Abstract
The aryl hydrocarbon receptor (AHR) is highly expressed in multiple organs and tissues, and there is increasing evidence that the AHR plays an important role in cellular homeostasis and disease. The AHR is expressed in multiple tumor types, in cancer cell lines, and in tumors from animal models, and the function of the AHR has been determined by RNA interference, overexpression, and inhibition studies. With few exceptions, knockdown of the AHR resulted in decreased proliferation and/or invasion and migration of cancer cell lines, and in vivo studies in mice overexpressing the constitutively active AHR exhibited enhanced stomach and liver cancers, suggesting a pro-oncogenic role for the AHR. In contrast, loss of the AHR in transgenic mice that spontaneously develop colonic tumors and in carcinogen-induced liver tumors resulted in increased carcinogenesis, suggesting that the receptor may exhibit antitumorigenic activity prior to tumor formation. AHR ligands also either enhanced or inhibited tumorigenesis, and these effects were highly tumor specific, demonstrating that selective AHR modulators that exhibit agonist or antagonist activities represent an important new class of anticancer agents that can be directed against multiple tumors.
Key Words: Ah receptor, agonist activity, antagonist activity, drug target.
Background
Poland first hypothesized that an intracellular binding protein or receptor may be the initial target for the highly toxic 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related halogenated aromatics (HAs), and his laboratory was the first to purify and characterize the mouse hepatic aryl hydrocarbon receptor (AHR) protein (Poland and Glover, 1973; Poland et al., 1976). Studies in several laboratories confirmed the role for the receptor in mediating the toxicities of HAs and polynuclear aromatic hydrocarbons (PAHs), and thus, the AHR became inextricably linked to two of the most prominent classes of environmental toxicants (Goldstein et al., 1989; Nebert et al., 1975; Poland and Knutson, 1982; Safe, 1986). The AHR is a ligand-activated transcription factor that forms a nuclear heterodimer with the AHR nuclear translocator (ARNT) protein to activate gene expression through interactions with cognate dioxin responsive elements (DREs) located on Ah-responsive gene promoters (reviewed in Gu et al., 2000; Whitlock, 1999). AHR ligands such as TCDD induce expression of a gene battery that catalyzes the metabolism and conjugation of xenobiotics (Köhle and Bock, 2007; Nebert et al., 2004), and early studies on the mechanism of AHR-mediated gene expression extensively used the CYP1A1 gene as a model (Whitlock, 1999). It is generally assumed that the classical mechanism of action derived from studies on the CYP1A1 gene is required for inducing the prototypical toxic effects of TCDD and structurally related HAs, even though the molecular mechanisms and genes associated with toxicities such as chloracne, wasting syndrome, tumor promotion, and others are not well defined (Poland and Knutson, 1982; Whitlock, 1999).
Receptor for TCDD and Related HAs
The linkage between the AHR and the toxicity of TCDD and related compounds was also confirmed by the cloning of the receptor (Burbach et al., 1992; Dolwick et al., 1993; Ema et al., 1992; Schmidt et al., 1993) and the subsequent generation of Ahr knockout (Ahr −/−) mice (Fernandez-Salguero et al., 1995; Mimura et al., 1997; Schmidt et al., 1996). Although there were some phenotypic differences in Ahr −/− mice generated in different laboratories, there was general agreement that most of the toxicities observed in wild-type mice treated with TCDD were not observed in Ahr −/− mice, thus confirming that this receptor was necessary for mediating the toxicity of TCDD and related HAs (Barouki et al., 2007; Fernandez-Salguero et al., 1995, 1996, 1997; Mimura et al., 1997; Schmidt et al., 1996). These results consolidated the strong association between the AHR and toxic HAs, even though several endogenous ligands and phytochemicals have subsequently been identified as AHR ligands (Pohjanvirta, 2012). Studies in Ahr −/− mice have unraveled many endogenous functions of the AHR (see below); however, unlike the nuclear hormone receptors such as the estrogen receptor (ER) (Jordan, 2009), the development and applications of specific drugs that target the AHR have been delayed due to its association with toxic HAs.
Endogenous Function of the AHR
The development of Ahr −/− mice has demonstrated that the function of this receptor is not just the mediation of the effects of HAs and PAHs (Barouki et al., 2007; McMillan and Bradfield, 2007). Ahr −/− mice exhibit decreased fertility, decreased liver size, and structural and functional deficits in several tissues, and these include failure of developmental closure of the ductus venosus in liver (Lahvis et al., 2000, 2005), vascular abnormalities in several organs including the cardiovascular system (Lund et al., 2003, 2006; Sauzeau et al., 2011), reproductive tract problems that include decreased levels of mature follicles (Abbott et al., 1999; Baba et al., 2005; Benedict et al., 2000, 2003), portal duct fibrosis (Fernandez-Salguero et al., 1997; Schmidt et al., 1996), oculomotor deficits (Chevallier et al., 2013), and formation of uric acid stones in the urinary bladder (Butler et al., 2012). Ahr −/− mice also exhibit abnormalities in stem cells and their function (Singh et al., 2011a; Wang et al., 2010). One of the hallmarks of TCDD toxicity is linked to its species-/tissue-specific immunomodulatory effects (Kerkvliet, 1995; Vos, 1977), and several recent studies have demonstrated critical roles for the AHR in the immune system and autoimmunity (Aguilera-Montilla et al., 2013; Apetoh et al., 2010; DiNatale et al., 2010; Esser, 2012; Esser et al., 2009; Funatake et al., 2004, 2005, 2008; Gandhi et al., 2010; Jin et al., 2010; Kadow et al., 2011; Kerkvliet, 1995; Kiss et al., 2011; Lee et al., 2012; Li et al., 2011; Marshall and Kerkvliet, 2010; Marshall et al., 2008; Mezrich et al., 2010; Nguyen et al., 2010; Quintana et al., 2008; Singh et al., 2011b; Stevens et al., 2009; Veldhoen et al., 2008; Vos, 1977; Wu et al., 2011a, b). For example, regulatory T cells (Treg) that express FoxP3 control immune autoreactivity, and the inverse relationship between Treg cells and proinflammatory T cells producing interleukin 17 (TH17) is a critical element in developing autoimmune diseases. The AHR and its ligands play a key role in controlling Treg cells and TH17 cell differentiation. In a mouse model for experimental autoimmune encephalomyelitis (EAE), the potent AHR agonist TCDD decreased, whereas the “endogenous” AHR agonist 6-formylindolo[3,2-b]carbazole (FICZ) increased, the severity of EAE in mice (Veldhoen et al., 2008). It was also reported that kynurenine, a tryptophan metabolite and AHR agonist (Opitz et al., 2011), but not TCDD or FICZ, induced FoxP3 Tregs in CD4+CD25− T cells from Ahr +/+ B6 mice but not in Ahr −/− mice (Kadow et al., 2011). Moreover, the AHR and its ligands may influence tumorigenesis not only by direct effects on the cancer cell but also by modulation of the immune system (Opitz et al., 2011). Results showing tissue-specific AHR agonist or antagonist activities of various AHR ligands in immune systems are consistent with observations for other ligand-activated intracellular receptors, and this is the basis for development of selective receptor modulators for treatment of multiple diseases including cancer in which a receptor such as the AHR plays a key role (Jordan, 2007; Jordan and O’Malley, 2007).
SELECTIVE AHR MODULATORS
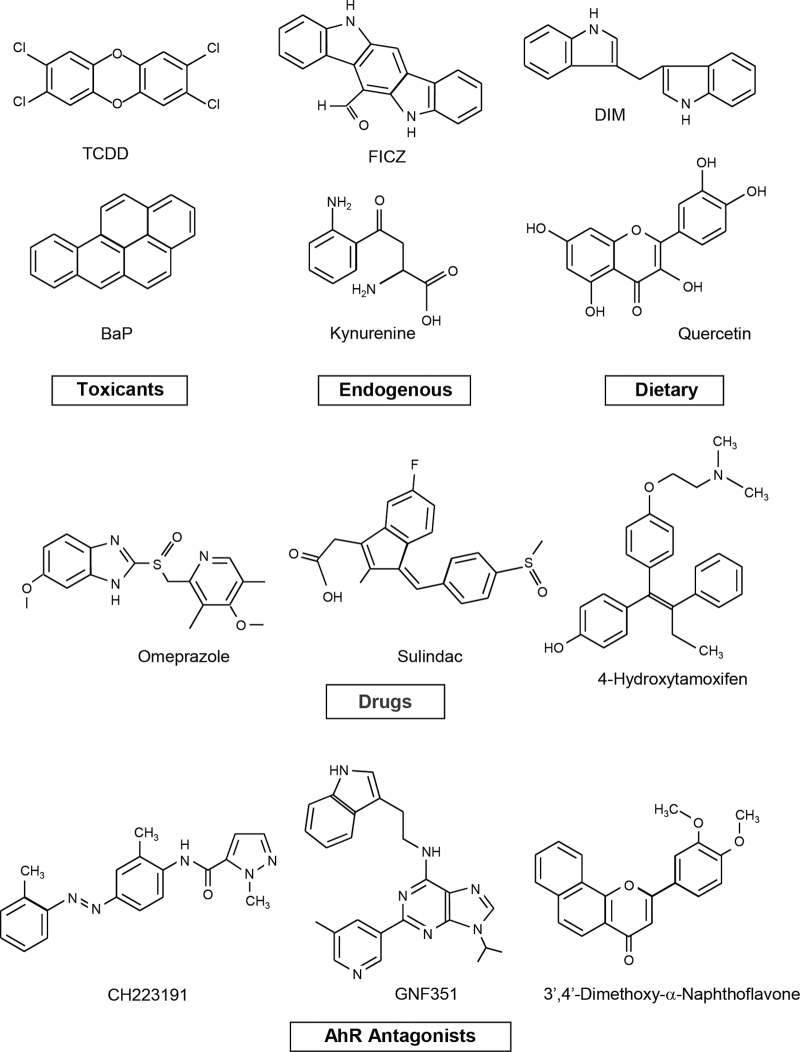
Initial skepticism regarding the AHR as a drug target was primarily due to the extensive literature demonstrating that most ligands for the receptor were toxic HAs and genotoxic PAHs such as TCDD and benzo[a]pyrene (BaP). However, the universe of AHR ligands has now greatly expanded and includes many other industrial compounds and byproducts, widely used pharmaceuticals, endogenous biochemicals including bilirubin, indigoids, FICZ and kynurenine, and several classes of chemoprotective phytochemicals such as the flavonoids, indole-3-carbinol, and related compounds (reviewed in Denison et al., 2011; Safe et al., 2012) (Fig. 1). The dramatic expansion of the number and classes of compounds that bind the AHR has clearly uncoupled the AHR from its function as an intracellular receptor for only toxic compounds, and like many other nuclear receptors (e.g., ERα), the AHR has important endogenous activity and ligands and interacts with structurally diverse chemicals.
Fig. 1.
Structures of different classes of AHR ligands. The AHR agonist activities have been reported for both kynurenine and kynurenic acid (DiNatale et al., 2010 and Opitz et al., 2011).
The development of drugs that target the AHR or other ligand-activated receptors is also dependent on the concept of selective AHR modulators (SahRMs) in which a receptor ligand exhibits tissue-specific AHR agonist or antagonist (Safe et al., 1999, 2012). This concept was observed and rationalized for drugs such as the ER ligand tamoxifen, which is an ER antagonist in breast tumors and used for treating ER-positive breast cancer but is an ER agonist in the uterus and a risk factor for uterine cancers (Jordan, 2007; Jordan and O’Malley, 2007). Tissue-specific differences in agonist or antagonist activity of a ligand are due to multiple factors that include ligand-induced conformational changes in the receptor and subsequent interactions with critical coactivators, corepressors, and other nuclear cofactors that exhibit tissue-specific expression (Katzenellenbogen et al., 1996). Studies in this laboratory initially characterized the SAhRM 6-methyl-1,3,8-trichlorodibenzofuran (MCDF) that was developed as an AHR antagonist and inhibited TCDD-induced CYP1A1, porphyria, immunotoxicity, and teratogenicity in mice (Astroff and Safe, 1989; Astroff et al., 1988; Bannister et al., 1989; Harris et al., 1989; Piskorska-Pliszczynska et al., 1991; Romkes et al., 1987; Santostefano et al., 1992; Yao and Safe, 1989; Zacharewski et al., 1992). In contrast, MCDF did not inhibit TCDD-induced antiestrogenic effects in the rodent uterus or breast cancer cells but was an AHR agonist and, like TCDD, exhibited antiestrogenic activity and inhibited mammary tumor growth in rodent models (McDougal et al., 1997, 2001). Other AHR ligands such as α-naphthoflavone and 3′-methoxy-4′-nitroflavone also exhibit both AHR agonist and antagonist activities (Gasiewicz and Rucci, 1991; Lu et al., 1996a; Santostefano et al., 1993; Zhou and Gasiewicz, 2003). It is also possible that the AHR agonist or antagonist activity of an AHR ligand will be dependent on tissue context but also vary with species (e.g., human vs. mouse). Recent studies have characterized new structural classes of AHR antagonists (Kim et al., 2006; Murray et al., 2011; Smith et al., 2011; Zhao et al., 2010), which may have clinical potential for treating diseases (including cancer) where AHR inhibition provides a therapeutic benefit. This has already been demonstrated in hemapoietic stem cells where a novel AHR antagonist promotes expansion of stem cells, which is critical for future development of clinical trials with stem cells (Boitano et al., 2010).
ROLE OF THE AHR IN CARCINOGENESIS
AHR Expression and Function
A recent study summarized AHR mRNA levels from a panel of 967 cancer cell lines from the Cancer Cell Line Encyclopedia. Chondrosarcomas and esophageal, upper aerodigestive, pancreatic, and liver cancer cell lines expressed relatively high levels, whereas many subtypes of leukemia cells expressed low AHR mRNA levels (O’Donnell et al., 2012). AHR mRNA levels in patient data sets were higher in thyroid, colon, pancreatic, and stomach tumors compared with nontumor tissue; however, Kaplan-Meier analysis of the data indicated that AHR mRNA levels were not prognostic for patient survival (http://www.ncbi.nlm.nih.gov/gds). A limited number of studies on AHR protein expression in cancer patients showed higher AHR expression in pancreatic, prostate, urinary tract, lung, and esophageal tumors but not in pituitary tumors, and the location of the receptor (i.e., cytosolic and/or nuclear) was variable in most tumors (Gluschnaider et al., 2010; Gramatzki et al., 2009; Ishida et al., 2010; Jaffrain-Rea et al., 2009; Koliopanos et al., 2002; Lin et al., 2003; Portal-Nunez et al., 2012; Zhang et al., 2012a) (Table 1). In upper urinary tract tumors, there was a correlation between increasing nuclear AHR protein expression and increasing tumor grade, suggesting that at least for these tumors nuclear AHR levels predict a higher tumor grade (Portal-Nunez et al., 2012).
Table 1.
AHR Protein Expression in Tumors
| Tissue (References) | AHR protein expression |
|---|---|
| Pancreatic (Koliopanos et al., 2002) | 14/15 (tumors, m to h, cytosolic) |
| 9/15 (nontumors, f) | |
| Prostate (Gluschnaider et al., 2010) | Tumors (h, cytosolic and nuclear) |
| Nontumors (w-nd, cytosolic > nuclear) | |
| Urinary tract (Ishida et al., 2010) | Tumors (h, % nuclear staining increased with increasing tumor grade) |
| Nontumor (w, cytosolic and nuclear) | |
| Lung (Portal-Nunez et al., 2012) | Adenocarcinomas (h, 56%) |
| Bronchioloalveolar carcinomas (h, 47%) | |
| Bronchiolar epithelium (h, 11%) | |
| Lung (Lin et al., 2003) | Tumors (h, 59/91; l, 32/91) |
| Nontumors (h, 7/31; l, 24/31) | |
| Esophagus (Zhang et al., 2012a) | Tumors/nontumors = 2.2/1 in protein expression |
| Pituitary (Jaffrain-Rea et al., 2009) | Tumors/nontumors: Weak staining, primarily cytosolic |
| Gliomas (Gramatzki et al., 2009) | Expression in tumor and nontumor tissues (23 autopsies) |
| WHO grade II astrocytomas > grade III anaplastic astrocytomas > grade IV glioblastomas (mean total AHR) | |
| Grade IV glioblastomas > grade III anaplastic astrocytomas ≅ grade II astrocytomas (nuclear AHR protein) |
Note. m to h, medium to high; f, faint; w-nd, weak to nondetectable; w, weak.
Genetic and mutagenesis studies on liver cancer cells have characterized cell lines with variable Ah responsiveness (and AHR expression), and these cells exhibit differences in cell proliferation and other responses (Ma and Whitlock, 1996; Reiners and Clift, 1999). For example, Ahr-D (defective) mouse Hepa1c1c7 cells appear less well differentiated and express low levels of albumin compared with wild-type cells, and loss of the AHR is associated with decreased rates of cell proliferation and an increased number of cells in G0/G1 phase of the cell cycle (Ma and Whitlock, 1996). Similar results have been reported for rat hepatoma cell lines (Weiss et al., 1996). In a series of elegant studies, it was shown that the AHR forms a complex with the retinoblastoma (Rb) tumor suppressor gene resulting in increased E2F-dependent gene expression and modulation of other cell cycle–related effects in liver cancer cells (Ge and Elferink, 1998; Huang and Elferink, 2005; Puga et al., 2000, 2009). RNA interference and knockdown of the AHR in human hepatoma HepG2 cells also resulted in growth inhibition, and this was accompanied by downregulation of several cell cycle–related genes including cyclins D1 and E, cdk2, and cdk4 (Abdelrahim et al., 2003). In contrast to the growth promoting effects of the AHR in liver cancer cell lines, diethylnitrosamine (DEN)–induced liver adenomas in Ahr −/− mice (male) were increased compared with wild-type mice, suggesting that the AHR exhibits tumor suppressor–like activity in vivo (Fan et al., 2010). These results contrast studies in liver cancer cell lines but may not necessarily be contradictory because the tumor suppressor–like activity of the AHR may be dominant in steps leading up to liver adenoma formation, and this function may change in adenomas and carcinomas.
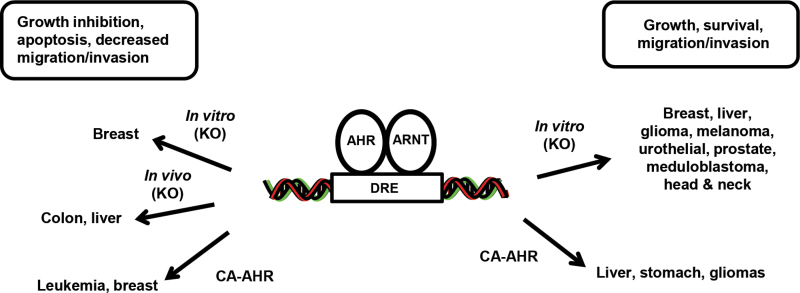
The role of the AHR in modulating growth and migration of cancer cells has also been investigated in cell lines derived from other tumors, and the receptor exhibits both tumor suppressive and oncogenic activity, which is cell context dependent (Fig. 2). For example, AHR silencing decreased growth of melanoma cancer cells overexpressing NRAS (Barretina et al., 2012), decreased urothelial cancer T24 cell invasion and MMP-9 expression (Portal-Nunez et al., 2012), decreased expression of fibroblast growth factor-9 and osteopontin in lung cancer cells (Chuang et al., 2012; Wang et al., 2009), decreased C4-2 (androgen independent) prostate cancer cell growth (Tran et al., 2013), decreased DAOY medulloblastoma cell growth, and induced G0/G1 arrest (p27 is also induced) (Dever and Opanashuk, 2012). AHR silencing in HN30 head and neck cancer cells decreased basal and serum-stimulated cell migration, and this has been linked to downregulation of constitutive interleukin-6 (IL-6), which exhibits promigratory and growth promoting activity in many cancer cell lines (DiNatale et al., 2011, 2012). Knockdown of the AHR in H508 colon cancer cells did not significantly affect growth (Xie et al., 2012); and in vivo studies with Ahr −/− mice showed that loss of the AHR resulted in enhanced formation of spontaneous colonic polyps and cecal tumors (Kawajiri et al., 2009). APC min/+ mice express adenomatous poyposis coli (APC) gene mutations and spontaneously develop colonic and intestinal tumors, and loss of one Ahr allele (APC min/+/Ahr +/−) enhanced this response (note: APC min/+/Ahr −/− mice were not good breeders). Thus, the constitutive Ahr exhibited tumor suppressor–like activity in a colonic tumor model, and this was similar to that observed in a mouse model for carcinogen-induced liver cancer (Fan et al., 2010).
Fig. 2.
AHR: endogenous functions in cancer.
AHR silencing by RNAi in MCF-7 and BT474 cancer cells enhanced cell proliferation, and in the former cell line, the percentages of cells in G2/M and G0/G1 phases of the cell cycle were increased and decreased, respectively (Zhang et al., 2009). Similar results were observed in BaP-resistant T47D cells that express low AHR levels (Moore et al., 1996), whereas BaP-resistant MCF-7 cells (low AHR expression) exhibited slower growth (Moore et al., 1994). In contrast, knockdown of the AHR in MDA-MB-468 cells did not affect cell proliferation (Zhang et al., 2009). Immortalized mammary tumor fibroblasts derived from Ahr +/+ and Ahr −/− mice were used as models to show that the AHR was required for tumor growth in a sc mouse xenograft model, and AHR loss was associated with decreased migration and angiogenesis (VEGFR1) (Mulero-Navarro et al., 2005). Thus, the role of AHR expression in breast cancer is variable and cell context dependent, and results of in vivo studies with Ahr −/− mice crossed with a transgenic mammary tumor model have not been reported.
AHR function has also been investigated by overexpression of a constitutively active AHR (Ca-AHR) in which amino acids 288–421 of the ligand binding domain have been deleted. Ca-AHR expressing mice rapidly developed stomach lesions and stomach cancer in both male and female mice (Andersson et al., 2002). It was also reported that liver tumor prevalence and multiplicity were higher in DEN-initiated Ca-AHR versus wild-type mice, suggesting that the AHR enhanced hepatocarcinogenesis in mice (Moennikes et al., 2004), whereas this contrasted to the tumor suppressor–like activity in DEN-initiated mice with or without AHR loss (Fan et al., 2010). In contrast, Ca-AHR overexpression in MCF-7 breast cancer cells and Jurkat T cells inhibited growth in both cell lines and also induced apoptosis in Jurkat cells (Ito et al., 2004; Köhle et al., 2002). The variable in vitro and in vivo effects of Ca-AHR are somewhat contradictory, and the utility of the Ca-AHR in predicting the role of the AHR in carcinogenesis requires further validation.
TCDD as a Carcinogen
The carcinogenicity of TCDD and its role as a tumor promoter have been extensively investigated in long-term feeding studies and in shorter term two-stage carcinogen-induced models (reviewed in Bock and Köhle, 2005; Knerr and Schrenk, 2006). The dietary studies invariably show development of hepatocellular preneoplastic nodules, adenomas or carcinomas in female and/or male rats and mice and, depending on the rodent strain/species, this may be accompanied by thyroid, thymus, skin, lung, nasal turbinate, tongue, and other oral cancers. There is also general consensus that TCDD acts as a tumor promoter, and this has been confirmed in several animal studies; however, the mechanisms of TCDD-induced hepatocellular carcinomas are not fully understood. The International Agency for Research on Cancer (IARC) has classified TCDD as a Group I human carcinogen (IARC, 1997) based, in part, on increased overall cancer rates in exposed cohorts; however, this designation is disputed by others (Cole et al., 2003) and may be resolved with time.
The effects of TCDD on breast cancer were first reported in female Sprague Dawley rats administered TCDD in the diet (Kociba et al., 1978) for 2 years. The observed increase in hepatocellular carcinomas in female rats was accompanied by decreased rates of both age-dependent uterine and mammary tumors, which develop in these animals. The inhibition of two estrogen-dependent tumors in this rat model suggested that TCDD activation of the AHR inhibited 17β-estradiol (E2)–induced genes and responses, and the potential antiestrogenic activity of TCDD has subsequently been confirmed in the rodent uterus, breast, and endometrial cancer cells and mammary tumors (reviewed in Safe and Wormke, 2003). The effects of TCDD on mammary tumorigenesis are highly variable and dependent on the model and the timing of exposure. For example, prenatal exposure to 1 µg/kg TCDD on gestational day 15 increased mammary terminal end bud formation and enhanced susceptibility to carcinogen-induced mammary tumor formation (Brown et al., 1998). TCDD inhibits pregnancy-induced mammary gland development in mice; however, in parous or nulliparous mice treated with TCDD during pregnancy, there was a significant delay and decreased incidence in 7/12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumor formation (Wang et al., 2011a). The animal models do not definitively link exposure to TCDD and other exposed populations with increased incidence of breast cancer (Safe et al., 2011); however, further long-term monitoring of the Seveso population accidentally exposed to TCDD will provide more definite data on the human breast cancer risks associated with exposure to TCDD.
Although the effects of different concentrations and the timing of exposure to TCDD and other relevant AHR agonists on tumor development have been reported, most studies have focused on using cancer cells as models for determining the mechanisms and pathways activated by TCDD and other AHR ligands. Results clearly demonstrate the complexity of AHR-mediated pathways and cell- and tumor-type-dependent differences in their mechanisms of action, and this is consistent with observation for other ligand-activated receptors. Furthermore, because the AHR plays a role in multiple tumor types, the identification of SAhRMs that exhibit tissue-specific AHR agonist or antagonist activities will lead to future clinical applications for AHR ligands in cancer treatment, and the therapeutic potential for these compounds will be emphasized in discussing results reported for effects of AHR ligands on various tumor types.
AHR IN CARCINOGENESIS: OPPORTUNITIES FOR CHEMOTHERAPEUTIC DRUG DEVELOPMENT
ER-Positive Breast Cancer
The antiestrogenic activity of TCDD observed in the long-term dietary feeding study in female Sprague Dawley rats (Kociba et al., 1978) has been extensively investigated in MCF-7 and other ER-positive breast cancer cells. Based on initial studies with TCDD, two major pathways were reported, and these included (1) induction of CYP1A1/CYP1B1 which in turn increased oxidative metabolism of E2 (Spink et al., 1990, 1992) and (2) activation of proteasomes by the liganded AHR and downregulation of ERα. The latter pathway showed TCDD-induced interaction of the ligand-bound AHR with ERα, which was followed by increased ubiquitination of ERα and degradation by proteasomes (Wormke et al., 2000b, 2003). Both pathways result in depletion of ERα and E2; however, their importance is ligand dependent because MCDF that also exhibits antiestrogenic activity in MCF-7 cells has minimal effects on induction of CYP1A1/CYP1B1 (Zacharewski et al., 1992). Therefore, CYP-dependent metabolism of E2 does not play a role in the antiestrogenic activity of MCDF.
The antiestrogenic activity of TCDD and other AHR ligands including MCDF and related alkyl chlorinated dibenzofurans, PAHs, and coplanar PCBs have also been reported in MCF-7 and other ER-positive breast cancer cells. For example, TCDD inhibits expression of E2-induced postconfluent focus production, cell cycle progression, plasminogen activator activities, cathepsin D, c-Fos, pS2, heat shock protein 27 (Hsp27), receptor-interacting protein 140 (RIP140), prolactin receptor, progesterone receptor, and carbamoylphosphate synthetase/aspartate transcarbamylase/dihydroorotase and BRCA-1 (Augereau et al., 2006; Biegel and Safe, 1990; Duan et al., 1999; Gierthy and Lincoln, 1988; Gierthy et al., 1987; Gillesby et al., 1997; Harper et al., 1994; Hockings et al., 2006; Khan et al., 2006; Krishnan and Safe, 1993; Krishnan et al., 1994, 1995; Lu et al., 1996b; Porter et al., 2001; Wang et al., 1998, 2001; Zacharewski et al., 1994). Several mechanisms have been described for ligand-activated inhibitory AHR-ERα cross talk, and these include direct binding of the AHR complex to inhibitory DRE (iDREs) cis-elements (promoters containing the core GCGTG AHR/ARNT binding motif (such as cathepsin D, pS2, Hsp27, and c-Fos); similar observations have been reported for RIP140 where a DRE and ERE overlap (Augereau et al., 2006). iDREs are located at various positions in the proximal gene promoters and may interfere with ERα-DNA binding or assembly of pol-ll and associated nuclear factors required for gene expression (Duan et al., 1999; Gillesby et al., 1997; Krishnan et al., 1995; Porter et al., 2001). A recent study also reported that inhibition of hormone regulation of cathepsin D expression was ARNT independent (Labrecque et al., 2012), and this pathway may also be important for other genes affected by inhibitory AHR-ERα cross talk. Hormonal activation of several E2-responsive genes in ER-positive breast cancer cells also involves ERα-Sp1 bound to GC-rich promoter sequences, and TCDD inhibits ERα/Sp1-mediated transaction through competitive dissociation of the ERα/Sp1 complex because the AHR binds both proteins (Khan et al., 2006; Safe and Kim, 2008). Another mechanism may be due, in part, to competition (squelching) by the liganded AHR and ERα complexes for common coactivators and possibly other nuclear cofactors (Kumar and Perdew, 1999; Kumar et al., 1999; Nguyen et al., 1999). Chromatin immunoprecipitation (ChIP) assays have also provided important new insights on ERα-AHR cross talk and the corecruitment of both transcription factors to the same gene promoters (Beischlag and Perdew, 2005; Matthews et al., 2005). TCDD treatment recruited ERα and the AHR to the CYP1A1 promoter, and ERα contributed to estrogen-dependent regulation of Ah responsiveness. Ahmed et al. (2009) also showed by ChIP-seq that TCDD enhanced AHR/ARNT-ERα interactions at multiple human gene promoters, and a recent study showed novel gene-specific recruitment of both receptor complexes along with the nuclear cofactor RIP140 (Madak-Erdogan and Katzenellenbogen, 2012). This study showed that ERα-mediated gene activation is regulated, in part, through AHR-dependent modulation of RIP140 recruitment to ERα binding sites (Madak-Erdogan and Katzenellenbogen, 2012). Inhibitory AHR-ERβ cross talk has also been reported, and there is evidence that competition by the liganded AHR for ARNT decreases ERβ and to a lesser extent ERα-mediated transactivation because ARNT is a coactivator of ER (Rüegg et al., 2008).
Inhibitory AHR-ERα cross talk clearly plays a role in the antiestrogenic activity of TCDD and other AHR ligands; however, the liganded AHR also modulates many other genes and pathways that inhibit ER-positive breast cancer growth. For example, TCDD induces tumor growth factor β, tumor necrosis factor α, IL-1B (Vogel and Abel, 1995), IL-6 (which may be due to IL-1B induction) (Hollingshead et al., 2008), cyclin G2 (Ahmed et al., 2012), the human breast cancer resistance protein (BCRP/ABCG2) (Tan et al., 2010), and COX-2 (Degner et al., 2009). The AHR also interacts with CDK4 and RB, and TCDD causes dissociation of CDK4 from this complex, enhancing RB-dependent repression of E2F1 and inhibition of G0/G1 to S-phase progression (Barhoover et al., 2010; Huang and Elferink, 2005). It was also reported that TCDD or constitutively activated AHR enhanced phospho-JNK, decreased E-cadherin, and increased MCF-7 cell motility (EMT-like) (Diry et al., 2006). However, another study showed that AHR agonists or constitutively active AHR decreased mammosphere formation and inhibited Wnt/β-catenin signaling (Zhao et al., 2012). The rationale for these opposing observations is unclear. TCDD also decreased expression of the G-protein-coupled receptor CXCR4 and its chemokine ligand CXCL12 and also blocked E2-induced activation of CXCR4 (Hsu et al., 2007). This response has been linked to inhibition of MCF-7 cell migration by TCDD, and similar results have been observed for 3,3′-diindolymethane (DIM) (Hsu et al., 2007, 2008). TCDD also inhibited colonization of MCF-7 and ZR-75 cells in soft agar and cell invasion, and this was associated with induction of cell differentiation and differentiation markers such as K-casein (Hall et al., 2010).
The effects of AHR agonists on mammary tumor growth in vivo complement the in vitro data and confirm the antiestrogenic and antitumorigenic activity of these compounds. TCDD inhibits carcinogen-induced mammary tumor development and also inhibits growth of human tumors in a xenograft model, and similar results have been observed for MCDF and related compounds (Gierthy and Lincoln, 1988; Holcomb and Safe, 1994; McDougal et al., 1997, 2001). Moreover, 3,3′,4,4′-tetrachlorobiphenyl also inhibited carcinogen-induced mammary tumor formation (Ramamoorthy et al., 1999) and growth, and similar results were observed for DIM and substituted DIMs (Chen et al., 1998; McDougal et al., 2000); however, these compounds also act through other pathways.
ER-Negative Breast Cancer
Early studies with ER-negative breast cancer cells suggested that Ah responsiveness was dependent on expression of ERα (Vickers et al., 1989); however, it was subsequently shown that the AHR is expressed in most ER-negative breast cancer cells (Wang et al., 1995), although with the exception of the MDA-MB-468 cells, the fold induction of CYP1A1 (by TCDD) is decreased (Wang et al., 1997; Zhang et al., 2009). Both TCDD and MCDF inhibit growth of MDA-MB-468 cells, and this is due to induction of TGFα which is growth inhibitory in this cell line (Wang et al., 1997). There is evidence that the AHR may repress c-MYC in Hs578T cells (Yang et al., 2005). The AHR-Rb-mediated repression of E2F1 (Barhoover et al., 2010), induction of differentiation markers, inhibition of cell invasion (Hall et al., 2010), and downregulation of CXCR4 (Hsu et al., 2007, 2008) are observed in ER-positive MCF-7 and ER-negative MDA-MB-231 and other cell lines. MCDF and TCDD also decreased invasion of MDA-MB-231 cells, and this was due to AHR-mediated upregulation of microRNA-335 (miR-335), which in turn suppresses expression of prometastatic genes such as SOX-4 (Zhang et al., 2012b). MCDF also inhibits metastasis of MDA-MB-231 cells to the lung after tail vein injection, and these results are consistent with the antimetastatic effects of TCDD using metastatic 4T1.2 mouse mammary tumor cells in an orthotopic model (Wang et al., 2011b).
Structurally diverse chemicals that exhibit AHR agonist activity include several widely used pharmaceuticals (Hu et al., 2007) such as the antiallergic drug tranilast. Tranilast is an AHR agonist in MDA-MB-231 cells and inhibited cell growth migration, colony formation, mammosphere formation, and metastasis of MDA-MB-231 cells to the lung after tail vein injection (Prud’homme et al., 2010; Subramaniam et al., 2011). Several SERMs including 4-hydroxytamoxifen (4-OHT) exhibit AHR agonist activity in breast cancer cells, and it was also shown the 4-OHT blocks osteoclast differentiation (AHR dependent) and this may contribute to bone preservation associated with use of tamoxifen for breast cancer therapy (DuSell et al., 2010). Recent studies in this laboratory have screened eight AHR-active pharmaceuticals as inducers of CYP1A1/CYP1B1 and inhibitors of BT474 and MDA-MB-468 breast cancer cell migration (Jin et al., 2012). The effects of these compounds were structure, cell context, and response dependent; mexiletine is an AHR agonist in liver cancer cells (Hu et al., 2007) and in BT474 cells but was an AHR antagonist in MDA-MB-468 cells. Among the eight AHR-active pharmaceuticals, flutamide, leflunomide, nimodipine, omeprazole, sulindac, and tranilast, but not 4-OHT or mexiletine, inhibited MDA-MB-468 cell migration (Jin et al., 2012), and current studies are further investigating these pharmaceuticals for their applications in ER-negative breast cancer chemotherapy. It is evident from the extensive research on both ER-positive and ER-negative breast cancer cells that the AHR is a highly relevant drug target. At present, at least one compound (“aminoflavone”) that binds the AHR is in phase II clinical trials for breast cancer (Loaiza-Perez et al., 2004). This compound is a prodrug and AHR agonist that induces AHR-dependent CYP1A1/1B1, which in turn activates the drug through oxidative metabolism.
Endometrial and Ovarian Cancer
The role of the AHR and AHR agonists have not been extensively investigated in endometrial and ovarian cancer cell lines; however, there is evidence that comparable AHR-ERα cross talk and growth inhibitory pathways are operative (Castro-Rivera et al., 1999; Rogers and Denison, 2002; Rowlands et al., 1993; Wormke et al., 2000a) and require further investigation.
Liver Cancer
Treatment of rat and mouse liver cancer cell lines with TCDD results in the inhibition of G0/G1 to S-phase progression and accumulation of cells in G0/G1 (Elferink et al., 2001; Ge and Elferink, 1998; Huang and Elferink, 2005; Marlowe et al., 2004; Puga et al., 2000, 2009). These effects are accompanied by the induction of the cyclin-dependent kinase inhibitor p27 (Kolluri et al., 1999; Levine-Fridman et al., 2004) and inhibition of E2F1-regulated gene expression, which is due, in part, to interactions with RB. Some of the responses are similar in liver and breast cancer cell lines, and ligand activation of the AHR enhances interactions with RB, resulting in decreased E2F1-dependent gene expression, and this may also result in displacement of p300 (Marlowe et al., 2004). Detailed mechanism studies are contradictory because one report showed that the DNA binding and transactivation domains of the AHR and ARNT were not required for repression of E2F1-regulated transaction in mouse hepatoma Hep1c1 cells (Marlowe et al., 2004). In contrast, ARNT was required for E2F repression in rat hepatoma BP8 cells (Ah nonresponsive) transfected with an AHR expression plasmid (Huang and Elferink, 2005). It has been suggested that these cell context–dependent differences may be due to the requirement for ARNT in dissociating HSP90 from the AHR (Puga et al., 2002). In HepG2 cells, TCDD induces plasminogen activator inhibitor type 2 (PAI-2) mRNA (Gohl et al., 1996) and the anterior gradient 2 (AGR2) metastasis marker (Gohl et al., 1996) and enhances cellular migration via an AHR-dependent nongenomic focal adhesion kinase/Src pathway (Ambolet-Camoit et al., 2010). In contrast to human HepG cells, TCDD induces cancer cell growth in rat and mouse hepatoma cells, and therefore, potential application of SAhRMs for liver cancer therapy must be further investigated.
Colon, Gastric, and Pancreatic Tumors
TCDD and other AHR agonists induce proliferation of several colon cancer cell lines, and this involves extranuclear AHR-mediated activation of Src and the EGFR pathway (Tomkiewicz et al., 2013). AHR agonists not only enhance growth but also induce proinflammatory IL-1β and MMP-9, calcium ion flux, and the ABCG2 drug transporter in colon cancer cells (Le Ferrec et al., 2002; Tompkins et al., 2010; Villard et al., 2007). TCDD also induces gastric cancer cell growth and invasion and MMP-9 expression (Peng et al., 2009), and a report showing that DIM inhibits gastric cancer cell growth (Yin et al., 2012) is probably due to AHR-independent pathways. Thus, AHR agonists enhance colon and gastric cancer cell growth, suggesting a possible therapeutic role for SAhRMs that exhibit antagonist activities. In contrast, the AHR is expressed in most pancreatic tumors (14/15), and TCDD, MCDF, and related SAhRMs induce p21 and inhibit pancreatic cancer cell proliferation and anchorage-independent growth (Koliopanos et al., 2002), suggesting that SAhRM agonists may have clinical applications for pancreatic cancer therapy.
Prostate and Urothelial Cancers
Initial studies investigated AHR-androgen receptor (AR) cross talk in prostate cancer cells and showed that TCDD inhibited basal and androgen-induced growth and cell cycle progression (G0/G1 to S-phase arrest) (Barnes-Ellerbe et al., 2004; Jana et al., 1999, 2000; Morrow et al., 2004). β-TrCP is an E3-ligase, and depletion of this gene results in AHR upregulation and growth inhibition; TCDD did not enhance growth inhibition (Gluschnaider et al., 2010) nor did TCDD affect Wnt/β-catenin-AHR interactions in prostate cancer cells (Chesire et al., 2004). AHR agonists induced MMP-9 in androgen-insensitive PC3 and DU145 cells (Haque et al., 2005), suggesting that the chemotherapeutic activity of AHR agonists may primarily be associated with androgen-sensitive prostate cancer. In T24 urothelial cancer cells, TCDD induced MMP-1 and MMP-9 and enhanced cell invasion, suggesting that SAhRM antagonists may have some therapeutic activity, and this is supported by AHR silencing in these cells, which resulted in decreased invasion and MMP expression (Ishida et al., 2010).
Head and Neck and Lung Cancers
The AHR regulates IL-6 expression in head and neck cancers, and TCDD alone or in combination with IL-β enhances proinflammatory IL-6 expression in head and neck cancer cell lines (DiNatale et al., 2011, 2012). However, treatment of these cells with the AHR antagonists 6,2′,4′-trimethoxyflavone or [N-(2-(1H-indol-3-yl)ethyl)-9-isopropyl-2-(5-methylpyridin-3-yl)-9H-purin-6-amine] inhibited head and neck cancer cell migration. AHR antagonists also inhibited BaP induction of the drug transporter ABCG2, demonstrating a potential clinical application for SAhRM antagonists in treatment of head and neck cancers (DiNatale et al., 2012).
The AHR is highly expressed in lung cancer patients (Lin et al., 2003; Portal-Nunez et al., 2012), and several reports show that various AHR agonists including tobacco smoke extracts (rich in PAHs), β-naphthoflavone, PAHs, TCDD, and related AHR agonists induce lung cancer cell growth through activation of multiple pathways (Chuang et al., 2012; Lin et al., 2003; Shimba et al., 2002; Wang et al., 2009). For example, AHR agonists induce fibroblast growth factor-9 (Wang et al., 2009) and growth promoting genes including PCNA and DP2 (Shimba et al., 2002), osteopontin (Chuang et al., 2012), and adrenomedullin (Portal-Nunez et al., 2012), which contribute to lung cancer cell growth/migration and tumor progression, respectively. Moreover, both adrenomedullin and osteopontin expression in tumors correlated with expression of AHR or AHR-regulated genes. Thus, the AHR and AHR agonists play a role in lung and head and neck cancer growth/progression, and as demonstrated for head and neck cancers, AHR antagonists may have therapeutic benefits for treating both cancers.
Melanoma, Esophageal, and Pituitary tumors
TCDD and other AHR agonists induced several MMPs and also increased invasion/migration in melanoma A2058 cells (Villano et al., 2006). The anti-inflammatory drug leflunomide was characterized as an AHR agonist, and inhibition of A375 melanoma cell growth and induction of p21 by leflunomide were AHR dependent (O’Donnell et al., 2012). In contrast, TCDD did not inhibit growth of this cell line, and further studies on the differences between TCDD versus leflunomide in these cells require further investigation.
The AHR protein is highly expressed in esophageal tumors and cancer cell lines (e.g., Eca 109 and TE-13), and the AHR agonist β-naphthoflavone inhibits invasion of esophageal cancer cells (Zhang et al., 2012a). Another report shows that BaP induced and AHR antagonists (salicylamide and kaempferol) inhibited expression of the ABCG2 drug transporter in cisplatin-resistant cell lines, suggesting that the use of SAhRM agonists/antagonists may be cell context dependent. Expression of the AHR and AHR interacting protein has been reported in pituitary adenomas; however, effects of SAhRMs have not been determined (Jaffrain-Rea et al., 2009).
Lymphomas and Leukemia
Exposure to HAs has been associated with increased lymphomas, and studies with lymphoma cancer cell lines showed that TCDD decreased apoptosis, and this was accompanied by induction of COX-2, C/EBPβ, and bcl-xl (Vogel et al., 2007). Moreover, AHR antagonists reversed the prosurvival effects of TCDD, suggesting that SAhRM antagonists may be useful for treating lymphoma. Retinoic acid–induced differentiation of HL-60 promyelocytic leukemia cells was due, in part, to AHR-mediated downregulation of the stem cell transcription factor Oct4 (Bunaciu and Yen, 2011). The effects of SAhRM agonists/antagonists were not determined; however, it is likely that an AHR ligand may have clinical application for some leukemias.
Neuronal Cancers
The AHR promotes proliferation of human DAOY medulloblastoma cells (Dever and Opanashuk, 2012), and TCDD induces CYP1A1 in these cells; however, the functional effects of TCDD or other AHR ligands in this cell line have not been investigated (Dever and Opanashuk, 2012). The AHR is expressed in human gliomas and glioblastoma cell lines, and treatment with 3-MC enhanced G0/G1 to S-phase progression in LN-308 but not LNT-229 cells (Gramatzki et al., 2009). The AHR antagonist CH-223191 did not affect cell cycle progression, but this compound or AHR silencing decreased the clonogenicity of these cells. CH-223191 also decreased invasiveness of the glioblastoma cell lines, demonstrating potential clinical application for SAhRM antagonists. Subsequent studies have linked tryptophan-2,3-dioxygenase-2-mediated metabolism of tryptophan to the AHR agonist kynurenine as a critical event in promoting the progression and survival of brain tumors (Adams et al., 2012; Opitz et al., 2011). Kynurenine activation of the AHR not only promotes tumor cell survival and motility but also inhibits protective immune response pathways, and it was suggested that the TDO-2-kynurenine-AHR pathway may play a role in formation of multiple tumors (Adams et al., 2012). These results also confirm the previous report (Gramatzki et al., 2009), which suggested a role for SAhRM antagonists for brain tumor chemotherapy. Interestingly, it has also been reported that indirubins (AHR ligands) decrease glioma invasion (Williams et al., 2011) by inhibition of GSK3, and the activity of indirubins as SAhRM antagonists in gliomas needs to be reinvestigated.
SUMMARY
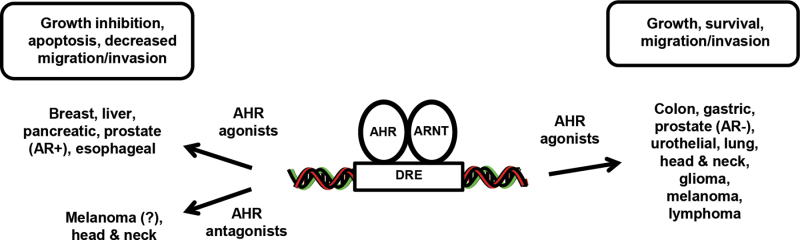
There is increasing evidence that the AHR and its ligands play an important role in carcinogenesis (Figs. 2 and 3), confirming the potential for the AHR as a drug target. Many tumors express the AHR (mRNA and/or protein) and, although there may be some inconsistencies regarding the tumor suppressor or pro-oncogenic functions of the AHR (Fig. 2), it is clear that the endogenous receptor influences tumor growth, survival, migration, and invasion. There is also tumor-specific variability with respect to the effects of AHR ligands (agonists vs. antagonists) on carcinogenesis; however, in most tumors, it is clear that these ligands affect tumor growth, survival, migration, and invasion (Fig. 3). In tumors where an AHR agonist or antagonist exhibits pro-oncogenic activity, it should be feasible to develop a SAhRM with the reverse activity that will inhibit tumorigenesis. It has already been shown that SAhRM agonists inhibit mammary carcinogenesis (McDougal et al., 2001; Zhang et al., 2012b) and SAhRM antagonists inhibit head and neck cancer and glioblastomas (DiNatale et al., 2011, 2012; Gramatzki et al., 2009), and a similar approach can be used for treating other AHR-dependent diseases. Thus, the AHR is like many other receptors (e.g., ERα) that mediate ligand-dependent toxic and therapeutic responses, indicating the importance for continued development of new SAhRMs for clinical applications including cancer chemotherapy.
Fig. 3.
Activities of AHR ligands in cancer.
FUNDING
National Institutes of Health (R01 CA142697).
REFERENCES
- Abbott B. D., Schmid J. E., Pitt J. A., Buckalew A. R., Wood C. R., Held G. A., Diliberto J. J. (1999). Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol. Appl. Pharmacol. 155, 62–70 [DOI] [PubMed] [Google Scholar]
- Abdelrahim M., Smith R., 3rd, Safe S. (2003). Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Mol. Pharmacol. 63, 1373–1381 [DOI] [PubMed] [Google Scholar]
- Adams S., Braidy N., Bessede A., Bessesde A., Brew B. J., Grant R., Teo C., Guillemin G. J. (2012). The kynurenine pathway in brain tumor pathogenesis. Cancer Res. 72, 5649–5657 [DOI] [PubMed] [Google Scholar]
- Aguilera-Montilla N., Chamorro S., Nieto C., Sánchez-Cabo F., Dopazo A., Fernández-Salguero P. M., Rodríguez-Fernández J. L., Pello O. M., Andrés V., Cuenda A., et al. (2013). Aryl hydrocarbon receptor contributes to the MEK/ERK-dependent maintenance of the immature state of human dendritic cells. Blood. 121, e108–e117 [DOI] [PubMed] [Google Scholar]
- Ahmed S., Al-Saigh S., Matthews J. (2012). FOXA1 is essential for aryl hydrocarbon receptor-dependent regulation of cyclin G2. Mol. Cancer Res. 10, 636–648 [DOI] [PubMed] [Google Scholar]
- Ahmed S., Valen E., Sandelin A., Matthews J. (2009). Dioxin increases the interaction between aryl hydrocarbon receptor and estrogen receptor alpha at human promoters. Toxicol. Sci. 111, 254–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambolet-Camoit A., Bui L. C., Pierre S., Chevallier A., Marchand A., Coumoul X., Garlatti M., Andreau K., Barouki R., Aggerbeck M. (2010). 2,3,7,8-Tetrachlorodibenzo-p-dioxin counteracts the p53 response to a genotoxicant by upregulating expression of the metastasis marker agr2 in the hepatocarcinoma cell line HepG2. Toxicol. Sci. 115, 501–512 [DOI] [PubMed] [Google Scholar]
- Andersson P., McGuire J., Rubio C., Gradin K., Whitelaw M. L., Pettersson S., Hanberg A., Poellinger L. (2002). A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc. Natl. Acad. Sci. U. S. A. 99, 9990–9995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L., Quintana F. J., Pot C., Joller N., Xiao S., Kumar D., Burns E. J., Sherr D. H., Weiner H. L., Kuchroo V. K. (2010). The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11, 854–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astroff B., Safe S. (1989). 6-Substituted-1,3,8-trichlorodibenzofurans as 2,3,7,8-tetrachlorodibenzo-p-dioxin antagonists in the rat: Structure activity relationships. Toxicology. 59, 285–296 [DOI] [PubMed] [Google Scholar]
- Astroff B., Zacharewski T., Safe S., Arlotto M. P., Parkinson A., Thomas P., Levin W. (1988). 6-Methyl-1,3,8-trichlorodibenzofuran as a 2,3,7,8-tetrachlorodibenzo-p-dioxin antagonist: Inhibition of the induction of rat cytochrome P-450 isozymes and related monooxygenase activities. Mol. Pharmacol. 33, 231–236 [PubMed] [Google Scholar]
- Augereau P., Badia E., Fuentes M., Rabenoelina F., Corniou M., Derocq D., Balaguer P., Cavailles V. (2006). Transcriptional regulation of the human NRIP1/RIP140 gene by estrogen is modulated by dioxin signalling. Mol. Pharmacol. 69, 1338–1346 [DOI] [PubMed] [Google Scholar]
- Baba T., Mimura J., Nakamura N., Harada N., Yamamoto M., Morohashi K., Fujii-Kuriyama Y. (2005). Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol. Cell. Biol. 25, 10040–10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister R., Biegel L., Davis D., Astroff B., Safe S. (1989). 6-Methyl-1,3,8-trichlorodibenzofuran (MCDF) as a 2,3,7,8-tetrachlorodibenzo-p-dioxin antagonist in C57BL/6 mice. Toxicology. 54, 139–150 [DOI] [PubMed] [Google Scholar]
- Barhoover M. A., Hall J. M., Greenlee W. F., Thomas R. S. (2010). Aryl hydrocarbon receptor regulates cell cycle progression in human breast cancer cells via a functional interaction with cyclin-dependent kinase 4. Mol. Pharmacol. 77, 195–201 [DOI] [PubMed] [Google Scholar]
- Barnes-Ellerbe S., Knudsen K. E., Puga A. (2004). 2,3,7,8-Tetrachlorodibenzo-p-dioxin blocks androgen-dependent cell proliferation of LNCaP cells through modulation of pRB phosphorylation. Mol. Pharmacol. 66, 502–511 [DOI] [PubMed] [Google Scholar]
- Barouki R., Coumoul X., Fernandez-Salguero P. M. (2007). The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 581, 3608–3615 [DOI] [PubMed] [Google Scholar]
- Barretina J., Caponigro G., Stransky N., Venkatesan K., Margolin A. A., Kim S., Wilson C. J., Lehár J., Kryukov G. V., Sonkin D., et al. (2012). The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 483, 603–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag T. V., Perdew G. H. (2005). ER alpha-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J. Biol. Chem. 280, 21607–21611 [DOI] [PubMed] [Google Scholar]
- Benedict J. C., Lin T. M., Loeffler I. K., Peterson R. E., Flaws J. A. (2000). Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol. Sci. 56, 382–388 [DOI] [PubMed] [Google Scholar]
- Benedict J. C., Miller K. P., Lin T. M., Greenfeld C., Babus J. K., Peterson R. E., Flaws J. A. (2003). Aryl hydrocarbon receptor regulates growth, but not atresia, of mouse preantral and antral follicles. Biol. Reprod. 68, 1511–1517 [DOI] [PubMed] [Google Scholar]
- Biegel L., Safe S. (1990). Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on cell growth and the secretion of the estrogen-induced 34-, 52- and 160-kDa proteins in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 37, 725–732 [DOI] [PubMed] [Google Scholar]
- Bock K. W., Köhle C. (2005). Ah receptor- and TCDD-mediated liver tumor promotion: Clonal selection and expansion of cells evading growth arrest and apoptosis. Biochem. Pharmacol. 69, 1403–1408 [DOI] [PubMed] [Google Scholar]
- Boitano A. E., Wang J., Romeo R., Bouchez L. C., Parker A. E., Sutton S. E., Walker J. R., Flaveny C. A., Perdew G. H., Denison M. S., et al. (2010). Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 329, 1345–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. M., Manzolillo P. A., Zhang J. X., Wang J., Lamartiniere C. A. (1998). Prenatal TCDD and predisposition to mammary cancer in the rat. Carcinogenesis. 19, 1623–1629 [DOI] [PubMed] [Google Scholar]
- Bunaciu R. P., Yen A. (2011). Activation of the aryl hydrocarbon receptor AhR Promotes retinoic acid-induced differentiation of myeloblastic leukemia cells by restricting expression of the stem cell transcription factor Oct4. Cancer Res. 71, 2371–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach K. M., Poland A., Bradfield C. A. (1992). Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc. Natl. Acad. Sci. U. S. A. 89, 8185–8189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R., Inzunza J., Suzuki H., Fujii-Kuriyama Y., Warner M., Gustafsson J. Å. (2012). Uric acid stones in the urinary bladder of aryl hydrocarbon receptor (AhR) knockout mice. Proc. Natl. Acad. Sci. U. S. A. 109, 1122–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Rivera E., Wormke M., Safe S. (1999). Estrogen and aryl hydrocarbon responsiveness of ECC-1 endometrial cancer cells. Mol. Cell. Endocrinol. 150, 11–21 [DOI] [PubMed] [Google Scholar]
- Chen I., McDougal A., Wang F., Safe S. (1998). Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 19, 1631–1639 [DOI] [PubMed] [Google Scholar]
- Chesire D. R., Dunn T. A., Ewing C. M., Luo J., Isaacs W. B. (2004). Identification of aryl hydrocarbon receptor as a putative Wnt/beta-catenin pathway target gene in prostate cancer cells. Cancer Res. 64, 2523–2533 [DOI] [PubMed] [Google Scholar]
- Chevallier A., Mialot A., Petit J. M., Fernandez-Salguero P., Barouki R., Coumoul X., Beraneck M. (2013). Oculomotor deficits in aryl hydrocarbon receptor null mouse. PLoS One. 8, e53520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C. Y., Chang H., Lin P., Sun S. J., Chen P. H., Lin Y. Y., Sheu G. T., Ko J. L., Hsu S. L., Chang J. T. (2012). Up-regulation of osteopontin expression by aryl hydrocarbon receptor via both ligand-dependent and ligand-independent pathways in lung cancer. Gene. 492, 262–269 [DOI] [PubMed] [Google Scholar]
- Cole P., Trichopoulos D., Pastides H., Starr T., Mandel J. S. (2003). Dioxin and cancer: A critical review. Regul. Toxicol. Pharmacol. 38, 378–388 [DOI] [PubMed] [Google Scholar]
- Degner S. C., Papoutsis A. J., Selmin O., Romagnolo D. F. (2009). Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3’-diindolylmethane in breast cancer cells. J. Nutr. 139, 26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M. S., Soshilov A. A., He G., DeGroot D. E., Zhao B. (2011). Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 124, 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever D. P., Opanashuk L. A. (2012). The aryl hydrocarbon receptor contributes to the proliferation of human medulloblastoma cells. Mol. Pharmacol. 81, 669–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale B. C., Murray I. A., Schroeder J. C., Flaveny C. A., Lahoti T. S., Laurenzana E. M., Omiecinski C. J., Perdew G. H. (2010). Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 115, 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale B. C., Schroeder J. C., Perdew G. H. (2011). Ah receptor antagonism inhibits constitutive and cytokine inducible IL6 production in head and neck tumor cell lines. Mol. Carcinog. 50, 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale B. C., Smith K., John K., Krishnegowda G., Amin S. G., Perdew G. H. (2012). Ah receptor antagonism represses head and neck tumor cell aggressive phenotype. Mol. Cancer Res. 10, 1369–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diry M., Tomkiewicz C., Koehle C., Coumoul X., Bock K. W., Barouki R., Transy C. (2006). Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene. 25, 5570–5574 [DOI] [PubMed] [Google Scholar]
- Dolwick K. M., Schmidt J. V., Carver L. A., Swanson H. I., Bradfield C. A. (1993). Cloning and expression of a human Ah receptor cDNA. Mol. Pharmacol. 44, 911–917 [PubMed] [Google Scholar]
- Duan R., Porter W., Samudio I., Vyhlidal C., Kladde M., Safe S. (1999). Transcriptional activation of c-fos protooncogene by 17beta-estradiol: Mechanism of aryl hydrocarbon receptor-mediated inhibition. Mol. Endocrinol. 13, 1511–1521 [DOI] [PubMed] [Google Scholar]
- DuSell C. D., Nelson E. R., Wittmann B. M., Fretz J. A., Kazmin D., Thomas R. S., Pike J. W., McDonnell D. P. (2010). Regulation of aryl hydrocarbon receptor function by selective estrogen receptor modulators. Mol. Endocrinol. 24, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink C. J., Ge N. L., Levine A. (2001). Maximal aryl hydrocarbon receptor activity depends on an interaction with the retinoblastoma protein. Mol. Pharmacol. 59, 664–673 [DOI] [PubMed] [Google Scholar]
- Ema M., Sogawa K., Watanabe N., Chujoh Y., Matsushita N., Gotoh O., Funae Y., Fujii-Kuriyama Y. (1992). cDNA cloning and structure of mouse putative Ah receptor. Biochem. Biophys. Res. Commun. 184, 246–253 [DOI] [PubMed] [Google Scholar]
- Esser C. (2012). Biology and function of the aryl hydrocarbon receptor: Report of an international and interdisciplinary conference. Arch. Toxicol. 86, 1323–1329 [DOI] [PubMed] [Google Scholar]
- Esser C., Rannug A., Stockinger B. (2009). The aryl hydrocarbon receptor in immunity. Trends Immunol. 30, 447–454 [DOI] [PubMed] [Google Scholar]
- Fan Y., Boivin G. P., Knudsen E. S., Nebert D. W., Xia Y., Puga A. (2010). The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res. 70, 212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salguero P. M., Hilbert D. M., Rudikoff S., Ward J. M., Gonzalez F. J. (1996). Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol. Appl. Pharmacol. 140, 173–179 [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P., Pineau T., Hilbert D. M., McPhail T., Lee S. S., Kimura S., Nebert D. W., Rudikoff S., Ward J. M., Gonzalez F. J. (1995). Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 268, 722–726 [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P. M., Ward J. M., Sundberg J. P., Gonzalez F. J. (1997). Lesions of aryl-hydrocarbon receptor-deficient mice. Vet. Pathol. 34, 605–614 [DOI] [PubMed] [Google Scholar]
- Funatake C. J., Dearstyne E. A., Steppan L. B., Shepherd D. M., Spanjaard E. S., Marshak-Rothstein A., Kerkvliet N. I. (2004). Early consequences of 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on the activation and survival of antigen-specific T cells. Toxicol. Sci. 82, 129–142 [DOI] [PubMed] [Google Scholar]
- Funatake C. J., Marshall N. B., Kerkvliet N. I. (2008). 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters the differentiation of alloreactive CD8+ T cells toward a regulatory T cell phenotype by a mechanism that is dependent on aryl hydrocarbon receptor in CD4+ T cells. J. Immunotoxicol. 5, 81–91 [DOI] [PubMed] [Google Scholar]
- Funatake C. J., Marshall N. B., Steppan L. B., Mourich D. V., Kerkvliet N. I. (2005). Cutting edge: Activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J. Immunol. 175, 4184–4188 [DOI] [PubMed] [Google Scholar]
- Gandhi R., Kumar D., Burns E. J., Nadeau M., Dake B., Laroni A., Kozoriz D., Weiner H. L., Quintana F. J. (2010). Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat. Immunol. 11, 846–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiewicz T. A., Rucci G. (1991). Alpha-naphthoflavone acts as an antagonist of 2,3,7, 8-tetrachlorodibenzo-p-dioxin by forming an inactive complex with the Ah receptor. Mol. Pharmacol. 40, 607–612 [PubMed] [Google Scholar]
- Ge N. L., Elferink C. J. (1998). A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle. J. Biol. Chem. 273, 22708–22713 [DOI] [PubMed] [Google Scholar]
- Gene Expression Omnibus (GEO) Datasets: NCBI gene expression and hybridization array data repository. http://www.ncbi.nlm.nih.gov/gds [DOI] [PMC free article] [PubMed]
- Gierthy J. F., Lincoln D. W., 2nd (1988). Inhibition of postconfluent focus production in cultures of MCF-7 human breast cancer cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Breast Cancer Res. Treat. 12, 227–233 [DOI] [PubMed] [Google Scholar]
- Gierthy J. F., Lincoln D. W., Gillespie M. B., Seeger J. I., Martinez H. L., Dickerman H. W., Kumar S. A. (1987). Suppression of estrogen-regulated extracellular tissue plasminogen activator activity of MCF-7 cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Res. 47, 6198–6203 [PubMed] [Google Scholar]
- Gillesby B. E., Stanostefano M., Porter W., Safe S., Wu Z. F., Zacharewski T. R. (1997). Identification of a motif within the 5’ regulatory region of pS2 which is responsible for AP-1 binding and TCDD-mediated suppression. Biochemistry. 36, 6080–6089 [DOI] [PubMed] [Google Scholar]
- Gluschnaider U., Hidas G., Cojocaru G., Yutkin V., Ben-Neriah Y., Pikarsky E. (2010). beta-TrCP inhibition reduces prostate cancer cell growth via upregulation of the aryl hydrocarbon receptor. PLoS One. 5, e9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohl G., Lehmköster T., Münzel P. A., Schrenk D., Viebahn R., Bock K. W. (1996). TCDD-inducible plasminogen activator inhibitor type 2 (PAI-2) in human hepatocytes, HepG2 and monocytic U937 cells. Carcinogenesis. 17, 443–449 [DOI] [PubMed] [Google Scholar]
- Goldstein J. A., Safe S., Kimbrough R. D., Jensen A. A. (1989). Mechanism of action and structure-activity relationships for the chlorinated dibenzo-p-dioxins and related compounds. In Halogenated Biphenyls, Naphthalenes, Dibenzodioxins and Related Compounds., Vol. 2, pp. 239–293 Elsevier-North Holland, Amsterdam, The Netherlands: [Google Scholar]
- Gramatzki D., Pantazis G., Schittenhelm J., Tabatabai G., Köhle C., Wick W., Schwarz M., Weller M., Tritschler I. (2009). Aryl hydrocarbon receptor inhibition downregulates the TGF-beta/Smad pathway in human glioblastoma cells. Oncogene. 28, 2593–2605 [DOI] [PubMed] [Google Scholar]
- Gu Y. Z., Hogenesch J. B., Bradfield C. A. (2000). The PAS superfamily: Sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. 40, 519–561 [DOI] [PubMed] [Google Scholar]
- Hall J. M., Barhoover M. A., Kazmin D., McDonnell D. P., Greenlee W. F., Thomas R. S. (2010). Activation of the aryl-hydrocarbon receptor inhibits invasive and metastatic features of human breast cancer cells and promotes breast cancer cell differentiation. Mol. Endocrinol. 24, 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M., Francis J., Sehgal I. (2005). Aryl hydrocarbon exposure induces expression of MMP-9 in human prostate cancer cell lines. Cancer Lett. 225, 159–166 [DOI] [PubMed] [Google Scholar]
- Harper N., Wang X., Liu H., Safe S. (1994). Inhibition of estrogen-induced progesterone receptor in MCF-7 human breast cancer cells by aryl hydrocarbon (Ah) receptor agonists. Mol. Cell. Endocrinol. 104, 47–55 [DOI] [PubMed] [Google Scholar]
- Harris M., Zacharewski T., Astroff B., Safe S. (1989). Partial antagonism of 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated induction of aryl hydrocarbon hydroxylase by 6-methyl-1,3,8-trichlorodibenzofuran: Mechanistic studies. Mol. Pharmacol. 35, 729–735 [PubMed] [Google Scholar]
- Hockings J. K., Thorne P. A., Kemp M. Q., Morgan S. S., Selmin O., Romagnolo D. F. (2006). The ligand status of the aromatic hydrocarbon receptor modulates transcriptional activation of BRCA-1 promoter by estrogen. Cancer Res. 66, 2224–2232 [DOI] [PubMed] [Google Scholar]
- Holcomb M., Safe S. (1994). Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumor growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Lett. 82, 43–47 [DOI] [PubMed] [Google Scholar]
- Hollingshead B. D., Beischlag T. V., Dinatale B. C., Ramadoss P., Perdew G. H. (2008). Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res. 68, 3609–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E. L., Chen N., Westbrook A., Wang F., Zhang R., Taylor R. T., Hankinson O. (2008). CXCR4 and CXCL12 down-regulation: A novel mechanism for the chemoprotection of 3,3’-diindolylmethane for breast and ovarian cancers. Cancer Lett. 265, 113–123 [DOI] [PubMed] [Google Scholar]
- Hsu E. L., Yoon D., Choi H. H., Wang F., Taylor R. T., Chen N., Zhang R., Hankinson O. (2007). A proposed mechanism for the protective effect of dioxin against breast cancer. Toxicol. Sci. 98, 436–444 [DOI] [PubMed] [Google Scholar]
- Hu W., Sorrentino C., Denison M. S., Kolaja K., Fielden M. R. (2007). Induction of cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: Results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol. Pharmacol. 71, 1475–1486 [DOI] [PubMed] [Google Scholar]
- Huang G., Elferink C. J. (2005). Multiple mechanisms are involved in Ah receptor-mediated cell cycle arrest. Mol. Pharmacol. 67, 88–96 [DOI] [PubMed] [Google Scholar]
- IARC (1997). Polychlorinated Dibenzo-para-dioxins and Polychlorinated Dibenzofurans. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Lyon, France: [PMC free article] [PubMed] [Google Scholar]
- Ishida M., Mikami S., Kikuchi E., Kosaka T., Miyajima A., Nakagawa K., Mukai M., Okada Y., Oya M. (2010). Activation of the aryl hydrocarbon receptor pathway enhances cancer cell invasion by upregulating the MMP expression and is associated with poor prognosis in upper urinary tract urothelial cancer. Carcinogenesis. 31, 287–295 [DOI] [PubMed] [Google Scholar]
- Ito T., Tsukumo S., Suzuki N., Motohashi H., Yamamoto M., Fujii-Kuriyama Y., Mimura J., Lin T. M., Peterson R. E., Tohyama C., et al. (2004). A constitutively active arylhydrocarbon receptor induces growth inhibition of jurkat T cells through changes in the expression of genes related to apoptosis and cell cycle arrest. J. Biol. Chem. 279, 25204–25210 [DOI] [PubMed] [Google Scholar]
- Jaffrain-Rea M. L., Angelini M., Gargano D., Tichomirowa M. A., Daly A. F., Vanbellinghen J. F., D’Innocenzo E., Barlier A., Giangaspero F., Esposito V., et al. (2009). Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: Pathological and clinical implications. Endocr. Relat. Cancer. 16, 1029–1043 [DOI] [PubMed] [Google Scholar]
- Jana N. R., Sarkar S., Ishizuka M., Yonemoto J., Tohyama C., Sone H. (1999). Cross-talk between 2,3,7,8-tetrachlorodibenzo-p-dioxin and testosterone signal transduction pathways in LNCaP prostate cancer cells. Biochem. Biophys. Res. Commun. 256, 462–468 [DOI] [PubMed] [Google Scholar]
- Jana N. R., Sarkar S., Ishizuka M., Yonemoto J., Tohyama C., Sone H. (2000). Comparative effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on MCF-7, RL95-2, and LNCaP cells: Role of target steroid hormones in cellular responsiveness to CYP1A1 induction. Mol. Cell Biol. Res. Commun. 4, 174–180 [DOI] [PubMed] [Google Scholar]
- Jin G. B., Moore A. J., Head J. L., Neumiller J. J., Lawrence B. P. (2010). Aryl hydrocarbon receptor activation reduces dendritic cell function during influenza virus infection. Toxicol. Sci. 116, 514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin U. H., Lee S. O., Safe S. (2012). Aryl hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR modulators in MDA-MB-468 and BT474 breast cancer cells. J. Pharmacol. Exp. Ther. 343, 333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan V. C. (2007). SERMs: Meeting the promise of multifunctional medicines. J. Natl. Cancer Inst. 99, 350–356 [DOI] [PubMed] [Google Scholar]
- Jordan V. C. (2009). A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: The origins of targeted therapy and chemoprevention. Cancer Res. 69, 1243–1254 [DOI] [PubMed] [Google Scholar]
- Jordan V. C., O’Malley B. W. (2007). Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J. Clin. Oncol. 25, 5815–5824 [DOI] [PubMed] [Google Scholar]
- Kadow S., Jux B., Zahner S. P., Wingerath B., Chmill S., Clausen B. E., Hengstler J., Esser C. (2011). Aryl hydrocarbon receptor is critical for homeostasis of invariant gammadelta T cells in the murine epidermis. J. Immunol. 187, 3104–3110 [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen J. A., O’Malley B. W., Katzenellenbogen B. S. (1996). Tripartite steroid hormone receptor pharmacology: Interaction with multiple effector sites as a basis for the cell- and promoter-specific action of these hormones. Mol. Endocrinol. 10, 119–131 [DOI] [PubMed] [Google Scholar]
- Kawajiri K., Kobayashi Y., Ohtake F., Ikuta T., Matsushima Y., Mimura J., Pettersson S., Pollenz R. S., Sakaki T., Hirokawa T., et al. (2009). Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc. Natl. Acad. Sci. U. S. A. 106, 13481–13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet N. I. (1995). Immunological effects of chlorinated dibenzo-p-dioxins. Environ. Health Perspect. 103(Suppl. 9)47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Barhoumi R., Burghardt R., Liu S., Kim K., Safe S. (2006). Molecular mechanism of inhibitory aryl hydrocarbon receptor-estrogen receptor/Sp1 cross talk in breast cancer cells. Mol. Endocrinol. 20, 2199–2214 [DOI] [PubMed] [Google Scholar]
- Kim S. H., Henry E. C., Kim D. K., Kim Y. H., Shin K. J., Han M. S., Lee T. G., Kang J. K., Gasiewicz T. A., Ryu S. H., et al. (2006). Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol. Pharmacol. 69, 1871–1878 [DOI] [PubMed] [Google Scholar]
- Kiss E. A., Vonarbourg C., Kopfmann S., Hobeika E., Finke D., Esser C., Diefenbach A. (2011). Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 334, 1561–1565 [DOI] [PubMed] [Google Scholar]
- Knerr S., Schrenk D. (2006). Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol. Nutr. Food Res. 50, 897–907 [DOI] [PubMed] [Google Scholar]
- Kociba R. J., Keyes D. G., Beyer J. E., Carreon R. M., Wade C. E., Dittenber D. A., Kalnins R. P., Frauson L. E., Park C. N., Barnard S. D., et al. (1978). Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol. Appl. Pharmacol. 46, 279–303 [DOI] [PubMed] [Google Scholar]
- Köhle C., Bock K. W. (2007). Coordinate regulation of phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem. Pharmacol. 73, 1853–1862 [DOI] [PubMed] [Google Scholar]
- Köhle C., Hassepass I., Bock-Hennig B. S., Walter Bock K., Poellinger L., McGuire J. (2002). Conditional expression of a constitutively active aryl hydrocarbon receptor in MCF-7 human breast cancer cells. Arch. Biochem. Biophys. 402, 172–179 [DOI] [PubMed] [Google Scholar]
- Koliopanos A., Kleeff J., Xiao Y., Safe S., Zimmermann A., Büchler M. W., Friess H. (2002). Increased arylhydrocarbon receptor expression offers a potential therapeutic target for pancreatic cancer. Oncogene. 21, 6059–6070 [DOI] [PubMed] [Google Scholar]
- Kolluri S. K., Weiss C., Koff A., Göttlicher M. (1999). p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev. 13, 1742–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Porter W., Santostefano M., Wang X., Safe S. (1995). Molecular mechanism of inhibition of estrogen-induced cathepsin D gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in MCF-7 cells. Mol. Cell. Biol. 15, 6710–6719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Safe S. (1993). Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), and dibenzofurans (PCDFs) as antiestrogens in MCF-7 human breast cancer cells: Quantitative structure-activity relationships. Toxicol. Appl. Pharmacol. 120, 55–61 [DOI] [PubMed] [Google Scholar]
- Krishnan V., Wang X., Safe S. (1994). Estrogen receptor-Sp1 complexes mediate estrogen-induced cathepsin D gene expression in MCF-7 human breast cancer cells. J. Biol. Chem. 269, 15912–15917 [PubMed] [Google Scholar]
- Kumar M. B., Perdew G. H. (1999). Nuclear receptor coactivator SRC-1 interacts with the Q-rich subdomain of the AhR and modulates its transactivation potential. Gene Expr. 8, 273–286 [PMC free article] [PubMed] [Google Scholar]
- Kumar M. B., Tarpey R. W., Perdew G. H. (1999). Differential recruitment of coactivator RIP140 by Ah and estrogen receptors. Absence of a role for LXXLL motifs. J. Biol. Chem. 274, 22155–22164 [DOI] [PubMed] [Google Scholar]
- Labrecque M. P., Takhar M. K., Hollingshead B. D., Prefontaine G. G., Perdew G. H., Beischlag T. V. (2012). Distinct roles for aryl hydrocarbon receptor nuclear translocator and ah receptor in estrogen-mediated signaling in human cancer cell lines. PLoS One. 7, e29545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis G. P., Lindell S. L., Thomas R. S., McCuskey R. S., Murphy C., Glover E., Bentz M., Southard J., Bradfield C. A. (2000). Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 97, 10442–10447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis G. P., Pyzalski R. W., Glover E., Pitot H. C., McElwee M. K., Bradfield C. A. (2005). The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol. Pharmacol. 67, 714–720 [DOI] [PubMed] [Google Scholar]
- Le Ferrec E., Lagadic-Gossmann D., Rauch C., Bardiau C., Maheo K., Massiere F., Le Vee M., Guillouzo A., Morel F. (2002). Transcriptional induction of CYP1A1 by oltipraz in human Caco-2 cells is aryl hydrocarbon receptor- and calcium-dependent. J. Biol. Chem. 277, 24780–24787 [DOI] [PubMed] [Google Scholar]
- Lee J. S., Cella M., McDonald K. G., Garlanda C., Kennedy G. D., Nukaya M., Mantovani A., Kopan R., Bradfield C. A., Newberry R. D., et al. (2012). AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 13, 144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine-Fridman A., Chen L., Elferink C. J. (2004). Cytochrome P4501A1 promotes G1 phase cell cycle progression by controlling aryl hydrocarbon receptor activity. Mol. Pharmacol. 65, 461–469 [DOI] [PubMed] [Google Scholar]
- Li Y., Innocentin S., Withers D. R., Roberts N. A., Gallagher A. R., Grigorieva E. F., Wilhelm C., Veldhoen M. (2011). Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 147, 629–640 [DOI] [PubMed] [Google Scholar]
- Lin P., Chang H., Tsai W. T., Wu M. H., Liao Y. S., Chen J. T., Su J. M. (2003). Overexpression of aryl hydrocarbon receptor in human lung carcinomas. Toxicol. Pathol. 31, 22–30 [DOI] [PubMed] [Google Scholar]
- Loaiza-Pérez A. I., Kenney S., Boswell J., Hollingshead M., Alley M. C., Hose C., Ciolino H. P., Yeh G. C., Trepel J. B., Vistica D. T., et al. (2004). Aryl hydrocarbon receptor activation of an antitumor aminoflavone: Basis of selective toxicity for MCF-7 breast tumor cells. Mol. Cancer Ther. 3, 715–725 [PubMed] [Google Scholar]
- Lu Y. F., Santostefano M., Cunningham B. D., Threadgill M. D., Safe S. (1996a). Substituted flavones as aryl hydrocarbon (Ah) receptor agonists and antagonists. Biochem. Pharmacol. 51, 1077–1087 [DOI] [PubMed] [Google Scholar]
- Lu Y. F., Sun G., Wang X., Safe S. (1996b). Inhibition of prolactin receptor gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin in MCF-7 human breast cancer cells. Arch. Biochem. Biophys. 332, 35–40 [DOI] [PubMed] [Google Scholar]
- Lund A. K., Goens M. B., Kanagy N. L., Walker M. K. (2003). Cardiac hypertrophy in aryl hydrocarbon receptor null mice is correlated with elevated angiotensin II, endothelin-1, and mean arterial blood pressure. Toxicol. Appl. Pharmacol. 193, 177–187 [DOI] [PubMed] [Google Scholar]
- Lund A. K., Goens M. B., Nuñez B. A., Walker M. K. (2006). Characterizing the role of endothelin-1 in the progression of cardiac hypertrophy in aryl hydrocarbon receptor (AhR) null mice. Toxicol. Appl. Pharmacol. 212, 127–135 [DOI] [PubMed] [Google Scholar]
- Ma Q., Whitlock J. P., Jr (1996). The aromatic hydrocarbon receptor modulates the Hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Mol. Cell. Biol. 16, 2144–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madak-Erdogan Z., Katzenellenbogen B. S. (2012). Aryl hydrocarbon receptor modulation of estrogen receptor α-mediated gene regulation by a multimeric chromatin complex involving the two receptors and the coregulator RIP140. Toxicol. Sci. 125, 401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlowe J. L., Knudsen E. S., Schwemberger S., Puga A. (2004). The aryl hydrocarbon receptor displaces p300 from E2F-dependent promoters and represses S phase-specific gene expression. J. Biol. Chem. 279, 29013–29022 [DOI] [PubMed] [Google Scholar]
- Marshall N. B., Kerkvliet N. I. (2010). Dioxin and immune regulation: Emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann. N. Y. Acad. Sci. 1183, 25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N. B., Vorachek W. R., Steppan L. B., Mourich D. V., Kerkvliet N. I. (2008). Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Immunol. 181, 2382–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J., Wihlén B., Thomsen J., Gustafsson J. A. (2005). Aryl hydrocarbon receptor-mediated transcription: Ligand-dependent recruitment of estrogen receptor alpha to 2,3,7,8-tetrachlorodibenzo-p-dioxin-responsive promoters. Mol. Cell. Biol. 25, 5317–5328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal A., Sethi Gupta M., Ramamoorthy K., Sun G., Safe S. H. (2000). Inhibition of carcinogen-induced rat mammary tumor growth and other estrogen-dependent responses by symmetrical dihalo-substituted analogs of diindolylmethane. Cancer Lett. 151, 169–179 [DOI] [PubMed] [Google Scholar]
- McDougal A., Wilson C., Safe S. (1997). Inhibition of 7,12-dimethylbenz[a]anthracene-induced rat mammary tumor growth by aryl hydrocarbon receptor agonists. Cancer Lett. 120, 53–63 [DOI] [PubMed] [Google Scholar]
- McDougal A., Wormke M., Calvin J., Safe S. (2001). Tamoxifen-induced antitumorigenic/antiestrogenic action synergized by a selective aryl hydrocarbon receptor modulator. Cancer Res. 61, 3902–3907 [PubMed] [Google Scholar]
- McMillan B. J., Bradfield C. A. (2007). The aryl hydrocarbon receptor sans xenobiotics: Endogenous function in genetic model systems. Mol. Pharmacol. 72, 487–498 [DOI] [PubMed] [Google Scholar]
- Mezrich J. D., Fechner J. H., Zhang X., Johnson B. P., Burlingham W. J., Bradfield C. A. (2010). An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185, 3190–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura J., Yamashita K., Nakamura K., Morita M., Takagi T. N., Nakao K., Ema M., Sogawa K., Yasuda M., Katsuki M., et al. (1997). Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 2, 645–654 [DOI] [PubMed] [Google Scholar]
- Moennikes O., Loeppen S., Buchmann A., Andersson P., Ittrich C., Poellinger L., Schwarz M. (2004). A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res. 64, 4707–4710 [DOI] [PubMed] [Google Scholar]
- Moore M., Ruh M., Steinberg M., Safe S. (1996). Isolation and characterization of variant benzo[a]pyrene-resistant T47D human breast-cancer cells. Int. J. Cancer. 66, 117–123 [DOI] [PubMed] [Google Scholar]
- Moore M., Wang X., Lu Y. F., Wormke M., Craig A., Gerlach J. H., Burghardt R., Barhoumi R., Safe S. (1994). Benzo[a]pyrene-resistant MCF-7 human breast cancer cells. A unique aryl hydrocarbon-nonresponsive clone. J. Biol. Chem. 269, 11751–11759 [PubMed] [Google Scholar]
- Morrow D., Qin C., Smith R., 3rd, Safe S. (2004). Aryl hydrocarbon receptor-mediated inhibition of LNCaP prostate cancer cell growth and hormone-induced transactivation. J. Steroid Biochem. Mol. Biol. 88, 27–36 [DOI] [PubMed] [Google Scholar]
- Mulero-Navarro S., Pozo-Guisado E., Pérez-Mancera P. A., Alvarez-Barrientos A., Catalina-Fernández I., Hernández-Nieto E., Sáenz-Santamaria J., Martínez N., Rojas J. M., Sánchez-García I., et al. (2005). Immortalized mouse mammary fibroblasts lacking dioxin receptor have impaired tumorigenicity in a subcutaneous mouse xenograft model. J. Biol. Chem. 280, 28731–28741 [DOI] [PubMed] [Google Scholar]
- Murray I. A., Flaveny C. A., Chiaro C. R., Sharma A. K., Tanos R. S., Schroeder J. C., Amin S. G., Bisson W. H., Kolluri S. K., Perdew G. H. (2011). Suppression of cytokine-mediated complement factor gene expression through selective activation of the Ah receptor with 3’,4’-dimethoxy-α-naphthoflavone. Mol. Pharmacol. 79, 508–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Dalton T. P., Okey A. B., Gonzalez F. J. (2004). Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 279, 23847–23850 [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Robinson J. R., Niwa A., Kumaki K., Poland A. P. (1975). Genetic expression of aryl hydrocarbon hydroxylase activity in the mouse. J. Cell. Physiol. 85(2 Pt 2 Suppl 1)393–414 [DOI] [PubMed] [Google Scholar]
- Nguyen N. T., Kimura A., Nakahama T., Chinen I., Masuda K., Nohara K., Fujii-Kuriyama Y., Kishimoto T. (2010). Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. U. S. A. 107, 19961–19966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. A., Hoivik D., Lee J. E., Safe S. (1999). Interactions of nuclear receptor coactivator/corepressor proteins with the aryl hydrocarbon receptor complex. Arch. Biochem. Biophys. 367, 250–257 [DOI] [PubMed] [Google Scholar]
- O’Donnell E. F., Kopparapu P. R., Koch D. C., Jang H. S., Phillips J. L., Tanguay R. L., Kerkvliet N. I., Kolluri S. K. (2012). The aryl hydrocarbon receptor mediates leflunomide-induced growth inhibition of melanoma cells. PLoS One. 7, e40926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz C. A., Litzenburger U. M., Sahm F., Ott M., Tritschler I., Trump S., Schumacher T., Jestaedt L., Schrenk D., Weller M., et al. (2011). An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 478, 197–203 [DOI] [PubMed] [Google Scholar]
- Peng T. L., Chen J., Mao W., Song X., Chen M. H. (2009). Aryl hydrocarbon receptor pathway activation enhances gastric cancer cell invasiveness likely through a c-Jun-dependent induction of matrix metalloproteinase-9. BMC Cell Biol. 10, 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskorska-Pliszczynska J., Astroff B., Zacharewski T., Harris M., Rosengren R., Morrison V., Safe L., Safe S. (1991). Mechanism of action of 2,3,7,8-tetrachlorodibenzo-p-dioxin antagonists: Characterization of 6-[125I]methyl-8-iodo-1,3-dichlorodibenzofuran-Ah receptor complexes. Arch. Biochem. Biophys. 284, 193–200 [DOI] [PubMed] [Google Scholar]
- Pohjanvirta R. (2012). The Ah Receptor in Biology and Toxicology. John Wiler & Sons, Hoboken, NJ: [Google Scholar]
- Poland A., Glover E. (1973). Studies on the mechanism of toxicity of the chlorinated dibenzo-p-dioxins. Environ. Health Perspect. 5, 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A., Glover E., Kende A. S. (1976). Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 251, 4936–4946 [PubMed] [Google Scholar]
- Poland A., Knutson J. C. (1982). 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: Examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 22, 517–554 [DOI] [PubMed] [Google Scholar]
- Portal-Nuñez S., Shankavaram U. T., Rao M., Datrice N., Atay S., Aparicio M., Camphausen K. A., Fernández-Salguero P. M., Chang H., Lin P., et al. (2012). Aryl hydrocarbon receptor-induced adrenomedullin mediates cigarette smoke carcinogenicity in humans and mice. Cancer Res. 72, 5790–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter W., Wang F., Duan R., Qin C., Castro-Rivera E., Kim K., Safe S. (2001). Transcriptional activation of heat shock protein 27 gene expression by 17beta-estradiol and modulation by antiestrogens and aryl hydrocarbon receptor agonists. J. Mol. Endocrinol. 26, 31–42 [DOI] [PubMed] [Google Scholar]
- Prud’homme G. J., Glinka Y., Toulina A., Ace O., Subramaniam V., Jothy S. (2010). Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS One. 5, e13831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A., Barnes S. J., Dalton T. P., Chang C. Y., Knudsen E. S., Maier M. A. (2000). Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest. J. Biol. Chem. 275, 2943–2950 [DOI] [PubMed] [Google Scholar]
- Puga A., Ma C., Marlowe J. L. (2009). The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem. Pharmacol. 77, 713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A., Xia Y., Elferink C. (2002). Role of the aryl hydrocarbon receptor in cell cycle regulation. Chem. Biol. Interact. 141, 117–130 [DOI] [PubMed] [Google Scholar]
- Quintana F. J., Basso A. S., Iglesias A. H., Korn T., Farez M. F., Bettelli E., Caccamo M., Oukka M., Weiner H. L. (2008). Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 453, 65–71 [DOI] [PubMed] [Google Scholar]
- Ramamoorthy K., Gupta M. S., Sun G., McDougal A., Safe S. H. (1999). 3,3’4,4’-Tetrachlorobiphenyl exhibits antiestrogenic and antitumorigenic activity in the rodent uterus and mammary cells and in human breast cancer cells. Carcinogenesis. 20, 115–123 [DOI] [PubMed] [Google Scholar]
- Reiners J. J., Jr, Clift R. E. (1999). Aryl hydrocarbon receptor regulation of ceramide-induced apoptosis in murine hepatoma 1c1c7 cells. A function independent of aryl hydrocarbon receptor nuclear translocator. J. Biol. Chem. 274, 2502–2510 [DOI] [PubMed] [Google Scholar]
- Rogers J. M., Denison M. S. (2002). Analysis of the antiestrogenic activity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in human ovarian carcinoma BG-1 cells. Mol. Pharmacol. 61, 1393–1403 [DOI] [PubMed] [Google Scholar]
- Romkes M., Piskorska-Pliszczynska J., Safe S. (1987). Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on hepatic and uterine estrogen receptor levels in rats. Toxicol. Appl. Pharmacol. 87, 306–314 [DOI] [PubMed] [Google Scholar]
- Rowlands C., Krishnan V., Wang X., Santostefano M., Safe S., Miller W. R., Langdon S. (1993). Characterization of the aryl hydrocarbon receptor and aryl hydrocarbon responsiveness in human ovarian carcinoma cell lines. Cancer Res. 53, 1802–1807 [PubMed] [Google Scholar]
- Rüegg J., Swedenborg E., Wahlström D., Escande A., Balaguer P., Pettersson K., Pongratz I. (2008). The transcription factor aryl hydrocarbon receptor nuclear translocator functions as an estrogen receptor beta-selective coactivator, and its recruitment to alternative pathways mediates antiestrogenic effects of dioxin. Mol. Endocrinol. 22, 304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S., Chadalapaka G., Jutooru I. (2012). AHR-reactive compounds in the human diet. In The Ah Receptor in Biology and Toxicology. (Pohjanvirta R, Ed.), pp. 331–342 John Wiler & Sons, Hoboken, NJ: [Google Scholar]
- Safe S., Kim K. (2008). Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J. Mol. Endocrinol. 41, 263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S., Qin C., McDougal A. (1999). Development of selective aryl hydrocarbon receptor modulators for treatment of breast cancer. Expert Opin. Investig. Drugs. 8, 1385–1396 [DOI] [PubMed] [Google Scholar]
- Safe S., Walker K., Zhang S. (2011). Dioxin as an environmental pollutant and its role in breast cancer. In Environment and Breast Cancer. (Russo J, Ed.), pp. 127–146 Spring-Verlag, New York, NY: [Google Scholar]
- Safe S., Wormke M. (2003). Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem. Res. Toxicol. 16, 807–816 [DOI] [PubMed] [Google Scholar]
- Safe S. H. (1986). Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu. Rev. Pharmacol. Toxicol. 26, 371–399 [DOI] [PubMed] [Google Scholar]
- Santostefano M., Merchant M., Arellano L., Morrison V., Denison M. S., Safe S. (1993). Alpha-Naphthoflavone-induced CYP1A1 gene expression and cytosolic aryl hydrocarbon receptor transformation. Mol. Pharmacol. 43, 200–206 [PubMed] [Google Scholar]
- Santostefano M., Piskorska-Pliszczynska J., Morrison V., Safe S. (1992). Effects of ligand structure on the in vitro transformation of the rat cytosolic aryl hydrocarbon receptor. Arch. Biochem. Biophys. 297, 73–79 [DOI] [PubMed] [Google Scholar]
- Sauzeau V., Carvajal-González J. M., Riolobos A. S., Sevilla M. A., Menacho-Márquez M., Román A. C., Abad A., Montero M. J., Fernández-Salguero P., Bustelo X. R. (2011). Transcriptional factor aryl hydrocarbon receptor (Ahr) controls cardiovascular and respiratory functions by regulating the expression of the Vav3 proto-oncogene. J. Biol. Chem. 286, 2896–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. V., Carver L. A., Bradfield C. A. (1993). Molecular characterization of the murine Ahr gene. Organization, promoter analysis, and chromosomal assignment. J. Biol. Chem. 268, 22203–22209 [PubMed] [Google Scholar]
- Schmidt J. V., Su G. H., Reddy J. K., Simon M. C., Bradfield C. A. (1996). Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. U. S. A. 93, 6731–6736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimba S., Komiyama K., Moro I., Tezuka M. (2002). Overexpression of the aryl hydrocarbon receptor (AhR) accelerates the cell proliferation of A549 cells. J. Biochem. 132, 795–802 [DOI] [PubMed] [Google Scholar]
- Singh K. P., Garrett R. W., Casado F. L., Gasiewicz T. A. (2011a). Aryl hydrocarbon receptor-null allele mice have hematopoietic stem/progenitor cells with abnormal characteristics and functions. Stem Cells Dev. 20, 769–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. P., Singh U. P., Singh B., Price R. L., Nagarkatti M., Nagarkatti P. S. (2011b). Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One. 6, e23522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., Murray I. A., Tanos R., Tellew J., Boitano A. E., Bisson W. H., Kolluri S. K., Cooke M. P., Perdew G. H. (2011). Identification of a high-affinity ligand that exhibits complete aryl hydrocarbon receptor antagonism. J. Pharmacol. Exp. Ther. 338, 318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink D. C., Eugster H. P., Lincoln D. W., 2nd., Schuetz J. D., Schuetz E. G., Johnson J. A., Kaminsky L. S., Gierthy J. F. (1992). 17 Beta-estradiol hydroxylation catalyzed by human cytochrome P450 1A1: A comparison of the activities induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in MCF-7 cells with those from heterologous expression of the cDNA. Arch. Biochem. Biophys. 293, 342–348 [DOI] [PubMed] [Google Scholar]
- Spink D. C., Lincoln D. W., 2nd, Dickerman H. W., Gierthy J. F. (1990). 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes an extensive alteration of 17 beta-estradiol metabolism in MCF-7 breast tumor cells. Proc. Natl. Acad. Sci. U. S. A. 87, 6917–6921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens E. A., Mezrich J. D., Bradfield C. A. (2009). The aryl hydrocarbon receptor: A perspective on potential roles in the immune system. Immunology. 127, 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam V., Ace O., Prud’homme G. J., Jothy S. (2011). Tranilast treatment decreases cell growth, migration and inhibits colony formation of human breast cancer cells. Exp. Mol. Pathol. 90, 116–122 [DOI] [PubMed] [Google Scholar]
- Tan K. P., Wang B., Yang M., Boutros P. C., Macaulay J., Xu H., Chuang A. I., Kosuge K., Yamamoto M., Takahashi S., et al. (2010). Aryl hydrocarbon receptor is a transcriptional activator of the human breast cancer resistance protein (BCRP/ABCG2). Mol. Pharmacol. 78, 175–185 [DOI] [PubMed] [Google Scholar]
- Tomkiewicz C., Herry L., Bui L. C., Métayer C., Bourdeloux M., Barouki R., Coumoul X. (2013). The aryl hydrocarbon receptor regulates focal adhesion sites through a non-genomic FAK/Src pathway. Oncogene. 32, 1811–1820 [DOI] [PubMed] [Google Scholar]
- Tompkins L. M., Li H., Li L., Lynch C., Xie Y., Nakanishi T., Ross D. D., Wang H. (2010). A novel xenobiotic responsive element regulated by aryl hydrocarbon receptor is involved in the induction of BCRP/ABCG2 in LS174T cells. Biochem. Pharmacol. 80, 1754–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C., Richmond O., Aaron L., Powell J. B. (2013). Inhibition of constitutive aryl hydrocarbon receptor (AhR) signaling attenuates androgen independent signaling and growth in (C4-2) prostate cancer cells. Biochem. Pharmacol. 85, 753–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M., Hirota K., Westendorf A. M., Buer J., Dumoutier L., Renauld J. C., Stockinger B. (2008). The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 453, 106–109 [DOI] [PubMed] [Google Scholar]
- Vickers P. J., Dufresne M. J., Cowan K. H. (1989). Relation between cytochrome P450IA1 expression and estrogen receptor content of human breast cancer cells. Mol. Endocrinol. 3, 157–164 [DOI] [PubMed] [Google Scholar]
- Villano C. M., Murphy K. A., Akintobi A., White L. A. (2006). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces matrix metalloproteinase (MMP) expression and invasion in A2058 melanoma cells. Toxicol. Appl. Pharmacol. 210, 212–224 [DOI] [PubMed] [Google Scholar]
- Villard P. H., Caverni S., Baanannou A., Khalil A., Martin P. G., Penel C., Pineau T., Seree E., Barra Y. (2007). PPARalpha transcriptionally induces AhR expression in Caco-2, but represses AhR pro-inflammatory effects. Biochem. Biophys. Res. Commun. 364, 896–901 [DOI] [PubMed] [Google Scholar]
- Vogel C., Abel J. (1995). Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on growth factor expression in the human breast cancer cell line MCF-7. Arch. Toxicol. 69, 259–265 [DOI] [PubMed] [Google Scholar]
- Vogel C. F., Li W., Sciullo E., Newman J., Hammock B., Reader J. R., Tuscano J., Matsumura F. (2007). Pathogenesis of aryl hydrocarbon receptor-mediated development of lymphoma is associated with increased cyclooxygenase-2 expression. Am. J. Pathol. 171, 1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. G. (1977). Immune suppression as related to toxicology. CRC Crit. Rev. Toxicol. 5, 67–101 [DOI] [PubMed] [Google Scholar]
- Wang C. K., Chang H., Chen P. H., Chang J. T., Kuo Y. C., Ko J. L., Lin P. (2009). Aryl hydrocarbon receptor activation and overexpression upregulated fibroblast growth factor-9 in human lung adenocarcinomas. Int. J. Cancer. 125, 807–815 [DOI] [PubMed] [Google Scholar]
- Wang F., Samudio I., Safe S. (2001). Transcriptional activation of cathepsin D gene expression by 17beta-estradiol: Mechanism of aryl hydrocarbon receptor-mediated inhibition. Mol. Cell. Endocrinol. 172, 91–103 [DOI] [PubMed] [Google Scholar]
- Wang T., Gavin H. M., Arlt V. M., Lawrence B. P., Fenton S. E., Medina D., Vorderstrasse B. A. (2011a). Aryl hydrocarbon receptor activation during pregnancy, and in adult nulliparous mice, delays the subsequent development of DMBA-induced mammary tumors. Int. J. Cancer. 128, 1509–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Wyrick K. L., Meadows G. G., Wills T. B., Vorderstrasse B. A. (2011b). Activation of the aryl hydrocarbon receptor by TCDD inhibits mammary tumor metastasis in a syngeneic mouse model of breast cancer. Toxicol. Sci. 124, 291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Smith R., 3rd, Safe S. (1998). Aryl hydrocarbon receptor-mediated antiestrogenicity in MCF-7 cells: Modulation of hormone-induced cell cycle enzymes. Arch. Biochem. Biophys. 356, 239–248 [DOI] [PubMed] [Google Scholar]
- Wang W. L., Porter W., Burghardt R., Safe S. H. (1997). Mechanism of inhibition of MDA-MB-468 breast cancer cell growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Carcinogenesis. 18, 925–933 [DOI] [PubMed] [Google Scholar]
- Wang X., Thomsen J. S., Santostefano M., Rosengren R., Safe S., Perdew G. H. (1995). Comparative properties of the nuclear aryl hydrocarbon (Ah) receptor complex from several human cell lines. Eur. J. Pharmacol. 293, 191–205 [DOI] [PubMed] [Google Scholar]
- Wang Y., Fan Y., Puga A. (2010). Dioxin exposure disrupts the differentiation of mouse embryonic stem cells into cardiomyocytes. Toxicol. Sci. 115, 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Kolluri S. K., Kiefer F., Göttlicher M. (1996). Complementation of Ah receptor deficiency in hepatoma cells: Negative feedback regulation and cell cycle control by the Ah receptor. Exp. Cell Res. 226, 154–163 [DOI] [PubMed] [Google Scholar]
- Whitlock J. P., Jr (1999). Induction of cytochrome P4501A1. Annu. Rev. Pharmacol. Toxicol. 39, 103–125 [DOI] [PubMed] [Google Scholar]
- Williams S. P., Nowicki M. O., Liu F., Press R., Godlewski J., Abdel-Rasoul M., Kaur B., Fernandez S. A., Chiocca E. A., Lawler S. E. (2011). Indirubins decrease glioma invasion by blocking migratory phenotypes in both the tumor and stromal endothelial cell compartments. Cancer Res. 71, 5374–5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormke M., Castro-Rivera E., Chen I., Safe S. (2000a). Estrogen and aryl hydrocarbon receptor expression and crosstalk in human Ishikawa endometrial cancer cells. J. Steroid Biochem. Mol. Biol. 72, 197–207 [DOI] [PubMed] [Google Scholar]
- Wormke M., Stoner M., Saville B., Safe S. (2000b). Crosstalk between estrogen receptor alpha and the aryl hydrocarbon receptor in breast cancer cells involves unidirectional activation of proteasomes. FEBS Lett. 478, 109–112 [DOI] [PubMed] [Google Scholar]
- Wormke M., Stoner M., Saville B., Walker K., Abdelrahim M., Burghardt R., Safe S. (2003). The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol. Cell. Biol. 23, 1843–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Li W., Lok P., Matsumura F., Vogel C. F. (2011a). AhR deficiency impairs expression of LPS-induced inflammatory genes in mice. Biochem. Biophys. Res. Commun. 410, 358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. Y., Quintana F. J., da Cunha A. P., Dake B. T., Koeglsperger T., Starossom S. C., Weiner H. L. (2011b). In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS One. 6, e23618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G., Peng Z., Raufman J. P. (2012). Src-mediated aryl hydrocarbon and epidermal growth factor receptor cross talk stimulates colon cancer cell proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1006–G1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Liu D., Murray T. J., Mitchell G. C., Hesterman E. V., Karchner S. I., Merson R. R., Hahn M. E., Sherr D. H. (2005). The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene. 24, 7869–7881 [DOI] [PubMed] [Google Scholar]
- Yao C., Safe S. (1989). 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced porphyria in genetically inbred mice: Partial antagonism and mechanistic studies. Toxicol. Appl. Pharmacol. 100, 208–216 [DOI] [PubMed] [Google Scholar]
- Yin X. F., Chen J., Mao W., Wang Y. H., Chen M. H. (2012). A selective aryl hydrocarbon receptor modulator 3,3’-Diindolylmethane inhibits gastric cancer cell growth. J. Exp. Clin. Cancer Res. 31, 46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharewski T., Harris M., Biegel L., Morrison V., Merchant M., Safe S. (1992). 6-Methyl-1,3,8-trichlorodibenzofuran (MCDF) as an antiestrogen in human and rodent cancer cell lines: Evidence for the role of the Ah receptor. Toxicol. Appl. Pharmacol. 113, 311–318 [DOI] [PubMed] [Google Scholar]
- Zacharewski T. R., Bondy K. L., McDonell P., Wu Z. F. (1994). Antiestrogenic effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on 17 beta-estradiol-induced pS2 expression. Cancer Res. 54, 2707–2713 [PubMed] [Google Scholar]
- Zhang J., Zong H., Li S., Zhang D., Zhang L., Xia Q. (2012a). Activation of aryl hydrocarbon receptor suppresses invasion of esophageal squamous cell carcinoma cell lines. Tumori. 98, 152–157 [DOI] [PubMed] [Google Scholar]
- Zhang S., Kim K., Jin U. H., Pfent C., Cao H., Amendt B., Liu X., Wilson-Robles H., Safe S. (2012b). Aryl hydrocarbon receptor agonists induce microRNA-335 expression and inhibit lung metastasis of estrogen receptor negative breast cancer cells. Mol. Cancer Ther. 11, 108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Lei P., Liu X., Li X., Walker K., Kotha L., Rowlands C., Safe S. (2009). The aryl hydrocarbon receptor as a target for estrogen receptor-negative breast cancer chemotherapy. Endocr. Relat. Cancer. 16, 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Degroot D. E., Hayashi A., He G., Denison M. S. (2010). CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol. Sci. 117, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Kanno Y., Nakayama M., Makimura M., Ohara S., Inouye Y. (2012). Activation of the aryl hydrocarbon receptor represses mammosphere formation in MCF-7 cells. Cancer Lett. 317, 192–198 [DOI] [PubMed] [Google Scholar]
- Zhou J., Gasiewicz T. A. (2003). 3’-Methoxy-4’-nitroflavone, a reported aryl hydrocarbon receptor antagonist, enhances Cyp1a1 transcription by a dioxin responsive element-dependent mechanism. Arch. Biochem. Biophys. 416, 68–80 [DOI] [PubMed] [Google Scholar]