Abstract
Both epidemiological and empirical studies have indicated that nickel (Ni) may play an important role in PM2.5 exposure–induced adverse cardiovascular effects. However, the underlying mechanism remains unclear. In the present study, we exposed mice to concentrated ambient PM2.5 (CAP), Ni, or coexposure to both CAP + Ni in a specially designed whole-body exposure system for a duration of 3 months and investigated their effects on vascular function, oxidative stress, and vascular inflammation. CAP + Ni exposure induced greater endothelial dysfunction compared with CAP or Ni alone. Ni exposure decreased endothelial nitric oxide synthase (eNOS) dimers in the aorta, which was potentiated by coexposure with CAP. CAP alone did not reduce NOS dimers but was more effective than Ni in decreasing phosphorylation of eNOS (S1177) and Akt (T308). Ni had minimal effects on the expression of vascular inflammatory genes but synergized with CAP in marked upregulation of tumor necrosis factor-alpha and monocyte chemotactic protein-1. The effects of Ni on NOS monomer formation in endothelial cells were redox dependent as evidenced by attenuation of effects by Tiron in cultured endothelial cells. Ni synergized with lipopolysaccharide, another bioactive component of CAP in reducing eNOS dimerization in cultured endothelial cells. Ni exposure induces endothelial dysfunction through oxidative stress-dependent inhibition of eNOS dimerization. Its interaction with other components of CAP may significantly contribute to the adverse cardiovascular effects of CAP exposure.
Key Words: Nickel, PM2.5, eNOS.
Exposure to airborne particulate matter with a diameter less than 2.5 μm (PM2.5) has been associated with excess risk for cardiovascular diseases, such as hypertension, atherosclerosis, and type 2 diabetes mellitus (Brook et al., 2004; Pope et al., 2004). Although it is of both scientific and socioeconomic importance to determine the components responsible for PM2.5 exposure–induced cardiovascular morbidity and mortality, they remain elusive, as PM2.5 have very complicated compositions with marked geographical and temporal variations. Nickel (Ni) is a transitional metal identified in PM2.5 from diverse sources (Baceva et al., 2012; Barandovski et al., 2012; Slezakova et al., 2012; Tian et al., 2012) and primarily produced by the combustion of fossil fuels (Grandjean, 1984). Epidemiological studies have shown that the levels of Ni in PM2.5 are associated with all-cause and/or cardiovascular mortality (Hernandez-Mena et al., 2011; Huang et al., 2012; Zhang et al., 2009). We previously found that the levels of Ni in concentrated ambient PM2.5 (CAP) were strongly correlated to acute changes in heart rates and their variability (Lippmann et al., 2006). Furthermore, acute inhalation exposure to Ni induces bradycardia and arrhythmogenesis in rats (Campen et al., 2001), and long-term inhalation exposure to Ni exacerbates atherosclerosis in ApoE−/− mice (Kang et al., 2011). Taken together, these studies strongly support that Ni may play an important role in PM2.5 exposure–associated cardiovascular diseases.
Endothelial dysfunction, characterized by a reduced nitric oxide (NO)-mediated vasorelaxation, is implicated in the pathophysiology of various cardiovascular diseases (Ignarro et al., 1999; Schalkwijk and Stehouwer, 2005). A rapidly increasing number of studies have demonstrated that CAP exposure results in marked endothelial dysfunction (Allen et al., 2011; Poursafa et al., 2011), indicating that PM2.5 exposure–associated cardiovascular morbidity and mortality may be mediated by endothelial dysfunction. Notably, inhalation exposure to Ni also reduces NO-mediated vasorelaxation (Cuevas et al., 2010), suggesting that PM2.5 exposure–induced endothelial dysfunction may be partly attributable to Ni.
Endothelial nitric oxide synthase (eNOS) is the key enzyme for vascular-regulated NO production. The active form of eNOS exists as two identical subunits that form a head to tail homodimer, a process known as dimerization. The full activation of eNOS requires other signaling such as phosphorylation/dephosphorylation at several sites and interaction with other proteins (Rafikov et al., 2011). We previously demonstrated that CAP exposure decreased eNOS phosphorylation at a proactive site (Sun et al., 2008). However, in spite of the essential role of eNOS dimerization in the regulation of eNOS activity, the effect of CAP exposure on eNOS dimerization has not yet been investigated.
Like exposure to PM2.5, occupational exposure to Ni induced marked oxidative stress, and the latter is believed to mediate Ni exposure–induced allergic reactions and carcinogenesis (Zhao et al., 2009). Oxidative stress is central in the pathophysiology of diverse cardiovascular diseases through disruption of vascular homeostasis (Forstermann and Munzel, 2006; Forstermann and Sessa, 2012; Rafikov et al., 2011). For example, in addition to directly antagonizing the effects of NO, it has been shown to potently inhibit eNOS dimerization and thus decrease NO production (Rafikov et al., 2011). However, there are few studies investigating the role of oxidative stress in CAP and/or Ni exposure–induced endothelial dysfunction. In the present study, we exposed mice to CAP and/or Ni and analyzed their effects on vascular function, oxidative stress induction, and eNOS dimerization/phosphorylation. The role of oxidative stress in Ni and/or CAP-induced eNOS dimerization was further assessed in cultured endothelial cells.
MATERIALS AND METHODS
Animals.
The animals and CAP exposure protocol were previously described (Xu et al., 2012). Briefly, 24 male ApoE−/− mice (8 weeks old) were obtained from the Jackson Laboratory and allowed to acclimate for 2 weeks. They were then exposed to filter air (FA), CAP, FA + Ni, and CAP + Ni using a versatile aerosol concentration enrichment system for 6h/day, 5 days/week between 8 September and 17 December 2009. The ambient mean daily PM2.5 concentration at the study site was 7.4±4.4 μg/m3. Mean daily concentrations of PM2.5 in the exposure chamber were 69.6±48.4 and 66.5±44.6 μg/m3, CAP and CAP + Ni, respectively (≈ninefold concentration from the ambient levels). The control mice were exposed to an identical treatment except for a high-efficiency particulate air filter (FA, Pall Life Sciences, East Hills, NY) that was positioned in the inlet valve to the exposure system to remove all of the particles from the airstream (Sun et al., 2005). Because the mice were exposed for 6h/day, 5 days a week, the equivalent PM2.5 concentrations to which the mice were exposed were 12.4 and 11.9 μg/m3, CAP and CAP + Ni, respectively, which is within the annual average PM2.5 National Ambient Air Quality Standard of 15 μg/m3.
Ni particles were produced by opposing metallic Ni electrodes (99.995% purity, ESPI, Ashland, OR) in an ultrapure argon chamber (Model GFG-1000, Karlsruhe, Germany) as described previously (Xu et al., 2012). Ultrapure oxygen was added to filtered dilution air in order to keep the exposure atmosphere at 20% oxygen. Generated particles were then admixed with CAP in a VACES system. Animals were exposed to nano-Ni(OH)2 in a whole-body exposure chamber. Wipe tests of chamber surfaces showed little surface deposition of Ni nanoparticles and, therefore, oral dose was expected to be negligible as compared with inhaled dose. The mean concentrations of Ni in these exposure atmospheres were 0.9±5.5, 440.6±557.3, and 467.9±601.1ng/m3 in the CAP, FA + Ni, and CAP + Ni groups, respectively. The concentration of Ni used in the present study was selected according to our previous 6-month CAP exposure study showing that the Ni concentration in CAP was about 200ng/m3 (Lippmann et al., 2006).
Western blot analysis.
Tissues or cell lysates were prepared using radioimmunoprecipitation analysis (RIPA) buffer supplemented with protease and phosphatase inhibitors. Protein samples were then separated by 10% SDS-polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene fluoride membranes. Target proteins were detected by primary antibodies as follows: mouse anti-β-actin (Sigma), mouse anti-eNOS (BD Transduction Laboratories), rabbit anti-phospho-eNOS (Ser1177) (Cell Signaling Technology), rabbit anti-phospho-Akt (Thr308) (Cell Signaling Technology), and rabbit anti-heme oxygenase-1 (HO-1; Abcam). Secondary antibodies conjugated with horseradish peroxidase and chemiluminescence reagent (Amersham) were used to visualize the target proteins. Densities of target protein bands were determined with Quantity One 4.4.1 (Bio-Rad). The internal control, β-actin, was used to normalize loading variations.
Real-time RT-PCR.
Total RNA was extracted with TRI-Reagent (Sigma, St Louis, MO) per the instruction. Real-time PCR was carried out using SYBR Green as we described previously (Ying et al., 2009). 2ΔCt [target genes relative to the endogenous glyceraldehyde-3-phosphate dehydrogenase control] were calculated as suggested by Schmittgen and Zakrajsek (2000).
Vascular function assay.
Briefly, mice were anesthetized with pentobarbital sodium, and the thoracic aorta was quickly removed and cleaned in physiological salt solution (PSS) containing (mM): NaCl, 130; NaHCO3, 14.9; KCl, 4.7; KH2PO4, 1.18; MgSO4·7H2O, 1.18; CaCl2·2H2O, 1.56; EDTA, 0.026; and glucose, 5.5. The aorta was cut into 2-mm rings. The aortic rings were then mounted in a muscle bath containing PSS at 37°C and bubbled with 95% O2-5% CO2. Isometric force generation was recorded with a Multi Myograph System (Danish Myo Technology A/S, Aarhus N, Denmark). A resting tension of 4 mN was imposed on each ring, and the rings were allowed to equilibrate for 1h.
Arterial integrity was assessed first by stimulation of vessels with 120mM KCl. No significant difference in contractions induced by 120mM KCl was observed (5.9±1.2, 6.4±1.4, 6.2±2.1, and 5.4±1.3 mN; FA, CAP, FA + Ni, and CAP + Ni, respectively). To test the effect of exposures on vascular function, the contractile responses of aortic rings to phenylephrine (PE) in the absence or presence of a NOS inhibitor ω-nitro-L-arginine methyl ester (L-NAME 100μM) were assessed in an accumulative manner. To analyze the endothelial function, aortic rings were precontracted with PE (1μM), and acetylcholine (ACh) or sodium nitroprusside (SNP) was added in an accumulative manner.
Cell culture experiments.
Human umbilical vein endothelial cells (HUVECs) were obtained from American Type Cell Culture (Manassas, VA) and maintained in endothelial cell basic medium supplemented with 10% fetal bovine serum (FBS) (Promocell, Heidelberg, Germany). Before treatment with Ni or other chemicals, cells were grown to 90% confluence in 60-mm dishes and starved in endothelial cell basic medium supplemented with 0.5% FBS overnight. After treatments, cells were immediately placed on ice and washed twice with ice-cold PBS. All subsequent manipulations were then performed on ice. Cells were lysed with 100 μl of ice-cold RIPA buffer (Upstate, Jaffrey, NH: 50mM Tris-HCl [pH 7.4], 150mM NaCl, 50mM β-glycerophosphate, 50mM NaF, 1mM EGTA, 1mM Na3VO4, 1% NP-40, 0.25% sodium deoxycholate, 1mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 5 μg/ml leupeptin, and 2 μg/ml pepstatin). Cells were scraped with a rubber policeman, rocked for 30min at 4°C, and centrifuged at 14,000 × g for 30min at 4°C. The supernatants were transfer to new tubes. Protein concentration in each sample was determined with BCA protein assay reagents (Pierce, Rockford, IL).
Statistical analysis.
Probability values less than 0.05 were considered significant. Student’s t-test or ANOVA will be used for statistical analysis with Graphpad Instat 5 software (Graphpad Instat Software, San Diego, CA).
RESULTS
A Synergistic Action of Ni and CAP on Vascular Functions
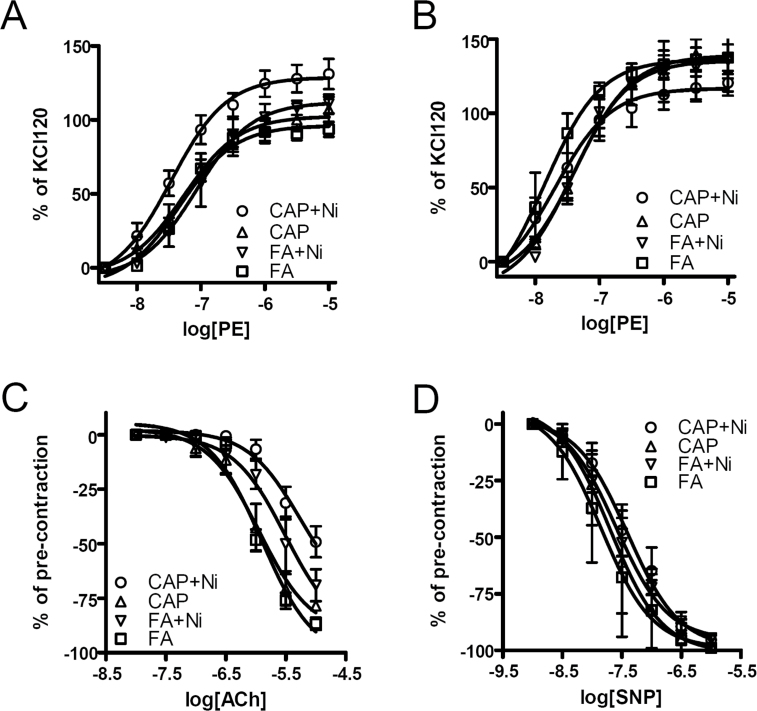
Figure 1A and Table 1 reveal that although CAP and FA + Ni groups had aortic contractile response to PE similar to that of FA group, CAP + Ni group had significantly increased aortic contractile response to PE. This increased aortic contractile response appeared to result from endothelial dysfunction, as there was no such a significant difference in the presence of the NOS inhibitor L-NAME (Fig. 1B and Table 1). Notably, L-NAME markedly increased contraction in FA, CAP, and FA + Ni groups but not CAP + Ni group, further supporting endothelial dysfunction in CAP + Ni group. Consistent with contractile response results, CAP + Ni significantly decreased the aortic dilator response to ACh (Fig. 1C and Table 1). Ni alone exposure also significantly decreased aortic relaxation response to ACh. In contrast, CAP exposure did not alter aortic relaxation response. Figure 1D and Table 1 show that all groups had similar response to an endothelium-independent dilator SNP, supporting that the vascular effects of CAP and Ni are primarily attributed to endothelial dysfunction.
Fig. 1.
Ni and CAP synergistically induce endothelial dysfunction. ApoE−/− mice were exposed to FA/CAP for a duration of 3 months, and then aortic rings were prepared and mounted onto myography. (A) PE was added in an accumulative manner, and the stable contractions were presented. (B) PE was added in an accumulative manner in the presence of L-NAME (200μM), and the stable contractions were presented. (C) Aortic rings were contracted with PE (0.3μM) and then relaxed by the indicated concentration of ACh. The peak relaxations were obtained and presented. (D) Aortic rings were contracted with PE (0.3μM) and then relaxed by the indicated concentration of NO donor SNP. The peak relaxations were obtained and presented. n = 5–6/group.
Table 1.
The log EC50s and Maximal Effects of Vascular Responses
| FA | CAP | FA + Ni | CAP + Ni | |||||
|---|---|---|---|---|---|---|---|---|
| log IC50 | Maximal | log IC50 | Maximal | log IC50 | Maximal | log IC50 | Maximal | |
| PE | −7.1±0.1 | 96±3 | −7.3±0.1 | 103±5 | −7.1±0.1 | 112±5 | −7.5±0.1*,& | 129±5*,& |
| PE/L-NAME | −7.7±0.2 | 136±5 | −7.4±0.1 | 139±8 | −7.4±0.2 | 136±8 | −7.7±0.2 | 119±12 |
| ACh | −5.9±0.1 | −102±6 | −6.0±0.1 | −91±7 | −5.5±0.2* | −92±7 | −5.3±0.2*,# | −73±12* |
| SNP | −7.8±0.2 | −99±9 | −7.7±0.2 | −101±8 | −7.6±0.08 | −95±3 | −7.5±0.1 | −99±6 |
Note. *p < 0.05 versus FA, # p < 0.05 versus CAP, & p < 0.05 versus FA + Ni.
A Synergistic Action of Ni and CAP on eNOS Dimerization
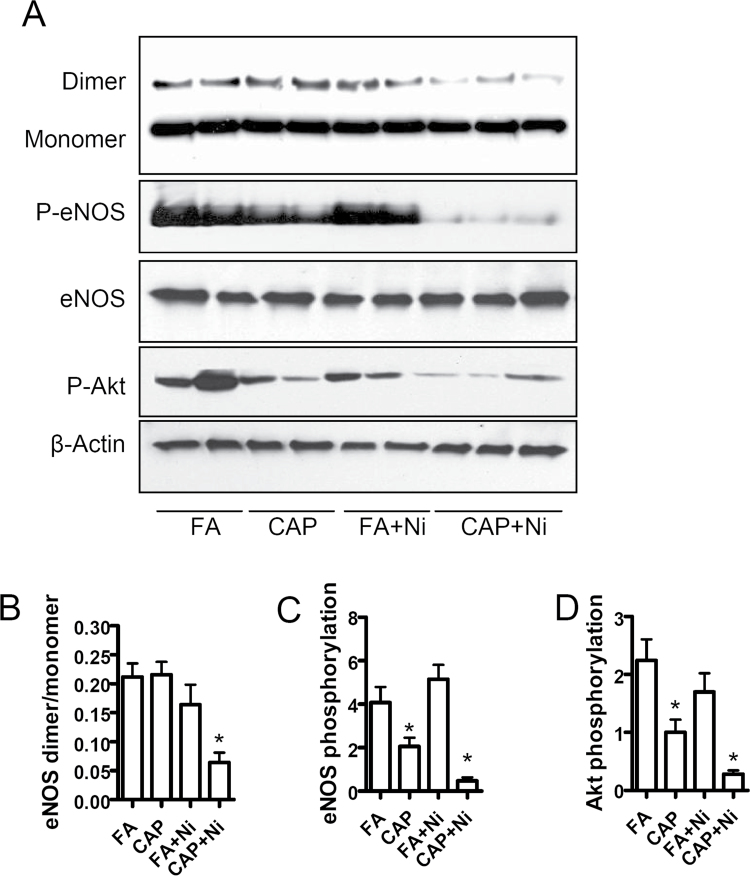
To investigate the mechanism whereby CAP and Ni induce endothelial dysfunction, we assessed the aortic eNOS dimerization in these mice. Figure 2 demonstrates that although CAP or Ni alone exposure did not significantly decrease the dimerization of eNOS, CAP + Ni exposure markedly decreased the level of eNOS dimer. Notably, although CAP alone had no effect on eNOS dimerization, it significantly decreased eNOS phosphorylation at Ser1177, a proactive phosphorylation site. In contrast, Ni did not affect eNOS phosphorylation at this proactive site, whereas it markedly enhanced the inhibition of eNOS phosphorylation induced by CAP. Akt is the primary kinase responsible for eNOS phosphorylation at this site (Fulton et al., 1999). Figure 2 shows that consistent with eNOS phosphorylation analysis, CAP exposure significantly decreased Akt activation, whereas Ni itself did not reduce Akt activation but markedly enhanced the effect of CAP on Akt activation.
Fig. 2.
Ni and CAP synergistically inhibit eNOS dimerization. Aorta from FA/CAP-exposed ApoE−/− mice were isolated and lyzed in RIPA buffer. The dimerization of eNOS and the phosphorylation of eNOS and Akt were analyzed with nondenaturing and denaturing gel electrophoresis. (A) The representative images of Western blot analysis. (B–D) The quantification of eNOS dimerization (B), eNOS phosphorylation (C), and Akt phosphorylation (D). n = 5–6/group. *p < 0.05 versus control; one-way ANOVA.
CAP But Not Ni Increased Vascular Inflammatory Response
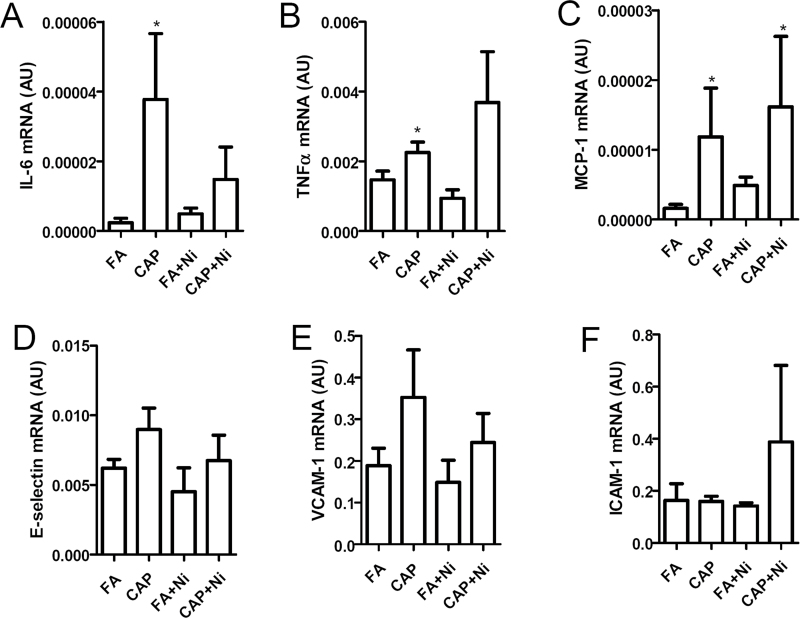
Because vascular inflammation may have marked effects on the endothelial function, we analyzed the proinflammatory cytokines in mesenteric arteries by real-time RT-PCR. Figure 3 reveals that CAP significantly increased mesenteric arterial mRNA expression of interleukin-6, tumor necrosis factor-alpha, and monocyte chemotactic protein-1. In contrast, Ni did not significantly increase the mRNA expression of any tested proinflammatory cytokines. The mesenteric arterial expression levels of proinflammatory cytokines in CAP + Ni group were similar to that in CAP group, further supporting that CAP but not Ni increased vascular inflammation. Compared with FA group, no group had significant difference in the mRNA expression levels of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 (Figs. 3D–F).
Fig. 3.
CAP but not Ni induces vascular inflammation. The mRNA expression of proinflammatory genes, including IL-6 (A), TNF-α (B), MCP-1 (C), E-selectin (D), VCAM-1 (E), and ICAM-1 (F), in mesenteric arteries were analyzed by real-time RT-PCR. n = 5–6/group. *p < 0.05 versus control; one-way ANOVA.
A Synergistic Action of CAP and Ni on Systemic and Vascular Oxidative Stress
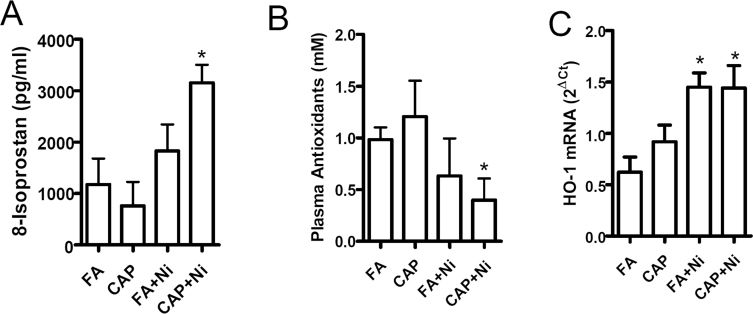
Various studies have demonstrated that Ni induces marked oxidative stress, and the latter plays a critical role in the regulation of eNOS dimerization (Rafikov et al., 2011). We therefore assessed plasma 8-isoprostane, a systemic indicator of oxidative stress. Figure 4A reveals that CAP + Ni significantly increased plasma 8-isoprostane, indicating the presence of systemic oxidative stress in this group. Exposure to Ni alone showed a nonsignificant trend of increase in plasma 8-isoprostane. In contrast, exposure to CAP did not have significant effects on the 8-isoprostane. The increased oxidative stress in animals exposed to both CAP and Ni was further supported by the plasma total antioxidant capacity analysis (Fig. 4B). HO-1 is upregulated in response to oxidative stress. Real-time RT-PCR analysis of HO-1 mRNA in mesenteric arteries demonstrated markedly increased expression of HO-1 mRNA in FA + Ni and CAP + Ni groups (Fig. 4C).
Fig. 4.
Ni and CAP synergistically increase systemic and vascular oxidative stress. (A and B) Plasma from FA/CAP-exposed ApoE−/− mice were isolated and 8-isoprostane (A) and antioxidants (B) in plasma were analyzed with commercially available kits. (C) HO-1, mRNA expression levels in mesenteric arteries of FA/PM-exposed ApoE−/− mice were analyzed by real-time RT-PCR. n = 5–6/group. *p < 0.05 versus control; one-way ANOVA.
Ni Inhibited eNOS Dimerization Through Induction of Oxidative Stress
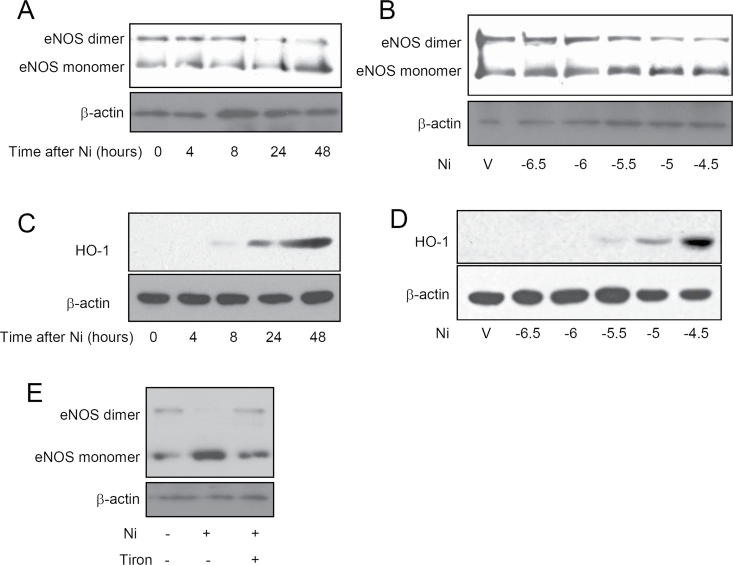
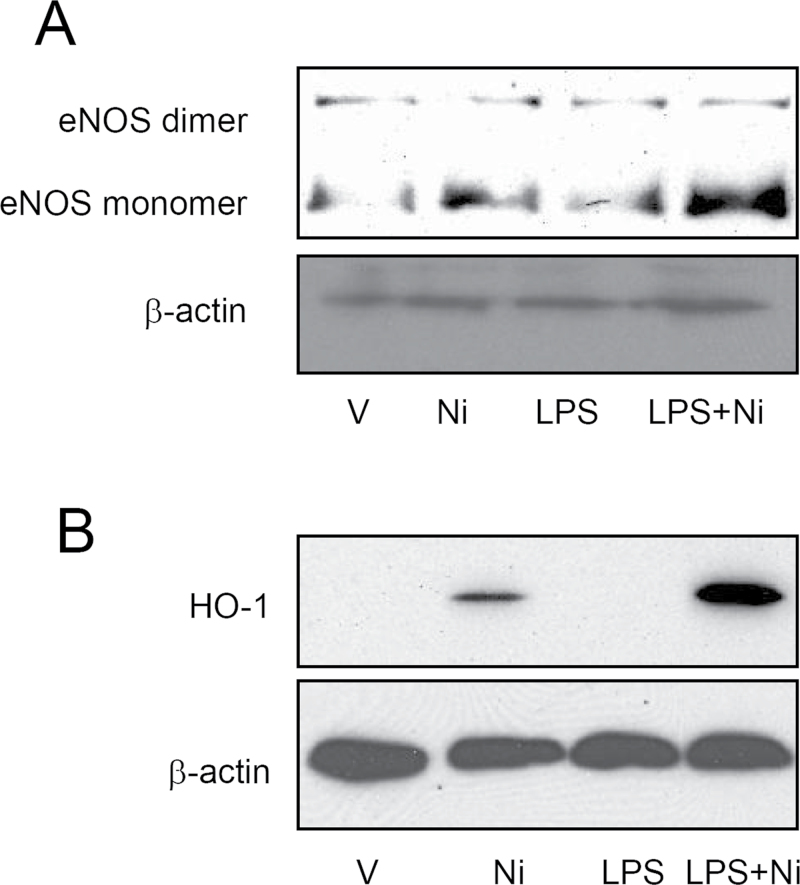
Figures 5A and 5B show that consistent with its in vivo effects, Ni time- and concentration-dependently reduced eNOS dimerization in cultured HUVECs. Consistent with previous reports showing that exposure to Ni induces marked oxidative stress (Zhao et al., 2009). Figures 5C and 5D reveal that Ni time- and concentration-dependently increased HO-1 protein expression. The similarity in timing and dosing suggested that the effect of Ni on eNOS dimerization may be mediated by oxidative stress. Therefore, we next assessed if Tiron, a potent antioxidant, could reverse the inhibitory effect of Ni on eNOS dimerization. Supporting the proposed role of oxidative stress, Figure 5E demonstrates that Tiron was sufficient to abolish the effect of Ni on eNOS dimerization. CAP exposure has also been shown to induce oxidative stress, and we previously demonstrated that the induction of systemic oxidative stress by CAP exposure is mediated by TLR4 (Kampfrath et al., 2011). Lipopolysaccharide (LPS) is a classic ligand of TLR4 and an important bioactive component of CAP. We therefore assessed if LPS and Ni have a synergic action on eNOS dimerization. Figures 6A and 6B demonstrate that although LPS alone did not significantly inhibit eNOS dimerization in HUVECs, it markedly exacerbated the effect of Ni on eNOS dimerization.
Fig. 5.
Ni reduces eNOS dimerization through induction of oxidative stress in HUVECs. HUVECs were treated with Ni (10μM) for the indicated time (A and C) or the indicated concentration of Ni for 48h (B and D). The eNOS dimerization (A and B) and the expression levels of HO-1 protein (C and D) were analyzed by Western blot. (E) HUVECs were treated with Ni (10μM) and/or Tiron (200mM), and eNOS dimerization was analyzed by Western blot. A representative image of two or three independent experiments is presented.
Fig. 6.
Ni and LPS synergistically induce oxidative stress and inhibit eNOS dimerization in HUVECs. HUVECs were treated with Ni (10μM) and/or LPS for 48h, and then eNOS dimerization (A) and HO-1 protein expression (B) were analyzed by Western blot.
DISCUSSION
Most recently, we showed that Ni enhanced CAP exposure–associated metabolic disorders and mitochondrial dysfunction (Xu et al., 2012). In the present study, we demonstrate that it may also play a critical role in CAP exposure–associated cardiovascular diseases. The main findings in the present study include that (1) Ni exposure exacerbated CAP exposure–associated endothelial dysfunction, (2) the synergic vascular action of Ni and CAP exposures were paralleled by their synergic effect on eNOS dimerization and phosphorylation, (3) Ni and CAP synergistically increased systemic and vascular oxidative stress, and (4) Ni inhibited eNOS dimerization through induction of oxidative stress in cultured endothelial cells.
Ni Exposure and Vascular Function
In the present study, we demonstrate that long-term inhalation exposure to Ni led to markedly reduced response to ACh. This is consistent with previous studies showing that a short-term inhalation exposure to Ni is sufficient to reduce ACh-induced vasorelaxation (Cuevas et al., 2010). ACh-induced vasorelaxation is one of the most frequently used parameter for assessing endothelial function, and reduced ACh-induced vasorelaxation has been correlated to diverse human diseases such as hypertension, atherosclerosis, and insulin resistance (Ignarro et al., 1999; Schalkwijk and Stehouwer, 2005). Our results thus provide evidence that exposure to Ni may link to these cardiovascular diseases by targeting endothelium. Endothelial dysfunction is also one of the adverse cardiovascular effects of CAP exposure (Ying et al., 2009). In the present study, CAP only exposure did not induce evident endothelial dysfunction in aorta, which may be attributed to the relatively low concentration of CAP (69.6±48.4 vs. 138.4±83.7 μg/m3) (Ying et al., 2009). In contrast, the same treatment was sufficient to induce significant microcirculatory dysfunction in the cremaster muscle (Xu et al., 2012), reflecting a vascular bed-dependent sensitivity to CAP exposure. Notably, it is known that the relative contribution of eNOS, PGI2, and endothelium-derived hyperpolarizing factor-mediated relaxation differs depending on vessel type and size (Shimokawa et al., 1996). Endothelium of small arteries regulates vascular tone dependent less on eNOS than that of larger arteries (Hilgers et al., 2006). Therefore, these data together suggest that eNOS may be relatively resistant to CAP exposure.
However, long-term exposure to even this relatively low concentration of CAP was sufficient to decrease eNOS phosphorylation at a proactive site. In contrast to the reduced contractile response to PE in the short-term exposure to Ni (Cuevas et al., 2010), our present data reveal that long-term exposure to Ni did not alter the aortic contractile response to PE, suggesting an exposure duration–dependent effect of Ni on vascular smooth muscle cells. However, the molecular basis for this exposure duration–dependent effect remains to be determined.
A number of studies have demonstrated that perivascular adipose tissue plays an important role in the regulation of vascular function (Aghamohammadzadeh and Heagerty, 2012). A most recent study further demonstrated that perivascular adipose tissue is critical for a normal eNOS function in human vessels (Margaritis et al., 2013). Notably, we previously demonstrated that CAP and Ni coexposure significantly altered the function of perivascular adipose tissue as evidenced by reduced expression of uncoupling protein-1 (Xu et al., 2012). In the future, it is interesting to determine whether this alteration in perivascular adipose tissue is attributed to endothelial dysfunction shown in the present study.
Ni Exposure and eNOS Dimerization
ACh-dependent aortic relaxation is primarily mediated by eNOS-dependent NO production (Massion and Balligand, 2003). Our data reveal that long-term inhalation exposure to Ni markedly reduced aortic eNOS dimerization. Given the essential role of eNOS dimerization in eNOS activation, these data indicate that Ni inhalation exposure–induced endothelial dysfunction may be through inhibition of eNOS dimerization. To our knowledge, this is the first study showing airborne metal may inhibit eNOS dimerization. Notably, in the present study, CAP exposure did not reduce eNOS dimerization, but eNOS phosphorylation at a proactive site, which may be subsequent to a decrease in Akt signaling, demonstrating a unique effect of Ni. This is consistent with the synergistic action of Ni and CAP on endothelial function, as it suggests different mechanisms for CAP- and Ni-induced eNOS dysfunction.
Oxidative Stress and Ni-Induced Endothelial Function
Ni is one of the transition metal that can induce marked oxidative stress in vivo (Costa et al., 2002). Consistent with this property of Ni, our data demonstrate that Ni exposure significantly increase mouse systemic oxidative stress. Critical components of oxidative stress such as superoxide can antagonize the effect of NO (Muller and Morawietz, 2009) and play an important role in eNOS dimerization (Rafikov et al., 2011), indicating that Ni exposure inhibits eNOS dimerization probably through induction of oxidative stress. Supporting this concept, Ni induced marked oxidative stress in cultured endothelial cells, and an antioxidant Tiron almost normalizes Ni-induced inhibition on eNOS dimerization. However, further studies are needed to determine if Ni inhalation exposure inhibits eNOS dimerization in vivo though induction of oxidative stress.
Is Ni the Catalyst for the Cardiovascular Effects of CAP?
To determine the chemical components of CAP responsible for their adverse cardiovascular effects is one of the important goals of environmental cardiovascular research. However, it currently remains elusive due to the complexity and variation of CAP composition. It is believed that interplay among the different components of CAP may be also critical for CAP exposure–associated cardiovascular diseases. Our data reveal that long-term exposure to Ni and CAP had a synergistic action on endothelial function as evidenced by the much more severe endothelial dysfunction in CAP + Ni group. This is consistent with previous study showing that the toxicity of Ni hydroxide nanoparticles is not driven solely by released Ni ions from deposited particles but also particle-dependent effects (Gillespie et al., 2010). Ni is one of the most frequently identified metals in airborne particulate matters (Baceva et al., 2012; Barandovski et al., 2012; Slezakova et al., 2012; Tian et al., 2012). Multiple epidemiological studies show that Ni concentration in PM2.5 is correlated to PM2.5 exposure–associated mortality (Hernandez-Mena et al., 2011; Huang et al., 2012; Zhang et al., 2009). Notably, we previously demonstrated that Ni in CAP is most strongly correlated to the cardiac function (Lippmann et al., 2006). Our present data not only confirm the role of Ni in CAP-associated adverse cardiovascular effects but also suggest a novel role of Ni in these adverse effects of CAP exposure: a synergistic action of CAP and Ni on endothelial function indicates that Ni may work as a catalyst in CAP-associated adverse cardiovascular effects. However, the mechanism underlying the synergic action of CAP and Ni remains to be determined.
Limitations
Although our study provides definitive evidence that Ni may be critical in mediating the adverse cardiovascular effects of CAP, there are limitations necessitating further studies. As we previously discussed (Xu et al., 2012), our “overexpression” design by adding Ni to CAP may be different from the real world scenarios, and a “knockout” design involving the elimination of certain metals such as Ni from ambient air for an inhalation exposure will be important to confirm the role of Ni in PM2.5-associated adverse cardiovascular effects, although which is technically challenging. In addition, we used only one dose of Ni and CAP, and a dose dependency study in the future may be necessary to verify the synergistic action of Ni and CAP.
CONCLUSIONS
Ni exposure induces endothelial dysfunction through oxidative stress-dependent inhibition of eNOS dimerization. Its interaction with other components of PM2.5 may significantly contribute to the adverse cardiovascular effects of PM2.5 exposure.
FUNDING
National Institutes of Health (R01ES013406, R01ES015146, R21 DK088522 to S.R., ES016588, ES017412, ES018900 to Q.S., R01ES015495, U01ES020126 to L.C.C.); New York University Center Grant (ES00260); American Heart Association (11POST7640030 to Z.Y.); National Natural Science Foundation of China (81270342 to Z.Y.).
REFERENCES
- Aghamohammadzadeh R., Heagerty A. M. (2012). Obesity-related hypertension: Epidemiology, pathophysiology, treatments, and the contribution of perivascular adipose tissue. Ann. Med. 44(Suppl. 1), S74–S84 [DOI] [PubMed] [Google Scholar]
- Allen R. W., Carlsten C., Karlen B., Leckie S., van Eeden S., Vedal S., Wong I., Brauer M. (2011). An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am. J. Respir. Crit. Care Med. 183, 1222–1230 [DOI] [PubMed] [Google Scholar]
- Baceva K., Stafilov T., Sajn R., Tănăselia C. (2012). Moss biomonitoring of air pollution with heavy metals in the vicinity of a ferronickel smelter plant. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 47, 645–656 [DOI] [PubMed] [Google Scholar]
- Barandovski L., Frontasyeva M. V., Stafilov T., Sajn R., Pavlov S., Enimiteva V. (2012). Trends of atmospheric deposition of trace elements in Macedonia studied by the moss biomonitoring technique. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 47, 2000–2015 [DOI] [PubMed] [Google Scholar]
- Brook R. D., Franklin B., Cascio W., Hong Y., Howard G., Lipsett M., Luepker R., Mittleman M., Samet J., Smith S. C., Jr, et al. (2004). Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109, 2655–2671 [DOI] [PubMed] [Google Scholar]
- Campen M. J., Nolan J. P., Schladweiler M. C., Kodavanti U. P., Evansky P. A., Costa D. L., Watkinson W. P. (2001). Cardiovascular and thermoregulatory effects of inhaled PM-associated transition metals: A potential interaction between nickel and vanadium sulfate. Toxicol. Sci. 64, 243–252 [DOI] [PubMed] [Google Scholar]
- Costa M., Salnikow K., Sutherland J. E., Broday L., Peng W., Zhang Q., Kluz T. (2002). The role of oxidative stress in nickel and chromate genotoxicity. Mol. Cell. Biochem. 234–235, 265–275 [PubMed] [Google Scholar]
- Cuevas A. K., Liberda E. N., Gillespie P. A., Allina J., Chen L. C. (2010). Inhaled nickel nanoparticles alter vascular reactivity in C57BL/6 mice. Inhal. Toxicol. 22(Suppl. 2), 100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstermann U., Munzel T. (2006). Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 113, 1708–1714 [DOI] [PubMed] [Google Scholar]
- Forstermann U., Sessa W. C. (2012). Nitric oxide synthases: Regulation and function. Eur. Heart J. 33, 829–837, 837a–837b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. (1999). Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. A., Kang G. S., Elder A., Gelein R., Chen L., Moreira A. L., Koberstein J., Tchou-Wong K. M., Gordon T., Chen L. C. (2010). Pulmonary response after exposure to inhaled nickel hydroxide nanoparticles: Short and long-term studies in mice. Nanotoxicology 4, 106–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P. (1984). Human exposure to nickel. IARC Sci. Publ. 53, 469–485 [PubMed] [Google Scholar]
- Hernandez-Mena L., Murillo-Tovar M., Ramírez-Muñíz M., Colunga-Urbina E., de la Garza-Rodríguez I., Saldarriaga-Noreña H. (2011). Enrichment factor and profiles of elemental composition of PM 2.5 in the city of Guadalajara, Mexico. Bull. Environ. Contam. Toxicol. 87, 545–549 [DOI] [PubMed] [Google Scholar]
- Hilgers R. H., Todd J., Jr., Webb R. C. (2006). Regional heterogeneity in acetylcholine-induced relaxation in rat vascular bed: Role of calcium-activated K+ channels. Am. J. Physiol. Heart Circ. Physiol. 291, H216–H222 [DOI] [PubMed] [Google Scholar]
- Huang W., Cao J., Tao Y., Dai L., Lu S. E., Hou B., Wang Z., Zhu T. (2012). Seasonal variation of chemical species associated with short-term mortality effects of PM(2.5) in Xi’an, a Central City in China. Am. J. Epidemiol. 175, 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Cirino G., Casini A., Napoli C. (1999). Nitric oxide as a signaling molecule in the vascular system: An overview. J. Cardiovasc. Pharmacol. 34, 879–886 [DOI] [PubMed] [Google Scholar]
- Kampfrath T., Maiseyeu A., Ying Z., Shah Z., Deiuliis J. A., Xu X., Kherada N., Brook R. D., Reddy K. M., Padture N. P., et al. (2011). Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 108, 716–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G. S., Gillespie P. A., Gunnison A., Moreira A. L., Tchou-Wong K. M., Chen L. C. (2011). Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. Environ. Health Perspect. 119, 176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M., Ito K., Hwang J. S., Maciejczyk P., Chen L. C. (2006). Cardiovascular effects of nickel in ambient air. Environ. Health Perspect. 114, 1662–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaritis M., Antonopoulos A. S., Digby J., Lee R., Reilly S., Coutinho P., Shirodaria C., Sayeed R., Petrou M., De Silva R., et al. (2013). Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 127, 2209–2221 [DOI] [PubMed] [Google Scholar]
- Massion P. B., Balligand J. L. (2003). Modulation of cardiac contraction, relaxation and rate by the endothelial nitric oxide synthase (eNOS): Lessons from genetically modified mice. J. Physiol. 546(Pt 1), 63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller G., Morawietz H. (2009). Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid. Redox Signal. 11, 1711–1731 [DOI] [PubMed] [Google Scholar]
- Pope C. A., III, Burnett R. T., Thurston G. D., Thun M. J., Calle E. E., Krewski D., Godleski J. J. (2004). Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 109, 71–77 [DOI] [PubMed] [Google Scholar]
- Poursafa P., Kelishadi R., Lahijanzadeh A., Modaresi M., Javanmard S. H., Assari R., Amin M. M., Moattar F., Amini A., Sadeghian B. (2011). The relationship of air pollution and surrogate markers of endothelial dysfunction in a population-based sample of children. BMC Public Health 11, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafikov R., Fonseca F. V., Kumar S., Pardo D., Darragh C., Elms S., Fulton D., Black S. M. (2011). eNOS activation and NO function: Structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J. Endocrinol. 210, 271–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalkwijk C. G., Stehouwer C. D. (2005). Vascular complications in diabetes mellitus: The role of endothelial dysfunction. Clin. Sci. (Lond). 109, 143–159 [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D., Zakrajsek B. A. (2000). Effect of experimental treatment on housekeeping gene expression: Validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 46, 69–81 [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Yasutake H., Fujii K., Owada M. K., Nakaike R., Fukumoto Y., Takayanagi T., Nagao T., Egashira K., Fujishima M., et al. (1996). The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J. Cardiovasc. Pharmacol. 28, 703–711 [DOI] [PubMed] [Google Scholar]
- Slezakova K., Alvim-Ferraz M. d. a. C., Pereira M. d. o. C. (2012). Elemental characterization of indoor breathable particles at a Portuguese urban hospital. J. Toxicol. Environ. Health A 75, 909–919 [DOI] [PubMed] [Google Scholar]
- Sun Q., Wang A., Jin X., Natanzon A., Duquaine D., Brook R. D., Aguinaldo J. G., Fayad Z. A., Fuster V., Lippmann M., et al. (2005). Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 294, 3003–3010 [DOI] [PubMed] [Google Scholar]
- Sun Q., Yue P., Ying Z., Cardounel A. J., Brook R. D., Devlin R., Hwang J. S., Zweier J. L., Chen L. C., Rajagopalan S. (2008). Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler. Thromb. Vasc. Biol. 28, 1760–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H. Z., Lu L., Cheng K., Hao J. M., Zhao D., Wang Y., Jia W. X., Qiu P. P. (2012). Anthropogenic atmospheric nickel emissions and its distribution characteristics in China. Sci. Total Environ. 417–418, 148–157 [DOI] [PubMed] [Google Scholar]
- Xu X., Rao X., Wang T. Y., Jiang S. Y., Ying Z., Liu C., Wang A., Zhong M., Deiuliis J. A., Maiseyeu A., et al. (2012). Effect of co-exposure to nickel and particulate matter on insulin resistance and mitochondrial dysfunction in a mouse model. Part. Fibre Toxicol. 9, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z., Giachini F. R., Tostes R. C., Webb R. C. (2009). PYK2/PDZ-RhoGEF links Ca2+ signaling to RhoA. Arterioscler. Thromb. Vasc. Biol. 29, 1657–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z., Kampfrath T., Thurston G., Farrar B., Lippmann M., Wang A., Sun Q., Chen L. C., Rajagopalan S. (2009). Ambient particulates alter vascular function through induction of reactive oxygen and nitrogen species. Toxicol. Sci. 111, 80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Chau P. Y., Lai H. K., Wong C. M. (2009). A review of effects of particulate matter-associated nickel and vanadium species on cardiovascular and respiratory systems. Int. J. Environ. Health Res. 19, 175–185 [DOI] [PubMed] [Google Scholar]
- Zhao J., Shi X., Castranova V., Ding M. (2009). Occupational toxicology of nickel and nickel compounds. J. Environ. Pathol. Toxicol. Oncol. 28, 177–208 [DOI] [PubMed] [Google Scholar]