Abstract
Multiple sclerosis (MS), a demyelinating immune-mediated central nervous system disease characterized by increasing female penetrance, is the leading cause of disability in young adults in the developed world. Epidemiological data strongly implicate an environmental factor, acting at the population level during gestation, in the increasing incidence of female MS observed over the last 50 years, yet the identity of this factor remains unknown. Gestational exposure to bisphenol A (BPA), an endocrine disruptor used in the manufacture of polycarbonate plastics since the 1950s, has been reported to alter a variety of physiological processes in adulthood. BPA has estrogenic activity, and we hypothesized that increased gestational exposure to environmental BPA may therefore contribute to the increasing female MS risk. To test this hypothesis, we utilized two different mouse models of MS, experimental autoimmune encephalomyelitis (EAE) in C57BL/6J mice (chronic progressive) and in SJL/J mice (relapsing-remitting). Dams were exposed to physiologically relevant levels of BPA in drinking water starting 2 weeks prior to mating and continuing until weaning of offspring. EAE was induced in adult offspring. No significant changes in EAE incidence, progression, or severity were observed with BPA exposure, despite changes in cytokine production by autoreactive T cells. However, endocrine disruption was evidenced by changes in testes development, and transcriptomic profiling revealed that BPA exposure altered the expression of several genes important for testes development, including Pdgfa, which was downregulated. Overall, our results do not support gestational BPA exposure as a significant contributor to the increasing female MS risk.
Key Words: multiple sclerosis, experimental autoimmune encephalomyelitis, central nervous system, T cell, Bisphenol A, environmental exposure, gestational, developmental, autoimmunity, testes, endocrine disruption, PDGF.
Bisphenol A (BPA) is an organic compound used to manufacture polycarbonate plastics and epoxy resins, many of which are used in food packaging (e.g., in can linings and polycarbonate plastic containers). BPA was first synthesized in 1891, and evidence of its estrogenicity came from experiments in the 1930s (Dodds and Lawson, 1938). In fact, BPA was intended for clinical use, but because it was less potent than diethylstilbestrol (DES, which was formulated around the same time), it was never used clinically. Subsequently, polymer chemists discovered that it could be polymerized to form polycarbonate plastic, and it has been in commercial use since 1957. Unfortunately, the ester bond that links BPA monomers to one another to form a polymer is not stable and hence the polymer decays with time, releasing BPA into materials with which it comes into contact, including food or water. Today, it can be consistently detected in the circulation and tissues of humans (Bushnik et al., 2010). Multiple studies have shown that at low levels of exposure BPA can act as an endocrine disruptor, altering many physiological processes (Soto and Sonnenschein, 2010). BPA exposure during development (in utero or neonatal) can have particularly profound effects, many of which are manifested in adulthood. Importantly, maternal exposure to BPA has been shown to suppress DNA methylation in developing pups and change offspring phenotypes by stably altering the epigenome (Dolinoy et al., 2007).
There is some evidence that developmental BPA exposure can alter the immune system in the adult state, although this has not been explored in detail. In general, BPA exposure in mice is associated with enhanced cytokine and antibody production, and decreased numbers of regulatory T cells (Alizadeh et al., 2006; Ohshima et al., 2007; Yan et al., 2008; Yoshino et al., 2004; Youn et al., 2002; Yurino et al., 2004), although many of these reports focused on adult, as opposed to gestational, exposure to BPA. Importantly, BPA exposure exacerbates the development of experimental asthma (Bauer et al., 2012; Midoro-Horiuti et al., 2010), and higher urinary levels of BPA were associated with increased asthma risk in humans (Donohue et al., 2013; Spanier et al., 2012; Vaidya and Kulkarni, 2012). Whether gestational BPA exposure can play a role in autoimmune disease remains almost completely unexplored.
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) characterized by demyelination, axonal damage, and progressive neurological dysfunction (Noseworthy et al., 2000). MS is the most common disabling neurologic disease of young adults and adolescents, affecting ~400,000 individuals in the United States and ~2.5 million individuals worldwide (Courtney et al., 2009). MS susceptibility is characterized by a maternal parent-of-origin (MPOO) effect and increased female penetrance. The MPOO effect has been well documented in studies of half-siblings (Ebers et al., 2004), sibships including dizygotic twins (Willer et al., 2003), a large extended Dutch pedigree (Hoppenbrouwers et al., 2008), avuncular pairs (Herrera et al., 2008), and Caucasian-North American Aboriginal admixture matings (Ramagopalan et al., 2009). The incidence of female MS has been increasing over the last 50 years (Orton et al., 2006) to the current female:male MS sex ratio of 3–4:1 in Caucasians and 6:1 in admixed African Americans (Weinstock-Guttman et al., 2003). The temporal increase in female MS has been relatively rapid, suggesting that the increasing female MS risk may be due to environmental factors acting at the population level during gestation (MPOO effect) in an ethnic-specific manner, that is, gene-by-environment interactions occurring during gestation differentially affect MS risk more in female than in male offspring.
The increasing female MS incidence temporally coincides with increased exposure to BPA, and because gestational BPA exposure can have a profound impact that lasts through adulthood, it is a plausible environmental factor that could contribute to the MPOO effects in MS. Furthermore, susceptibility to MS and its primary animal model, experimental autoimmune encephalomyelitis (EAE) is strongly influenced by sex hormones, in particular estrogen (Ramagopalan et al., 2009) and estrogen receptor signaling (Polanczyk et al., 2003, 2004). Finally, the findings that BPA can enhance immune responses also support its potential role as an environmental factor influencing autoimmunity.
We hypothesized that developmental exposure to environmental endocrine disruptors such as BPA may contribute to the increasing female MS risk. Importantly, the increase in the female:male MS sex ratio has recently been shown to be due to an increase in relapsing-remitting (RR) MS (Ramagopalan et al., 2010), which would be consistent with differential gene-by-BPA interactions specific to RR disease. In this regard, we and others have shown that responsiveness to estrogens and estrogen disruptors is genetically controlled (Griffith et al., 1997; Nakai et al., 1999; Roper et al., 1999; Spearow et al., 1999, 2001; Wall et al., 2013; Zheng et al., 1989). Hence, we investigated whether gestational exposure to BPA differentially influenced the susceptibility of adult C57BL/6J (B6) mice to chronic progressive EAE and SJL/JCrHsd (SJL) mice to RR EAE, two different genetic models of MS. Although we observed evidence of mild endocrine disruption in BPA-exposed mice and alternations in immune responses, we found no significant effect on EAE susceptibility in either model, suggesting that BPA is not a good candidate for an environmental agent influencing MS susceptibility.
MATERIALS AND METHODS
Animals.
C57BL/6J (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). SJL/JCrHsd (SJL) mice were purchased from Harlan Laboratories. All animals were maintained under specific pathogen-free conditions on a 12:12 light-dark cycle and were fed Purina mouse pellets (Ralston-Purina, St Louis, MO) and water ad libitum, except where noted. The experimental procedures performed in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Vermont; IACUC protocol number 12-031, approved April 2012.
BPA exposure.
Dams were purchased from the animal vendor at 6 weeks of age and rested for 2 weeks after arrival at the animal facility at the University of Vermont. Subsequently, the dams were switched to the appropriate diet, either standard mouse chow (Purina) or casein-based phytoestrogen-free (PF) diet (AIN-93G Rodent Diets; Research Diet, Inc.) for 1 week, followed by introduction of BPA at 10 µg/ml in 1% ethanol in the drinking water, or control 1% ethanol drinking water. The dose of BPA was chosen because it has been shown to result in BPA accumulation in mouse tissues at concentrations comparable with that in humans (Kabuto et al., 2004). Stock BPA (Sigma-Aldrich, St Louis, MO) was dissolved at 1mg/ml in 100% ethanol and diluted 1:100 in drinking water. Drinking water was purified using the Milli-Q Academic A10 system (Millipore) and was provided in glass water bottles with rubber stoppers. Water was replaced 2–3 times per week. Animals were housed in polysulfone cages, which do not leach significant amounts of BPA (Midoro-Horiuti et al., 2010). Two weeks following the start of BPA exposure, breeder males were introduced for up to 4 weeks. BPA, control water, and special chow were maintained until the pups were weaned at 3 weeks of age, at which point the pups were switched to standard mouse chow and drinking water. Anogenital distance (AGD) and weight were measured at weaning. Weight-adjusted AGD was calculated by dividing AGD by the pup weight (which was normalized by the average pup weight for a given sex in the normal diet control group).
Induction and evaluation of EAE.
EAE was induced and evaluated in B6 and SJL mice essentially as described previously (Noubade et al., 2011). Briefly, 8- to 12-week-old mice were injected sc with an emulsion containing 100 μg of myelin oligodendrocyte glycoprotein 35–55 (MOG35–55) peptide (B6) or 100 μg of proteolipid protein 139–151 ( PLP139–151) peptide (SJL) and complete Freund’s adjuvant (CFA) (Sigma-Aldrich) supplemented with 200 μg of Mycobacterium tuberculosis H37RA (Difco Laboratories, Detroit, MI) in the posterior right and left flank; 1 week later, all mice were similarly injected at two sites on the right and left flank anterior of the initial injection sites (2× MOG35–55 or PLP139–151 + CFA). Mice were scored daily starting at day 10 postinjection for clinical signs of EAE according to the following scale: 0 = normal; 1 = limp tail; 2 = moderate hind limb weakness or mild ataxia; 3 = hind limb paralysis; 4 = hind limb paralysis with urinary or fecal incontinence; 5 = hind limb paralysis with front limb weakness or paralysis, or death. Clinical quantitative trait variables were generated as previously described (Noubade et al., 2011).
Cytokine quantification by ELISA.
Mice were immunized with 2× MOG35–55 (B6) or PLP135–151 (SJL) + CFA, and spleen and draining lymph node (DLN) were harvested on day 30 postimmunization. Red blood cells were lysed by hypotonic lysis with Gey’s solution. Single-cell suspensions were prepared at 1×106 cells/ml in RPMI containing 10% fetal bovine serum (Hyclone, Logan, UT) and stimulated with 50 μg/ml of MOG35–55 or PLP135–151. Cell culture supernatants were collected at 72h and cytokine levels were measured by ELISA as described below.
For the detection of cytokines in the cell culture supernatants, ELISAs were performed as described previously (Noubade et al., 2011), using the capture mAbs: anti-IFNγ and anti-IL-17A and the corresponding biotinylated mAbs (BioLegend). Other ELISA reagents included: horseradish peroxidase–conjugated avidin D (Vector Laboratories, Burlingame, CA), TMB microwell peroxidase substrate and stop solution (Kirkegaard and Perry Laboratories, Gaithersburg, MD). rIFNγ and rIL-17A (BioLegend) were used as standards.
Proliferation assays.
Mice were immunized with 2× MOG35–55 (B6) or PLP135–151 (SJL) + CFA, and spleen and DLN cells were collected on day 10 postimmunization. Single-cell suspensions of spleen and DLN were prepared at 2.5×105 cells/well in RPMI medium and stimulated in a 96-well plate with different concentrations of MOG35–55 or PLP135–151 for 72h. During the last 18h of culture, 1 μCi of 3H-thymidine (PerkinElmer) was added. Cells were harvested onto glass fiber filters and thymidine uptake was determined with a liquid scintillation counter.
Tissue collection.
For collection of uterus, testes, and ovaries, mice were terminally anesthetized with ketamine and intracardially perfused with PBS. For each animal, one of the testes or ovaries, and one of the uterine horns was snap frozen in liquid nitrogen for RNA extraction. The other uterine horn, ovary, or testis was fixed in 10% neutral buffered formalin for 24h, then stored in 100% ethanol until further processing. The tissues were subsequently processed for paraffin embedding and hematoxylin and eosin (H&E) staining.
Microscopic evaluation of tissues.
At least two 5-μm sections of testes, ovaries, or uterus were stained with H&E and examined by a board-certified veterinary pathologist (BD) under light microscopy. Tissues were examined without knowledge of treatment. Sections were excluded from evaluation if there were tissue or sectioning artifacts related to processing. Alterations observed were graded in severity representing minimal, mild, moderate, or severe.
RNA extraction and cDNA synthesis.
RNA was extracted from frozen tissues using TRIzol reagent (Invitrogen) in accordance with manufacturer’s instructions. Quantitative and qualitative analysis of RNA was performed using Nanodrop1000 spectrophotometer (ThermoScientific) and the Agilent 2100Bioanalyzer (Agilent Technologies, Santa Clara, CA), respectively. One microgram of the extracted total RNA was used for cDNA synthesis. First-strand cDNA was synthesized using SuperScript III and random hexamers (Life Technologies Corporation, Grand Island, NY) according to manufacturer’s instructions.
Quantitative real-time PCR analysis of mRNA expression.
Quantitative real-time PCR (qRT-PCR) was performed essentially as previously described (Williams et al., 2008). Briefly, each reaction was performed using 10ng cDNA, 1 pmol each of forward and reverse primers, and SYBR green PCR master mix (Life Technologies Corporation) in a 10 μl final reaction volume. Mouse 18S rRNA was used for normalization of mRNA expression. All runs were performed using the Applied Biosystems 7500 Fast Real-Time PCR System (Life Technologies Corporation), and all amplification products were checked with dissociation-curve analysis. Only amplifications with one defined melting curve were used for further analysis. Relative expression and SD were calculated using the comparative threshold cycle (ΔΔCt) method.
Microarray experiment and analysis and bioinformatics analysis.
Two-color comparative microarray technique and Operon’s 70-mer-oligonucleotide arrays (Microarray Inc., Huntsville, AL) were used, covering the mouse transcriptome of 37,000 genes and variants. Analysis was performed essentially as previously described (Williams et al., 2008). RNA from 15 control mice was pooled and compared with a pool of RNA from 7 BPA-treated mice, and the comparison was replicated and dye swapped totaling four replicates. From each pool, 15–20 µg of total RNA were used per cDNA synthesis and labeling reaction (eight in total). Labeled cDNA was hybridized to arrays for 35–40h at 42°C, washed, and scanned at 10 µm resolution using the Axon GenePix 4400A (Molecular Devices, LLC, Sunnyvale, CA) scanner. The obtained Tiff images were analyzed using the GenePix Pro 6.1 software (Molecular Devices, LLC). All data analysis steps were performed in the R environment as previously described (Richter et al., 2006). Differentially expressed genes were identified using the empirical Bayes moderated t-test within the Limma package, which generated a list of differentially expressed genes with p values and B scores. Genes were considered differentially expressed when p < 0.05 and log2 fold change > |0.45|. Microarray data are available on NCBI’s GEO under accession GSE45917.
Gene ontology and subnetwork enrichment analysis were performed for differentially expressed genes using the Ariadne Pathway Studios program (Elsevier Inc., Rockville, MD). Because this type of analysis performs better with a large number of genes and tolerates false positives, a less stringent cutoff of p < 0.05 combined with log2 fold change > |0.2| was used for the gene list of differentially expressed genes subjected to this analysis. p Values indicating overrepresentation using the Ariadne Pathway Studios Gene Ontology analysis were calculated with Fisher’s exact test and considered significant when p < 0.05.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism 6 software (GraphPad software Inc.). Significance of differences was determined using one-way or two-way ANOVA or t-test, as described in the figure legends. For all analyses, p < 0.05 was considered significant. Numerical data are displayed as mean ± SE of the mean throughout.
RESULTS
We utilized two different mouse models of MS: chronic progressive EAE in B6 mice and RR-EAE in SJL mice. In order to assess the effect of gestational BPA exposure, B6 and SJL dams were exposed to 10 µg/ml BPA in drinking water beginning 2 weeks prior to mating and continuing until the offspring were weaned. This dose was chosen because it has been shown to result in BPA accumulation in mouse tissues (Kabuto et al., 2004) at concentrations comparable with that in humans (summarized in Midoro-Horiuti et al., 2010). Furthermore, this dose of BPA was sufficient to cause enhanced murine asthma (Midoro-Horiuti et al., 2010). BPA-exposed mice were fed a casein-based diet free of soy protein, the major source of dietary phytoestrogens, such as genistein (Barnes, 2010). An extra control group receiving normal diet was included to test for effects of the PF diet. Another control group received normal diet and normal water to test for the effects of 1% ethanol in the drinking water of the other groups. This group did not show any significant difference from the normal diet + 1% ethanol drinking water group in any of the measured parameters (data not shown).
Effect of BPA Exposure on EAE
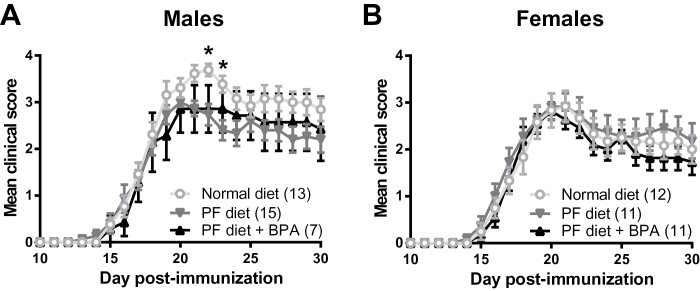
EAE was induced in adult (8–12 weeks) offspring from BPA-exposed and control B6 mothers by immunization with MOG35–55 emulsified in CFA on D0 and D7 (Noubade et al., 2011). This EAE induction protocol results in less severe EAE compared with the standard 1× MOG35–55 + CFA + pertussis toxin protocol used in most EAE studies (Stromnes and Goverman, 2006), hence readily allowing for detection of disease exacerbation, that is, onset, severity, and duration of clinical disease course. Immunized B6 mice showed typical chronic progressive EAE, which in males was modestly more severe in the normal diet group compared with PF diet (Fig. 1). However, the BPA-exposed group did not show a difference in EAE severity compared with the PF diet control group (Fig. 1). No differences in EAE were observed with either treatment in female B6 mice. EAE incidence in B6 mice was 100%.
Fig. 1.
Effect of gestational BPA exposure on EAE in B6 mice. EAE was induced in male (A) and female (B) B6 mice using the 2× MOG35–55 + CFA protocol. Data were analyzed two-way ANOVA and post hoc comparison. *indicates significant difference from PF diet, p < 0.05. The numbers in parentheses indicate the number of animals in each group.
Immunized SJL mice showed EAE remission after the first acute attack, followed by a second episode of lesser severity. The time of onset for the first and second EAE episodes was quite variable (Figs. 2A and 2B); hence, cumulative disease score (CDS) (Noubade et al., 2011) was used as the primary quantitative measure of disease severity for each episode (Figs. 2C–F). None of the treatments affected CDS in either sex in SJL mice (Fig. 2). We also found that although the incidence of acute phase disease was high (90–100%), the incidence of relapse was ~60% (Figs. 2G and 2H). Most importantly, BPA treatment did not significantly affect incidence of relapse; therefore, the incidence of RR disease was not significantly different among treatment groups. Taken together, these results suggest that gestational BPA exposure does not affect the clinical course of EAE in the adult mouse.
Fig. 2.
Effect of gestational BPA exposure on EAE in SJL mice. EAE was induced in male (A, C, E, and G) and female (B, D, F, and H) B6 mice using the 2× or PLP139–151 + CFA protocol. CDS was calculated by adding the mean clinical scores for days 10–21 (first episode, C and D) or days 22–32 (second episode/relapse, E and F). Percentage of SJL mice exhibiting relapse is shown in (G and H). EAE course (A and B) was analyzed by two-way ANOVA with post hoc comparisons. CDS (C–F) was analyzed by one-way ANOVA with post hoc comparisons. Incidence of relapse (G and H) was analyzed by the chi-square and Fisher’s exact tests. No significant differences were found among treatment groups. The numbers in parentheses indicate the number of animals in each group.
Effect of BPA Exposure on Antigen-Specific Cytokine Responses
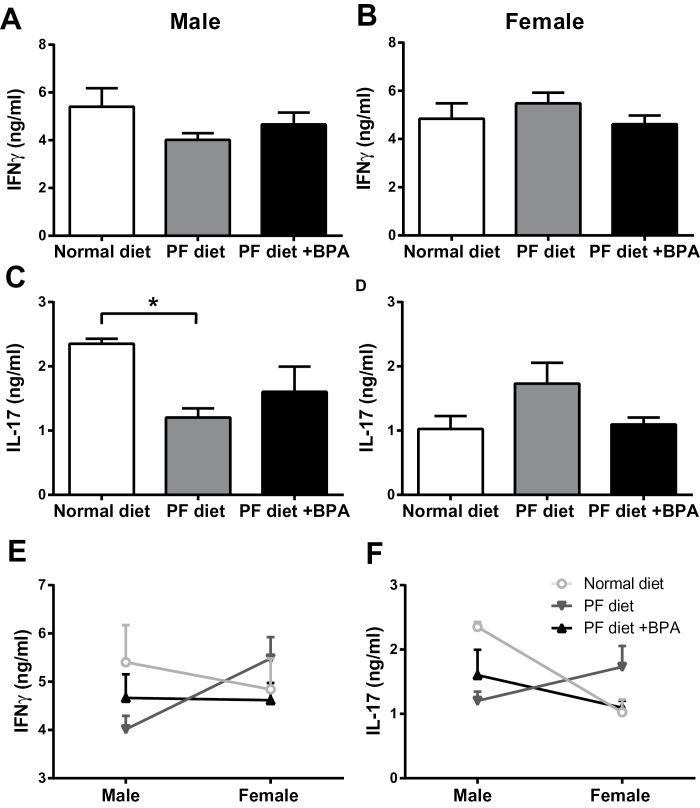
BPA has been reported to lead to dysregulated cytokine responses. To address this possibility in an organ-specific autoimmune disease setting, we measured MOG35–55-specific responses in peripheral lymphoid organs by ELISA for IFNγ and IL-17, which are the signature cytokines produced by encephalitogenic Th1 and Th17 cells, respectively. No significant changes were observed in male B6 mice (Figs. 3A and 3C). In female B6 mice, the PF diet resulted in a significant upregulation of IL-17, an effect that was reversed by BPA exposure (Fig. 3D), suggesting that xeno- and phytoestrogens may suppress IL-17 production in a sex-specific fashion in B6 mice. These data were reanalyzed by comparing cytokine production by males versus females in each group to look for sexual dimorphisms and the effects of exposures on them. B6 males in the regular diet control group produced more IFNγ and IL-17 than females, consistent with our previous unpublished observations (Krementsov, Case, Saligrama, and Teuscher, unpublished data). However, this sexual dimorphism was reversed by BPA exposure for IFNγ and by PF diet for IL-17 (Figs. 3E and 3F).
Fig. 3.
Effect of gestational BPA exposure on antigen-specific cytokine production in B6 mice. Cells from spleen and LN were isolated from male (A, C, and E) and female (B, D, and F) B6 mice immunized with the 2× MOG35–55 + CFA protocol and restimulated with 50 µg/ml MOG35–55 for 72h. Cytokine production in the supernatants was measured by ELISA and analyzed by one-way ANOVA with post hoc comparisons. *indicates p < 0.05. n = 5 for all groups.
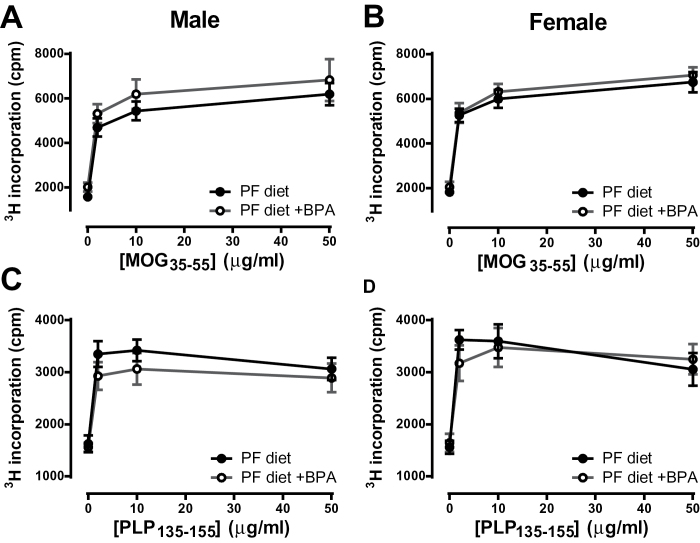
In male SJL mice, the PF diet resulted in a significant decrease in IL-17 production, but this was not reversed by BPA treatment (Fig. 4C). No significant changes were observed in female SJL mice with any treatment, although the pattern of IL-17 production (Fig. 4D) was remarkably similar to B6 mice (Fig. 3D), where xenoestrogens suppressed IL-17 production. In terms of sexual dimorphisms, IL-17 production was higher in males than in females, as in B6 mice. This dimorphism was eliminated by the PF diet, but only partially restored by BPA treatment (Fig. 4F). Antigen-specific proliferative responses were also measured, and these did not differ for any of the treatments in either B6 or SJL mice (Fig. 5). Taken together, our results show that gestational xenoestrogen exposure can modulate adult cytokine responses in a gene-by-sex-specific manner, but these effects are not sufficient to affect EAE severity.
Fig. 4.
Effect of gestational BPA exposure on antigen-specific cytokine production in SJL mice. Cells from spleen and LN were isolated from male (A, C, and E) and female (B, D, and F) SJL mice immunized with the 2× PLP135–151 + CFA protocol and restimulated with 50 µg/ml PLP135–151 for 72h. Cytokine production in the supernatants was measured by ELISA and analyzed by one-way ANOVA with post hoc comparisons. *indicates p < 0.05. n = 5 for all groups, except the male PF diet group, where n = 4.
Fig. 5.
Effect of gestational BPA exposure on antigen-specific proliferative responses. Cells from spleen and LN were isolated from male (A and C) and female (B and D) B6 mice (A and B) or SJL mice (C and D) immunized with the 2× MOG35–55 or PLP135–151 + CFA protocols, respectively. Cells were restimulated with the indicated dose of peptide for 72h and 3H-thymindine incorporation during the last 18h was measured. Data were analyzed by two-way ANOVA. No significant differences were found. n = 5 for all groups, except the SJL male PF diet group, where n = 4.
Secondary Indicators of Endocrine Disruption
In order to confirm that our BPA exposure protocol produced the desired endocrine-disruptive effects, we tested multiple parameters associated with endocrine disruption, including body weight, AGD, adult reproductive organ weight and histology, and global gene expression in the gonad. Changes in AGD can serve as a marker of endocrine disruption, and potent estrogens such as DES can cause changes in AGD (LaRocca et al., 2011). Pups born to control and BPA-exposed dams were weighed at weaning (3 weeks of age) and their AGD and weight were measured. We found no effect of either treatment on weight-corrected AGD in either strain (Figs. 6A–D). Pup weight was also not affected by either treatment in B6 mice (Figs. 6E and 6F); however, in female SJL mice, PF diet caused an increase in pup weight, an effect that was reversed by BPA exposure (Figs. 6G and 6H), a finding consistent with a previous report showing reduced body weight at postnatal day 22 due to gestational BPA exposure (Honma et al., 2002).
Fig. 6.
Effect of gestational BPA on weight and AGD. AGD (A–D) and weight (E and F) were measured at weaning (3 weeks of age) in B6 mice (A, B, E, and F) or SJL mice (C, D, G, and H). AGD was adjusted by weight as described in the Materials and Methods section. Data were analyzed by one-way ANOVA. *indicates p < 0.05, **indicates p < 0.01. Numbers above the bars indicate the number of animals in each group. The number of animals for weight measurements was the same as what is listed for AGD measurements.
Gestational BPA exposure has been reported to affect sexual differentiation in mice (Honma et al., 2002; Xi et al., 2011) and in humans (Miao et al., 2011), particularly in males. Thus, we tested whether gestational BPA exposure was associated with changes in adult gonads in B6 and SJL mice. No significant changes in testis or uterus wet weight were found with either treatment (data not shown). At the histological level, uteri and ovaries from BPA-exposed mice had no remarkable lesions, although quantitative oocyte counts were not performed (data not shown). In testes, a basal low level of individual germ cell apoptosis was observed in both control and BPA-treated mice (Table 1). This observation was consistent with a background level of germ cell apoptosis typical in mouse testes in either mitotic or meiotic stages of spermatogenesis. A diagnosis of degeneration was used when there was greater level of germ cell apoptosis compared with baseline, and a notable loss of germ cells or a general disruption of normal maturation observed in individual cross-sections of seminiferous tubules. Degeneration in the testes was diagnosed more often in BPA-exposed mice and with a slight increase in severity (Table 1) compared with other control groups. Degeneration was most common in late stages of spermatogenesis, VIII–XII.
Table 1.
Effect of BPA Exposure on Testes Histology
| B6 | SJL | |||||
|---|---|---|---|---|---|---|
| Normal diet | PF diet | PF diet + BPA | Normal diet | PF diet | PF diet + BPA | |
| Mice/group | 13 | 15 | 7 | 10 | 4 | 8 |
| No. of testes examineda | 7 | 10 | 5 | 9 | 3 | 8 |
| Diagnosis | ||||||
| Degenerationb | 1 | 0 | 2 | 0 | 0 | 5 |
| Severity gradec | ||||||
| 1 | 1 | 2 | 3 | |||
| 2 | 2 | |||||
| 3 | ||||||
| 4 | ||||||
| Apoptosis | 0 | 3 | 0 | 2 | 3 | 3 |
| Severity gradec | ||||||
| 1 | 3 | 2 | 3 | 3 | ||
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
| Tubular atrophyd | 0 | 1 | 1 | 0 | 0 | 0 |
Note. At least two 5-μm sections of testes, ovaries, or uterus were stained with H&E and examined by a board-certified veterinary pathologist under light microscopy.
aTestes from some mice were not examined due to sectioning artifacts, etc.
bDegeneration was used when there was greater than individual cell apoptosis and notable loss of germ cells or a general disruption of normal maturation process in individual cross-sections of seminiferous tubules.
cSeverity grades 1–4 correlate to minimal, mild, moderate, or severe, respectively. The grade represents the extent or distribution (focal, multifocal, locally extensive, diffuse).
dAtrophy is defined as complete loss of spermatogenesis, this could be in one to several tubules, and tubules were lined by Sertoli cells only.
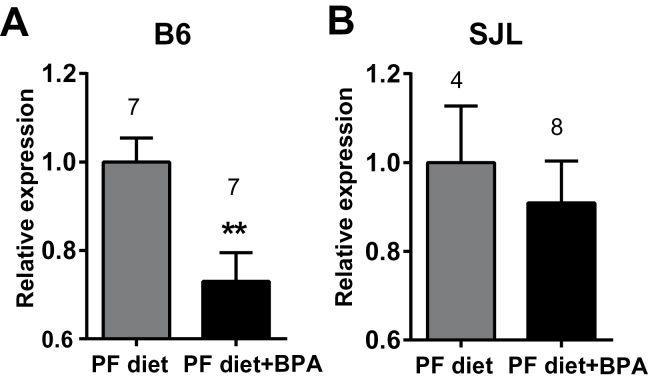
Based on these findings, we hypothesized that gestational BPA exposure resulted in changes in gene expression that persisted in the adult testes. To test this hypothesis, RNA was extracted from testes of B6 mice from the BPA-treated and PF diet control–treated groups, and subjected to microarray analysis. Results from the microarray experiments indicated that BPA exposure resulted in subtle, but detectable changes in gene expression (Table 2). Pathway analysis of differentially expressed genes suggested involvement of the innate immune response, as well as dysregulation of cell proliferation, spermatogenesis, and apoptosis, the latter being consistent with histopathological analyses (Table 3). Furthermore, in our microarray results, we observed downregulation of Pdgfa (platelet-derived growth factor, alpha), a gene well documented to be critical for proper testes development and function (Basciani et al., 2010). This downregulation was confirmed by qRT-PCR in B6 mice, yet was absent in SJL mice (Fig. 7), suggesting a genetic component in the response to BPA, consistent with other’s observations (Spearow et al., 1999, 2001) and our reports on genetic control of responsiveness to estrogen (Griffith et al., 1997; Wall et al., 2013). Taken together, these results indicate that BPA exposure caused subtle, but detectable degeneration in the adult testes in our study.
Table 2.
List of Differentially Expressed Genes in BPA-Treated Mice Versus PF Diet Control
| Genes downregulated by BPA | Genes upregulated by BPA | ||||
|---|---|---|---|---|---|
| Gene | LogFC | p Value | Gene | LogFC | p Value |
| Lars2 | −1.47 | 0.000 | Ikbip | 1.14 | 0.035 |
| Nap1l1 | −1.29 | 0.002 | Fam82a1 | 0.95 | 0.041 |
| Vrk3 | −1.08 | 0.001 | Eno2 | 0.83 | 0.042 |
| Ropn1l | −0.91 | 0.009 | 1700025E21Rik | 0.83 | 0.011 |
| Irgc1 | −0.85 | 0.000 | Serpina3n | 0.82 | 0.010 |
| Dpcd | −0.84 | 0.005 | Tmx3 | 0.81 | 0.004 |
| Eif2b1 | −0.77 | 0.022 | Slit1 | 0.77 | 0.046 |
| Mocos | −0.75 | 0.006 | Msln | 0.75 | 0.024 |
| Efcab8 | −0.74 | 0.003 | C3 | 0.70 | 0.002 |
| Gm5617 | −0.70 | 0.010 | Tmem50a | 0.68 | 0.015 |
| Git1 | −0.67 | 0.008 | Dcun1d4 | 0.65 | 0.027 |
| Tnp2 | −0.64 | 0.027 | Ssxb2 | 0.62 | 0.016 |
| Rap1gds1 | −0.64 | 0.022 | Gpx3 | 0.61 | 0.002 |
| Eprs | −0.64 | 0.038 | Sdcbp | 0.61 | 0.012 |
| Hapln1 | −0.63 | 0.048 | Txndc9 | 0.60 | 0.014 |
| Prm1 | −0.63 | 0.025 | Pdhb | 0.60 | 0.028 |
| Prss55 | −0.62 | 0.006 | Txndc9 | 0.59 | 0.020 |
| Odf2 | −0.61 | 0.004 | Olfr706 | 0.58 | 0.016 |
| Lrch4 | −0.61 | 0.012 | Gnpda2 | 0.55 | 0.003 |
| Ttbk1 | −0.61 | 0.014 | Gramd4 | 0.54 | 0.028 |
| 2610318N02Rik | −0.59 | 0.002 | 4930522H14Rik | 0.53 | 0.011 |
| Ubc | −0.57 | 0.007 | Cenpp | 0.52 | 0.021 |
| Iars2 | −0.57 | 0.044 | Prpf40a | 0.52 | 0.008 |
| Mpg | −0.56 | 0.049 | Sall4 | 0.50 | 0.043 |
| Cisd2 | −0.55 | 0.021 | C1qc | 0.50 | 0.015 |
| Cdc42ep3 | −0.53 | 0.009 | Smoc2 | 0.49 | 0.012 |
| Col15a1 | −0.52 | 0.048 | Serping1 | 0.47 | 0.002 |
| Fam108a | −0.52 | 0.002 | |||
| Cdk2 | −0.52 | 0.003 | |||
| Gapdhs | −0.52 | 0.016 | |||
| Rnf181 | −0.51 | 0.033 | |||
| Zfp219 | −0.51 | 0.003 | |||
| Cypt4 | −0.51 | 0.031 | |||
| Pdgfa | −0.49 | 0.047 | |||
| Clpb | −0.49 | 0.006 | |||
| Amhr2 | −0.49 | 0.012 | |||
| Trim36 | −0.46 | 0.005 | |||
| 1810013L24Rik | −0.46 | 0.005 | |||
| Larp1 | −0.45 | 0.010 | |||
Note. RNA from testes from mice gestationally exposed to BPA or PF diet controls was subjected to microarray analysis as detailed in the Materials and Methods section. To generate this list, a cutoff of p < 0.05 and log2 fold change (LogFC) > |0.45| was used.
Table 3.
Pathway Analysis of Differentially Expressed Genes in BPA-Treated Mice
| Category | p Value |
|---|---|
| Gene set enrichment analysis | |
| Innate immune response | 0.0003 |
| Translation | 0.0009 |
| Complement activation | 0.006 |
| Carbohydrate metabolic process | 0.021 |
| Antiapoptosis | 0.033 |
| Negative regulation of cell proliferation | 0.039 |
| Spermatid development | 0.049 |
| Pathway | p Value |
| Subnetworks enrichment analysis | |
| Expression targets of IL1 family | 0.010 |
| Expression targets of TGFA | 0.011 |
| Expression targets of IL1A | 0.021 |
| Expression targets of CEBPA | 0.041 |
| Expression targets of HIF1A | 0.043 |
Note. Gene ontology and subnetwork enrichment analysis were performed for differentially expressed genes using the Ariadne Pathway Studios program as detailed in the Materials and Methods section. p Values < 0.05 were considered significant.
Fig. 7.
Effect of gestational BPA exposure on Pdgfa expression in the adult testis. RNA was isolated from B6 (A) and SJL (B) testes and subjected to qRT-PCR analysis for Pdgfa expression. 18S RNA served as an endogenous control. Data were analyzed using unpaired t-test. **indicates p < 0.01. Numbers above the bars indicate the number of animals in each group.
DISCUSSION
Based on several lines of evidence, we hypothesized that gestational BPA exposure may be responsible for the increased MS risk observed in females over the past decades. This hypothesis was tested using the EAE model in mice exposed to BPA during gestation and early development. Although BPA and dietary phytoestrogens affected antigen-specific production of some of the cytokines associated with EAE, these effects did not translate into significant changes in EAE severity. Given the fact that the efficacy of our BPA exposure protocol was confirmed by alterations in several indicators of endocrine disruption, including pup weight and reproductive organ development, our results do not support BPA as a modifier of CNS autoimmunity.
Multiple reports examined immune function and cytokine production by T lymphocytes after gestational BPA exposure. Holladay et al. (2010) reported an increase in concanavalin A–induced IL-17, granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, and IL-4 production in male C57B6/129svj mice exposed to BPA in utero, although this study was statistically underpowered and has not been reproduced. Other reports observed enhanced Th1 and Th2 responses in DBA/1 and BALB/c mice (Yan et al., 2008; Yoshino et al., 2004). Additional reports showed diminished T regulatory cell (Treg) numbers (Ohshima et al., 2007; Yan et al., 2008), in one case together with enhanced Th2 but diminished Th1 responses (Ohshima et al., 2007). Consistent with enhanced Th2 responses and diminished Treg numbers, gestational BPA exposure resulted in exacerbated experimental asthma in mice (Bauer et al., 2012; Midoro-Horiuti et al., 2010; Nakajima et al., 2012). Importantly, the asthma findings in mice are also supported by several recent human studies that show associations between BPA levels in urine and asthma incidence (Donohue et al., 2013; Spanier et al., 2012; Vaidya and Kulkarni, 2012). In contrast, with regard to autoimmune disease, there is one recent report that shows a lack of effect of developmental or adult BPA exposure in an experimental colitis model, a model of tissue-specific autoimmunity driven by Th1/17 cells (Roy et al., 2013), and another report examining the role of adult BPA exposure in a lupus model, where BPA actually dampened Th1 responses and delayed autoimmunity (Sawai et al., 2003). Lastly, in full agreement with our results, the aforementioned study also reported a lack of an effect of gestational BPA exposure on EAE, although experimental data pertaining to this observation or information regarding the EAE model used were not shown (Roy et al., 2013). Taken together with our results demonstrating no effect of gestational BPA exposure in a Th1/17-driven EAE model, these findings suggest a more prominent role for BPA in allergic responses, with no effect on Th1/17-mediated autoimmunity. Similarly, gestational BPA exposure did not affect adaptive antiviral responses or virus clearance in an influenza infection model in mice (Roy et al., 2012).
Our findings that gestational BPA exposure influenced gene expression in the adult testes are in line with previous studies demonstrating various effects of similar exposure on male gonad development (Honma et al., 2002; Miao et al., 2011; vom Saal et al., 1998; Xi et al., 2011), although to our knowledge, a genome-wide transcriptomic analysis has never been carried out. Thus, our analysis not only generates a useful database for comparisons with effects of other endocrine disruptors on testes but also generates insights into the mechanism of action of BPA. Gene pathway analysis suggests dysregulated proliferation and apoptosis, and this is supported by histological observations. We also observed strain-specific BPA-induced downregulation of Pdgfa, suggesting that the endocrine-disrupting effects of BPA on the testes in B6 and SJL mice may involve different molecular pathways. PDGF signaling is well documented to be critical for testes development and function. Previous reports show that BPA and other estrogens can stimulate testicular gonocyte proliferation in a PDGF-dependent manner in vitro (Thuillier et al., 2010). It is likely that in our in vivo experiments, this process is dysregulated by continuous gestational BPA exposure, which in the end leads to downregulation of PDGF and germ cell apoptosis. The finding that BPA exposure can alter the expression of receptors for PDGF in neonatal testes (Thuillier et al., 2003) further supports dysregulation of the PDGF signaling pathway as a general theme in BPA-mediated endocrine disruption in the testes. Other differentially expressed genes of interest included cell cycle/apoptosis-related genes: Vrk3, c3, Cdk2, Cdc42ep3, Ikbip, Nap1l1, Smoc2 (Cerione, 2004; Kang and Kim, 2008; Lee et al., 2007; Liu et al., 2008; Park and Luger, 2006; Ricklin et al., 2010); or genes with documented or hypothetical function in the testes or sperm development/motility: Ropn1l/ASP, Dpcd, Ssxb2, Tnp2, Prm1, Odf2, Smoc2 (Chen et al., 2003; Fiedler et al., 2013; Haueter et al., 2010; Hoyer-Fender et al., 1998; Pazin and Albrecht, 2009; Zariwala et al., 2004; Zhao et al., 2004). These results are also consistent with dysregulated apoptosis in the testes.
The observation that BPA-enhanced degeneration in the testes was most often associated with late stages of spermatogenesis suggests potential developmentally induced alterations in Sertoli cell–dependent germ cell maturation. The results from the testes microarray studies are consistent with the histopathologic observations and provide further support that this BPA exposure paradigm appears to affect Sertoli cells. A limitation of this analysis was the few testes available from diet-matched controls and exclusion of testes due to substantial artifact from fixation or tearing of tissue from sectioning (Table 1). Our results are consistent with multiple previous reports showing effects of BPA on male gonad development and sperm production (Honma et al., 2002; Miao et al., 2011; vom Saal et al., 1998; Xi et al., 2011) but are at odds with a different study showing a lack of effect of BPA on Sertoli cells (LaRocca et al., 2011). However, the latter report used a much shorter BPA exposure protocol in C57BL/6N mice (gestational days 10–16), whereas in our study exposure began prior to pregnancy. Moreover, C57BL/6N mice, a substrain of C57BL/6J mice that have been separated since 1951, have extensive genetic differences (http://phenome.jax.org) that most certainly contribute to phenotypic differences between the substrains (Bryant, 2011). Repeated studies and follow-up studies focusing specifically on Sertoli cell function will be necessary to confirm our observations.
Many recent reports have implicated BPA exposure in a wide variety of physiological abnormalities, and, as a result of this, many countries have banned the use of BPA in food packaging (Erler and Novak, 2010). Yet, compared with other xenoestrogens, for example, DES, there is often a lack of consensus on the effects of BPA. This may be in part due to the fact that many different exposure protocols and animal models are used and the doses of BPA vary widely from study to study (Richter et al., 2007). It is now recognized that some physiological responses to BPA and other environmental agents do not always show classical dose dependence (Fagin, 2012). This is also a limitation in the interpretation of our study, as only one dose of BPA was used, although this exact dose and exposure protocol was sufficient to exacerbate experimental asthma in mice in several studies (Midoro-Horiuti et al., 2010; Nakajima et al., 2012) and affected brain and testes development in another study (Kabuto et al., 2004). Another potential limitation of our study is that animals were maintained on a normal diet (containing phytoestrogens) after developmental BPA exposure; hence, it is possible that this adulthood exposure to phytoestrogens may have negated any effects of developmental BPA exposure on EAE. However, this is not supported by our data because maintaining the animals on normal chow after weaning did not mask the effects of BPA on cytokine responses (Figs. 3 and 4). Although BPA binds to and activates estrogen receptors 1000- to 10,000-fold less efficiently than estradiol (Kuiper et al., 1998), BPA can pass the placenta and result in unparalleled exposure to fetuses (Balakrishnan et al., 2010; Nishikawa et al., 2010). In addition, many reports suggest that it may activate nonclassical estrogen signaling pathways, or affect other nuclear receptors, such as the arylhydrocarbon receptor and peroxisome proliferator–activated receptors (Richter et al., 2007; Rogers et al., 2013). Although this may be another explanation for the lack of consistent results with BPA studies, it is also possible that BPA exposure alone may not be sufficient to affect the complex physiological processes that culminate in autoimmune disease. In combination with exposure to other environmental agents, whether estrogenic (e.g., equol) (Shor et al., 2012) or not (e.g., vitamin D) (Handunnetthi et al., 2010), gestational BPA exposure may indeed influence the incidence of adult onset disease. Further studies will be needed to address these possibilities. At present, our data suggest that gestational BPA exposure does not affect EAE development, and thus do not support gestational BPA exposure as a significant contributor to the increasing female MS risk.
FUNDING
National Institute of Health’s National Institute of Neurological Disorders and Stroke (AI041747, NS036526, NS060901 to C.T.); Pilot Research Award (PP1728) from the National Multiple Sclerosis Society to C.T.; postdoctoral fellowship (FG 1911-A-1) from the National Multiple Sclerosis Society to D.N.K.
ACKNOWLEDGMENTS
The authors declare no competing financial interests.
REFERENCES
- Alizadeh M., Ota F., Hosoi K., Kato M., Sakai T., Satter M. A. (2006). Altered allergic cytokine and antibody response in mice treated with Bisphenol A. J. Med. Invest. 53, 70–80 [DOI] [PubMed] [Google Scholar]
- Balakrishnan B., Henare K., Thorstensen E. B., Ponnampalam A. P., Mitchell M. D. (2010). Transfer of bisphenol A across the human placenta. Am. J. Obstet. Gynecol. 202, 393.e1–393.e7 [DOI] [PubMed] [Google Scholar]
- Barnes S. (2010). The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol. 8, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basciani S., Mariani S., Spera G., Gnessi L. (2010). Role of platelet-derived growth factors in the testis. Endocr. Rev. 31, 916–939 [DOI] [PubMed] [Google Scholar]
- Bauer S. M., Roy A., Emo J., Chapman T. J., Georas S. N., Lawrence B. P. (2012). The effects of maternal exposure to bisphenol A on allergic lung inflammation into adulthood. Toxicol. Sci. 130, 82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant C. D. (2011). The blessings and curses of C57BL/6 substrains in mouse genetic studies. Ann. N.Y. Acad. Sci. 1245, 31–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnik T., Haines D., Levallois P., Levesque J., Van Oostdam J., Viau C. (2010). Lead and bisphenol A concentrations in the Canadian population. Health Rep. 21, 7–18 [PubMed] [Google Scholar]
- Cerione R. A. (2004). Cdc42: New roads to travel. Trends Cell Biol. 14, 127–132 [DOI] [PubMed] [Google Scholar]
- Chen Y. T., Alpen B., Ono T., Gure A. O., Scanlan M. A., Biggs W. H., III, Arden K., Nakayama E., Old L. J. (2003). Identification and characterization of mouse SSX genes: A multigene family on the X chromosome with restricted cancer/testis expression. Genomics. 82, 628–636 [DOI] [PubMed] [Google Scholar]
- Courtney A. M., Treadaway K., Remington G., Frohman E. (2009). Multiple sclerosis. Med. Clin. North Am. 93, 451–476, ix–x. [DOI] [PubMed] [Google Scholar]
- Dodds E. C., Lawson W. (1938). Molecular structure in relation to oestrogenic activity. Compounds without a phenanthrene nucleus. Proc. R. Soc. Lond. B Biol. Sci. 125, 222–232 [Google Scholar]
- Dolinoy D. C., Huang D., Jirtle R. L. (2007). Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. U.S.A. 104, 13056–13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue K. M., Miller R. L., Perzanowski M. S., Just A. C., Hoepner L. A., Arunajadai S., Canfield S., Resnick D., Calafat A. M., Perera F. P., et al. (2013). Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J. Allergy Clin. Immunol. 131, 736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebers G. C., Sadovnick A. D., Dyment D. A., Yee I. M., Willer C. J., Risch N. (2004). Parent-of-origin effect in multiple sclerosis: Observations in half-siblings. Lancet. 363, 1773–1774 [DOI] [PubMed] [Google Scholar]
- Erler C., Novak J. (2010). Bisphenol a exposure: Human risk and health policy. J. Pediatr. Nurs. 25, 400–407 [DOI] [PubMed] [Google Scholar]
- Fagin D. (2012). Toxicology: The learning curve. Nature. 490, 462–465 [DOI] [PubMed] [Google Scholar]
- Fiedler S. E., Dudiki T., Vijayaraghavan S., Carr D. W. (2013). Loss of R2D2 proteins ROPN1 and ROPN1L causes defects in murine sperm motility, phosphorylation, and fibrous sheath integrity. Biol. Reprod. 88, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. S., Jensen S. M., Lunceford J. K., Kahn M. W., Zheng Y., Falase E. A., Lyttle C. R., Teuscher C. (1997). Evidence for the genetic control of estradiol-regulated responses. Implications for variation in normal and pathological hormone-dependent phenotypes. Am. J. Pathol. 150, 2223–2230 [PMC free article] [PubMed] [Google Scholar]
- Handunnetthi L., Ramagopalan S. V., Ebers G. C. (2010). Multiple sclerosis, vitamin D, and HLA-DRB1*15. Neurology. 74, 1905–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haueter S., Kawasumi M., Asner I., Brykczynska U., Cinelli P., Moisyadi S., Bürki K., Peters A. H., Pelczar P. (2010). Genetic vasectomy-overexpression of Prm1-EGFP fusion protein in elongating spermatids causes dominant male sterility in mice. Genesis. 48, 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera B. M., Ramagopalan S. V., Lincoln M. R., Orton S. M., Chao M. J., Sadovnick A. D., Ebers G. C. (2008). Parent-of-origin effects in MS: Observations from avuncular pairs. Neurology. 71, 799–803 [DOI] [PubMed] [Google Scholar]
- Holladay S. D., Xiao S., Diao H., Barber J., Nagy T., Ye X., Gogal R. M., Jr (2010). Perinatal bisphenol A exposure in C57B6/129svj male mice: Potential altered cytokine/chemokine production in adulthood. Int. J. Environ. Res. Public Health. 7, 2845–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S., Suzuki A., Buchanan D. L., Katsu Y., Watanabe H., Iguchi T. (2002). Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod. Toxicol. 16, 117–122 [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers I. A., Liu F., Aulchenko Y. S., Ebers G. C., Oostra B. A., van Duijn C. M., Hintzen R. Q. (2008). Maternal transmission of multiple sclerosis in a dutch population. Arch. Neurol. 65, 345–348 [DOI] [PubMed] [Google Scholar]
- Hoyer-Fender S., Petersen C., Brohmann H., Rhee K., Wolgemuth D. J. (1998). Mouse Odf2 cDNAs consist of evolutionary conserved as well as highly variable sequences and encode outer dense fiber proteins of the sperm tail. Mol. Reprod. Dev. 51, 167–175 [DOI] [PubMed] [Google Scholar]
- Kabuto H., Amakawa M., Shishibori T. (2004). Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 74, 2931–2940 [DOI] [PubMed] [Google Scholar]
- Kang T. H., Kim K. T. (2008). VRK3-mediated inactivation of ERK signaling in adult and embryonic rodent tissues. Biochim. Biophys. Acta. 1783, 49–58 [DOI] [PubMed] [Google Scholar]
- Kuiper G. G., Lemmen J. G., Carlsson B., Corton J. C., Safe S. H., van der Saag P. T., van der Burg B., Gustafsson J. A. (1998). Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 139, 4252–4263 [DOI] [PubMed] [Google Scholar]
- LaRocca J., Boyajian A., Brown C., Smith S. D., Hixon M. (2011). Effects of in utero exposure to Bisphenol A or diethylstilbestrol on the adult male reproductive system. Birth Defects Res. B Dev. Reprod. Toxicol. 92, 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. H., Jeon Y. T., Kim S. H., Song Y. S. (2007). NF-kappaB as a potential molecular target for cancer therapy. Biofactors. 29, 19–35 [DOI] [PubMed] [Google Scholar]
- Liu P., Lu J., Cardoso W. V., Vaziri C. (2008). The SPARC-related factor SMOC-2 promotes growth factor-induced cyclin D1 expression and DNA synthesis via integrin-linked kinase. Mol. Biol. Cell. 19, 248–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao M., Yuan W., He Y., Zhou Z., Wang J., Gao E., Li G., Li D. K. (2011). In utero exposure to bisphenol-A and anogenital distance of male offspring. Birth Defects Res. A Clin. Mol. Teratol. 91, 867–872 [DOI] [PubMed] [Google Scholar]
- Midoro-Horiuti T., Tiwari R., Watson C. S., Goldblum R. M. (2010). Maternal bisphenol a exposure promotes the development of experimental asthma in mouse pups. Environ. Health Perspect. 118, 273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai M., Uchida K., Teuscher C. (1999). The development of male reproductive organ abnormalities after neonatal exposure to tamoxifen is genetically determined. J. Androl. 20, 626–634 [PubMed] [Google Scholar]
- Nakajima Y., Goldblum R. M., Midoro-Horiuti T. (2012). Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: An animal model study. Environ. Health. 11, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M., Iwano H., Yanagisawa R., Koike N., Inoue H., Yokota H. (2010). Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ. Health Perspect. 118, 1196–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy J. H., Lucchinetti C., Rodriguez M., Weinshenker B. G. (2000). Multiple sclerosis. N. Engl. J. Med. 343, 938–952 [DOI] [PubMed] [Google Scholar]
- Noubade R., Krementsov D. N., Del Rio R., Thornton T., Nagaleekar V., Saligrama N., Spitzack A., Spach K., Sabio G., Davis R. J., et al. (2011). Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood. 118, 3290–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Yamada A., Tokuriki S., Yasutomi M., Omata N., Mayumi M. (2007). Transmaternal exposure to bisphenol a modulates the development of oral tolerance. Pediatr. Res. 62, 60–64 [DOI] [PubMed] [Google Scholar]
- Orton S. M., Herrera B. M., Yee I. M., Valdar W., Ramagopalan S. V., Sadovnick A. D., Ebers G. C. (2006). Sex ratio of multiple sclerosis in Canada: A longitudinal study. Lancet Neurol. 5, 932–936 [DOI] [PubMed] [Google Scholar]
- Park Y. J., Luger K. (2006). Structure and function of nucleosome assembly proteins. Biochem. Cell Biol. 84, 549–558 [DOI] [PubMed] [Google Scholar]
- Pazin D. E., Albrecht K. H. (2009). Developmental expression of Smoc1 and Smoc2 suggests potential roles in fetal gonad and reproductive tract differentiation. Dev. Dyn. 238, 2877–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk M., Yellayi S., Zamora A., Subramanian S., Tovey M., Vandenbark A. A., Offner H., Zachary J. F., Fillmore P. D., Blankenhorn E. P., et al. (2004). Estrogen receptor-1 (Esr1) and -2 (Esr2) regulate the severity of clinical experimental allergic encephalomyelitis in male mice. Am. J. Pathol. 164, 1915–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk M., Zamora A., Subramanian S., Matejuk A., Hess D. L., Blankenhorn E. P., Teuscher C., Vandenbark A. A., Offner H. (2003). The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am. J. Pathol. 163, 1599–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopalan S. V., Byrnes J. K., Orton S. M., Dyment D. A., Guimond C., Yee I. M., Ebers G. C., Sadovnick A. D. (2010). Sex ratio of multiple sclerosis and clinical phenotype. Eur. J. Neurol. 17, 634–637 [DOI] [PubMed] [Google Scholar]
- Ramagopalan S. V., Yee I. M., Dyment D. A., Orton S. M., Marrie R. A., Sadovnick A. D., Ebers G. C. (2009). Parent-of-origin effect in multiple sclerosis: Observations from interracial matings. Neurology. 73, 602–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. A., Birnbaum L. S., Farabollini F., Newbold R. R., Rubin B. S., Talsness C. E., Vandenbergh J. G., Walser-Kuntz D. R., vom Saal F. S. (2007). In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 24, 199–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Wirta V, Dahl L, Bruce S, Lundeberg J, Carlsson L, Williams C. (2006). Global gene expression analyses of hematopoietic stem cell-like cell lines with inducible Lhx2 expression BMC Genomics. 7, 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D., Hajishengallis G., Yang K., Lambris J. D. (2010). Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. A., Metz L., Yong V. W. (2013). Review: Endocrine disrupting chemicals and immune responses: A focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 53, 421–430 [DOI] [PubMed] [Google Scholar]
- Roper R. J., Griffith J. S., Lyttle C. R., Doerge R. W., McNabb A. W., Broadbent R. E., Teuscher C. (1999). Interacting quantitative trait loci control phenotypic variation in murine estradiol-regulated responses. Endocrinology. 140, 556–561 [DOI] [PubMed] [Google Scholar]
- Roy A., Bauer S. M., Lawrence B. P. (2012). Developmental exposure to bisphenol A modulates innate but not adaptive immune responses to influenza A virus infection. PLoS One. 7, e38448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Gaylo A., Cao W., Saubermann L. J., Lawrence B. P. (2013). Neither direct nor developmental exposure to bisphenol A alters the severity of experimental inflammatory colitis in mice [Epub ahead of print]. J. Immunotoxicol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai C., Anderson K., Walser-Kuntz D. (2003). Effect of bisphenol A on murine immune function: Modulation of interferon-gamma, IgG2a, and disease symptoms in NZB X NZW F1 mice. Environ. Health Perspect. 111, 1883–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor D., Sathyapalan T., Atkin S. L., Thatcher N. J. (2012). Does equol production determine soy endocrine effects? Eur. J. Nutr. 51, 389–398 [DOI] [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C. (2010). Environmental causes of cancer: Endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 6, 363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier A. J., Kahn R. S., Kunselman A. R., Hornung R., Xu Y., Calafat A. M., Lanphear B. P. (2012). Prenatal exposure to bisphenol A and child wheeze from birth to 3 years of age. Environ. Health Perspect. 120, 916–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearow J. L., Doemeny P., Sera R., Leffler R., Barkley M. (1999). Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science. 285, 1259–1261 [DOI] [PubMed] [Google Scholar]
- Spearow J. L., O’Henley P., Doemeny P., Sera R., Leffler R., Sofos T., Barkley M. (2001). Genetic variation in physiological sensitivity to estrogen in mice. APMIS. 109, 356–364 [DOI] [PubMed] [Google Scholar]
- Stromnes I. M., Goverman J. M. (2006). Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 1, 1810–1819 [DOI] [PubMed] [Google Scholar]
- Thuillier R., Mazer M., Manku G., Boisvert A., Wang Y., Culty M. (2010). Interdependence of platelet-derived growth factor and estrogen-signaling pathways in inducing neonatal rat testicular gonocytes proliferation. Biol. Reprod. 82, 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuillier R., Wang Y., Culty M. (2003). Prenatal exposure to estrogenic compounds alters the expression pattern of platelet-derived growth factor receptors alpha and beta in neonatal rat testis: Identification of gonocytes as targets of estrogen exposure. Biol. Reprod. 68, 867–880 [DOI] [PubMed] [Google Scholar]
- Vaidya S. V., Kulkarni H. (2012). Association of urinary bisphenol A concentration with allergic asthma: Results from the National Health and Nutrition Examination Survey 2005-2006. J. Asthma. 49, 800–806 [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Cooke P. S., Buchanan D. L., Palanza P., Thayer K. A., Nagel S. C., Parmigiani S., Welshons W. V. (1998). A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol. Ind. Health. 14, 239–260 [DOI] [PubMed] [Google Scholar]
- Wall E. H., Hewitt S. C., Liu L., del Rio R., Case L. K., Lin C. Y., Korach K. S., Teuscher C. (2013). Genetic control of estrogen-regulated transcriptional and cellular responses in mouse uterus. FASEB J. 27, 1874–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock-Guttman B., Jacobs L. D., Brownscheidle C. M., Baier M., Rea D. F., Apatoff B. R., Blitz K. M., Coyle P. K., Frontera A. T., Goodman A. D., et al. (2003). Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Mult. Scler. 9, 293–298 [DOI] [PubMed] [Google Scholar]
- Willer C. J., Dyment D. A., Risch N. J., Sadovnick A. D., Ebers G. C. (2003). Twin concordance and sibling recurrence rates in multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 100, 12877–12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C., Edvardsson K., Lewandowski S. A., Ström A., Gustafsson J. A. (2008). A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 27, 1019–1032 [DOI] [PubMed] [Google Scholar]
- Xi W., Wan H. T., Zhao Y. G., Wong M. H., Giesy J. P., Wong C. K. (2011). Effects of perinatal exposure to bisphenol A and di(2-ethylhexyl)-phthalate on gonadal development of male mice. Environ. Sci. Pollut. Res. Int. 19, 2515–2527 [DOI] [PubMed] [Google Scholar]
- Yan H., Takamoto M., Sugane K. (2008). Exposure to Bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Environ. Health Perspect. 116, 514–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S., Yamaki K., Li X., Sai T., Yanagisawa R., Takano H., Taneda S., Hayashi H., Mori Y. (2004). Prenatal exposure to bisphenol A up-regulates immune responses, including T helper 1 and T helper 2 responses, in mice. Immunology. 112, 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J. Y., Park H. Y., Lee J. W., Jung I. O., Choi K. H., Kim K., Cho K. H. (2002). Evaluation of the immune response following exposure of mice to bisphenol A: Induction of Th1 cytokine and prolactin by BPA exposure in the mouse spleen cells. Arch. Pharm. Res. 25, 946–953 [DOI] [PubMed] [Google Scholar]
- Yurino H., Ishikawa S., Sato T., Akadegawa K., Ito T., Ueha S., Inadera H., Matsushima K. (2004). Endocrine disruptors (environmental estrogens) enhance autoantibody production by B1 cells. Toxicol. Sci. 81, 139–147 [DOI] [PubMed] [Google Scholar]
- Zariwala M., O’Neal W. K., Noone P. G., Leigh M. W., Knowles M. R., Ostrowski L. E. (2004). Investigation of the possible role of a novel gene, DPCD, in primary ciliary dyskinesia. Am. J. Respir. Cell Mol. Biol. 30, 428–434 [DOI] [PubMed] [Google Scholar]
- Zhao M., Shirley C. R., Mounsey S., Meistrich M. L. (2004). Nucleoprotein transitions during spermiogenesis in mice with transition nuclear protein Tnp1 and Tnp2 mutations. Biol. Reprod. 71, 1016–1025 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Sundstrom S. A., Lyttle C. R., Teuscher C. (1989). Differential expression of estrogen-regulated CD4 and Ia positive cells in the immature rat uterus. J. Leukoc. Biol. 46, 493–496 [DOI] [PubMed] [Google Scholar]