0006-3223(95)00316-9. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Fukuzako T, Takeuchi K, Ohbo Y, Ueyama K, Takigawa M, Fujimoto T. Phosphorus magnetic resonance spectroscopy in schizophrenia: correlation between membrane phospholipid metabolism in the temporal lobe and positive symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:629–640. doi: 10.1016/0278-5846(96)00036-x. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Kodama S, Fukuzako T, Yamada K, Doi W, Sato D, Takigawa M. Subtype-associated metabolite differences in the temporal lobe in schizophrenia detected by proton magnetic resonance spectroscopy. Psychiatry Res. 1999;92:45–56. doi: 10.1016/s0925-4927(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Takeuchi K, Hokazono Y, Fukuzako T, Yamada K, Hashiguchi T, Obo Y, Ueyama K, Takigawa M, Fujimoto T. Proton magnetic resonance spectroscopy of the left medial temporal and frontal lobes in chronic schizophrenia: preliminary report. Psychiatry Res. 1995;61:193–200. doi: 10.1016/0925-4927(95)02622-5. [DOI] [PubMed] [Google Scholar]
- Galinska B, Szulc A, Tarasow E, Kubas B, Dzienis W, Czernikiewicz A, Walecki J. Duration of untreated psychosis and proton magnetic resonance spectroscopy (1H-MRS) findings in first-episode schizophrenia. Med Sci Monit. 2009;15:CR82–88. [PubMed] [Google Scholar]
- Goto S, Umehara J, Aizawa T, Kokubun S. Comparison of cervical spinal canal diameter between younger and elder generations of Japanese. J Orthop Sci. 15:97–103. doi: 10.1007/s00776-009-1427-7. [DOI] [PubMed] [Google Scholar]
- Hagino H, Suzuki M, Mori K, Nohara S, Yamashita I, Takahashi T, Kurokawa K, Matsui M, Watanabe N, Seto H, Kurachi M. Proton magnetic resonance spectroscopy of the inferior frontal gyrus and thalamus and its relationship to verbal learning task performance in patients with schizophrenia: a preliminary report. Psychiatry Clin Neurosci. 2002;56:499–507. doi: 10.1046/j.1440-1819.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- Heimberg C, Komoroski RA, Lawson WB, Cardwell D, Karson CN. Regional proton magnetic resonance spectroscopy in schizophrenia and exploration of drug effect. Psychiatry Res. 1998;83:105–115. doi: 10.1016/s0925-4927(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Hendren RL, Hodde-Vargas J, Yeo RA, Vargas LA, Brooks WM, Ford C. Neuropsychophysiological study of children at risk for schizophrenia: a preliminary report. J Am Acad Child Adolesc Psychiatry. 1995;34:1284–1291. doi: 10.1097/00004583-199510000-00013. [DOI] [PubMed] [Google Scholar]
- Jakary A, Vinogradov S, Feiwell R, Deicken RF. N-acetylaspartate reductions in the mediodorsal and anterior thalamus in men with schizophrenia verified by tissue volume corrected proton MRSI. Schizophr Res. 2005;76:173–185. doi: 10.1016/j.schres.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240:318–332. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- Jessen F, Scherk H, Traber F, Theyson S, Berning J, Tepest R, Falkai P, Schild HH, Maier W, Wagner M, Block W. Proton magnetic resonance spectroscopy in subjects at risk for schizophrenia. Schizophr Res. 2006;87:81–88. doi: 10.1016/j.schres.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Shungu DC, Anjilvel S, Chan S, Ellis SP, Xanthopoulos E, Malaspina D, Gorman JM, Mann JJ, Laruelle M, Kaufmann CA. Hippocampal pathology in schizophrenia: magnetic resonance imaging and spectroscopy studies. Psychiatry Res. 2000;98:163–175. doi: 10.1016/s0925-4927(00)00044-5. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Dick RM, Diwadkar VA, Montrose DM, Prasad KM, Stanley JA. Striatal metabolic alterations in non-psychotic adolescent offspring at risk for schizophrenia: a (1)H spectroscopy study. Schizophr Res. 2009;115:88–93. doi: 10.1016/j.schres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Montrose DM, Pierri JN, Dick EL, Rosenberg D, Talagala L, Sweeney JA. Magnetic resonance imaging and spectroscopy in offspring at risk for schizophrenia: preliminary studies. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1285–1295. doi: 10.1016/s0278-5846(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Klar AA, Ballmaier M, Leopold K, Hake I, Schaefer M, Bruhl R, Schubert F, Gallinat J. Interaction of hippocampal volume and N-acetylaspartate concentration deficits in schizophrenia: a combined MRI and 1H-MRS study. Neuroimage. 2010;53:51–57. doi: 10.1016/j.neuroimage.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Li BS, Wang H, Gonen O. Metabolite ratios to assumed stable creatine level may confound the quantification of proton brain MR spectroscopy. Magn Reson Imaging. 2003;21:923–928. doi: 10.1016/s0730-725x(03)00181-4. [DOI] [PubMed] [Google Scholar]
- Lim KO, Adalsteinsson E, Spielman D, Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Proton magnetic resonance spectroscopic imaging of cortical gray and white matter in schizophrenia. Arch Gen Psychiatry. 1998;55:346–352. doi: 10.1001/archpsyc.55.4.346. [DOI] [PubMed] [Google Scholar]
- Lutkenhoff ES, van Erp TG, Thomas MA, Therman S, Manninen M, Huttunen MO, Kaprio J, Lonnqvist J, O’Neill J, Cannon TD. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 15:308–318. doi: 10.1038/mp.2008.87. [DOI] [PubMed] [Google Scholar]
- Maier M, Ron MA. Hippocampal age-related changes in schizophrenia: a proton magnetic resonance spectroscopy study. Schizophr Res. 1996;22:5–17. doi: 10.1016/0920-9964(96)00044-8. [DOI] [PubMed] [Google Scholar]
- Maier M, Ron MA, Barker GJ, Tofts PS. Proton magnetic resonance spectroscopy: an in vivo method of estimating hippocampal neuronal depletion in schizophrenia. Psychol Med. 1995;25:1201–1209. doi: 10.1017/s0033291700033171. [DOI] [PubMed] [Google Scholar]
- Maier M, Mellers J, Toone B, Trimble M, Ron MA. Schizophrenia, temporal lobe epilepsy and psychosis: an in vivo magnetic resonance spectroscopy and imaging study of the hippocampus/amygdala complex. Psychol Med. 2000;30:571–581. doi: 10.1017/s0033291799001993. [DOI] [PubMed] [Google Scholar]
- Martinez-Granados B, Brotons O, Martinez-Bisbal MC, Celda B, Marti-Bonmati L, Aguilar EJ, Gonzalez JC, Sanjuan J. Spectroscopic metabolomic abnormalities in the thalamus related to auditory hallucinations in patients with schizophrenia. Schizophr Res. 2008;104:13–22. doi: 10.1016/j.schres.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Miyaoka T, Yasukawa R, Mizuno S, Sukegawa T, Inagaki T, Horiguchi J, Seno H, Oda K, Kitagaki H. Proton magnetic resonance spectroscopy (1H-MRS) of hippocampus, basal ganglia, and vermis of cerebellum in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome) J Psychiatr Res. 2005;39:29–34. doi: 10.1016/j.jpsychires.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Molina V, Sanz J, Sarramea F, Luque R, Benito C, Palomo T. No association between dorsolateral prefrontal gray matter deficit and N-acetyl aspartate ratios in schizophrenia. Neuropsychobiology. 2006;54:171–178. doi: 10.1159/000098653. [DOI] [PubMed] [Google Scholar]
- Molina V, Sanchez J, Sanz J, Reig S, Benito C, Leal I, Sarramea F, Rebolledo R, Palomo T, Desco M. Dorsolateral prefrontal N-acetyl-aspartate concentration in male patients with chronic schizophrenia and with chronic bipolar disorder. Eur Psychiatry. 2007;22:505–512. doi: 10.1016/j.eurpsy.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Molina V, Sanchez J, Reig S, Sanz J, Benito C, Santamarta C, Pascau J, Sarramea F, Gispert JD, Misiego JM, Palomo T, Desco M. N-acetyl-aspartate levels in the dorsolateral prefrontal cortex in the early years of schizophrenia are inversely related to disease duration. Schizophr Res. 2005;73:209–219. doi: 10.1016/j.schres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Skinner TE, Schmalbrock P, Robitaille PM. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994;165:481–485. doi: 10.1192/bjp.165.4.481. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Levitt J, Caplan R, Asarnow R, McCracken JT, Toga AW, Alger JR. 1H MRSI evidence of metabolic abnormalities in childhood-onset schizophrenia. Neuroimage. 2004;21:1781–1789. doi: 10.1016/j.neuroimage.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Ohara K, Isoda H, Suzuki Y, Takehara Y, Ochiai M, Takeda H, Hattori K, Igarashi Y. Proton magnetic resonance spectroscopy of lenticular nuclei in simple schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:507–519. doi: 10.1016/s0278-5846(00)00089-0. [DOI] [PubMed] [Google Scholar]
- Ohrmann P, Siegmund A, Suslow T, Pedersen A, Spitzberg K, Kersting A, Rothermundt M, Arolt V, Heindel W, Pfleiderer B. Cognitive impairment and in vivo metabolites in first-episode neuroleptic-naive and chronic medicated schizophrenic patients: a proton magnetic resonance spectroscopy study. J Psychiatr Res. 2007;41:625–634. doi: 10.1016/j.jpsychires.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Ohrmann P, Kugel H, Bauer J, Siegmund A, Kolkebeck K, Suslow T, Wiedl KH, Rothermundt M, Arolt V, Pedersen A. Learning potential on the WCST in schizophrenia is related to the neuronal integrity of the anterior cingulate cortex as measured by proton magnetic resonance spectroscopy. Schizophr Res. 2008;106:156–163. doi: 10.1016/j.schres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Olson DP, Hirashima F, Yurgelun-Todd D, Renshaw PF. Relaxation effects in spectroscopic studies of schizophrenia. In: Ng V, Barker GJ, Hendler T, editors. Psychiatric Neuroimaging: Proceedings of the NATO advanced research workshop on Psychiatric Neuroimaging. Amsterdam, the Netherlands: IOS Press; 2003. pp. 179–185. [Google Scholar]
- Omori M, Murata T, Kimura H, Koshimoto Y, Kado H, Ishimori Y, Ito H, Wada Y. Thalamic abnormalities in patients with schizophrenia revealed by proton magnetic resonance spectroscopy. Psychiatry Res. 2000;98:155–162. doi: 10.1016/s0925-4927(00)00049-4. [DOI] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010a;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, Renshaw PF. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, Jensen JE, Rouse ED, Cohen BM, Renshaw PF, Olson DP. T2 relaxation time abnormalities in bipolar disorder and schizophrenia. Magn Reson Med. 2010b;63:1–8. doi: 10.1002/mrm.22148. [DOI] [PubMed] [Google Scholar]
- Pae CU, Choe BY, Joo RH, Lim HK, Kim TS, Yoo SS, Choi BG, Kim JJ, Lee SJ, Lee C, Paik IH, Lee CU. Neuronal dysfunction of the frontal lobe in schizophrenia. Neuropsychobiology. 2004;50:211–215. doi: 10.1159/000079972. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Moseley M, Lim KO. Brain gray and white matter transverse relaxation time in schizophrenia. Psychiatry Res. 1999;91:93–100. doi: 10.1016/s0925-4927(99)00023-2. [DOI] [PubMed] [Google Scholar]
- Premkumar P, Parbhakar VA, Fannon D, Lythgoe D, Williams SC, Kuipers E, Kumari V. N-acetyl aspartate concentration in the anterior cingulate cortex in patients with schizophrenia: a study of clinical and neuropsychological correlates and preliminary exploration of cognitive behaviour therapy effects. Psychiatry Res. 2010;182:251–260. doi: 10.1016/j.pscychresns.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon SE, Valiakalayil A, Hanstock CC, Seres P, Tibbo P. Elevated 3T proton MRS glutamate levels associated with poor Continuous Performance Test (CPT-0X) scores and genetic risk for schizophrenia. Schizophr Res. 2008;99:218–224. doi: 10.1016/j.schres.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Reid MA, Stoeckel LE, White DM, Avsar KB, Bolding MS, Akella NS, Knowlton RC, den Hollander JA, Lahti AC. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2010;68:625–633. doi: 10.1016/j.biopsych.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw PF, Yurgelun-Todd DA, Tohen M, Gruber S, Cohen BM. Temporal lobe proton magnetic resonance spectroscopy of patients with first-episode psychosis. Am J Psychiatry. 1995;152:444–446. doi: 10.1176/ajp.152.3.444. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Spieker EA, Francis A, Barker PB, Carpenter WT, Buchanan RW. White matter alterations in deficit schizophrenia. Neuropsychopharmacology. 2009;34:1514–1522. doi: 10.1038/npp.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch N, Tebartz van Elst L, Valerius G, Buchert M, Thiel T, Ebert D, Hennig J, Olbrich HM. Neurochemical and structural correlates of executive dysfunction in schizophrenia. Schizophr Res. 2008;99:155–163. doi: 10.1016/j.schres.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Rutgers DR, van der Grond J. Relaxation times of choline, creatine and N-acetyl aspartate in human cerebral white matter at 1.5 T. NMR Biomed. 2002;15:215–221. doi: 10.1002/nbm.762. [DOI] [PubMed] [Google Scholar]
- Sanches RF, Crippa JA, Hallak JE, Araujo D, Zuardi AW. Proton magnetic resonance spectroscopy of the frontal lobe in schizophrenics: a critical review of the methodology. Rev Hosp Clin Fac Med Sao Paulo. 2004;59:145–152. doi: 10.1590/s0041-87812004000300010. [DOI] [PubMed] [Google Scholar]
- Sarramea Crespo F, Luque R, Prieto D, Sau P, Albert C, Leal I, de Luxan A, Osuna MI, Ruiz M, Galan R, Cabaleiro F, Molina V. Biochemical changes in the cingulum in patients with schizophrenia and chronic bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2008;258:394–401. doi: 10.1007/s00406-008-0808-9. [DOI] [PubMed] [Google Scholar]
- Schirmer T, Auer DP. On the reliability of quantitative clinical magnetic resonance spectroscopy of the human brain. NMR Biomed. 2000;13:28–36. doi: 10.1002/(sici)1099-1492(200002)13:1<28::aid-nbm606>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Sharma R, Venkatasubramanian PN, Barany M, Davis JM. Proton magnetic resonance spectroscopy of the brain in schizophrenic and affective patients. Schizophr Res. 1992;8:43–49. doi: 10.1016/0920-9964(92)90059-e. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Ochi S, Fukami G, Fujisaki M, Koike K, Okamura N, Ohgake S, Koizumi H, Matsuzawa D, Zhang L, Watanabe H, Nakazato M, Shinoda N, Komatsu N, Morita F, Iyo M. Posterior cingulate gyrus metabolic changes in chronic schizophrenia with generalized cognitive deficits. J Psychiatr Res. 2007;41:49–56. doi: 10.1016/j.jpsychires.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Shioiri T, Hamakawa H, Kato T, Murashita J, Fujii K, Inubushi T, Takahashi S. Proton magnetic resonance spectroscopy of the basal ganglia in patients with schizophrenia: a preliminary report. Schizophr Res. 1996;22:19–26. doi: 10.1016/0920-9964(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Obata T, Matsuzawa D, Nonaka H, Kanazawa Y, Yoshitome E, Ikehira H, Hashimoto K, Iyo M. Specific metabolites in the medial prefrontal cortex are associated with the neurocognitive deficits in schizophrenia: a preliminary study. Neuroimage. 2010;49:2783–2790. doi: 10.1016/j.neuroimage.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Sigmundsson T, Maier M, Toone BK, Williams SC, Simmons A, Greenwood K, Ron MA. Frontal lobe N-acetylaspartate correlates with psychopathology in schizophrenia: a proton magnetic resonance spectroscopy study. Schizophr Res. 2003;64:63–71. doi: 10.1016/s0920-9964(02)00533-9. [DOI] [PubMed] [Google Scholar]
- Stanley JA, Williamson PC, Drost DJ, Rylett RJ, Carr TJ, Malla A, Thompson RT. An in vivo proton magnetic resonance spectroscopy study of schizophrenia patients. Schizophr Bull. 1996;22:597–609. doi: 10.1093/schbul/22.4.597. [DOI] [PubMed] [Google Scholar]
- Stanley JA, Vemulapalli M, Nutche J, Montrose DM, Sweeney JA, Pettegrew JW, MacMaster FP, Keshavan MS. Reduced N-acetyl-aspartate levels in schizophrenia patients with a younger onset age: a single-voxel 1H spectroscopy study. Schizophr Res. 2007;93:23–32. doi: 10.1016/j.schres.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel RM, Bastin ME, McConnell S, Marshall I, Cunningham-Owens DG, Lawrie SM, Johnstone EC, Best JJ. Diffusion tensor imaging (DTI) and proton magnetic resonance spectroscopy (1H MRS) in schizophrenic subjects and normal controls. Psychiatry Res. 2001;106:161–170. doi: 10.1016/s0925-4927(01)00080-4. [DOI] [PubMed] [Google Scholar]
- Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, O’Gorman RL, Barker GJ, McGuire PK. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66:533–539. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Supprian T, Hofmann E, Warmuth-Metz M, Franzek E, Becker T. MRI T2 relaxation times of brain regions in schizophrenic patients and control subjects. Psychiatry Res. 1997;75:173–182. doi: 10.1016/s0925-4927(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Szulc A, Galinska B, Tarasow E, Kubas B, Dzienis W, Konarzewska B, Poplawska R, Tomczak AA, Czernikiewicz A, Walecki J. N-acetylaspartate (NAA) levels in selected areas of the brain in patients with chronic schizophrenia treated with typical and atypical neuroleptics: a proton magnetic resonance spectroscopy (1H MRS) study. Med Sci Monit. 2007;13(Suppl 1):17–22. [PubMed] [Google Scholar]
- Tanaka Y, Obata T, Sassa T, Yoshitome E, Asai Y, Ikehira H, Suhara T, Okubo Y, Nishikawa T. Quantitative magnetic resonance spectroscopy of schizophrenia: relationship between decreased N-acetylaspartate and frontal lobe dysfunction. Psychiatry Clin Neurosci. 2006;60:365–372. doi: 10.1111/j.1440-1819.2006.01515.x. [DOI] [PubMed] [Google Scholar]
- Tang CY, Friedman J, Shungu D, Chang L, Ernst T, Stewart D, Hajianpour A, Carpenter D, Ng J, Mao X, Hof PR, Buchsbaum MS, Davis K, Gorman JM. Correlations between Diffusion Tensor Imaging (DTI) and Magnetic Resonance Spectroscopy (1H MRS) in schizophrenic patients and normal controls. BMC Psychiatry. 2007;7:25. doi: 10.1186/1471-244X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayoshi S, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J, Nakataki M, Ueno S, Harada M, Ohmori T. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS) Schizophr Res. 2009;108:69–77. doi: 10.1016/j.schres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Terpstra M, Vaughan TJ, Ugurbil K, Lim KO, Schulz SC, Gruetter R. Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: application to schizophrenia. MAGMA. 2005;18:276–282. doi: 10.1007/s10334-005-0012-0. [DOI] [PubMed] [Google Scholar]
- Theberge J, Al-Semaan Y, Williamson PC, Menon RS, Neufeld RW, Rajakumar N, Schaefer B, Densmore M, Drost DJ. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–2233. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, Neufeld RW, Rogers J, Pavlosky W, Schaefer B, Densmore M, Al-Semaan Y, Williamson PC. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- Theberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, Northcott S, Menon RS, Neufeld RW, Rajakumar N, Pavlosky W, Densmore M, Schaefer B, Williamson PC. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- Thomas MA, Ke Y, Levitt J, Caplan R, Curran J, Asarnow R, McCracken J. Preliminary study of frontal lobe 1H MR spectroscopy in childhood-onset schizophrenia. J Magn Reson Imaging. 1998;8:841–846. doi: 10.1002/jmri.1880080413. [DOI] [PubMed] [Google Scholar]
- Tibbo P, Hanstock CC, Asghar S, Silverstone P, Allen PS. Proton magnetic resonance spectroscopy (1H-MRS) of the cerebellum in men with schizophrenia. J Psychiatry Neurosci. 2000;25:509–512. [PMC free article] [PubMed] [Google Scholar]
- Traber F, Block W, Lamerichs R, Gieseke J, Schild HH. 1H metabolite relaxation times at 3.0 tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19:537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- Tunc-Skarka N, Weber-Fahr W, Hoerst M, Meyer-Lindenberg A, Zink M, Ende G. MR spectroscopic evaluation of N-acetylaspartate’s T2 relaxation time and concentration corroborates white matter abnormalities in schizophrenia. Neuroimage. 2009;48:525–531. doi: 10.1016/j.neuroimage.2009.06.061. [DOI] [PubMed] [Google Scholar]
- van Elst LT, Valerius G, Buchert M, Thiel T, Rusch N, Bubl E, Hennig J, Ebert D, Olbrich HM. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:724–730. doi: 10.1016/j.biopsych.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Venkatraman TN, Hamer RM, Perkins DO, Song AW, Lieberman JA, Steen RG. Single-voxel 1H PRESS at 4.0 T: precision and variability of measurements in anterior cingulate and hippocampus. NMR Biomed. 2006;19:484–491. doi: 10.1002/nbm.1055. [DOI] [PubMed] [Google Scholar]
- Weber-Fahr W, Ende G, Braus DF, Bachert P, Soher BJ, Henn FA, Buchel C. A fully automated method for tissue segmentation and CSF-correction of proton MRSI metabolites corroborates abnormal hippocampal NAA in schizophrenia. Neuroimage. 2002;16:49–60. doi: 10.1006/nimg.2002.1057. [DOI] [PubMed] [Google Scholar]
- Williamson P, Pelz D, Merskey H, Morrison S, Karlik S, Drost D, Carr T, Conlon P. Frontal, temporal, and striatal proton relaxation times in schizophrenic patients and normal comparison subjects. Am J Psychiatry. 1992;149:549–551. doi: 10.1176/ajp.149.4.549. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Kamer T, Roy A, Vogeley K, Schneider-Axmann T, Wagner M, Maier W, Rietschel M, Schulze TG, Scherk H, Schild HH, Block W, Traber F, Tepest R, Honer WG, Falkai P. Reduction of the internal capsule in families affected with schizophrenia. Biol Psychiatry. 2008;63:65–71. doi: 10.1016/j.biopsych.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Berger G, Velakoulis D, Phillips LJ, McGorry PD, Yung AR, Desmond P, Pantelis C. Proton magnetic resonance spectroscopy in first episode psychosis and ultra high-risk individuals. Schizophr Bull. 2003;29:831–843. doi: 10.1093/oxfordjournals.schbul.a007049. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Yucel M, Wellard RM, Harrison BJ, Clarke K, Fornito A, Velakoulis D, Pantelis C. Evidence for neuronal dysfunction in the anterior cingulate of patients with schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. Schizophr Res. 2007;94:328–331. doi: 10.1016/j.schres.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Berger GE, Wellard RM, Proffitt T, McConchie M, Velakoulis D, McGorry PD, Pantelis C. A 1H-MRS investigation of the medial temporal lobe in antipsychotic-naive and early-treated first episode psychosis. Schizophr Res. 2008;102:163–170. doi: 10.1016/j.schres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Fukui T, Fukuda R, Yamada H, Yamasaki S, Kuroki N, Abe O, Kasai K, Tsujii K, Iwanami A, Aoki S, Ohtomo K, Kato N, Kato T. 1H-MR spectroscopy and gray matter volume of the anterior cingulate cortex in schizophrenia. Neuroreport. 2002;13:2133–2137. doi: 10.1097/00001756-200211150-00029. [DOI] [PubMed] [Google Scholar]
- Yasukawa R, Miyaoka T, Mizuno S, Inagaki T, Horiguchi J, Oda K, Kitagaki H. Proton magnetic resonance spectroscopy of the anterior cingulate gyrus, insular cortex and thalamus in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia (Gilbert’s syndrome) J Psychiatry Neurosci. 2005;30:416–422. [PMC free article] [PubMed] [Google Scholar]
- Yoo SY, Yeon S, Choi CH, Kang DH, Lee JM, Shin NY, Jung WH, Choi JS, Jang DP, Kwon JS. Proton magnetic resonance spectroscopy in subjects with high genetic risk of schizophrenia: investigation of anterior cingulate, dorsolateral prefrontal cortex and thalamus. Schizophr Res. 2009;111:86–93. doi: 10.1016/j.schres.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Renshaw PF, Gruber SA, Ed M, Waternaux C, Cohen BM. Proton magnetic resonance spectroscopy of the temporal lobes in schizophrenics and normal controls. Schizophr Res. 1996;19:55–59. doi: 10.1016/0920-9964(95)00071-2. [DOI] [PubMed] [Google Scholar]
- Zabala A, Sanchez-Gonzalez J, Parellada M, Moreno DM, Reig S, Burdalo MT, Robles O, Desco M, Arango C. Findings of proton magnetic resonance spectometry in the dorsolateral prefrontal cortex in adolescents with first episodes of psychosis. Psychiatry Res. 2007;156:33–42. doi: 10.1016/j.pscychresns.2006.12.016. [DOI] [PubMed] [Google Scholar]