Abstract
Providing sufficient islet mass is important for successful islet transplantation. Apoptosis plays a relevant role in post-isolation islet cell death, and prevention of apoptosis could improve transplant outcomes. The purpose of this study was to determine whether increased concentration of human albumin (HA) in pre-transplantation culture of human islets would allow for reducing apoptosis. Human islets were cultured in CMRL with 1.5 or 5% of HA for 24h and apoptosis was evaluated indirectly by measuring caspase 3 activity and directly by measuring tetramethylrhodamine-ethyl-ester (TMRE) in dissociated islets. Islet function and viability were evaluated. Islets cultured in higher albumin concentration presented with lower caspase 3 activity (43.9±3.9 vs. 67.4±11.1, p = 0.011), an early mediator of apoptosis, and had increased insulin secretory capacity (3.76±0.91 vs 1.23±0.21, p = 0.023). These findings may have implications for islet transplant outcomes, which ought to be investigated in vivo.
Keywords: human islet, apoptosis
INTRODUCTION
Pancreatic Islet transplantation is a promising approach for restoring normoglycemia in diabetic patients (1). However, islet transplantation has been hampered by difficulties in recovery, isolation, purification, and culture procedures. Two strategies following human islet isolation are available: the islets may be transplanted to the patient immediately or can be cultured for a period of time before transplantation. According to 2006 CITR data (2), out of 415 islet preparations, 43% were transplanted immediately and 57% were cultured for 30.1±1.3 h, then transplanted to the patients.
Culturing islets before transplantation has several advantages: it can reduce the immunogenicity of islets, facilitate islet purification, allow patients to be treated with immunodepleting agents (3–5), provide sufficient time for quality assessments and islet transportation between centers. In addition, pre-transplantation culture permits islets to recover from the stress of pancreas harvesting, cold storage and islet isolation. These stressful events lead to activation of apoptosis, necrosis and pro-inflammatory cascades resulting in a compromised islet yield and function.
However, islet culture can result in a loss of tissue mass that occurs over time (6–9). Although improvements in culture methods and conditions have resulted in considerable advancements in the long-term maintenance of islets (10–11), the longevity of human islet cells in culture has remained short (12–13).
For the last 30 years, numerous research groups have tried to find the perfect islet culture conditions by changing culture temperature and media formulation (7, 14–16). In this study, we focused on comparing the effect of human albumin (HA) concentration in pre-transplantation culture of human islets on apoptosis, viability and in vitro function. Our results show that in the presence of higher albumin concentration, mediators of apoptosis are reduced and islet function is improved.
MATERIAL AND METHODS
Human Islets
Human pancreata were retrieved from heart-beating donors at the time of multi-organ harvest for transplantation. Islets were isolated using the semi-automated method described by Ricordi et al., (17). Briefly, pancreas digestion was performed using Liberase-HI (Roche, Indianapolis, IN). Next, pancreata were manually perfused through the main pancreatic duct. After digestion, islets were purified in a COBE 2991 cell separator using continuous Ficoll gradients, and purity was assessed using dithizone staining.0
Culture Conditions
Aliquots of human islet were cultured in CMRL 1066 (Gibco, Invitrogen, Carlsbad, CA) supplemented with 1.5 or 5% HA (Grifols, Los Angeles, CA), 100units/ml penicillin, 0.1mg/ml streptomycin, 2mM glutamax and ITS (Gibco, Invitrogen, Carlsbad, CA). Islets were cultured at a density of 1,000 islet equivalent (IE) per ml at 37 °C in 5 humidified % CO2 atmosphere for 24h.
Apoptosis
Caspase 3 activity was evaluated using a colorimetric kit (Sigma, St. Louis, MO). 2,000IE were spun at 400×g for 1′ at 4°C, washed twice in cold PBS and dissolved in lysis buffer. The samples were sonicated and spun down for 10′ at 10,000×g at 4°C, the supernatants were kept for analysis. 50μg of total protein were incubated for 90′ in dark in presence of 200μM of Ac-DEVD-pNA at 37°C. The release of pNA was measured by using a Thermo Microplate Reader.
FACS Viability Assessment
For assessment of fractional β-cell viability the method of Ichii et al. was applied (18). A single cell suspension was created by incubating 1,000 islets in 2ml Accutase (Innovative Cell Technologies Inc., San Diego, CA) for 7′ at 37°C followed by 3′ of pipetting. Cells were then incubated with 1μM Newport-Green-PDX (NG) and 100ng/ml tetramethylrhodamine-ethyl-ester (TMRE) in PBS for 1h at 37°C. After washing, cells were stained with 5ug/ml 7-aminoactinomycin-D (7AAD; dyes from Molecular Probes, Invitrogen, Carlsbad, CA). These cells were analyzed using Cell Quest software and the LSR by Becton Dickinson (Mountainview, CA). Gating for NG was performed by side scatter and FL1.
Viability
In order to assess islet viability, 200IE were collected and washed in PBS and resupended in a solution of Trypan-blue (1:1 with PBS) (Gibco, Invitrogen, Carlsbad, CA). Viability was estimated under microscopic visualization of the proportion of non staining cells.
Function
Islet function was assessed by static glucose incubation and expressed in terms of stimulation index (SI) by calculating insulin secretion of islets challenged with Krebs-Ringer bicarbonate buffer (KRBB) containing 0.5% BSA with high glucose concentration (16.7mM) and dividing it by insulin secretion under low glucose conditions (1.6mM). Briefly, groups of ten handpicked islets were incubated with 1ml of low or high glucose solution for 1h. Supernatants were collected and insulin concentration measured by human insulin ELISA (Mercodia, Winston Salem, NC). Total insulin content was obtained by lysis of the islets with acid-alcohol and normalized to protein content.
Statistical Analysis
All values were expressed as mean ± SE. Caspase 3 activity was compared between the two groups using Paired t-test. Wilcoxon Signed Rank Sum Test was used to compare stimulation index. Viability data were evaluated using Fisher’s Exact Test. P values of <0.05 were considered statistically significant.
RESULTS
The effect of human albumin on caspase 3 activity in human islets
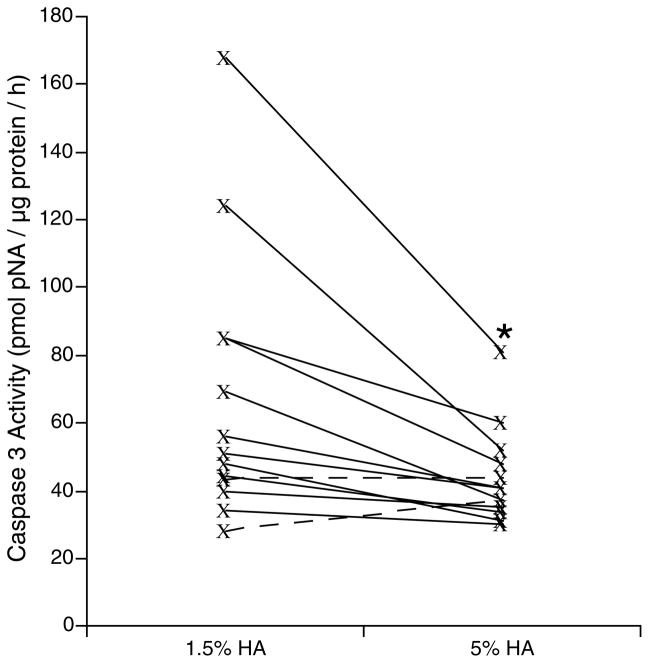
Apoptosis was measured as a marker of cell-death by measuring caspase 3 activity in thirteen human islet preparations after 24h of culture in different culture conditions (1.5% or 5% of HA). The apoptosis level in 5% of HA group was significantly lower than the 1.5% HA group (fig. 1, p = 0.011). Significant difference in apoptotic level of the two groups is evident when the caspase 3 activity level is higher (preparations with caspase 3 activity higher than 70 unit). Since DNA damage and fragmentation post-isolation were reported for all of these preparations, the difference in caspase 3 activity seemed to be related to the outcome of isolation. These results suggest a better islet recovery after culture in 5% HA from a particularly stressful pancreas harvest, cold preservation or islet isolation.
Figure 1. Caspase 3 Activity of 13 human islet preparations following 24h culture in presence of 1.5 or 5% of human Albumin.
Solid lines represent pancreata with higher caspase 3 activity in 1.5% HA group (11 out of 13) and dashed lines represent pancerata with higher higher caspase 3 activity in 5% HA group (2 out of 13). (Θ p = 0.011, compared to FW group).
The effect of human albumin on islet mitochondrial stability and viability
Apoptosis can be triggered at two sites either mitochondrially or extracellularly through death domain containing receptors. We examined the mitochondrial contribution to apoptotic activation in three different preparations cultured in different media. This method included the application of three fluorescent dies. The first 7AAD, is used to determine cell membrane integrity; the second TMRE, was used to monitor mitochondrial membrane stability and the third, NG, which binds zinc, was used as a specific marker for β-cells. After gating out the non-viable cells, the remaining cells were divided into four quadrants depending on their TMRE and NG staining properties. While mitochondrial stability or viability (cell membrane stability) of the total population in both groups did not show any significant change, mitochondrial stability in β-cells tended to be better preserved if human islets were cultured in 5% rather than 1.5 % HA. (Fig. 2A).
Figure 2. FACS and conventional viability.
A) FACS Viability Assessment: mitochondrial membrane stability of total cells (p = 0.67), mitochondrial membrane stability of β-cells (p = 0.157) and 7- aminoactinomycin viability (p = 0.73). B) Trypan blue viability (p = 0.122)
Viability was further evaluated after 24h culture using Trypan-blue dye on a 200IE aliquot. No statistical differences between different culture conditions were observed in islet viability (fig. 2B).
The effect of human albumin on islet functionality
In vitro islet function was assessed by calculation of Stimulation Index. As shown in figure 3A, a significantly higher SI was calculated for islets cultured in 5% HA compared to 1.5% HA group (p=0.0.023). Total content of insulin was maintained similar between the two groups (fig. 3B). These results together showed better preservation of islet functionality in 5% HA culture.
Figure 3. Islet functionality.
A) Stimulation index (Θ p = 0.023, compared to 1.5% HA group). B) Total insulin content normalized to protein (p = 0.97).
DISCUSSION
Annsufficient amount of grafted islets appeared to be the major reason for the poor early outcome of clinical islet transplantation relative to whole pancreas transplants. Some investigators have, recently reported that islet cell death, especially of β-cells is triggered by apoptosis soon after isolation (19–21). During the entire isolation process, islets are subjected to extreme conditions that represent significant cellular stress that elicit biochemical responses that may lead to cell death (22–23). Furthermore, the destruction or removal of peri-insular basement membrane (24) and cell-matrix relationship lead to the induction of apoptosis (25). Apoptosis inhibition has been demonstrated to be crucial for graft survival in different animal models (18, 26). Culturing human islets in the presence of a caspase 3 inhibitor prevented apoptosis and improved islet graft function (26). Therefore, any factor that could help to protect the islets against apoptosis, would be beneficial.
Our results showed great difference in apoptosis signaling for different preparations, probably due to a higher stress in harvesting, cold storage or isolation. Interestingly we noticed a greater caspase 3 activity reduction when highly stressed islet preparations were cultured in 5% HA compared to the islets cultured in 1.5% HA. Some benefits of human albumin are well documented; a primary function of albumin is to bind, sequester and stabilize a range of important small molecules and ions, acting as a multifaceted antioxidant. It has been recently shown that human serum albumin and human recombinant albumin are capable of inhibiting apoptosis (27–28). Zoellner and collaborators showed a loss of activity following beta-mercapto-ethanol reduction, which suggest the presence of an active site in the molecule of albumin that is responsible for mediating the anti-apoptotic effect (29). The same group also suggested that albumin inhibits cell apoptosis by preventing mitochondrial depolarization (30).
Optimization of the islet isolation process and consecutive islet culture is one way to make islet transplantation accessible to a larger number of patients. We showed that it is possible to reduce islet apoptosis by optimization of albumin concentration in culture medium.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.2006 CITR Annual Report wco.
- 3.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, Hunter DW, Sutherland DE. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. Jama. 2005;293(7):830–5. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 4.Lacy PE, Finke EH, Codilla RC. Cinemicrographic studies on beta granule movement in monolayer culture of islet cells. Lab Invest. 1975;33(5):570–6. [PubMed] [Google Scholar]
- 5.Kuttler B, Hartmann A, Wanka H. Long-term culture of islets abrogates cytokine-induced or lymphocyte-induced increase of antigen expression on beta cells. Transplantation. 2002;74(4):440–5. doi: 10.1097/00007890-200208270-00003. [DOI] [PubMed] [Google Scholar]
- 6.Weber M, Deng S, Kucher T, Shaked A, Ketchum RJ, Brayman KL. Adenoviral transfection of isolated pancreatic islets: a study of programmed cell death (apoptosis) and islet function. J Surg Res. 1997;69(1):23–32. doi: 10.1006/jsre.1997.4995. [DOI] [PubMed] [Google Scholar]
- 7.Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia. 1978;14(6):397–404. doi: 10.1007/BF01228134. [DOI] [PubMed] [Google Scholar]
- 8.Fraga DW, Sabek O, Hathaway DK, Gaber AO. A comparison of media supplement methods for the extended culture of human islet tissue. Transplantation. 1998;65(8):1060–6. doi: 10.1097/00007890-199804270-00009. [DOI] [PubMed] [Google Scholar]
- 9.Ilieva A, Yuan S, Wang RN, Agapitos D, Hill DJ, Rosenberg L. Pancreatic islet cell survival following islet isolation: the role of cellular interactions in the pancreas. J Endocrinol. 1999;161(3):357–64. doi: 10.1677/joe.0.1610357. [DOI] [PubMed] [Google Scholar]
- 10.Schmied BM, Ulrich A, Matsuzaki H, Ding X, Ricordi C, Moyer MP, Batra SK, Adrian TE, Pour PM. Maintenance of human islets in long-term culture. Differentiation. 2000;66(4–5):173–80. doi: 10.1046/j.1432-0436.2000.660403.x. [DOI] [PubMed] [Google Scholar]
- 11.Rush BT, Fraga DW, Kotb MY, Sabek OM, Lo A, Gaber LW, Halim AB, Gaber AO. Preservation of human pancreatic islet in vivo function after 6-month culture in serum-free media. Transplantation. 2004;77(8):1147–54. doi: 10.1097/01.tp.0000116769.94299.f4. [DOI] [PubMed] [Google Scholar]
- 12.Linetsky E, Bottino R, Lehmann R, Alejandro R, Inverardi L, Ricordi C. Improved human islet isolation using a new enzyme blend, liberase. Diabetes. 1997;46(7):1120–3. doi: 10.2337/diab.46.7.1120. [DOI] [PubMed] [Google Scholar]
- 13.Yuan S, Rosenberg L, Paraskevas S, Agapitos D, Duguid WP. Transdifferentiation of human islets to pancreatic ductal cells in collagen matrix culture. Differentiation. 1996;61(1):67–75. doi: 10.1046/j.1432-0436.1996.6110067.x. [DOI] [PubMed] [Google Scholar]
- 14.Rastellini C, Shapiro R, Corry R, Fung JJ, Starzl TE, Rao AS. Treatment of isolated pancreatic islets to reverse pancreatectomy- induced and insulin-dependent type I diabetes in humans: a 6-year experience. Transplant Proc. 1997;29(1–2):746–7. doi: 10.1016/s0041-1345(96)00449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes MA, Clayton HA, Chadwick DR, Bell PR, London NJ, James RF. Functional studies of rat, porcine, and human pancreatic islets cultured in ten commercially available media. Transplantation. 1995;60(8):854–60. [PubMed] [Google Scholar]
- 16.Matsumoto S, Goel S, Qualley S, Strong DM, Reems JA. A comparative evaluation of culture conditions for short-term maintenance (<24 hr) of human islets isolated using the Edmonton protocol. Cell Tissue Bank. 2003;4(2/4):85–93. doi: 10.1023/b:catb.0000007043.15164.8a. [DOI] [PubMed] [Google Scholar]
- 17.Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes. 1989;38 (Suppl 1):140–2. doi: 10.2337/diab.38.1.s140. [DOI] [PubMed] [Google Scholar]
- 18.Ichii H, Inverardi L, Pileggi A, Molano RD, Cabrera O, Caicedo A, Messinger S, Kuroda Y, Berggren PO, Ricordi C. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5(7):1635–45. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 19.Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20(3):270–6. doi: 10.1097/00006676-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Paraskevas S, Duguid WP, Maysinger D, Feldman L, Agapitos D, Rosenberg L. Apoptosis occurs in freshly isolated human islets under standard culture conditions. Transplant Proc. 1997;29(1–2):750–2. doi: 10.1016/s0041-1345(96)00452-6. [DOI] [PubMed] [Google Scholar]
- 21.Cattan P, Berney T, Schena S, Molano RD, Pileggi A, Vizzardelli C, Ricordi C, Inverardi L. Early assessment of apoptosis in isolated islets of Langerhans. Transplantation. 2001;71(7):857–62. doi: 10.1097/00007890-200104150-00006. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg L. Clinical islet cell transplantation. Are we there yet? Int J Pancreatol. 1998;24(3):145–68. doi: 10.1007/BF02788418. [DOI] [PubMed] [Google Scholar]
- 23.Canman CE, Kastan MB. Signal transduction. Three paths to stress relief. Nature. 1996;384(6606):213–4. doi: 10.1038/384213a0. [DOI] [PubMed] [Google Scholar]
- 24.Meredith JESM. Integrins, adhesion and apoptosis. Trends Cell Biol. 1997;7:146–50. doi: 10.1016/S0962-8924(97)01002-7. [DOI] [PubMed] [Google Scholar]
- 25.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124(4):619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano M, Matsumoto I, Sawada T, Ansite J, Oberbroeckling J, Zhang HJ, Kirchhof N, Shearer J, Sutherland DE, Hering BJ. Caspase-3 inhibitor prevents apoptosis of human islets immediately after isolation and improves islet graft function. Pancreas. 2004;29(2):104–9. doi: 10.1097/00006676-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Zoellner H, Hofler M, Beckmann R, Hufnagl P, Vanyek E, Bielek E, Wojta J, Fabry A, Lockie S, Binder BR. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J Cell Sci. 1996;109 (Pt 10):2571–80. doi: 10.1242/jcs.109.10.2571. [DOI] [PubMed] [Google Scholar]
- 28.Hsu SL, Wu WS, Tyan YS, Chou CK. Retinoic acid-induced apoptosis is prevented by serum albumin and enhanced by Lipiodol in human hepatoma Hep3B cells. Cancer Lett. 1998;129(2):205–14. doi: 10.1016/s0304-3835(98)00100-1. [DOI] [PubMed] [Google Scholar]
- 29.Zoellner H, Hou JY, Lovery M, Kingham J, Srivastava M, Bielek E, Vanyek E, Binder BR. Inhibition of microvascular endothelial apoptosis in tissue explants by serum albumin. Microvasc Res. 1999;57(2):162–73. doi: 10.1006/mvre.1998.2126. [DOI] [PubMed] [Google Scholar]
- 30.Gallego-Sandin S, Novalbos J, Rosado A, Cano-Abad MF, Arias E, Abad-Santos F, Garcia AG. Albumin prevents mitochondrial depolarization and apoptosis elicited by endoplasmic reticulum calcium depletion of neuroblastoma cells. Eur J Pharmacol. 2005;520(1–3):1–11. doi: 10.1016/j.ejphar.2005.06.044. [DOI] [PubMed] [Google Scholar]