Abstract
Activation of the aryl hydrocarbon receptor (AhR) by its prototypic ligand, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), induces potent suppression of an acute graft-versus-host (GVH) response and prevents GVH disease (GVHD). Suppression is associated with development of a regulatory population of donor CD4+ CD25+T-cells that express high levels of cytotoxic T-lymphocyte antigen 4 (CTLA-4). However, a direct link between these AhR-induced Tregs (AhR-Tregs) and suppression of GVHD remains to be shown. CTLA-4 is a negative regulator of T-cell responses and is associated with the induction of tolerogenic dendritic cells (DCs) that produce indoleamine 2,3-dioxygenase (IDO). We hypothesized that AhR-Tregs mediate suppression via their enhanced expression of CTLA-4, which, in turn, induces IFN-γ and IDO in host DCs. Subsequent depletion of tryptophan by IDO leads to termination of the donor T-cell response prior to development of effector CTL. Here, we show that despite increased expression of Ifng, Irf3, Irf7, Ido1, and Ido2 in the lymph nodes of TCDD-treated host mice, inhibition of IDO enzyme activity by 1-methyl-tryptophan was unable to relieve TCDD-mediated suppression of the GVH response. Furthermore, treatment with an anti-CTLA-4 antibody that blocks CTLA-4 signaling was also unable to alleviate TCDD-mediated suppression. Alternatively, we investigated the possibility that donor-derived AhR-Tregs produce IFN-γ to suppress effector CTL development. However, suppression of GVHD by TCDD was not affected by the use of Ifng-deficient donor cells. Together, these results indicate that neither overexpression of CTLA-4 nor production of IFN-γ by AhR-Tregs plays a major role in the manifestation of their immunosuppressive function in vivo.
Key Words: TCDD, IDO, CTLA-4, GVHD, T-regulatory cell.
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that is known to mediate potent immunosuppression upon activation by the prototypic ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Marshall and Kerkvliet, 2010). The ability of TCDD to suppress the development of several autoimmune diseases in association with an increase in the frequency of Foxp3+ Tregs has sparked an interest in the AhR as a therapeutic target (Benson and Shepherd, 2011; Kerkvliet et al., 2009; Quintana et al., 2008; Zhang et al., 2010). Direct activation of AhR in CD4+ T cells also induces Foxp3-negative Tregs (AhR-Tregs). These AhR-Tregs were first described in an acute parent-into-F1 graft-versus-host (GVH) model and were characterized by their high levels of CD25 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) and potent in vitro suppressive activity (Funatake et al., 2005). Additional studies have shown that AhR-Tregs express higher levels of several genes that have been associated with Treg function such as IL-10, granzyme B, and CD39 (Marshall et al., 2008). These AhR-Tregs are postulated to mediate suppression of the allo-CTL response and protection from GVH disease (GVHD) in TCDD-treated animals. However, the mechanisms of suppression used by AhR-Tregs remain poorly characterized.
CTLA-4 is an inhibitory receptor that shares 30% homology with CD28, a costimulatory receptor on T cells. Reaching maximal expression 48h after T-cell activation (Walunas et al., 1994), CTLA-4 competes with CD28 for the shared ligands CD80 and CD86, expressed on dendritic cells (DCs). As CTLA-4 has a higher affinity for these ligands relative to CD28, CTLA-4 may prevent CD28 signaling (Alegre et al., 2001). Therefore, although engagement of CD28 promotes T-cell proliferation and differentiation, engagement of CTLA-4 attenuates T-cell responses. Animals deficient in CTLA-4 succumb to a severe T-dependent lymphoproliferative disease, resulting in death at 3–4 weeks of age (Alegre et al., 2001) underscoring the necessity for CTLA-4 in T-cell regulation.
In addition to T-cell regulation, CTLA-4 can also influence DC function by interaction with CD80/86. This interaction can induce a tolerogenic phenotype through the induction of IFN-γ and indoleamine 2,3-dioxygenase (IDO) (Munn et al., 2004). IDO catalyzes the rate-limiting step of tryptophan degradation to kynurenine, resulting in depleted tryptophan levels and suppression of T-cell responses (Mellor and Munn, 2004). Interestingly, TCDD has been shown to alter IDO expression and function. For example, increased expression of the genes Ido1 and Ido2 has been observed in several DC subsets cultured with TCDD in vitro, including DCs derived from the human U937 monocytic cell line, murine bone marrow–derived DCs, and plasmacytoid DCs (pDCs) (Bankoti et al., 2010; Benson and Shepherd, 2011; Mezrich et al., 2010; Vogel et al., 2008). This process appeared to be dependent on AhR, as use of BMDC from AhR−/− mice, or use of an AhR antagonist prevented the TCDD-dependent increase in IDO expression (Bankoti et al., 2010; Vogel et al., 2008). IDO enzyme activity was also increased by TCDD in U937-derived DCs (Vogel et al., 2008). In other studies, IDO was implicated in the induction of Foxp3+ Tregs by kynurenine, another known AhR ligand (Mezrich et al., 2010; Nguyen et al., 2010). In one study, AhR in the DCs was required for Treg induction (Nguyen et al., 2010), whereas in the other, AhR expression in the CD4+ T cell was necessary (Mezrich et al., 2010).
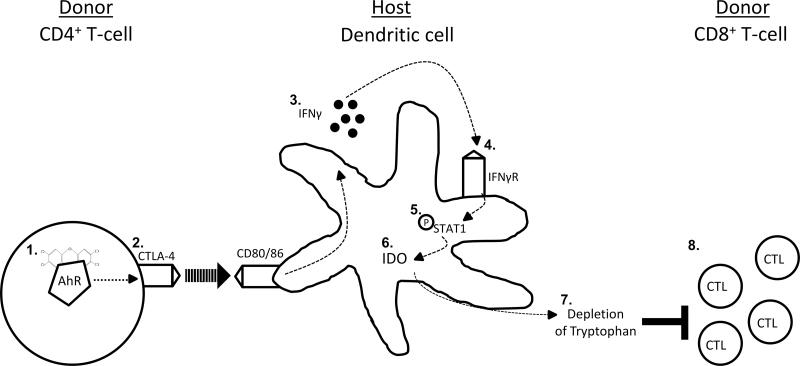
The studies reported here have addressed the hypothesis that the TCDD-mediated increase in CTLA-4 expression on donor CD4+ T cells induces IFN-γ and ultimately IDO in host DCs, thereby suppressing the allospecific CTL response (see Fig. 1). To address this hypothesis, we examined the effect of TCDD on the CTL response while independently blocking CTLA-4 and inhibiting IDO enzyme activity.
Fig. 1.
Depiction of TCDD-induced CTLA-4 on AhR-Treg activating the tolerogenic IDO pathway and suppressing the acute GVH response. AhR is activated by TCDD in donor CD4+ T cells (1). CTLA-4 is upregulated in an AhR-dependent manner in donor CD4+ T cells and ligates CD80/86 on host DCs (2). The activated DCs produce IFN-γ (3). IFN-γ feeds back in an autocrine manner through the IFN-γ receptor (IFN-γR) (4). IFN-γ signaling through the IFN-γR phosphorylates STAT1 (5). Phosphorylated STAT1 directly induces IDO (6), which catalyzes the rate-limiting step of l-tryptophan metabolism to l-kynurenine, depleting local tryptophan levels (7). Lack of tryptophan suppresses the development of effector CTL (8).
MATERIALS AND METHODS
Animals.
B6 (H-2b/b), B6.129s7-Ifng tm1Ts/J (Ifng −/−, H-2b/b), and B6D2F1 (H-2b/d) mice were purchased from the Jackson Laboratory. B6.PL-Thy1a/CyJ (Thy1.1+, H-2b/b) mice (originally purchased from the Jackson Laboratory) were maintained as a breeding colony on-site. Animals were housed at the specific pathogen-free Laboratory Animals Resource Center. All experimental procedures and treatments were approved by the Institutional Animal Care and Use Committee at Oregon State University.
TCDD preparation.
TCDD (Cambridge Isotope Laboratories; 99% purity) was dissolved in anisole (J.T. Baker) and further diluted in peanut oil. Host B6D2F1 mice were given 15 µg/kg of TCDD or vehicle control following adoptive transfer of donor cells on day 0.
Preparation of donor cells.
Spleens and lymph nodes from donor C57Bl/6 (H-2b/b) mice were aseptically removed and processed into single-cell suspensions by pressing the organs through a 70-µm nylon mesh cell strainer (BD Falcon). Red blood cells were removed via hypotonic water lysis. For experiments involving injection of whole splenocytes, CD4+ and CD8+ T-cell purity was determined by flow cytometry. Splenocyte suspensions were resuspended in injection buffer (Hank's balanced salt solution [HBSS], 1.5mM HEPES, and 50 µg/ml gentamicin). A maximum of 4×107 donor T cells were injected by tail vein injection into B6D2F1 (H-2b/d) hosts.
Where noted, CD4+ and CD8+ T cells were isolated from donor splenocytes using a negative selection PanT kit with autoMACS magnetic isolation (Miltenyi autoMACS). Purity was routinely above 90%. In some experiments, the donor T cells were labeled with 2µM 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (Molecular Probes) prior to adoptive transfer.
Anti-CTLA-4 treatment.
LEAF-purified anti-mouse CD152 (CTLA-4) (9H10) and LEAF-purified Syrian hamster immunoglobulin G (IgG) isotype control (SHG-1) were obtained from BioLegend. Following adoptive transfer, host mice received 100 µg of either anti-CTLA-4 antibody or isotype control via ip injection. Injections were repeated on days 1 and 2 after adoptive transfer. A similar treatment regimen resulted in increased proliferation of antigen-specific T cells following immunization with A20HA tumor cells (Sotomayor et al., 1999).
1-Methyl-tryptophan treatment.
1-Methyl-tryptophan (1-MT) was prepared as previously described (Saxena et al., 2007). Briefly, 1-MT was dissolved in 0.05N NaOH to a concentration of 20mM. The solution was adjusted to pH 7 with concentrated HCl. To achieve a final concentration of 2mg/ml, the 1-MT solution was diluted in autoclaved, distilled water, sweetened with one packet aspartame per 2 l of water. Beginning on day 3, all animals received sweetened water ad libitum. 1-MT supplemented water was administered in light-protected water bottles and given ad libitum to mice a day prior to adoptive transfer. Control animals received sweetened water. Water bottles were checked daily to ensure mice were consuming approximately 4ml (8mg) of 1-MT daily.
Quantification of IFN-γ production.
Splenocytes were cultured in RPMI 1640 medium containing 10% fetal bovine serum, 50 µg/ml gentamicin, 10mM HEPES, and 50µM β-mercaptoethanol. The cultures were incubated overnight (~18h) at 37°C and 5% CO2 and supernatants collected for ELISA analysis. IFN-γ was detected using the IFN-γ Ready-Set-Go ELISA kit from eBioscience.
Flow cytometry.
Host splenocytes were processed as described for donor cell preparation. Following red blood cell lysis, splenocytes were aliquoted into 96-well V-bottom plates (Corning) and washed twice with PAB (PBS, 1% bovine serum albumin, and 0.1% sodium azide). Samples were resuspended in rat IgG (Jackson ImmunoResearch) and incubated on ice prior to surface staining. For detection of donor cells, cells were stained with monoclonal antibodies (mAbs) to Thy1.1 (OX-7 or HIS51; BD PharMingen or eBioscience) or H-2Dd (KH95; BioLegend) in conjunction with either CD4 (GK1.5) or CD8 (53-6.7) (BD PharMingen). mAbs to CD25 (PC61.5), CD19 (eBio1D3), and CTLA-4 (UC10-4B9) were purchased from eBioscience. For detection of CTLA-4, samples were resuspended in fixable viability dye efluor 780 (eBioscience) for 20min following surface staining for CD4, CD8, Thy1.1, H-2Dd, CD25, and CD19. Intracellular staining was performed following fixation and permeabilization protocols from BD Biosciences.
Fluorescence-minus-one samples, in which all antibodies except the one of interest are included, were used as staining controls. A minimum of 10,000 donor CD4+ events or 1×106 total cells were collected per sample on a Beckman Coulter FC-500 flow cytometer. Data were compensated and analyzed using WinList (Verity Software, Version 6.0).
RNA extraction and qPCR.
RNA was isolated from pooled axial, brachial, and cervical lymph nodes of host mice using the RNeasy Mini Kit #74104 (Qiagen), with the on-column DNase digestion repeated twice to ensure removal of genomic DNA. RNA integrity was assessed by using a Bioanalyzer 2100 (Agilent). Reverse transcription was performed using the Superscript III first-strand synthesis supermix (Invitrogen), following the manufacturer’s instructions. For all qPCR reactions, SYBR Green/Rox qPCR Master Mix (SA Bioscience) was mixed with 10ng cDNA per reaction, plus the appropriate primers. An ABI PRISM 7500 Real-Time PCR system (Applied Biosystems) was used for all qPCR reactions. Primers for Ido1 (NM_008324.1), Ido2 (NM_145949.2), and Ifng (NM_008337.1) were obtained from SA Biosciences and used according to the manufacturer’s instructions. All other primer sequences were obtained from PrimerBank (http://pga.mgh.harvard.edu/primerbank). Forward and reverse primers were purchased from Invitrogen and validated. The PrimerBank ID codes were as follows: E2-2: 1001888a1; Irf3: 8393627a1; Irf7: 8567364a1; Lag3: 6678654a1; Siglech: 30520121a1; Tlr7: 18875360a1; Tlr9: 13626030a1. Data were analyzed using 7500 Software (v2.0.1) (Applied Biosystems).
Quantification of IDO enzyme activity.
Splenocytes (1×107) were cultured in 0.5ml 1× HBSS without phenol red (Sigma), containing 100µM l-tryptophan (Alfa Aesar, CAS #73-22-3), and incubated at 37°C and 5% CO2 as previously described (Hwu et al., 2000). After 4h, the supernatant was removed and stored at −80°C for analysis of l-kynurenine. A standard curve of l-kynurenine (Sigma Aldrich, CAS #2922-83-0) (0.1nM–10µM) was prepared and run in tandem with the experimental samples. An Agilent SB-C18 RRHD 1.8 µm (2.1mm × 150mm) column was used for UPLC analysis on a Shimdazu Nexera Liquid Chromatograph, using the following eluents: H2O + 0.1% formic acid (solvent A) and acetonitrile (solvent B). The gradient elution was 5% B for 2min, followed by 90% B for 4min, and 5% B for 2min for a total duration of 8min. The eluents were directed to an ABSciex Triple TOF 5600 for MS analysis of l-kynurenine.
Statistical analysis.
All results are presented as the mean ± SEM of biological replicates (n = 3–5). For comparisons between two treatment groups, Students t-tests were used, with statistical significance as p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***). Where indicated, the Mixed procedure with the Satterthwaite option was performed in SAS (version 9.3).
RESULTS
Activation of AhR by TCDD Increases Expression of CTLA-4, IFN-γ, and IDO
Prior studies have shown that activation of AhR by TCDD during an acute GVH response induces a Treg phenotype (CD25+CTLA-4+) in alloresponding donor CD4+ T cells (Funatake et al., 2005). Based on additional studies that have linked TCDD with increased expression of IDO under various conditions, we hypothesized that increased expression of CTLA-4 on these AhR-Tregs drives IDO production in the DCs via induction of IFN-γ. Secretion of excessive IDO then leads to depletion of tryptophan and suppression of the differentiation of effector CD8+ CTL (Fig. 1). To test this hypothesis, we first determined if the increased expression of CTLA-4, known to occur on day 2 of the GVH response, was also observed on day 3. At the same time, we evaluated the expression levels of genes for Ifng, Ido1, and Ido2. Ido2 is a paralog of Ido1 (Ball et al., 2007) that has previously been shown to be upregulated by TCDD (Vogel et al., 2008). Because IDO production has been associated with pDCs, we also analyzed the expression of other genes associated with these cells, including E2-2, Tlr7, Siglech, Tlr9, Lag3, Irf3, and Irf7 to determine if additional functions of the pDCs may be affected by TCDD in vivo (Matta et al., 2010).
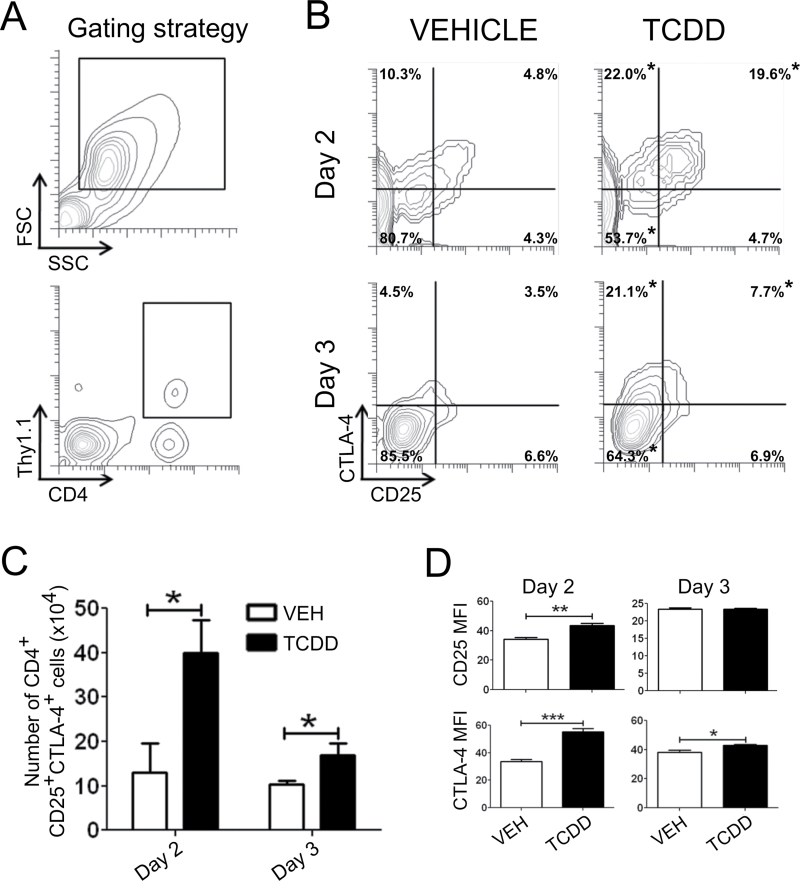
The gating strategy for identifying donor CD4+ T cells in host spleen by flow cytometry is shown in Figure 2A. The percentage and number of donor CD4+CD25+CTLA-4+ cells were significantly increased in TCDD-treated animals relative to vehicle on days 2 and 3 of the GVH response (Figs. 2B and 2C). The expression of CD25 was also significantly increased on a per cell basis (MFI) on day 2; however, this difference was lost on day 3 as the expression level of CD25 declined in both treatment groups (Fig. 2D). In contrast, the MFI of CTLA-4 was significantly increased by TCDD on both days (Fig. 2C).
Fig. 2.
Influence of TCDD on CD25 and CTLA-4 expression in donor CD4+ T cells on day 2 or 3 of the acute GVH response. GVHD was initiated by adoptive transfer of purified donor CD4+ and CD8+ T cells. (A) Using the forward scatter (FSC) versus side scatter (SSC) profile, lymphocytes were identified. Donor CD4+ T cells were identified by CFSE dilution (day 2) or the congenic marker Thy1.1 (day 3). (B) Flow cytometric analysis of CD25 and CTLA-4 expression on donor CD4+ T cells in vehicle- or TCDD-treated animals 2 or 3 days after adoptive transfer. (C) Total number of donor CD4+ T cells expressing CD25 and CTLA-4. (D) Median fluorescence intensity (MFI) of CD25 and CTLA-4 in donor CD4+ T cells. Statistical differences, calculated by Student’s t-test, are denoted by asterisks. Error bars represent the mean ± SEM, n = 4–5 biological replicates.
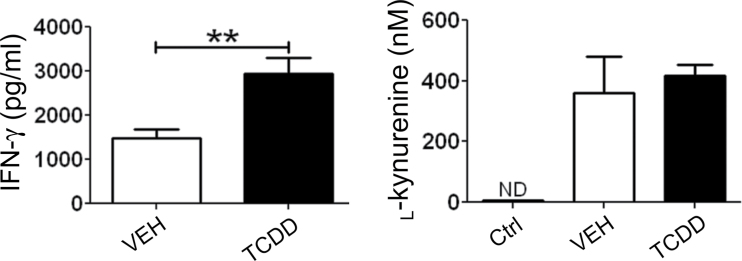
Expression levels of Ifng, Irf3, Irf7, and Ido2 were significantly upregulated in the lymph nodes of TCDD-treated host mice on day 2 (Table 1). On day 3, both Ido1 and Ido2 expression levels were increased over 10-fold in TCDD-treated mice, along with increased expression of Ifng and Irf7. IFN-γ protein levels were also significantly increased by TCDD as measured in supernatants of host splenocytes on day 3 (Fig. 3). On the other hand, the level of secreted l-kynurenine in day 3 supernatants was not significantly different between vehicle- and TCDD-treated cultures, suggesting that the overall enzymatic activity of IDO was not affected by TCDD.
Table 1.
Influence of TCDD on Expression of Genes Associated With the CTLA-4-IFN-γ-IDO Signaling Pathway
| Gene | Fold change (TCDD/VEH) | |
|---|---|---|
| Day 2 | Day 3 | |
| Cyp1a1 | 208* | 300* |
| Ido1 | 1.6 | 12.6* |
| Ido2 | 3.0* | 10.7* |
| Ifng | 3.0† | 1.9 |
| Irf3 | 1.9* | 0.8 |
| Irf7 | 2.6† | 2.7* |
Note. Expression of Cyp1a1 was queried as an indicator of AhR activation. Genes associated with pDCs were also evaluated, including E2-2, Siglech, Lag3, Irf3, Irf7, Tlr7, and Tlr9. RNA was isolated from the cervical, brachial, and axial lymph nodes from host mice on days 2 or 3 of the GVH response and used to query gene expression. Fold change (2−ΔΔCt) was calculated relative to vehicle (VEH). n = 4–5 biological replicates. Student’s t-test was used to calculate significance.
† p = 0.1, *p < 0.05.
Fig. 3.
Effect of TCDD on IFN-γ production and IDO enzyme activity. On day 3 of the GVH response, host splenocytes were isolated and cultured overnight for analysis of IFN-γ levels in the supernatant using an IFN-γ Ready-Set-Go ELISA (eBioscience). Host splenocytes were also prepared for quantification of IDO enzyme activity on day 3. Splenocytes were resuspended in 1× HBSS without phenol red and cultured with excess tryptophan (100µM) for 4h. The concentration of l-kynurenine was quantified using liquid chromatography-mass spectrometry as a measure of IDO enzyme function. CTRL = background level of l-kynurenine in 1× HBSS alone. Error bars represent the mean ± SEM, n = 4–5 biological replicates. Student’s t-test was used to determine significant differences between treatments. **p < 0.01. ND = not detected.
Role of IDO in TCDD-Mediated Suppression
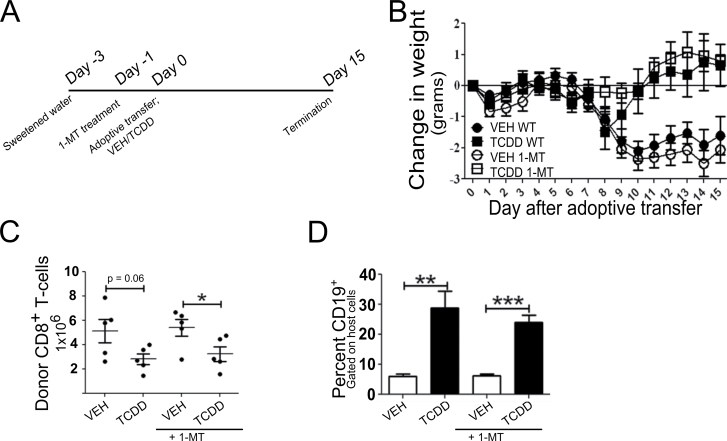
Although no difference in IDO activity was observed in spleen cell culture supernatants from vehicle- and TCDD-treated mice, the bulk measurement may not reveal differences in IDO activity at the interface of DC-T cell interaction. To directly evaluate the functional role of IDO in TCDD-mediated suppression of the GVH response, host mice were given 1-MT, a pharmacological inhibitor of IDO’s enzymatic activity, in the drinking water beginning 2 days before injection of donor cells and continuing for 15 days thereafter (Fig. 4A). Water consumption per cage was monitored daily to estimate intake of 1-MT. The average dose of 1-MT in vehicle-treated mice was 277mg/kg/day compared with 324mg/kg/day in TCDD-treated mice, both within the range of 1-MT doses shown to be effective at suppressing T-cell responses (Kwidzinski et al., 2005; Sakurai et al., 2002; Uyttenhove et al., 2003).
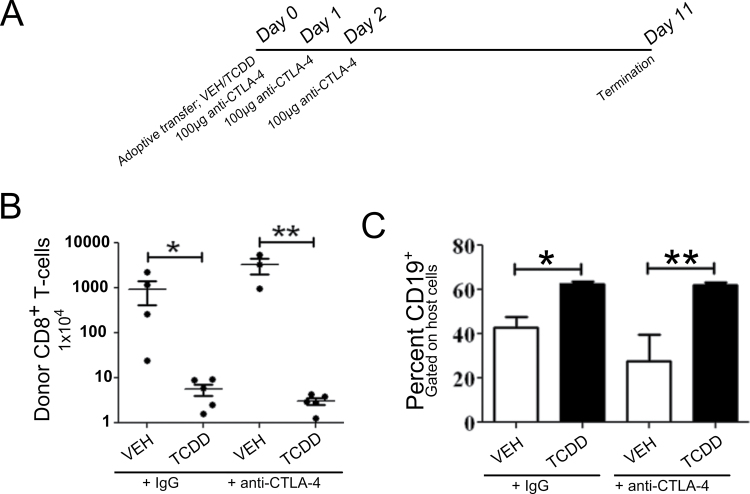
Fig. 4.
Effect of inhibition of IDO enzyme activity on suppression of the GVH response by TCDD. (A) Schematic of experimental design. Three days prior to adoptive transfer of donor splenocytes, mice were given autoclaved, distilled water sweetened with aspartame. On day 1, treatment with 1-MT, a pharmacological inhibitor of IDO, was begun. (B) Change in host body weight following adoptive transfer. (C) Using flow cytometry, the percent of H-2Dd negative donor cells was determined and used to calculate the total number of donor cells from the total number of splenoctyes. The percent of H-2DdnegCD8+ T cells was multiplied by the number of donor cells to calculate the total number of engrafted donor CD8+ T cells in the spleen of host animals. (D) CTL function was assessed as a measure of host Bcell depletion. Host B cells were identified by coexpression of H-2Dd and CD19. Error bars represent the mean ± SEM, n = 5 biological replicates. Treatment-related differences were determined using Student’s t-test. *p < 0.05, **<0.01, ***p < 0.001.
The effect of 1-MT on the GVH response was monitored by body weight loss and donor CD8+ T-cell engraftment in the spleen of host mice 15 days after adoptive transfer. Donor CD8+ T cells were identified by the absence of H-2Dd expression, and CTL activity was estimated based on the percentage of CD19+ host B cells in the spleen. As summarized in Figure 4, vehicle-treated mice lost a significant amount of body weight beginning on day 9 of the GVH response, and treatment with 1-MT did not influence this loss. In agreement with previous studies (Kerkvliet et al., 2002), TCDD treatment prevented the loss of body weight associated with the GVH response, and this was also unaffected by 1-MT. Donor CD8+ T-cell engraftment was reduced in TCDD-treated mice and the increased percentage of host B cells in the spleen of TCDD-treated mice validated suppression of the allo-CTL response. Treatment with 1-MT did not affect donor cell engraftment or CTL activity in either vehicle- or TCDD-treated mice. The absence of effects of 1-MT on these endpoints suggests that increased IDO activity is not a primary mechanism for suppression of the GVH response by TCDD. However, this conclusion must be tempered by the lack of an effect of 1-MT on the GVH response in vehicle-treated mice as well.
Functional Requirement for CTLA-4 in TCDD-Mediated Immunosuppression
Although the lack of effect of 1-MT on TCDD-induced suppression did not support the CTLA-4-IFN-γ-IDO hypothesis, CTLA-4 can also mediate suppression of T-cell responses by pathways independent of IDO. To determine if increased CTLA-4 expression was directly involved in the suppression of the GVH response by TCDD, we tested whether blockade of CTLA-4 could prevent TCDD-induced suppression of GVHD. Host mice were treated with anti-CTLA-4 antibody on days 0, 1, and 2 relative to donor T-cell transfer (Fig. 5A), and GVHD pathology was assessed on day 11 by analyzing donor CD8+ T-cell engraftment and host CD19+ B-cell depletion. Relative to vehicle-treated animals, treatment with TCDD suppressed donor T-cell engraftment and preserved the host B-cell population as expected. Anti-CTLA-4 treatment had no effect on GVHD pathology in either vehicle- or TCDD-treated animals (Figs. 5B and 5C). These results suggest that enhanced expression of CTLA-4 on donor CD4+ T cells following treatment with TCDD does not play a functional role in the suppression of GVHD by TCDD.
Fig. 5.
Effect of blocking CTLA-4 during donor CD4+ T-cell differentiation on suppression of GVHD by TCDD. (A) Schematic of experimental design. One hundred micrograms of anti-CTLA-4 or control IgG was administered on days 0, 1, and 2 following adoptive transfer of purified donor T cells and treatment with either vehicle or TCDD. (B) Using flow cytometry, the percent of Thy1.1+ donor cells was determined and used to calculate the total number of donor cells from the total number of splenocytes. The percent of Thy1.1+CD8+ T cells was multiplied by the number of donor cells to calculate the total number of engrafted donor CD8+ T cells in the spleen of host animals. (C) CTL function was assessed as a function of host B-cell depletion. Host B cells were tracked by expression of CD19. Error bars represent the mean ± SEM, n = 3–5 biological replicates. For statistical analysis, the mixed procedure with the Satterthwaite option was used. *p < 0.05 and **p < 0.01.
Effect of Donor-Derived IFN-γ in TCDD-Mediated Immunosuppression
Because neither IDO nor CTLA-4 appeared to be required for TCDD-mediated immunosuppression, a potential independent role for IFN-γ was investigated. Based on the fact that nTregs from IFN-γ-deficient animals have attenuated suppressive function (Koenecke et al., 2012; Markees et al., 1998; Sawitzki et al., 2005; Wei et al., 2010), it is possible that AhR-Tregs also utilize IFN-γ to mediate suppression of the CTL response. If true, lack of IFN-γ in the donor cells should result in evidence of GVHD in TCDD-treated mice. To directly evaluate the role of donor-derived IFN-γ, host mice were injected with donor splenocytes obtained from C57Bl/6 (WT) or IFN-γ-deficient (Ifng −/−) mice, which were then treated with vehicle or TCDD.
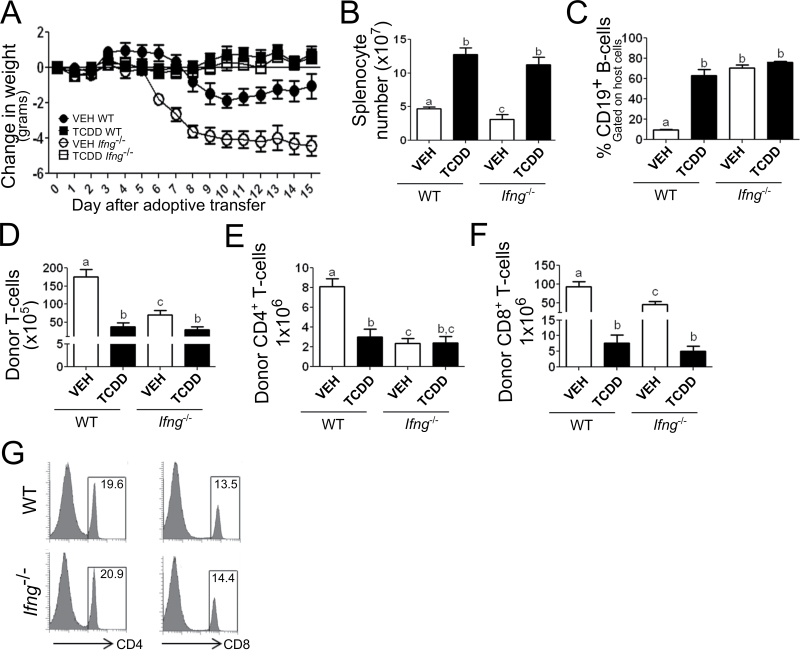
Vehicle-treated host mice that received Ifng −/− donor cells exhibited early onset and more severe body weight loss (Fig. 6A) consistent with previous results (Ellison et al., 1998). Mice that received Ifng −/− donor cells also had significantly fewer splenocytes than mice with WT donor cells (Fig. 6B) yet a much higher proportion of B cells (p < 0.001) (Fig. 6C). These results are consistent with a prior study showing a selective loss of Fas-mediated killing of host B cells that is promoted by IFN-γ (Puliaev et al., 2004). We also found that donor CD4+ and CD8+ T-cell engraftment was significantly reduced when the donor cells lacked IFN-γ (Figs. 6E and 6F). This was not due to a difference in the number of T cells injected in the initial donor cell inoculum (Fig. 6G).
Fig. 6.
Absence of IFN-γ in donor cells does not affect suppression of the CTL response by TCDD. (A) Change in host body weight following adoptive transfer. (B and C) Total splenocyte numbers on day 15. (C) Percent of CD19+ B cells remaining in the host (H-2Dd+) fraction. (D) Total number of donor (H-2Dd-neg) T cells present in the spleen of host animals. Number of donor (E) CD4+ or (F) CD8+ T cells in the spleen of host animals. (G) Percent of CD4+ and CD8+ T cells in the donor inocula prior to adaptive transfer of splenocytes on day 0. Significant differences between treatment groups were calculated using Student’s t-test (p < 0.05) and are denoted by different alphabetical characters. Error bars represent the mean ± SEM, n = 5 biological replicates.
Treatment of host mice with TCDD prevented body weight loss (Fig. 6A), decrease in splenocyte numbers (Fig. 6B), and donor T-cell engraftment (Figs. 6E and 6F) associated with GVHD, independent of the Ifng status of the donor cells. These results indicate that AhR-Tregs do not use IFN-γ for suppression of the GVH response.
DISCUSSION
Activation of AhR by TCDD during an acute GVH response induces Tregs with a suppressive capacity greater than that of natural Treg (Marshall et al., 2008). These AhR-Tregs do not express Foxp3, but express high levels of other Treg-associated markers such as CD25 and CTLA-4 (Funatake et al., 2005), suggesting that AhR functions as an autonomous ligand-dependent transcription factor for induction of Tregs. However, the mechanism by which AhR-Tregs mediate suppression of immune function remains unclear. The studies presented here were designed to test the hypothesis that the enhanced expression of CTLA-4 induced by TCDD on donor CD4+ T cells activates the IDO pathway, resulting in suppression of the CTL response (see Fig. 1). However, although treatment with TCDD significantly upregulated expression of Ifng, Ido1, and Ido2 in the lymph nodes and increased production of IFN-γ in spleen, blockade of CTLA-4 or IDO enzyme activity did not alleviate TCDD’s suppressive effects. Furthermore, the use of IFN-γ-deficient donor cells did not influence suppression suggesting that AhR-Tregs do not rely on IFN-γ for their immunosuppressive activity.
We initially tested the role of IDO by blocking its catalytic activity with the pharmacological inhibitor 1-MT. However, treatment with 1-MT had no effect on the day-15 GVH response in either control or TCDD-treated animals. The lack of effect of 1-MT in control animals was initially surprising because IDO-deficient animals were reported to show increased GVHD-associated mortality (Jasperson et al., 2008). However, the enhanced mortality in IDO-deficient mice was not due to excessive T-cell activation but rather to lack of upregulation of IDO in gut epithelial cells that reduced inflammatory injury in the colon. Taken together with our results, IDO and its associated depletion of tryptophan do not appear to play a prominent role in the regulation of T-cell responses in GVHD.
The inability of 1-MT to alter suppression of the GVH response in TCDD-treated animals indicates that the enzymatic activity of IDO is not involved in the suppressive mechanism of AhR-Tregs. This was surprising given the consistent upregulation of IDO by TCDD in this and other studies (Bankoti et al., 2010; Vogel et al., 2008). However, IDO has recently been shown to have signaling properties that are distinct from its catalytic function and are unaffected by 1-MT (Pallotta et al., 2011). In this study, TGF-β-conditioned pDCs were shown to use phosphorylated IDO as a signal transducer that activated p52-RelB to promote TGF-β production, which in turn promoted the induction of Foxp3+ Tregs. This IDO signaling pathway could be involved in the generation of Foxp3+ Tregs by TCDD-activated AhR that has been reported in several different autoimmune disease models (Benson and Shepherd, 2011; Kerkvliet et al., 2009; Quintana et al., 2008). Interestingly, we have recently observed that treatment with TCDD induces Foxp3 expression in both donor and host CD4+ T cells on day 15 of the GVH response (Diana Rohlman, Castle Funatake, Sumit Punj and Nancy I. Kerkvliet, in preparation). However, when the induction of Foxp3+ expression was blocked, it did not affect suppression of the GVH response by TCDD. Thus, although IDO may play a role as a signaling molecule to upregulate Foxp3 expression, these Foxp3+ T cells are not involved in TCDD-mediated suppression of acute GVH responses.
Because IDO has been associated primarily with a subset of tolerogenic pDCs, we looked at expression of several genes associated with pDC phenotype and function in the lymph nodes of TCDD-treated host mice. Of the genes examined, expression of Irf3 and Irf7 were significantly increased by TCDD. Irf3 and Irf7 code for transcription factors involved in regulation of expression of type I IFNs (IFN-α/β) (Honda et al., 2005). TCDD was previously shown to increase Irf3 expression in cultured U937 cells, and the mechanism was shown to be AhR and RelB dependent (Vogel et al., 2007). Increased production of type I IFNs by pDCs is a downstream consequence of IDO signaling (Pallotta et al., 2011), suggesting that increased expression of Irf3 and Ido may be linked in TCDD-treated pDCs. Although type I IFNs have long been known to play critical roles in virus defense, they have recently been shown to promote the development of Foxp3+ T cells and enhance their suppressive function (Gonzãlez-Navajas et al., 2012). The involvement of this pathway in AhR-mediated immunoregulation remains to be shown.
Apart from altered interactions with host DCs, upregulation of CTLA-4 on T cells could play a direct role in suppressing effector T-cell activation and/or proliferation by outcompeting CD28 signaling. However, when host mice were treated with an antibody that neutralizes the function of both the full-length and soluble isoforms of CTLA-4 (Ward et al., 2013), there was no effect on the suppression of the GVH response by TCDD. There was also no effect of antibody treatment on the GVH response in vehicle-treated mice. A similar treatment regimen in a murine tumor model resulted in increased expansion of antigen-specific T cells in response to HA-expressing tumor cells but did not prevent tolerance to tumor antigen (Sotomayor et al., 1999). In addition, although the same anti-CTLA-4 antibody has been shown to exacerbate symptoms of disease in colitis, diabetes, and experimental autoimmune encephalomyelitis (Luhder et al., 1998; Perrin et al., 1996; Watanabe et al., 2008), these studies used repeated dosing throughout the course of disease development. Because we specifically targeted CTLA-4 blockade to the first 3 days of the GVH response, during the window of susceptibility to TCDD and coincident with AhR-Treg differentiation, any effects of CTLA-4 blockade on the CTL effector phase of the immune response would have been spared. In fact, the half-life of the anti-CTLA-4 mAb (9H10) that we used has been shown to be 3–4 days (Allison, personal communication). This explanation is consistent with previous studies showing that antibody blockade of CTLA-4 in regulatory T cells alone was insufficient to enhance antitumor responses, whereas blockade of CTLA-4 in effector cells increased antitumor activity (Peggs et al., 2009). Thus, the inability of CTLA-4 blockade to affect TCDD-mediated suppression of GVHD indicates that increased expression of CTLA-4 on AhR-Treg is not involved in their suppressive function.
The apparent lack of involvement of CTLA-4 and IDO in TCDD-mediated suppression of GVHD led us to consider alternate pathways that would address the increase in IFN-γ seen in TCDD-treated mice (Fig. 3). Based on recent studies that demonstrated an autocrine role for IFN-γ in the suppressive mechanism of nTreg in Th1-mediated diseases (Beeston et al., 2010; Koenecke et al., 2012), we hypothesized that AhR-Tregs produce IFN-γ to mediate suppression of GVHD. Ifng −/− donor cells were used to initiate GVHD and the effects on the CTL response in the presence or absence of TCDD were examined. In vehicle-treated mice, GVHD was exacerbated by the lack of IFN-γ in the donor cells, in agreement with prior reports (Ellison et al., 1998; Koenecke et al., 2012; Yang et al., 1998). Splenocyte numbers were decreased, but percent of B cells was not diminished. This phenomenon has been attributed to the loss of IFN-γ-induced Fas expression on host B cells, preventing their selective targeting for FasL-mediated killing by CTL (Puliaev et al., 2004). Non-Fas-mediated pathways of cytotoxicity (i.e., perforin) were not affected by the lack of IFN-γ (Puliaev et al., 2004). However, our data indicate that donor T-cell engraftment is altered by the lack of IFN-γ, because significantly fewer CD4+ and CD8+ donor T cells were present in the spleen of mice receiving Ifng −/− donor cells. This is surprising, given their exacerbated symptoms of GVHD. The exacerbation of GVHD in mice that received Ifng −/− donor cells has been attributed to loss of suppressive activity of nTreg (Koenecke et al., 2012). Unlike the nTreg, AhR-Tregs apparently do not use IFN-γ as a suppressive mediator, because TCDD-treated animals did not lose weight and showed minimal evidence of a CTL response.
In summary, we found no evidence to support the hypothesis that AhR-Tregs induced by TCDD during a GVH response rely on the tolerogenic IDO pathway, CTLA-4 per se, or IFN-γ for suppression of the GVH response. This is in contrast to previous studies implicating IDO in Treg induction by several AhR ligands including TCDD (Mezrich et al., 2010; Nguyen et al., 2010; Vogel et al., 2008). However, these studies have focused on the expression of Foxp3 as a marker of Treg induction, which may arise from direct AhR-dependent induction of IDO in pDCs to drive Foxp3+ Treg expansion. The present study also confirms Irf3 and identifies Irf7 as potential AhR targets for induction of Foxp3+ Tregs. However, these Foxp3+ Tregs induced by TCDD are distinct from the Foxp3neg Tregs that emerge early in the GVH response and are dependent on AhR expression in the CD4+ T cell itself (Funatake et al., 2005). These AhR-Tregs share characteristics with Tr1-like Tregs including increased IL-10 and granzyme B expression (Gandhi et al., 2010; Marshall et al., 2008). The mechanisms of suppression used by AhR-Tregs in vivo remain to be defined.
FUNDING
National Institutes of Health (5RO1ES016651).
ACKNOWLEDGMENTS
We thank Dr Cliff Pereira for statistical assistance and the OSU Center for Genome Research and Biocomputing for technical assistance. The authors would like to acknowledge the services provided by the Cell and Tissue Analysis Facilities and Services Core of the Environmental Health Sciences Center, as well as the Mass Spectrometry Facilities and Services Core of the Environmental Health Sciences Center, Oregon State University, grant number P30ES000210-42, National Institute of Environmental Health Sciences, National Institutes of Health.
REFERENCES
- Alegre M. L., Frauwirth K. A., Thompson C. B. (2001). T-cell regulation by CD28 and CTLA-4. Nat. Rev. Immunol 1, 220–228 [DOI] [PubMed] [Google Scholar]
- Ball H. J., Sanchez-Perez A., Weiser S., Austin C. J. D., Astelbauer F., Miu J., McQuillan J. A., Stocker R., Jermiin L. S., Hunt N. H. (2007). Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene 396, 203–213 [DOI] [PubMed] [Google Scholar]
- Bankoti J., Rase B., Simones T., Shepherd D. M. (2010). Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol. Appl. Pharmacol 246, 18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeston T., Smith T. R. F., Maricic I., Tang X., Kumar V. (2010). Involvement of IFN-γ and perforin, but not Fas/FasL interactions in regulatory T cell-mediated suppression of experimental autoimmune encephalomyelitis. J. Neuroimmunol 229, 91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. M., Shepherd D. M. (2011). Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol. Sci 120, 68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison C. A., Fischer J. M. M., HayGlass K. T., Gartner J. G. (1998). Murine graft-versus-host disease in an F1-hybrid model using IFN-gamma gene knockout donors. J. Immunol 161, 631–640 [PubMed] [Google Scholar]
- Funatake C. J., Marshall N. B., Steppan L. B., Mourich D. V., Kerkvliet N. I. (2005). Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J. Immunol 175, 4184–4188 [DOI] [PubMed] [Google Scholar]
- Gandhi R., Kumar D., Burns E. J., Nadeau M., Dake B., Laroni A., Kozoriz D., Weiner H. L., Quintana F. J. (2010). Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat. Immunol 11, 846–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzãlez-Navajas J. M., Lee J., David M., Raz E. (2012). Immunomodulatory functions of type I interferons. Nat. Rev. Immunol 12, 125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Yanai H., Negishi H., Asagiri M., Sato M., Mizutani T., Shimada N., Ohba Y., Takaoka A., Yoshida N., et al. (2005). IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 [DOI] [PubMed] [Google Scholar]
- Hwu P., Du M. X., Lapointe R., Do M., Taylor M. W., Young H. A. (2000). Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol 164, 3596–3599 [DOI] [PubMed] [Google Scholar]
- Jasperson L. K., Bucher C., Panoskaltsis-Mortari A., Taylor P. A., Mellor A. L., Munn D. H., Blazar B. R. (2008). Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood 111, 3257–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet N. I., Shepherd D. M., Baecher-Steppan L. (2002). T lymphocytes are direct, aryl hydrocarbon receptor (AhR)-dependent targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): AhR expression in both CD4+ and CD8+ T cells is necessary for full suppression of a cytotoxic T lymphocyte response by TCDD. Toxicol. Appl. Pharmacol 185, 146–152 [DOI] [PubMed] [Google Scholar]
- Kerkvliet N. I., Steppan L. B., Vorachek W., Oda S., Farrer D., Wong C. P., Pham D., Mourich D. V. (2009). Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy 1, 539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenecke C., Lee C. W., Thamm K., Föhse L., Schafferus M., Mittrücker H. W., Floess S., Huehn J., Ganser A., Förster R., et al. (2012). IFN-γ production by allogeneic Foxp3+ regulatory T cells is essential for preventing experimental graft-versus-host disease. J. Immunol 189, 2890–2896 [DOI] [PubMed] [Google Scholar]
- Kwidzinski E., Bunse J., Aktas O., Richter D., Mutlu L., Zipp F., Nitsch R., Bechmann I. (2005). Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J 19, 1347–1349 [DOI] [PubMed] [Google Scholar]
- Luhder F., Höglund P., Allison J. P., Benoist C., Mathis D. (1998). Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J. Exp. Med 187, 427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markees T. G., Phillips N. E., Gordon E. J., Noelle R. J., Shultz L. D., Mordes J. P., Greiner D. L., Rossini A. A. (1998). Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4(+) T cells, interferon-gamma, and CTLA4. J. Clin. Invest 101, 2446–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N. B., Kerkvliet N. I. (2010). Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann. N. Y. Acad. Sci 1183, 25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N. B., Vorachek W. R., Steppan L. B., Mourich D. V., Kerkvliet N. I. (2008). Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Immunol 181, 2382–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta B. M., Castellaneta A., Thomson A. W. (2010). Tolerogenic plasmacytoid DC. Eur. J. Immunol 40, 2667–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor A. L., Munn D. H. (2004). IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol 4, 762–774 [DOI] [PubMed] [Google Scholar]
- Mezrich J. D., Fechner J. H., Zhang X., Johnson B. P., Burlingham W. J., Bradfield C. A. (2010). An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol 185, 3190–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn D. H., Sharma M. D., Mellor A. L. (2004). Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol 172, 4100–4110 [DOI] [PubMed] [Google Scholar]
- Nguyen N. T., Kimura A., Nakahama T., Chinen I., Masuda K., Nohara K., Fujii-Kuriyama Y., Kishimoto T. (2010). Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A 107, 19961–19966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta M. T., Orabona C., Volpi C., Vacca C., Belladonna M. L., Bianchi R., Servillo G., Brunacci C., Calvitti M., Bicciato S., et al. (2011). Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol 12, 870–878 [DOI] [PubMed] [Google Scholar]
- Peggs K. S., Quezada S. A., Chambers C. A., Korman A. J., Allison J. P. (2009). Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med 206, 1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin P., Maldonado J., Davis T., June C., Racke M. (1996). CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J. Immunol 157, 1333–1336 [PubMed] [Google Scholar]
- Puliaev R., Nguyen P., Finkelman F. D., Via C. S. (2004). Differential requirement for IFN-gamma in CTL maturation in acute murine graft-versus-host disease. J. Immunol 173, 910–919 [DOI] [PubMed] [Google Scholar]
- Quintana F. J., Basso A. S., Iglesias A. H., Korn T., Farez M. F., Bettelli E., Caccamo M., Oukka M., Weiner H. L. (2008). Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 [DOI] [PubMed] [Google Scholar]
- Sakurai K., Zou J. P., Tschetter J. R., Ward J. M., Shearer G. M. (2002). Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J. Neuroimmunol 129, 186–196 [DOI] [PubMed] [Google Scholar]
- Sawitzki B., Kingsley C. I., Oliveira V., Karim M., Herber M., Wood K. J. (2005). IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J. Exp. Med 201, 1925–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena V., Ondr J. K., Magnusen A. F., Munn D. H., Katz J. D. (2007). The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J. Immunol 179, 5041–5053 [DOI] [PubMed] [Google Scholar]
- Sotomayor E. M., Borrello I., Tubb E., Allison J. P., Levitsky H. I. (1999). In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc. Natl. Acad. Sci. U.S.A 96, 11476–11481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C., Pilotte L., Théate I., Stroobant V., Colau D., Parmentier N., Boon T., Van den Eynde B. J. (2003). Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med 9, 1269–1274 [DOI] [PubMed] [Google Scholar]
- Vogel C. F. A., Goth S. R., Dong B., Pessah I. N., Matsumura F. (2008). Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun 375, 331–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C. F. A., Sciullo E., Matsumura F. (2007). Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem. Biophys. Res. Commun 363, 722–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas T. L., Lenschow D. J., Bakker C. Y., Linsley P. S., Freeman G. J., Green J. M., Thompson C. B., Bluestone J. A. (1994). CTLA-4 can function as a negative regulator of T cell activation. Immunity 1, 405–413 [DOI] [PubMed] [Google Scholar]
- Ward F. J., Dahal L. N., Wijesekera S. K., Abdul-Jawad S. K., Kaewarpai T., Xu H., Vickers M. A., Barker R. N. (2013). The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur. J. Immunol 43, 1274–1285 [DOI] [PubMed] [Google Scholar]
- Watanabe K., Rao V. P., Poutahidis T., Rickman B. H., Ohtani M., Xu S., Rogers A. B., Ge Z., Horwitz B. H., Fujioka T., et al. (2008). Cytotoxic-T-lymphocyte-associated antigen 4 blockade abrogates protection by regulatory T cells in a mouse model of microbially induced innate immune-driven colitis. Infect. Immun 76, 5834–5842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B., Baker S., Wieckiewicz J., Wood K. J. (2010). IFN-gamma triggered STAT1-PKB/AKT signalling pathway influences the function of alloantigen reactive regulatory T cells. Am. J. Transplant 10, 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. G., Dey B. R., Sergio J. J., Pearson D. A., Sykes M. (1998). Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J. Clin. Invest 102, 2126–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ma J., Takeuchi M., Usui Y., Hattori T., Okunuki Y., Yamakawa N., Kezuka T., Kuroda M., Goto H. (2010). Suppression of experimental autoimmune uveoretinitis by inducing differentiation of regulatory T cells via activation of aryl hydrocarbon receptor. Invest. Ophthalmol. Vis. Sci 51, 2109–2117 [DOI] [PubMed] [Google Scholar]