Abstract
Recent epidemiological studies have demonstrated associations between air pollution and adverse effects that extend beyond respiratory and cardiovascular disease, including low birth weight, appendicitis, stroke, and neurological/neurobehavioural outcomes (e.g., neurodegenerative disease, cognitive decline, depression, and suicide). To gain insight into mechanisms underlying such effects, we mapped gene profiles in the lungs, heart, liver, kidney, spleen, cerebral hemisphere, and pituitary of male Fischer-344 rats immediately and 24h after a 4-h exposure by inhalation to particulate matter (0, 5, and 50mg/m3 EHC-93 urban particles) and ozone (0, 0.4, and 0.8 ppm). Pollutant exposure provoked differential expression of genes involved in a number of pathways, including antioxidant response, xenobiotic metabolism, inflammatory signalling, and endothelial dysfunction. The mRNA profiles, while exhibiting some interorgan and pollutant-specific differences, were remarkably similar across organs for a set of genes, including increased expression of redox/glucocorticoid-sensitive genes and decreased expression of inflammatory genes, suggesting a possible hormonal effect. Pollutant exposure increased plasma levels of adrenocorticotropic hormone and the glucocorticoid corticosterone, confirming activation of the hypothalamic-pituitary-adrenal axis, and there was a corresponding increase in markers of glucocorticoid activity. Although effects were transient and presumably represent an adaptive response to acute exposure in these healthy animals, chronic activation and inappropriate regulation of the hypothalamic-pituitary-adrenal axis are associated with adverse neurobehavioral, metabolic, immune, developmental, and cardiovascular effects. The experimental data are consistent with epidemiological associations of air pollutants with extrapulmonary health outcomes and suggest a mechanism through which such health effects may be induced.
Key Words: air pollution, particulate matter, ozone, glucocorticoid, hypothalamic-pituitary-adrenal axis, systemic effects, real-time PCR.
Variation in the levels of air pollutants has primarily been associated with increased risk of cardiovascular and respiratory morbidity and mortality (Burnett et al., 1995, 1997; Pope et al., 2004). Recent epidemiological studies have now also found associations of common air pollutants with a number of other adverse health effects. These include effects associated with long-term exposure to air pollutants such as neurodegenerative disease (Calderon-Garciduenas et al., 2004), cognitive impairment (Chen and Schwartz, 2009; Power et al., 2011; Ranft et al., 2009; Weuve et al., 2012), and low birth weight (Bell et al., 2007) and effects associated with short-term variation in pollutant levels such as stroke (Hong et al., 2002; Villeneuve et al., 2006), appendicitis (Kaplan et al., 2009), depression (Lim et al., 2012; Szyszkowicz et al., 2009), and suicide (Biermann et al., 2009; Kim et al., 2010; Szyszkowicz et al., 2010), among others. If substantiated, these studies suggest that the health burden attributable to air pollution may be considerably greater than conventional estimates. However, the biological processes underlying such health effects are not well understood.
If pollutant inhalation provokes acute biological responses that can precipitate adverse extracardiopulmonary health outcomes in susceptible individuals, it is reasonable to suppose that evidence of these responses can be found in altered gene expression. Perturbation of gene expression by air pollutants has been examined using targeted and global (e.g., microarray) approaches (Sen et al., 2007). Although microarrays provide a comprehensive analysis of the entire transcriptome and have been used effectively to distinguish among different pathologies, they may have insufficient power to detect subtle biologically significant responses arising from environmental exposures in vivo (Thomson et al., 2009b). Using real-time PCR, we have shown previously that expression of genes implicated in vasoregulation in the lungs, cerebral hemisphere, and pituitary is altered following exposure of rats by inhalation to two common pollutants implicated in the health effects of air pollution, particulate matter, and ozone (Thomson et al., 2005, 2006, 2007). It is clear even from this small set of genes that responses in the lungs, brain, and pituitary differ markedly from each other following exposure and that individual pollutants provoke distinct responses.
Our objective in this study was to gain insight into systemic effects of ozone and particulate matter exposure by “mapping” changes in gene expression across organs after pollutant inhalation. We hypothesized that monitoring the expression of genes involved in a number of biological pathways would generate a profile unique to the pollutant, time, and organ that could be used to understand processes underlying adverse health outcomes. Because evaluation of transcript levels in whole organs implies that any changes in gene expression in a subset of cells would be diluted by the mass of nonresponding cells, we reasoned that the pattern of responses may serve as a more useful measure of biological significance than the magnitude of response of a given gene or pathway. In addition, we anticipated that expression profiles would provide evidence of potential mediators of effects, the identity of which could then be confirmed through subsequent focussed analyses.
MATERIALS AND METHODS
Animals.
Specific pathogen-free Fischer-344 male rats (200–250g) were obtained from Charles River (St Constant, Québec, Canada). Animals were housed in individual plexiglass cages on wood-chip bedding under HEPA-filtered air and held to a 12-h dark/light cycle. Food and water were provided ad libitum. All experimental protocols were reviewed and approved by the Animal Care Committee of Health Canada.
Inhalation exposure to air pollutants.
Animals were exposed to EHC-93 urban particles (Bouthillier et al., 1998; Vincent et al., 1997a, b, 2001), ozone, or the combined pollutants using a nose-only inhalation system. Rats (n = 4–6 per treatment group) were trained in nose-only exposure tubes over 5 consecutive days and then exposed for 4h to clean air or to combinations of the individual pollutants EHC-93 (0, 5, and 50mg/m3) and ozone (0, 0.4, and 0.8 ppm) as previously described (Thomson et al., 2005; Vincent et al., 1997a, b). Animals were euthanized immediately after exposure or following 24-h recovery in filtered air. The particle size distribution of resuspended EHC-93 in our flow-past nose-only exposure system was multimodal, with two respirable modes at 1.3 μm aerodynamic diameter (DAE) and 3.6 μm DAE that together comprised 55% of the mass of the aerosol, and a nonrespirable mode at 15 μm DAE that comprised 45% of the mass (Vincent et al., 2001). Dosimetric analysis and comparison with a plausible human scenario performed previously (Thomson et al., 2005) indicated that the ratio of an experimental particle EHC-93 dose within the respiratory compartment of the rats during the 5mg/m3 exposure (2.5ng/cm2) and 50mg/m3 exposure (25ng/cm2) to the particle dose calculated for a plausible 24-h human exposure scenario (1.3ng/cm2) is 2-fold and 20-fold, respectively. The ratio of the centriacinar ozone dose at 0.4 ppm O3 (214ng O3/cm2) and 0.8 ppm O3 (427ng O3/cm2) to the estimated internal dose in a human subject under a plausible 24-h exposure scenario (127ng O3/cm2) is 1.7-fold and 3.4-fold, respectively. The dose rate in our study was obviously higher than for a 24-h environmental exposure, as the duration of nose-only exposure should be kept to a minimum for ethical reasons. Nevertheless, from a toxicological perspective, the pulmonary deposition of the pollutants are relevant to the human situation once uncertainty factors such as interspecies differences and the heightened sensitivity of a subset of the population are considered.
Biological samples.
Rats were anaesthetized by administration of sodium pentobarbital (60mg/kg, ip). Blood was collected from the abdominal aorta into vacutainer tubes containing the sodium salt of EDTA at 10mg/ml and PMSF at 1.7mg/ml, mixed gently, and placed on ice (Kumarathasan et al., 2001). Plasma was isolated by centrifugation (2000rpm for 10min), aliquoted, and frozen at −80°C. The lungs were washed by bronchoalveolar lavage with warm saline (37°C) at 30ml/kg body weight, then flash frozen in liquid nitrogen, and stored at −80°C. Heart, liver, kidney, and spleen were snap-frozen in liquid nitrogen. After exsanguination, the head was removed by decapitation at the first cervical vertebra. The brain was removed and the cerebral hemisphere was dissected, snap-frozen in liquid nitrogen, and stored at −80°C. The pituitary was removed and stored in the preservative solution RNAlater (Qiagen Inc., Mississauga, Ontario, Canada) at −20°C.
RNA isolation.
Frozen lung, heart, liver, kidney, spleen, and cerebral hemisphere samples were homogenized in TRIzol reagent (Invitrogen Canada Inc., Burlington, Ontario, Canada), and total RNA was isolated according to the manufacturer’s instructions. Pituitary total RNA was isolated using MiniPrep kits (Qiagen Inc.). Samples were aliquoted to avoid repeated freeze-thaw cycles and stored at −80°C. RNA was quantified in triplicate using the RiboGreen RNA Quantitation Reagent and Kit (Molecular Probes, Eugene, OR). Total RNA concentration was normalized for each sample and reverse transcribed using MuLV reverse transcriptase and random hexamers (Applied Biosystems, Mississauga, Ontario, Canada) according to the manufacturer’s instructions. Reactions were prepared using master mixes of reagents to minimize variation in reagent composition, and reactions were performed on all samples for a given tissue simultaneously under uniform conditions. Negative control samples run in parallel were generated by replacing the reverse transcriptase with DNAse/RNAse-free H2O.
Real-time PCR analysis.
Primers were designed to produce amplicons of less than 150 bases and an optimal annealing temperature of 60°C using the Universal Probe Library design software (Roche Diagnostics Canada, Laval, Quebec, Canada) and Primer-BLAST software (National Center for Biotechnology Information, Bethesda, MD) and are presented in Supplementary table 1. In silico testing was performed using mfold software (Zuker, 2003) to verify absence of secondary structure in the amplicon and surrounding template that might interfere with primer binding. Primers were validated for high (> 90%) efficiency using a dilution series of rat RNA. A robotic liquid handler (Caliper Zephyr Compact Liquid Handling Workstation, PerkinElmer, Woodridge, Ontario, Canada) was employed to dispense and mix reagents, prepared in bulk for uniform reagent composition, with cDNA. Forty nanograms of cDNA were incubated with iQ SYBR Green Supermix (Bio-Rad Laboratories (Canada) Ltd, Mississauga, Ontario, Canada) and 200 nM of each primer in a total volume of 20 μl/well. All reactions were performed in duplicate on 96-well plates in a spectrofluorometric thermal cycler (Lightcycler 480, Roche Diagnostics Canada). Uniform reaction conditions and reproducibility of results across the PCR plate were verified according to the method previously described (Thomson and Vincent, 2005). All time-matched samples from a given tissue were assessed on a single plate to eliminate any potential impact of plate-to-plate or run-to-run variability. Negative control samples from the reverse transcription reaction were included on all plates to test for the presence of contaminating genomic DNA. PCR runs were initiated by incubation at 95°C for 3min to activate the iTAQ polymerase followed by 50 cycles of denaturation at 95°C, annealing at 60°C, and elongation at 72°C, each for 10 s. Fluorescence was monitored at every cycle during the elongation step. A melt curve was conducted following each run to verify product purity.
Seven candidate reference genes (β-actin, glyceraldehyde-3-phosphate dehydrogenase, hypoxanthine guanine phosphoribosyl transferase, peptidylprolyl isomerase A, ribosomal protein L32, TATA box binding protein, and tyrosine-3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta polypeptide) were assessed for stability across treatment groups in each organ. RefFinder (Chen et al., 2011), incorporating four commonly used approaches to select the best reference gene (GeNorm [Vandesompele et al., 2002], BestFinder [Pfaffl et al., 2004], NormFinder [Andersen et al., 2004], and delta Ct [Silver et al., 2006]), was used to rank candidate genes. Statistical analyses (two-way and three-way ANOVAs) were then used to test for significant effects of treatment on the geometric mean of combinations of reference genes, followed by individual genes in order of rank. For the lungs, heart, spleen, cerebral hemisphere, and pituitary, β-actin was found to be the most stable gene, for the kidney, the geometric mean of β-actin, glyceraldehyde-3-phosphate dehydrogenase, and tyrosine-3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta polypeptide was used, whereas for the liver, peptidylprolyl isomerase A was selected. Expression was calculated relative to the reference gene(s) using the delta-delta Ct method (Livak and Schmittgen, 2001), and expressed as fold change relative to time-matched air control samples.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis of rat plasma for adrenocorticotropic hormone.
Rat plasma samples were purified using 10kDa molecular weight cutoff filters (Mohottalage et al., 2007). Purified samples were spotted on the AnchorChip target plate (600/384F, Bruker Daltonics Ltd, Milton, Ontario, Canada) following a dried droplet method with on-target washing (Kumarathasan et al., 2005). Washed spots were dried and analyzed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry using analysis parameters described previously (Mohottalage et al., 2007). Peak area values associated with the analyte peak at m/z 2093.1 assigned to the adrenocorticotropic hormone (ACTH) (1–17) fragment were normalized to the total ion peak area of the corresponding mass spectrum.
Corticosterone ELISA.
Corticosterone was assessed in 2 μl of plasma using the DetectX Corticosterone Enzyme Immunoassay Kit (Arbor Assays, Ann Arbor, MI) according to manufacturer’s instructions.
Statistical analyses.
Data are expressed as geometric mean ± geometric standard deviation. Where necessary, results were transformed to meet the requirements of normality and equal variance. Data were analyzed by two-way ANOVA (immediately after exposure) with ozone (0, 0.4, and 0.8 ppm) and EHC (0, 5, and 50mg/m3) as factors or three-way ANOVA with ozone (0 and 0.8 ppm), EHC (0 and 50mg/m3), and TIME (0 and 24h postexposure) as factors, followed by the Holm-Sidak multiple comparison procedure to elucidate the pattern of significant effects (α = 0.05; Sigma-Plot 12.3, Systat Software Inc., San Jose, CA). Effects of pollutants on endothelin-1, endothelin-3, iNOS, eNOS, and tumor necrosis factor (TNF) mRNAs in the lungs, cerebral hemisphere, and pituitary of the animals have previously been reported (Thomson et al., 2005, 2006, 2007) and are included here to allow for direct comparison with effects in other organs.
RESULTS
Mapping Transcriptional Impacts of Pollutant Exposure: Systemic Effects
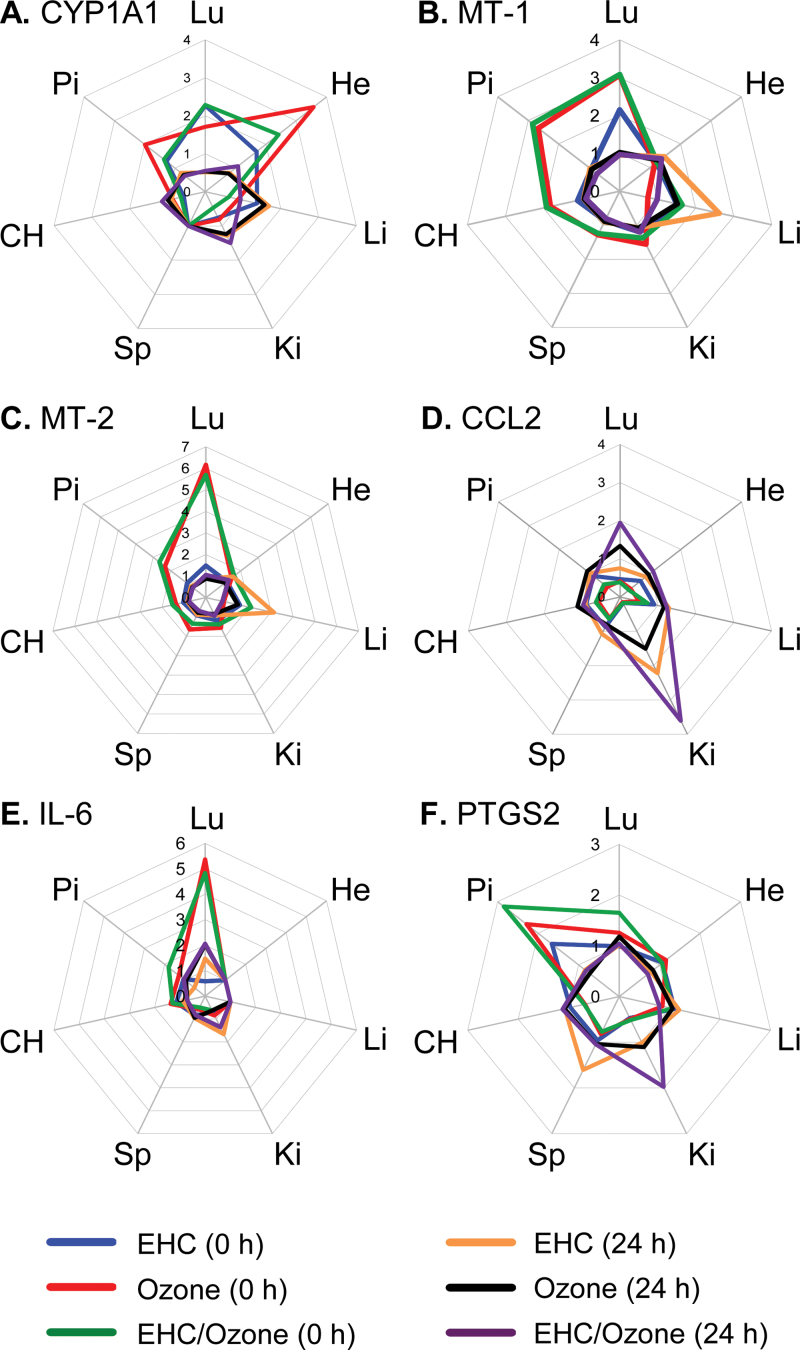
To examine systemic effects of pollutant exposure, we measured transcript levels of genes involved in a variety of biological pathways, notably antioxidant response, inflammatory response, xenobiotic metabolism, metal response, vasoregulation/endothelial dysfunction, and hypoxia and angiogenesis (genes and abbreviations are listed in the legend of Fig. 1). To facilitate comparison of treatment effects across organs, we created vector diagrams representing fold change relative to air-exposed control animals. Due to the large quantity of dose-response data, in these figures the results from the high-dose exposures alone were used to allow rapid visual comparison of effects. Displaying the fold change relative to the control air-exposed animals for each exposure (ozone, particles, or coexposure to ozone and particles) produced a profile in each organ, allowing effects of exposures to be compared directly within an organ according to differences in their shape and differences in response across organs to be quickly identified (Fig. 1). Vector diagrams were also generated that plot the effects of pollutants on a given gene across all organs, facilitating direct comparison of the pollutant effect on a given transcript among organs (Fig. 4). Because neither approach displays the variance in the data, all group means and statistical significance are presented in Supplementary table 2. Unless indicated otherwise, all effects discussed in text were statistically significant.
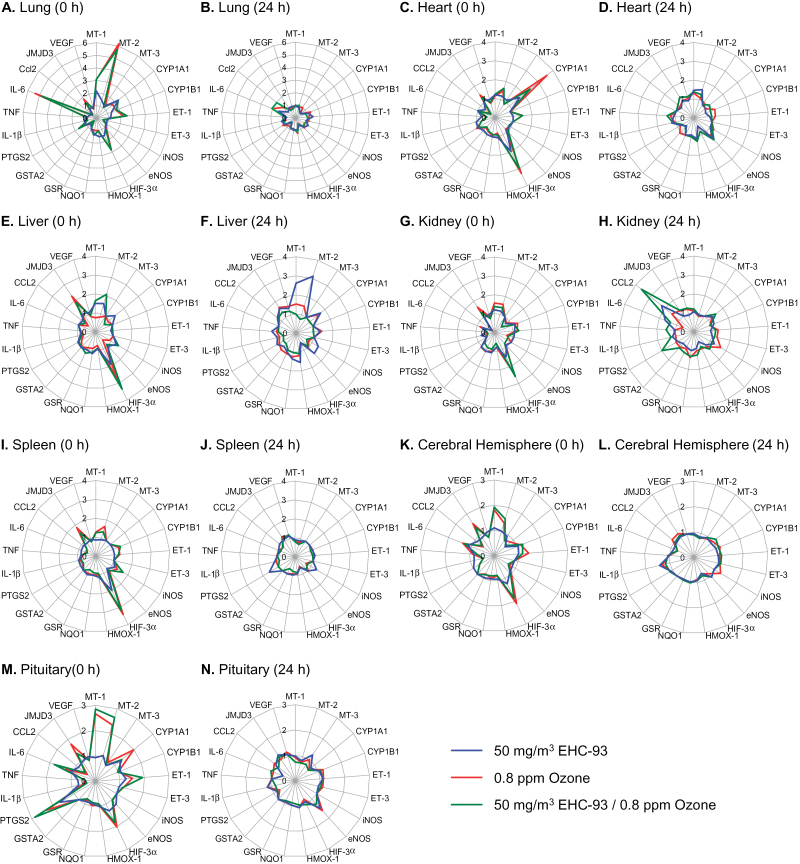
Fig. 1.
Mapping pollutant effects across organs. Gene expression was assessed in all organs 0 and 24h after exposure of rats (n = 4–6 per group) to 50mg/m3 particulate matter (blue line), 0.8 ppm ozone (red line), or coexposure to 50mg/m3 particulate matter and 0.8 ppm ozone (green line). Vector diagrams display the fold change in response to treatment relative to the mean of the air control group for each gene. Genes that fell below the level of detection were given a value of 1. Statistical analyses for all genes are presented in Supplementary table 2. Metallothionein (MT), cytochrome p450 family (CYP), endothelin (ET), inducible nitric oxide synthase (iNOS), endothelial nitric oxide synthase (eNOS), hypoxia inducible factor (HIF), heme oxygenase (HMOX), NAD(P)H dehydrogenase quinone 1 (NQO1), glutathione reductase (GSR), glutathione S-transferase A2 (GSTA2), prostaglandin endoperoxide synthase 2 (PTGS2), interleukin (IL), tumour necrosis factor (TNF), chemokine (CC motif) ligand 2 (CCL2), histone H3 lysine-27 demethylase JMJD3, and vascular endothelial growth factor (VEGF).
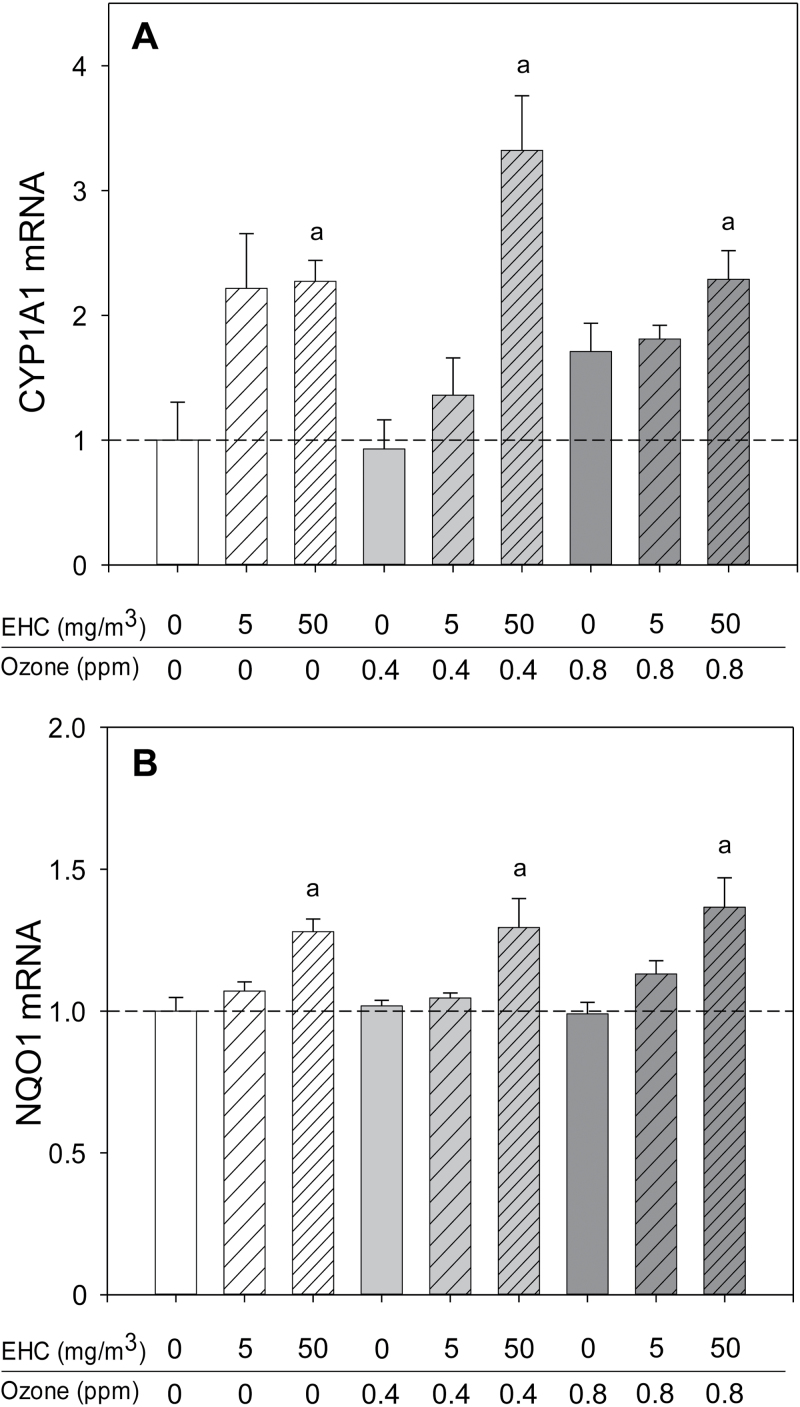
Fig. 4.
Xenobiotic and antioxidant gene response in the lungs after particulate matter inhalation. Animals (n = 4–6 per group) were exposed to particulate matter (0, 5, and 50mg/m3 EHC-93), ozone (0, 0.4, and 0.8 ppm), or combinations of particles and ozone for 4h and euthanized 0 or 24h after exposure. Results are presented as the geometric mean ± geometric standard deviation. Letters over bars indicate statistical significance (Holm-Sidak, p < 0.05). (A) Lung cytochrome P450 family 1 member A1 (CYP1A1) mRNA immediately after exposure. EHC main effect, p < 0.001. a0, 5 versus 50mg/m3. (B) Lung NAD(P)H dehydrogenase quinone 1 (NQO1) mRNA immediately after exposure. EHC main effect, p = 0.025. a0, 5 versus 50mg/m3.
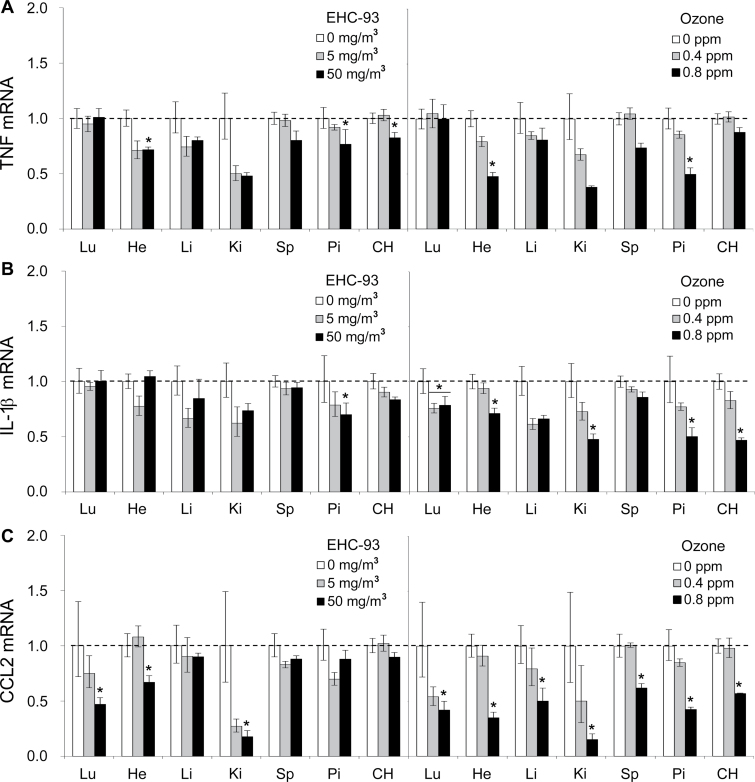
Pollutant inhalation modified gene expression in all organs examined, with the lungs, heart, and pituitary being particularly responsive (Fig. 1). In general, changes in gene expression were transient, returning to control levels after 24h, except where noted below. The “shape” of the response to both pollutants was remarkably similar in the liver, kidney, and spleen immediately after exposure and comprised a subset of responses observed in all organs. Commonly affected genes included a systemic increase in mRNA levels of the stress/metal-responsive metallothionein genes, with particularly prominent responses in the lungs and pituitary. Hypoxia-inducible factor (HIF)-3α, the transcriptionally regulated member of the HIF family of transcription factors, was increased two- to threefold in all organs. The histone H3 Lys27 demethylase JMJD3, an important regulator of transcription implicated in cell differentiation, was increased in all organs by ozone. Although particles and ozone increased ET-1 expression (vasoregulation/endothelial dysfunction) in the lungs (Thomson et al., 2005), ozone alone increased expression in the heart, kidney, and spleen but not in liver. Expression of the proinflammatory genes TNF, chemokine (C-C motif) ligand 2 (CCL-2)/monocyte chemotactic protein-1 (MCP-1), and interleukin (IL)-1β was decreased in most organs by particles and ozone. Although the effect was statistically significant predominantly at the high dose of particles or ozone, expression tended to be decreased in low-dose exposure groups for several organs, with the strongest effects measured in the kidney (Fig. 2).
Fig. 2.
Regulation of inflammatory genes immediately following pollutant inhalation. (A) TNF, (B) IL-1β, and (C) MCP-1 were measured in organs immediately after exposure to ozone and particulate matter (EHC-93). To facilitate comparison of effects across multiple organs for a given treatment, only effects of exposure to EHC-93 (0, 5, and 50mg/m3) or ozone (0, 0.4, and 0.8 ppm) are displayed (n = 4–6 per group). Results are presented as the geometric mean ± geometric standard deviation. Asterisks denote statistical significance relative to control (Holm-Sidak, p < 0.05) as guided by statistical analyses (two-way ANOVA) conducted on the entire data set that includes coexposure to both ozone and particulate matter. Complete statistical analyses are presented in Supplementary table 2.
Differential Effects of Ozone and Particulate Matter in the Lungs
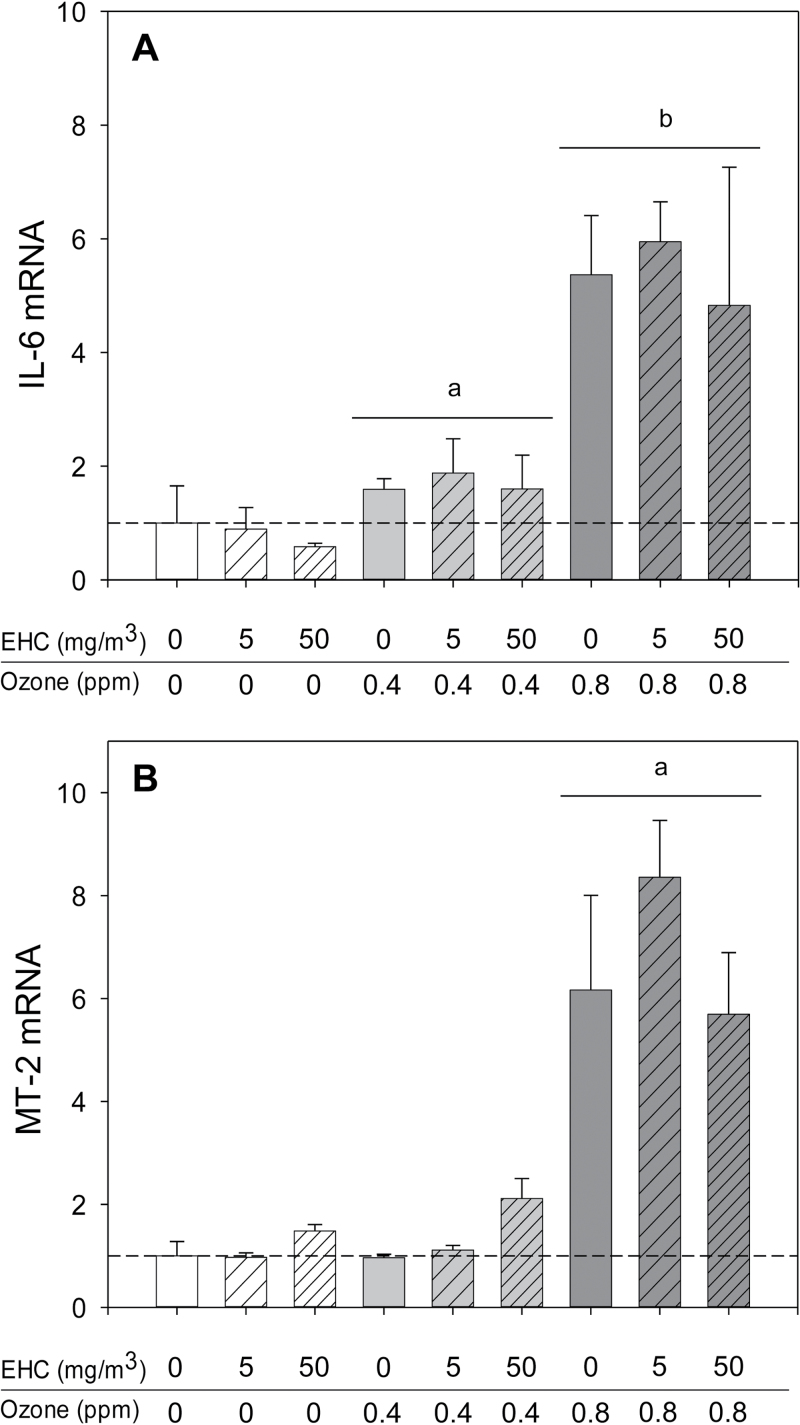
Notwithstanding the similar responses of several genes across organs, there were a number of organ-specific effects. This was particularly evident in the lungs. The pulmonary response to ozone was characterized by induction of antioxidant and inflammatory genes, notably increased metallothionein and IL-6 mRNA (Fig. 3). Ozone also provoked a modest increase of CYP1B1 and a delayed (24h only) increase in CCL-2/MCP-1 mRNA (Figs. 1A and B). In addition to nonsignificant impacts on lung metallothionein mRNA levels at the 50mg/m3 exposure (EHC main effect, p = 0.125; Fig. 3B), particulate matter inhalation resulted in modest increases in the antioxidant/xenobiotic response gene NAD(P)H dehydrogenase quinone 1 (NQO1) and the xenobiotic responsive cytochrome P450 family 1 member A1 (CYP1A1; Fig. 4).
Fig. 3.
Antioxidant and inflammatory responses in the lungs after ozone inhalation. Animals (n = 4–6 per group) were exposed to particulate matter (0, 5, and 50mg/m3 EHC-93), ozone (0, 0.4, and 0.8 ppm), or combinations of particles and ozone for 4h and euthanized 0 or 24h after exposure. Results are presented as the geometric mean ± geometric standard deviation. Letters over bars indicate statistical significance (Holm-Sidak, p < 0.05). (A) Lung IL-6 mRNA immediately after exposure. Ozone main effect, p < 0.001. a0 versus 0.4 ppm ozone; b0, 0.4 versus 0.8 ppm ozone. (B) Lung metallothionein (MT)-2 mRNA immediately after exposure. Ozone main effect, p < 0.001. a0, 0.4 versus 0.8 ppm ozone.
Extrapulmonary Effects of Pollutant Exposure
Comparison of expression across organs for a given gene highlighted differences among extrapulmonary responses (Fig. 5). In addition to a response in the lungs, CYP1A1 mRNA was increased in the heart and pituitary by both particles and ozone, but in contrast to pulmonary effects, the effect of ozone in both organs was of greater magnitude than the effect of particle exposure (Fig. 5A). Unlike the transient responses of metallothionein genes in most organs, there was a trend toward a sustained increase in the liver following particle exposure although this effect was not observed in the presence of ozone (three-way ANOVA, MT-1, EHC main effect, p = 0.068; MT-2, EHC × Ozone × Time interaction, p = 0.059; Figs. 5B and C). After initially decreasing in most organs, CCL-2/MCP-1 mRNA increased in the kidney 24h after exposure to the pollutants (Fig. 5D). The gene profiles of the cerebral hemisphere and pituitary displayed features similar to other organs, including transient increases of metallothionein genes, hypoxia-inducible factor-3α, and JMJD3 and decreased inflammatory gene expression (Fig. 1). In addition, there was a small immediate increase of pituitary IL-6 mRNA (Fig. 5E) and a prominent increase of PTGS2 mRNA (Fig. 5F), an effect that was not seen in other organs.
Fig. 5.
Mapping pollutant effects by gene. Gene expression was assessed in all organs 0 and 24h after exposure to 50mg/m3 particulate matter, 0.8 ppm ozone, or coexposure to 50mg/m3 particulate matter and 0.8 ppm ozone (n = 4–6 per group). Expression of a given gene across all organs was plotted to enable direct comparison of interorgan differences in response. Statistical analyses for all genes are presented in Supplementary table 2. Lung (Lu), heart (He), liver (Li), kidney (Ki), spleen (Sp), cerebral hemisphere (CH), pituitary (Pi), cytochrome P450 family 1 member A1 (CYP1A1), metallothionein (MT), chemokine (C-C motif) ligand 2(CCL2)/monocyte chemotactic protein1 (MCP1), interleukin (IL) and prostaglandin endoperoxide synthase 2 (PTGS2).
Activation of the Hypothalamic-Pituitary-Adrenal Axis and Corticosterone Release After Exposure to Ozone and Particulate Matter
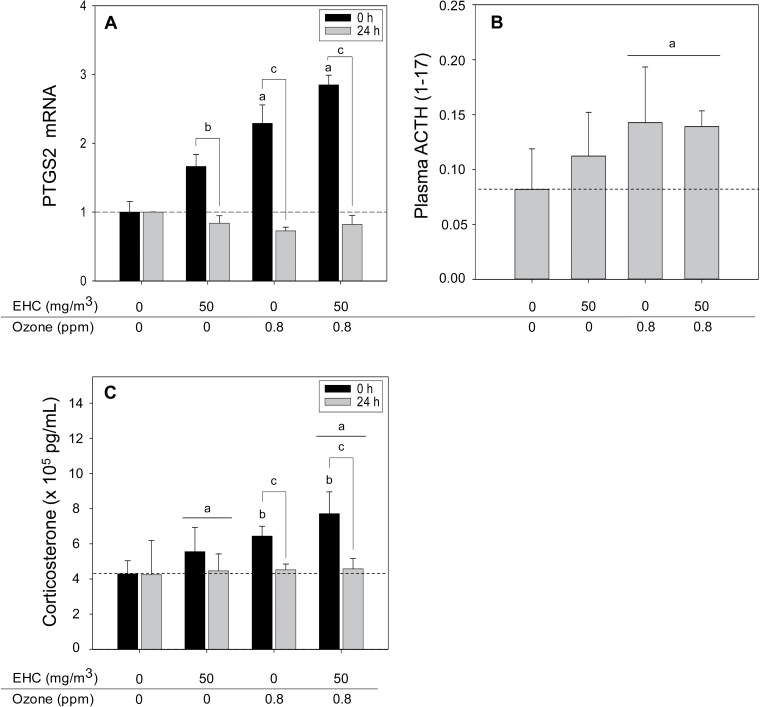
Given the pronounced response of the pituitary to pollutant exposure and the systemic decrease in expression of inflammatory genes, we hypothesized that at least a subset of effects represented a common response to activation of the hypothalamic-pituitary-adrenal (HPA) stress axis, resulting in glucocorticoid release. Moreover, MT-1 and MT-2, which exhibited systemic increases in response to pollutant exposure, are activated by glucocorticoids (Ghoshal et al., 1998). The increase of PTGS2 mRNA, a critical activator of the HPA axis (Bugajski et al., 2004; Reimsnider and Wood, 2004), by both particles and ozone, appeared to be additive, consistent with impacts on the HPA axis by both pollutants (Fig. 6A). To assess whether pollutant inhalation resulted in activation of the HPA axis, we measured ACTH levels in plasma. MALDI-TOF mass spectrometric analysis confirmed increased levels of a peak corresponding to the expected molecular weight of the ACTH (1–17) fragment (Ozone main effect, p = 0.015, two-way ANOVA, one-tailed α = 0.05; Fig. 6B). As ACTH signalling triggers release of the glucocorticoid corticosterone from the rat adrenal gland, we examined whether pollutant exposure increased plasma corticosterone. Plasma corticosterone was increased immediately after exposure, returning to control levels following 24-h recovery (Ozone × Time interaction, p = 0.009; EHC main effect, p = 0.041; three-way ANOVA, one tailed α = 0.05; Fig. 6C).
Fig. 6.
Activation of the hypothalamic-pituitary-adrenal axis. Letters over bars indicate statistical significance (Holm-Sidak, p < 0.05). (A) Pituitary prostaglandin endoperoxide synthase 2 (PTGS2) mRNA (n = 4–6 per group). Relative mRNA expression was assessed by real-time PCR in rats exposed to the indicated pollutant exposures. Results are presented as the geometric mean ± geometric standard deviation. Ozone × Time interation, p < 0.001. a0 versus 0.8 ppm within 0h; b0 versus 24h within 0 ppm ozone; c0 versus 24h within 0.8 ppm ozone. (B) Plasma ACTH levels (n = 4–6 per group). Plasma ACTH was assessed by integrating the peak area of the analyte peak at m/z 2093.1 assigned to the ACTH(1–17) fragment following MALDI-TOF mass spectrometric analysis (relative units). Ozone main effect, p = 0.015. a0 versus 0.8 ppm within OZONE; two-way ANOVA, one-tailed α = 0.05. (C) Plasma corticosterone (n = 4 per group). Immunoreactive corticosterone was measured by ELISA assay. Ozone × Time interaction, p = 0.018; EHC main effect, p = 0.041. a0 versus 50mg/m3 within EHC; b0 versus 0.8 ppm within 0h; c0 versus 24h within 0.8 ppm ozone; three-way ANOVA, one-tailed α = 0.05.
Pollutant-Induced Changes in the Expression of Glucocorticoid-Regulated Genes
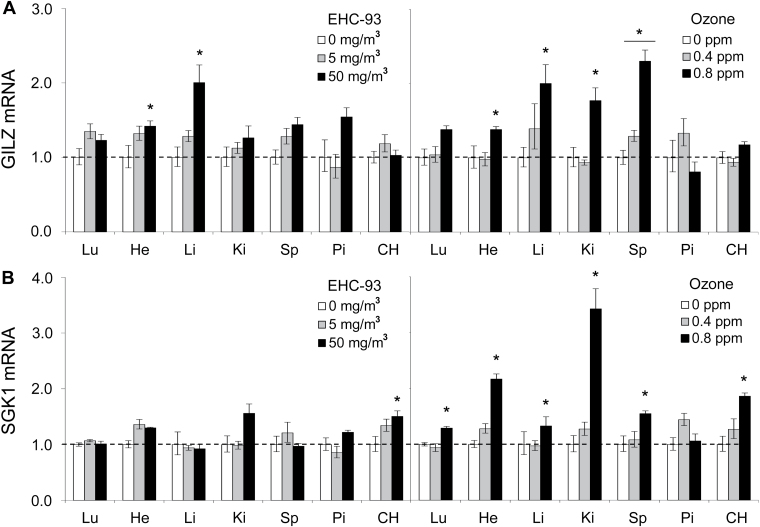
To determine whether the increase in corticosterone was accompanied by corresponding increases in known glucocorticoid-responsive genes, we examined the expression of glucocorticoid-inducible leucine zipper (GILZ), a marker and mediator of glucocorticoid activity (Ayroldi and Riccardi, 2009), and serum- and glucocorticoid-inducible kinase (SGK). Expression of both genes was increased in most organs immediately after particle and ozone exposure, including a trend toward increased expression at the lower level of exposure (e.g., 5mg/m3 EHC or 0.4 ppm ozone; Fig. 7).
Fig. 7.
Regulation of glucocorticoid-responsive genes by inhaled pollutants. (A) GILZ and (B) SGK mRNA levels were measured in organs immediately after exposure to ozone and particulate matter (EHC-93). To facilitate comparison of effects across multiple organs for a given treatment, only effects of exposure to EHC-93 (0, 5, and 50mg/m3) or ozone (0, 0.4, and 0.8 ppm) are displayed (n = 4–6 per group). Results are presented as the geometric mean ± geometric standard deviation. Asterisks denote statistical significance relative to control (Holm-Sidak, p < 0.05) as guided by statistical analyses (two-way ANOVA) conducted on the entire data set that includes coexposure to both ozone and particulate matter. Complete statistical analyses are presented in Supplementary table 2.
Activation of Distinct Signaling Pathways in the Lungs and Liver After Pollutant Inhalation
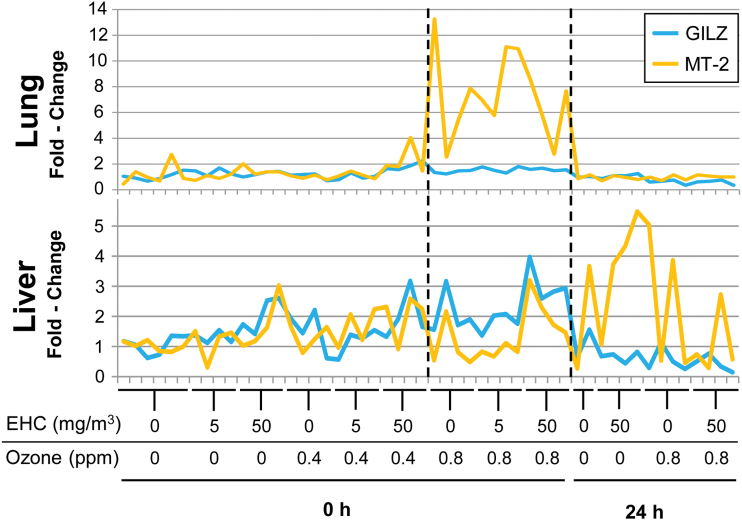
The promoter of the MT-2 gene possesses functional antioxidant response, metal response, and glucocorticoid response elements and is upregulated under conditions of oxidative stress, the presence of metals, and glucocorticoid stimulation (Ghoshal et al., 1998). It is, therefore, responsive to at least three biological pathways through which ozone and particulate matter may exert effects. To distinguish among effects of oxidative stress, metal response, and glucocorticoid action, we examined the relative transcript levels of GILZ, selected to represent glucocorticoid action (Ayroldi and Riccardi, 2009), and MT-2 in each sample. If glucocorticoids were driving MT-2 expression, levels of the two genes would be expected to covary across samples. After inhalation of 0.8 ppm ozone, MT-2 mRNA levels increased dramatically in the lungs without any significant change in the levels of GILZ mRNA (Fig. 8), suggesting that glucocorticoids are not responsible for this effect. In contrast, in the liver of these ozone-exposed animals, GILZ and MT-2 mRNA levels covaried, consistent with glucocorticoid regulation of both genes in this organ.
Fig. 8.
Distinct signalling pathways induced by pollutant exposure in the lungs and liver. Relative mRNA levels of GILZ and metallothionein (MT)-2 mRNA were plotted for each individual animal in the lungs and liver. Dotted lines indicate the group of animals exposed to 0.8 ppm ozone.
DISCUSSION
The HPA axis is a major part of the neuroendocrine system that controls homeostasis and primes the body for response to stress. Activation of the HPA axis leads to glucocorticoid-induced changes in a large number of genes involved in a variety of processes, including glucose metabolism, immune response, adipocyte differentiation, and hormone control (de Kloet, 2004; Wang, 2005). The increase of plasma ACTH and corticosterone, coupled with the significant systemic impacts of both pollutants on glucocorticoid-responsive genes, is consistent with activation of the HPA axis and subsequent effects on stress, immune, and metabolic functions (Ghoshal et al., 1998; O’Connor et al., 2000). Anti-inflammatory actions of glucocorticoids are mediated at least in part by GILZ (Ayroldi and Riccardi, 2009), which was increased across organs in response to both ozone and particulate matter. SGK, another classical glucocorticoid-inducible gene increased in this study primarily in the kidney, heart, and brain, is implicated in a host of (patho)physiological processes, including metabolism, kidney function, gastrointestinal function, and control of blood pressure (Lang et al., 2006). Metabolic disturbance may explain the systemic increase in HIF-3α following pollutant inhalation, as in addition to its responsiveness to hypoxia HIF-3α is sensitive to glucose and insulin (Heidbreder et al., 2007).
Notwithstanding the effects observed across organs consistent with glucocorticoid action, comparison of expression profiles revealed interorgan differences in the expression of genes involved in antioxidant response, xenobiotic metabolism, inflammatory signalling, and endothelial dysfunction, implying differential sensitivity or differential distribution of pollutants and mediators of effects. The pulmonary response to particles included increased expression of the nuclear factor erythroid 2-related factor 2-regulated genes NQO1 and heme oxygenase-1 mRNA and the aryl hydrocarbon receptor–regulated gene CYP1A1, consistent with activation of cytoprotective mechanisms in response to oxidative stress and xenobiotics (Xiao et al., 2003). The urban particles EHC-93 contain polycyclic aromatic hydrocarbons and transition metals and have been shown to activate xenobiotic response element, CYP1A1, MT-2, glutathione transferase, and heat shock response reporter genes in vitro (Vincent et al., 1997b). MT-2 and IL-6 play a critical protective role in the lungs following ozone inhalation (Inoue et al., 2008; McKinney et al., 1998), and the pronounced response of these genes to ozone but not particulate matter is in line with the acute lung injury and oxidative stress produced by ozone compared with the lack of acute structural damage to the lungs following particle inhalation (Vincent et al., 1997a). Ozone increased CYP1A1 mRNA in the heart, an effect generally considered cardiotoxic although the actual function of CYP1A1 in either a harmful or protective capacity is not well understood (Korashy and El-Kadi, 2006). Impacts of ozone inhalation on the liver have been noted previously, including exacerbation of acetaminophen-induced toxicity that correlated with reduced expression of inflammation and xenobiotic metabolism pathway genes (Aibo et al., 2010), but the factor(s) responsible for these effects are not known. Given the recent report that pretreatment with glucocorticoid receptor inhibitors protects mice from acetaminophen-induced liver injury (Masson et al., 2010), it is possible that ozone-induced corticosterone is at least partly responsible for the acetaminophen-ozone interactions observed in previous studies. We observed a trend toward increased metallothionein expression in the liver 24h after exposure to particles, consistent with circulation of particles (Furuyama et al., 2009) or soluble metals (Wallenborn et al., 2007), but it is unclear whether this was a direct effect. The observation of ozone-induced increases in ET-1 mRNA levels in the heart, kidney, and spleen builds upon our previous findings of increased circulating levels of endothelin-1 following exposure to particulate matter and ozone (Bouthillier et al., 1998; Thomson et al., 2005). Although the pattern of endothelin expression did not strictly track the expression of known glucocorticoid-responsive genes, it is possible that these effects are due, at least in part, to increased corticosterone (Spieker et al., 2002). Future studies employing glucocorticoid pathway inhibitors should clarify the extent to which glucocorticoids are implicated in these effects.
Effects on the HPA axis and on release or metabolism of glucocorticoids have been reported for a variety of environmental exposures, including polybrominated diphenyl ethers, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, and heavy metals (Odermatt and Gumy, 2008), but there has been little study of the involvement of this system in mediating health effects of ambient air pollutants. Exposure for 8h to 500 µg/m3 concentrated fine particles increased corticosterone levels in ovalbumin-sensitized Brown Norway rats and saline controls and produced higher levels of the neurotransmitter norepinephrine in the paraventricular nucleus of the hypothalamus and the olfactory bulb (Sirivelu et al., 2006). Prolonged exposure to ozone (6h/day for 15 days) resulted in behavioral changes and increased corticosterone levels consistent with a stress response (Martrette et al., 2011). The mechanism by which pollutants activate the HPA axis is unknown. Prostaglandins play a key role in stimulating the HPA axis, and our expression data, showing increased expression of prostaglandin endoperoxide synthase 2 mRNA in the pituitary but not in other organs immediately after exposure, suggest that local production of prostaglandins in the pituitary (Bugajski et al., 2004; Reimsnider and Wood, 2004) may be involved. Transit of particles or their soluble elements from the lungs to other organs, including the brain, has been described (Oberdörster et al., 2004); ozone, on the other hand, is entirely consumed in the lungs, suggesting that actions are through intermediary signals rather than direct effects. Short-term exposure to ozone can activate stress-responsive regions of the brain consistent with a reflexive response to systemic stress (Gackière et al., 2011). Both ozone and particulate matter can initiate Toll-like receptor and oxidative stress–mediated innate responses characterized by activation of the transcription factors NF-κB and activator protein-1 in the lungs (Connor et al., 2012; Miyata and Van Eeden, 2011), resulting in production of cytokines that can activate the HPA axis (Beishuizen and Thijs, 2003). Rather than being broadly immunosuppressive, glucocorticoids are now known to selectively repress Th1 cytokines and stimulate Th2 cytokines (Elenkov, 2004). This may explain the increased expression of JMJD3, which was recently shown to be involved in acquisition of the primarily Th2-driven M2 alternatively activated macrophage phenotype (Ishii et al., 2009) across organs despite a decrease of all other inflammatory mediators measured.
It is unclear whether the HPA axis activation is specific to ozone and particulate matter or rather represents a more generic stress response to inhalation of foreign material. Rodents exposed to ozone or particulate matter exhibit a number of similar responses, including decreases in markers of cardiac and thermoregulatory function as part of a hypothermic response, and it is speculated that such effects may arise from stimulation of pulmonary irritant receptors, resulting in parasympathetic nervous system activation (Watkinson et al., 2001). As in humans, responsiveness to stress differs across rat strains, with Fischer rats characterized as high responders (O’Connor et al., 2000). The nose-only inhalation model used in this study involves restraint of animals in nose-only tubes during the 4-h exposure, and despite training to tolerate immobilization, restraint has been shown to activate stress pathways in mice (Thomson et al., 2009a). As control animals were exposed to air by nose-only exposure, any stress associated with the method of exposure should be equivalent for all animals, and observed effects of the pollutants on the HPA axis are presumably additive to the stress of nose-only exposure (Ghoshal et al., 1998). Stress can modify responses to exogenous agents, and Stress × Pollutant interactions are an active area of research (Chen et al., 2008; Clougherty et al., 2007; Cooney, 2011). Several systems potentially impacted by air pollution, including the gastrointestinal (Kaplan et al., 2009) and reproductive (Bell et al., 2007) systems, were not evaluated, and analysis of effects in specific regions within the brain and other organs was beyond the scope of this study. The extent of extrapulmonary effects of particulate matter and ozone may therefore be greater than demonstrated here.
Acute activation of the HPA axis is generally characterized as an adaptive mechanism aimed at regaining homeostasis. Results from this study, in which healthy rats were exposed to a single acute exposure to ozone and particulate matter, indicate that HPA axis activation and glucocorticoid release were transient. It remains to be determined whether acute exposure of susceptible individuals or long-term exposure to ambient air pollution in general can increase the probability of HPA axis dysregulation. Although feedback inhibition generally protects the body from negative impacts of prolonged HPA axis activation, repeated or chronic exposure to stressors or dysregulation of feedback mechanisms can result in a variety of adverse effects. These can present as cognitive, emotional, and behavioral symptoms such as memory impairment, vulnerability to anxiety and depression, irritability, alteration of social behavior, and altered appetite and physical symptoms such as immune deficits, asthma, increased blood pressure, increased risk of cardiovascular and cerebrovascular disease, neurological disorders, metabolic syndrome, and accelerated aging (Anagnostis et al., 2009; Pompili et al., 2010). Repeated administration of corticosterone produces behavioral and neurobiological changes in rats consistent with depression in humans (Sterner and Kalynchuk, 2010), as does disruption of GR signalling in the brain resulting in HPA axis hyperactivity (Boyle et al., 2005), establishing a causal link between glucocorticoid elevation and depression. There is increasing evidence that exposure to environmental chemicals can impact glucocorticoid-regulated processes and thereby contribute to disease processes involving dysregulated metabolic processes, altered immune function, cardiovascular disease, mood disorders, impaired cognitive function, and cancer (Odermatt and Gumy, 2008). Epidemiological studies indicate that physical and social environments are modifiers of pollutant impacts on health, with heightened responses to air pollutants found in individuals exposed to higher stress environments (Chen et al., 2008; Clougherty et al., 2007). It is noteworthy that in addition to the considerable literature establishing a link between air pollution and cardiovascular and pulmonary diseases, recent studies show associations between air pollution and adverse neurobehavioral outcomes, including reduced cognitive ability (Chen and Schwartz, 2009; Power et al., 2011; Ranft et al., 2009; Weuve et al., 2012), memory impairment (van Kempen et al., 2012), increased irritability and aggression (Yolton et al., 2008), depression (Szyszkowicz et al., 2009), and suicide (Kim et al., 2010; Szyszkowicz et al., 2010). Supporting these associations, mice exposed for 10 months to fine airborne particulate matter show signs of cognitive impairment and depression (Fonken et al., 2011), and both acute and chronic exposure of rats to ozone results in neurobehavioral alterations (Dorado-Martínez et al., 2001; Rivas-Arancibia et al., 1998, 2010). Given the known impacts of HPA axis dysfunction on a cross-section of disease processes, notably depression, cardiovascular disease, and metabolic syndrome, the finding that both gaseous and particulate pollutants modulate HPA axis activity warrants further investigation as a potential mechanism underlying health effects of air pollutants in susceptible individuals.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Health Canada (ERHSD 859126 and 810409, Toxic Substances Research Initiative, Genomic Research and Development Initiative, Clean Air Regulatory Agenda).
Supplementary Material
ACKNOWLEDGMENTS
Thanks to Josée Guénette and DJ MacIntyre for conducting the exposures, Pat Goegan and Erica Blais for conducting the necropsies, and Andrew Ha and Cheuk Kei Yeung for technical assistance with the assays. Thanks to Dr Guillaume Pelletier and Dr Ella Atlas for review of the manuscript.
REFERENCES
- Aibo D. I., Birmingham N. P., Lewandowski R., Maddox J. F., Roth R. A., Ganey P. E., Wagner J. G., Harkema J. R. (2010). Acute exposure to ozone exacerbates acetaminophen-induced liver injury in mice. Toxicol. Sci. 115, 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostis P., Athyros V. G., Tziomalos K., Karagiannis A., Mikhailidis D. P. (2009). Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: A hypothesis. J. Clin. Endocrinol. Metab. 94, 2692–2701. [DOI] [PubMed] [Google Scholar]
- Andersen C. L., Jensen J. L., Ørntoft T. F. (2004). Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64, 5245–5250. [DOI] [PubMed] [Google Scholar]
- Ayroldi E., Riccardi C. (2009). Glucocorticoid-induced leucine zipper (GILZ): A new important mediator of glucocorticoid action. FASEB J. 23, 3649–3658. [DOI] [PubMed] [Google Scholar]
- Beishuizen A., Thijs L. G. (2003). Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J. Endotoxin Res. 9, 3–24. [DOI] [PubMed] [Google Scholar]
- Bell M. L., Ebisu K., Belanger K. (2007). Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ. Health Perspect. 115, 1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermann T., Stilianakis N., Bleich S., Thürauf N., Kornhuber J., Reulbach U. (2009). The hypothesis of an impact of ozone on the occurrence of completed and attempted suicides. Med. Hypotheses. 72, 338–341. [DOI] [PubMed] [Google Scholar]
- Bouthillier L., Vincent R., Goegan P., Adamson I. Y., Bjarnason S., Stewart M., Guénette J., Potvin M., Kumarathasan P. (1998). Acute effects of inhaled urban particles and ozone: Lung morphology, macrophage activity, and plasma endothelin-1. Am. J. Pathol. 153, 1873–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. P., Brewer J. A., Funatsu M., Wozniak D. F., Tsien J. Z., Izumi Y., Muglia L. J. (2005). Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc. Natl. Acad. Sci. U. S. A. 102, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugajski J., Gadek-Michalska A., Bugajski A. J. (2004). Nitric oxide and prostaglandin systems in the stimulation of hypothalamic-pituitary-adrenal axis by neurotransmitters and neurohormones. J. Physiol. Pharmacol. 55, 679–703. [PubMed] [Google Scholar]
- Burnett R. T., Brook J. R., Yung W. T., Dales R. E., Krewski D. (1997). Association between ozone and hospitalization for respiratory diseases in 16 Canadian cities. Environ. Res. 72, 24–31. [DOI] [PubMed] [Google Scholar]
- Burnett R. T., Dales R., Krewski D., Vincent R., Dann T., Brook J. R. (1995). Associations between ambient particulate sulfate and admissions to Ontario hospitals for cardiac and respiratory diseases. Am. J. Epidemiol. 142, 15–22. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L., Reed W., Maronpot R. R., Henríquez-Roldán C., Delgado-Chavez R., Calderón-Garcidueñas A., Dragustinovis I., Franco-Lira M., Aragón-Flores M., Solt A. C., et al. (2004). Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol. Pathol. 32, 650–658. [DOI] [PubMed] [Google Scholar]
- Chen D., Pan X., Xiao P., Farwell M. A., Zhang B. (2011). Evaluation and identification of reliable reference genes for pharmacogenomics, toxicogenomics, and small RNA expression analysis. J. Cell. Physiol. 226, 2469–2477. [DOI] [PubMed] [Google Scholar]
- Chen E., Schreier H. M., Strunk R. C., Brauer M. (2008). Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ. Health Perspect. 116, 970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. C., Schwartz J. (2009). Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology. 30, 231–239. [DOI] [PubMed] [Google Scholar]
- Clougherty J. E., Levy J. I., Kubzansky L. D., Ryan P. B., Suglia S. F., Canner M. J., Wright R. J. (2007). Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ. Health Perspect. 115, 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor A. J., Laskin J. D., Laskin D. L. (2012). Ozone-induced lung injury and sterile inflammation. Role of toll-like receptor 4. Exp. Mol. Pathol. 92, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney C. M. (2011). Stress-pollution interactions: An emerging issue in children’s health research. Environ. Health Perspect. 119, A431–A435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet E. R. (2004). Hormones and the stressed brain. Ann. N. Y. Acad. Sci. 1018, 1–15. [DOI] [PubMed] [Google Scholar]
- Dorado-Martínez C., Paredes-Carbajal C., Mascher D., Borgonio-Pérez G., Rivas-Arancibia S. (2001). Effects of different ozone doses on memory, motor activity and lipid peroxidation levels, in rats. Int. J. Neurosci. 108, 149–161. [DOI] [PubMed] [Google Scholar]
- Elenkov I. J. (2004). Glucocorticoids and the Th1/Th2 balance. Ann. N. Y. Acad. Sci. 1024, 138–146. [DOI] [PubMed] [Google Scholar]
- Fonken L. K., Xu X., Weil Z. M., Chen G., Sun Q., Rajagopalan S., Nelson R. J. (2011). Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol. Psychiatry. 16, 987–95, 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama A., Kanno S., Kobayashi T., Hirano S. (2009). Extrapulmonary translocation of intratracheally instilled fine and ultrafine particles via direct and alveolar macrophage-associated routes. Arch. Toxicol. 83, 429–437. [DOI] [PubMed] [Google Scholar]
- Gackière F., Saliba L., Baude A., Bosler O., Strube C. (2011). Ozone inhalation activates stress-responsive regions of the CNS. J. Neurochem. 117, 961–972. [DOI] [PubMed] [Google Scholar]
- Ghoshal K., Wang Y., Sheridan J. F., Jacob S. T. (1998). Metallothionein induction in response to restraint stress. Transcriptional control, adaptation to stress, and role of glucocorticoid. J. Biol. Chem. 273, 27904–27910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder M., Qadri F., Jöhren O., Dendorfer A., Depping R., Fröhlich F., Wagner K. F., Dominiak P. (2007). Non-hypoxic induction of HIF-3alpha by 2-deoxy-D-glucose and insulin. Biochem. Biophys. Res. Commun. 352, 437–443. [DOI] [PubMed] [Google Scholar]
- Hong Y. C., Lee J. T., Kim H., Ha E. H., Schwartz J., Christiani D. C. (2002). Effects of air pollutants on acute stroke mortality. Environ. Health Perspect. 110, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Takano H., Kaewamatawong T., Shimada A., Suzuki J., Yanagisawa R., Tasaka S., Ishizaka A., Satoh M. (2008). Role of metallothionein in lung inflammation induced by ozone exposure in mice. Free Radic. Biol. Med. 45, 1714–1722. [DOI] [PubMed] [Google Scholar]
- Ishii M., Wen H., Corsa C. A., Liu T., Coelho A. L., Allen R. M., Carson W. F., 4th, Cavassani K. A., Li X., Lukacs N. W., et al. (2009). Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 114, 3244–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G. G., Dixon E., Panaccione R., Fong A., Chen L., Szyszkowicz M., Wheeler A., MacLean A., Buie W. D., Leung T., et al. (2009). Effect of ambient air pollution on the incidence of appendicitis. CMAJ. 181, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Jung S. H., Kang D. R., Kim H. C., Moon K. T., Hur N. W., Shin D. C., Suh I. (2010). Ambient particulate matter as a risk factor for suicide. Am. J. Psychiatry. 167, 1100–1107. [DOI] [PubMed] [Google Scholar]
- Korashy H. M., El-Kadi A. O. (2006). The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab. Rev. 38, 411–450. [DOI] [PubMed] [Google Scholar]
- Kumarathasan P., Goegan P., Vincent R. (2001). An automated high-performance liquid chromatography fluorescence method for the analyses of endothelins in plasma samples. Anal. Biochem. 299, 37–44. [DOI] [PubMed] [Google Scholar]
- Kumarathasan P., Mohottalage S., Goegan P., Vincent R. (2005). An optimized protein in-gel digest method for reliable proteome characterization by MALDI-TOF-MS analysis. Anal. Biochem. 346, 85–89. [DOI] [PubMed] [Google Scholar]
- Lang F., Böhmer C., Palmada M., Seebohm G., Strutz-Seebohm N., Vallon V. (2006). (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 86, 1151–1178. [DOI] [PubMed] [Google Scholar]
- Lim Y. H., Kim H., Kim J. H., Bae S., Park H. Y., Hong Y. C. (2012). Air pollution and symptoms of depression in elderly adults. Environ. Health Perspect. 120, 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Martrette J. M., Thornton S. N., Trabalon M. (2011). Prolonged ozone exposure effects behaviour, hormones and respiratory muscles in young female rats. Physiol. Behav. 103, 302–307. [DOI] [PubMed] [Google Scholar]
- Masson M. J., Collins L. A., Carpenter L. D., Graf M. L., Ryan P. M., Bourdi M., Pohl L. R. (2010). Pathologic role of stressed-induced glucocorticoids in drug-induced liver injury in mice. Biochem. Biophys. Res. Commun. 397, 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney W. J., Jaskot R. H., Richards J. H., Costa D. L., Dreher K. L. (1998). Cytokine mediation of ozone-induced pulmonary adaptation. Am. J. Respir. Cell Mol. Biol. 18, 696–705. [DOI] [PubMed] [Google Scholar]
- Miyata R., van Eeden S. F. (2011). The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharmacol. 257, 209–226. [DOI] [PubMed] [Google Scholar]
- Mohottalage S., Karthikeyan S., Vincent R., Kumarathasan P. (2007). Impact of sample preparation process on protein/peptide marker identification: Shot-Gun screening by MALDI-TOF-MS. Can. J. Anal. Sci. Spectrosc. 52, 243–251. [Google Scholar]
- O’Connor T. M., O’Halloran D. J., Shanahan F. (2000). The stress response and the hypothalamic-pituitary-adrenal axis: From molecule to melancholia. QJM. 93, 323–333. [DOI] [PubMed] [Google Scholar]
- Oberdörster G., Sharp Z., Atudorei V., Elder A., Gelein R., Kreyling W., Cox C. (2004). Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 16, 437–445. [DOI] [PubMed] [Google Scholar]
- Odermatt A., Gumy C. (2008). Glucocorticoid and mineralocorticoid action: Why should we consider influences by environmental chemicals? Biochem. Pharmacol. 76, 1184–1193. [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W., Tichopad A., Prgomet C., Neuvians T. P. (2004). Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 26, 509–515. [DOI] [PubMed] [Google Scholar]
- Pompili M., Serafini G., Innamorati M., Möller-Leimkühler A. M., Giupponi G., Girardi P., Tatarelli R., Lester D. (2010). The hypothalamic-pituitary-adrenal axis and serotonin abnormalities: A selective overview for the implications of suicide prevention. Eur. Arch. Psychiatry Clin. Neurosci. 260, 583–600. [DOI] [PubMed] [Google Scholar]
- Pope C. A., 3rd, Burnett R. T., Thurston G. D., Thun M. J., Calle E. E., Krewski D., Godleski J. J. (2004). Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation. 109, 71–77. [DOI] [PubMed] [Google Scholar]
- Power M. C., Weisskopf M. G., Alexeeff S. E., Coull B. A., Spiro A., 3rd, Schwartz J. (2011). Traffic-related air pollution and cognitive function in a cohort of older men. Environ. Health Perspect. 119, 682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft U., Schikowski T., Sugiri D., Krutmann J., Krämer U. (2009). Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ. Res. 109, 1004–1011. [DOI] [PubMed] [Google Scholar]
- Reimsnider S. K., Wood C. E. (2004). Co-localisation of prostaglandin endoperoxide synthase and immunoreactive adrenocorticotrophic hormone in ovine foetal pituitary. J. Endocrinol. 180, 303–310. [DOI] [PubMed] [Google Scholar]
- Rivas-Arancibia S., Guevara-Guzmán R., López-Vidal Y., Rodríguez-Martínez E., Zanardo-Gomes M., Angoa-Pérez M., Raisman-Vozari R. (2010). Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol. Sci. 113, 187–197. [DOI] [PubMed] [Google Scholar]
- Rivas-Arancibia S., Vazquez-Sandoval R., Gonzalez-Kladiano D., Schneider-Rivas S., Lechuga-Guerrero A. (1998). Effects of ozone exposure in rats on memory and levels of brain and pulmonary superoxide dismutase. Environ. Res. 76, 33–39. [DOI] [PubMed] [Google Scholar]
- Sen B., Mahadevan B., DeMarini D. M. (2007). Transcriptional responses to complex mixtures: A review. Mutat. Res. 636, 144–177. [DOI] [PubMed] [Google Scholar]
- Silver N., Best S., Jiang J., Thein S. L. (2006). Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 7, 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirivelu M. P., MohanKumar S. M., Wagner J. G., Harkema J. R., MohanKumar P. S. (2006). Activation of the stress axis and neurochemical alterations in specific brain areas by concentrated ambient particle exposure with concomitant allergic airway disease. Environ. Health Perspect. 114, 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieker L. E., Hürlimann D., Ruschitzka F., Corti R., Enseleit F., Shaw S., Hayoz D., Deanfield J. E., Lüscher T. F., Noll G. (2002). Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 105, 2817–2820. [DOI] [PubMed] [Google Scholar]
- Sterner E. Y., Kalynchuk L. E. (2010). Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: Relevance to depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 34, 777–790. [DOI] [PubMed] [Google Scholar]
- Szyszkowicz M., Rowe B. H., Colman I. (2009). Air pollution and daily emergency department visits for depression. Int. J. Occup. Med. Environ. Health. 22, 355–362. [DOI] [PubMed] [Google Scholar]
- Szyszkowicz M., Willey J. B., Grafstein E., Rowe B. H., Colman I. (2010). Air pollution and emergency department visits for suicide attempts in vancouver, Canada. Environ. Health Insights. 4, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson E., Kumarathasan P., Goegan P., Aubin R. A., Vincent R. (2005). Differential regulation of the lung endothelin system by urban particulate matter and ozone. Toxicol. Sci. 88, 103–113. [DOI] [PubMed] [Google Scholar]
- Thomson E., Kumarathasan P., Vincent R. (2006). Pulmonary expression of preproET-1 and preproET-3 mRNAs is altered reciprocally in rats after inhalation of air pollutants. Exp. Biol. Med. (Maywood). 231, 979–984. [PubMed] [Google Scholar]
- Thomson E., Vincent R. (2005). Reagent volume and plate bias in real-time polymerase chain reaction. Anal. Biochem. 337, 347–350. [DOI] [PubMed] [Google Scholar]
- Thomson E. M., Kumarathasan P., Calderón-Garcidueñas L., Vincent R. (2007). Air pollution alters brain and pituitary endothelin-1 and inducible nitric oxide synthase gene expression. Environ. Res. 105, 224–233. [DOI] [PubMed] [Google Scholar]
- Thomson E. M., Williams A., Yauk C. L., Vincent R. (2009a). Impact of nose-only exposure system on pulmonary gene expression. Inhal. Toxicol. 21(Suppl. 1)74–82. [DOI] [PubMed] [Google Scholar]
- Thomson E. M., Williams A., Yauk C. L., Vincent R. (2009b). Toxicogenomic analysis of susceptibility to inhaled urban particulate matter in mice with chronic lung inflammation. Part. Fibre Toxicol. 6, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kempen E., Fischer P., Janssen N., Houthuijs D., van Kamp I., Stansfeld S., Cassee F. (2012). Neurobehavioral effects of exposure to traffic-related air pollution and transportation noise in primary schoolchildren. Environ. Res. 115, 18–25. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve P. J., Chen L., Stieb D., Rowe B. H. (2006). Associations between outdoor air pollution and emergency department visits for stroke in Edmonton, Canada. Eur. J. Epidemiol. 21, 689–700. [DOI] [PubMed] [Google Scholar]
- Vincent R., Bjarnason S. G., Adamson I. Y., Hedgecock C., Kumarathasan P., Guénette J., Potvin M., Goegan P., Bouthillier L. (1997a). Acute pulmonary toxicity of urban particulate matter and ozone. Am. J. Pathol. 151, 1563–1570. [PMC free article] [PubMed] [Google Scholar]
- Vincent R., Goegan P., Johnson G., Brook J. R., Kumarathasan P., Bouthillier L., Burnett R. T. (1997b). Regulation of promoter-CAT stress genes in HepG2 cells by suspensions of particles from ambient air. Fundam. Appl. Toxicol. 39, 18–32. [DOI] [PubMed] [Google Scholar]
- Vincent R., Kumarathasan P., Goegan P., Bjarnason S. G., Guenette J., Berube D., Adamson I. Y., Desjardins S., Burnett R. T., Miller F. J., et al. (2001). Inhalation toxicology of urban ambient particulate matter: Acute cardiovascular effects in rats. Res. Rep. Health Eff. Inst. 104, 5–54. [PubMed] [Google Scholar]
- Wallenborn J. G., McGee J. K., Schladweiler M. C., Ledbetter A. D., Kodavanti U. P. (2007). Systemic translocation of particulate matter-associated metals following a single intratracheal instillation in rats. Toxicol. Sci. 98, 231–239. [DOI] [PubMed] [Google Scholar]
- Wang M. (2005). The role of glucocorticoid action in the pathophysiology of the Metabolic Syndrome. Nutr. Metab. (Lond). 2, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson W. P., Campen M. J., Nolan J. P., Costa D. L. (2001). Cardiovascular and systemic responses to inhaled pollutants in rodents: Effects of ozone and particulate matter. Environ. Health Perspect. 109(Suppl. 4)539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J., Puett R. C., Schwartz J., Yanosky J. D., Laden F., Grodstein F. (2012). Exposure to particulate air pollution and cognitive decline in older women. Arch. Intern. Med. 172, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G. G., Wang M., Li N., Loo J. A., Nel A. E. (2003). Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J. Biol. Chem. 278, 50781–50790. [DOI] [PubMed] [Google Scholar]
- Yolton K., Khoury J., Hornung R., Dietrich K., Succop P., Lanphear B. (2008). Environmental tobacco smoke exposure and child behaviors. J. Dev. Behav. Pediatr. 29, 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. (2003). Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.