Abstract
The acute effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure have been well documented in many vertebrate species. However, less is known about the consequences in adulthood from sublethal exposure during development. To address this, we exposed zebrafish to sublethal levels of TCDD (1h; 50 pg/ml), either in early embryogenesis (day 0) or during sexual determination (3 and 7 weeks), and assessed the effects later in adulthood. We found that exposure during embryogenesis produced few effects on the adults themselves but did affect the offspring of these fish: Malformations and increased mortality were observed in the subsequent generation. Zebrafish exposed during sexual development showed defects in the cranial and axial skeleton as adults. This was most clearly manifested as scoliosis caused by malformation of individual vertebrae. These fish also showed defects in reproduction, producing fewer eggs with lower fertilization success. Both males and females were affected, with males contributing to the decrease in egg release from the females and exposed females contributing to fertilization failure. TCDD exposure at 3 and 7 weeks produced feminization of the population. Surprisingly, part of this was due to the appearance of fish with clearly female bodies, yet carrying testes in place of ovaries. Our results show that exposures that produce little if any impact during development can cause severe consequences during adulthood and present a model for studying this process.
Key Words: TCDD, dioxin, zebrafish, endocrine disruption, skeletal, reproductive, sexual differentiation, ovary.
There is mounting evidence that environmental exposures during development influence health outcomes later in life. Understanding how early exposure causes disease in adulthood is a significant challenge (Heindel, 2007; Skogen and Overland, 2012). Some of the obstacles to understanding this are the following: (1) The time lag between cause and effect is measured in decades; (2) genetic backgrounds of exposed individuals vary; (3) if the initial exposure produces only subtle effects, the exposure event may not be noted; (4) over the 20-year period of human development, each individual is exposed to a unique set of chemicals and pathogens.
One of the most studied toxicants is 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD). TCDD is a known endocrine disruptor and potent toxicant that is a prototype for a group of chemicals acting as agonists at the aryl hydrocarbon receptor (AHR) (Rowlands and Gustafsson, 1997; Safe et al., 1998). This group of chemicals can clearly be demonstrated as toxic, but it has been difficult to determine how subacute early-life exposure affects health in later life. Studies have suggested many different effects of TCDD and TCDD-like compounds in individuals exposed in utero, through lactation, or in childhood and adolescence; however, confounding factors are always present (Clapp and Ozonoff, 2000; Mocarelli et al., 2008; Verhofstad et al., 2011). Until we understand the links between early exposure and later disease, these uncertainties make risk-benefit decisions extremely difficult.
A solution to some of these difficulties is to use model systems with controlled exposures, consistent genetic background, and life spans that are short relative to that of the observer. The zebrafish is a very small vertebrate model that meets these requirements (Carney et al., 2006b).
We have previously focused on the acute effects of TCDD exposure on zebrafish embryos (Antkiewicz et al., 2005; Belair et al., 2001; Carney et al., 2006a; Xiong et al., 2008). Because it is clear that TCDD exposure alters developmental processes, it seems likely that even sublethal exposure could produce permanent effects.
Zebrafish develop as embryos in the egg during the first 2–3 days of life, a period of intense change. By the time of hatching, most of the major organs have formed, and the resulting larvae can move in response to stimulus. The primitive gonad forms as an immature ovary at 3 weeks. By 7 weeks of age, this develops into a maturing ovary or goes down an alternate route to form a testis (Jørgensen et al., 2008; Orban et al., 2009). The decision to form an ovary or a testis is determined by both chromosomal and environmental factors; however, in zebrafish, there are no identified sex determining regions known (Siegfried, 2010). For zebrafish, as with a great many other fish species, sex is defined by the gonads and secondary sex characteristics, rather than by genotype.
In this work, we tested the effects of sublethal TCDD exposures, testing both exposure during embryogenesis and exposure during the period of gonad formation and sex determination. These two types of experiments model exposure in utero and in adolescence. We find that concentrations that produce no acute toxicity at the time of exposure cause disease in later life.
MATERIALS AND METHODS
Fish husbandry.
Zebrafish embryos (AB strain) were kept at 27°C–30°C in lightly buffered water (60mg/l Instant Ocean Salts; Aquarium Systems, Mentor, OH) with a standard 14-h/10-h light/dark cycle as described by Westerfield (2000). Fish were fed twice per day. When needed, fish were euthanized with Tricaine methanesulfonate (1.67mg/ml). The protocol for zebrafish use and maintenance was approved by the Research Animal Resources Center of the University of Wisconsin-Madison, which follows the National Institutes of Health Guide to the Care and Use of Laboratory Animals (protocol no. M00489).
TCDD exposure.
TCDD (> 99% purity; Chemsyn) was used as a stock solution in dimethyl sulfoxide (DMSO). Zebrafish were exposed for 1h to waterborne TCDD or vehicle (0.1% DMSO). For embryonic exposure, newly fertilized eggs were exposed to TCDD or vehicle for 1h, with gentle rocking in glass scintillation vials containing 10 embryos/ml of water with the indicated concentration of dosing solution. For exposure during sexual differentiation, fish were exposed at 3 weeks postfertilization (wpf) and again at 7 wpf to TCDD or vehicle (0.1% DMSO) for 1h in small glass beakers with gentle rocking. The number of fish per volume of dosing solution was 1 fish/ml at 3 wpf and 1 fish/2ml at 7 wpf. This was changed due to growth between 3 and 7 wpf. TCDD exposures were done in three successive blocks (replicates). For the embryonic exposure, each replicate included eight vials of 10 embryos per milliliter of dosing solution, for a total of n = 24. For the sexual differentiation exposure, each replicate included eight vials of five fish, for a total n = 24. Mean percent survival (± SEM) was recorded.
Scoring for sex ratios and abnormalities.
At 16 wpf, fish were separated based on secondary sex characteristics (pigmentation and body shape), and the number of males and females was recorded. Skeletal abnormalities were scored visually for each fish and recorded, and examples were photographed. All surviving fish from all three blocks were analyzed: This included a total of 58 fish in the 4 hpf exposure group, 57 fish in the 4 hpf DMSO control, 50 fish in the 3 and 7 wpf exposure group, and 52 fish in the 3 and 7 wpf DMSO control. The visual criteria for scoring males were slim body and golden stripes, whereas females were scored by the rounded abdomen and silver stripes (Westerfield, 2000). Although subjective, this method proves very reliable in separating males from females for normal husbandry and reproduction and is used worldwide.
For gonad identification, all fish were euthanized with Tricaine when they were 1 year old. An incision was made on the ventral midline, and the gonads were identified using a dissecting microscope.
Reproduction.
At 20 wpf, fish were spawned in groups approximately once every 2 weeks over the course of 4 months to assess reproductive capacity (n = 30 spawns total for each experimental group). For all spawning experiments, fish were spawned in groups of three males and three females and were allowed to spawn for 3h after lights on in the morning.
After each of these spawns, the eggs were collected and counted. Eggs were also examined 24h after spawning to determine percentage fertilized. The embryos were also housed for 7 days to evaluate edema; skeletal, swim bladder, jaw, and heart abnormalities; and survival using a dissecting microscope. For these experiments, we randomly selected 100 embryos from each spawn. However, the spawns from the fish exposed at 3 and 7 wpf yielded an average of only 68 eggs per spawn, all of which were examined (n = 30).
To identify sex-specific effects of TCDD exposure, TCDD-exposed fish were matched with control fish of the opposite sex using the same male:female ratio of 3:3 as above. Throughout the spawning period (20–36 wpf), egg release, fertilization success, and survival of the offspring were determined as described above (12 spawns total for each experimental group).
Histopathology.
Fish were euthanized with Tricaine methanesulfonate, fixed in 10% Zn formalin, and decalcified with Cal-ExII, and the fish were bisected along the sagittal plane for fixing. The specimens were dehydrated in a graded series of ethanol, cleared in xylene, embedded in paraffin, sectioned (5mm), mounted onto slides, and stained with hematoxylin and eosin (H&E). Sections were imaged using an Olympus DP72 digital camera on an Olympus S2X16 microscope.
A total of 10 male and 10 female 1-year-old fish from each experimental group were analyzed. The gonads were evaluated for lesions by analyzing four sections (using the 10× objective) in two separate levels within each fish. In the ovary, the number of atretic or atrophying follicles per section was counted and recorded. Any section that had more than one atretic or atrophying follicle per 10× field was counted as being abnormal.
Bone staining.
The skeletons of 10 adult (1 year old) fish from each experimental group were stained and analyzed using methods described previously (Verheyden and Sun, 2008; Walker and Kimmel, 2007). Fish were euthanized and fixed in 95% ethanol for 2 days, stained in a solution containing alcian blue (0.015%) and alizarin red (0.01%) for 3 days, and neutralized in 4% sodium borate overnight. The specimens were then bleached with 3% hydrogen peroxide for 1h. Soft tissues were digested with 0.1% trypsin for 5 days, and the skeletons were cleared in 1% KOH and stored in glycerol. Stained zebrafish skeletons were imaged using a Zeiss Axiocam digital camera mounted on a Zeiss Axioplan microscope. For each skeleton, the images were used to measure total length of the fish (length from snout to base of the tail) and head size (length from snout to edge of operculum).
Statistical analysis.
Results are reported as mean ± SEM. Levene’s test was used as a preliminary screen for homogeneity of variances between or among groups with equal n values, and the Brown-Forsythe test was used for groups with unequal n values. When necessary, natural log or square root data transformations were used to maintain homogeneity of variances between or among blocks. If, using these tests, variances were equal between or among the groups that are being compared (p > 0.05), Student’s t-test was used to identify differences between two groups or one-way ANOVA to detect differences among more than two groups. Fisher’s Least Significant Test was then be used for multiple comparisons. A difference of p < 0.05 was arbitrarily considered significant.
RESULTS
Identifying Sublethal Doses
We were interested in two distinct periods of vulnerability: (1) Early development encompassing embryogenesis and early larval development and (2) the period of sexual differentiation and maturation, occurring between 3 and 7 wpf. For each of these phases of development, we used dose-response experiments to identify the highest exposure levels that would have no apparent effect on survival immediately following exposure. By aiming at the higher tolerated levels, we expected to increase our chances of recognizing consequences later in life.
For the first set of dose-response experiments, we exposed newly fertilized eggs at 4h postfertilization (hpf). For the second, we exposed embryos at 3 weeks and again at 7 weeks postfertilization (wpf) to cover the periods of gonad formation and sexual determination, respectively. All exposures were for 1h in water using our standardized laboratory protocol.
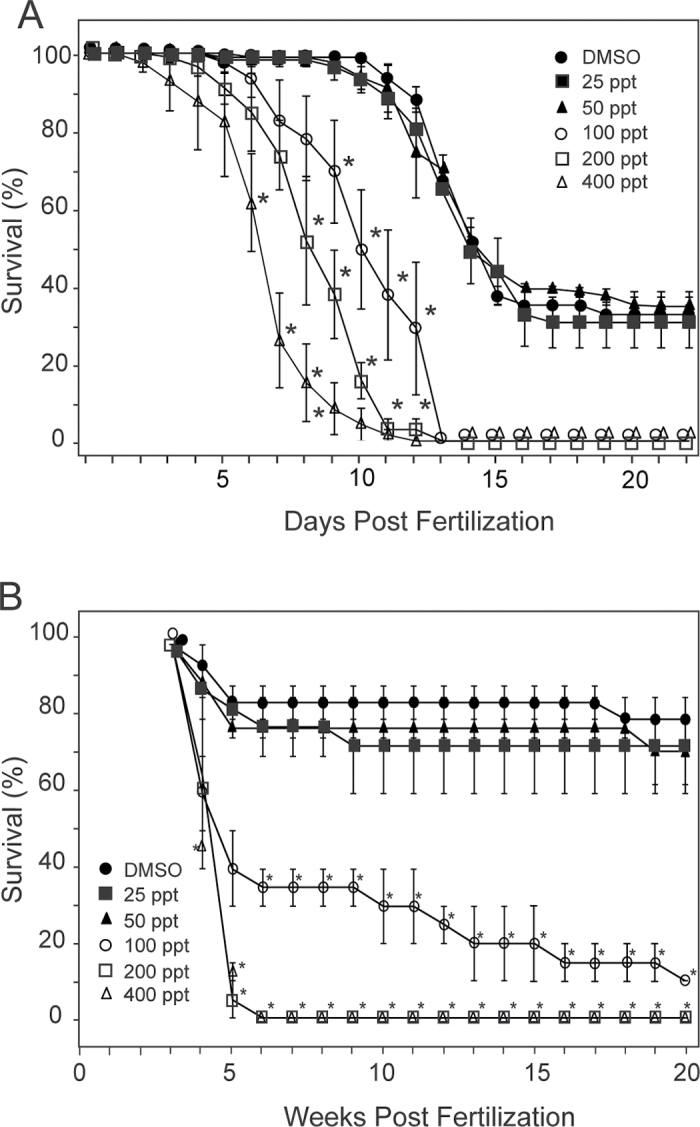
For the embryo exposure group, mortality was recorded until 22 days postfertilization (dpf), whereas the fish exposed at 3 and 7 wpf were followed until 20 wpf. In both cases, we ended the experiments after plateaus in effect were reached. It should be noted that zebrafish are very fecund, egg-scattering fish, producing larvae that frequently fail to survive the metamorphosis period at approximately 2 wpf. This is shown in the control group in Figure 1A. Because the second treatment group started with fish already at 3 wpf, the die-off had already occurred, making survival in Figure 1B look much higher. This difference in appearance is simply because of the different starting points for the two experiments.
Fig. 1.
Survival curves at different exposure levels. Zebrafish embryos or larvae were exposed to TCDD at the indicated concentrations as described in the Materials and Methods section. Surviving fish were counted at the indicated intervals. (A) Survival of fish exposed at 4 hpf. (B) Survival of fish exposed at 3 and 7 wpf. Fish were exposed and scored in individual replicate groups as described in the Materials and Methods section (n = 24 for each concentration). The asterisks indicate significant difference from the control value at that time point (p < 0.05).
At 50 pg/ml TCDD, we could not detect any impact on survival in the fish in either exposure group (Fig. 1). At the next higher dose, 100 pg/ml, mortality was significantly higher compared with controls for both types of exposure experiments, a trend that was repeated with all of the higher doses. We chose the 50 pg/ml (6.4nM) concentration for all subsequent experiments.
We set up experiments repeated in blocks exposing zebrafish to TCDD at 50 pg/ml at either 4 hpf or 3 and 7 wpf, as described above. We then followed the survival and development of the fish as they matured. The effects we found in the adults were most easily recognized in the fish that had been exposed at 3 and 7 wpf. These effects are described first.
Exposure at 3 and 7 wpf: Skeletal abnormalities.
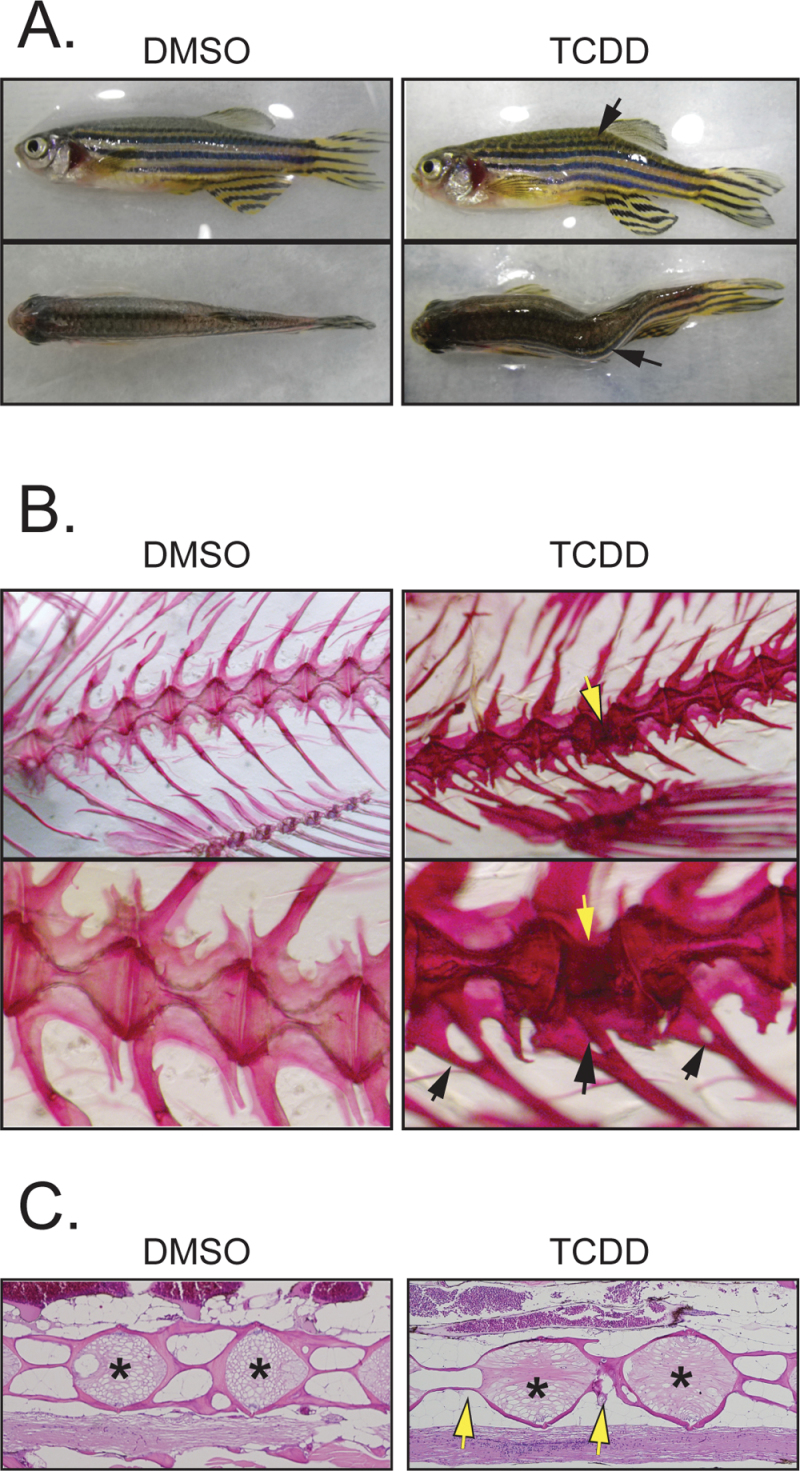
Sublethal TCDD exposure at 3 and 7 wpf caused craniofacial and spinal skeletal abnormalities, scored at 16 wpf. An example of these abnormalities is shown in Figure 2A, in which the axial skeleton is sharply angled. These kinks occurred in both the lateral and dorsal-ventral planes. The percentage of fish observed with at least one skeletal malformation was significantly increased in the treated fish at 84.6±7.4% (p < 0.001), whereas these abnormalities were only rarely seen in the controls at this age (1.8±0.9%).
Fig. 2.
Sublethal exposure causes abnormalities in the F0 axial skeleton. Zebrafish larvae were exposed to TCDD at 50 pg/ml (6.4nM) at 3 and 7 wpf and collected at 1 year of age as described in the Materials and Methods section. (A) Photographs of control and treated examples showing the lateral and dorsal views. The black arrows indicate a kink in the axial skeleton. (B) Alizarin red staining showing control and treated axial skeletons. The bottom row shows higher magnification of the images in the top row. The open arrows point to a defect in one of the vertebrae, whereas the black arrows point to malformed vertebral rays. (C) H&E-stained sagittal sections from control and treated fish. Arrows point to defects in the vertebral bodies; asterisks indicate region of the growth plate.
By staining the skeleton with alizarin red and digesting away and clarifying other tissues, we were able to obtain a detailed microscopic view of the vertebrae giving rise to the bent axis. The vertebrae in the control fish show a regular “hour glass”–like appearance, with widened end plates and narrowed vertebral bodies (Fig. 2B). Most of the vertebrae in the TCDD-exposed fish appeared normal as well. However, as indicated by the arrow in Figure 2B, it can be seen that one vertebra has been altered at the site of the axial kink. This malformed vertebra appeared to have relatively normal end plates, but the vertebral body was shortened and bent in the center, contributing to the malformed trunk. The skeletal defects observed with alizarin red staining corresponded to the kinks in the fish torsos observed prior to digestion.
We also observed defects in spinal processes indicated by the black arrows. The defects appear as holes at the base of the process as though either two spines had fused or the middle had been lost. In other examples (not shown), we observed bifurcations at the ends of ribs and spines.
The stained sections in Figure 2C show another view of the defects caused by exposure. The arrows indicate malformed vertebral bodies, in which the developing calcified walls have failed to form. The end plates, indicated by asterisks on the figure, appeared normal in the deformed spines.
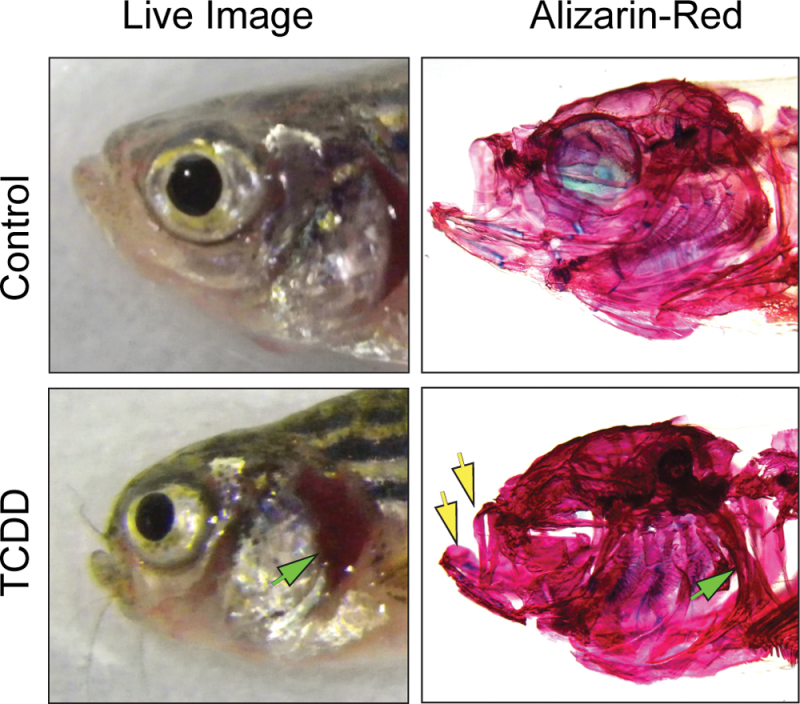
The exposure at 3 and 7 wpf also produced craniofacial malformations similar to those noted in other studies (Hornung et al., 1999; Xiong et al., 2008). The cartilages and bones making up the jaw were reduced in size, making the mouth smaller (Fig. 3). In this figure, images of individual control and exposed fish are shown together with their digested and stained skulls, showing the underlying bone structure. From this, it can be seen that the branchial arches and the operculum are shortened in the TCDD-exposed fish, whereas the bone at the top of the skull is thicker than normal. These effects produce a characteristic bump at the top of the head and expose the gills, both visible in the live fish.
Fig. 3.
Sublethal exposure causes abnormalities in bones of the F0 zebrafish head. Zebrafish larvae were exposed to TCDD at 50 pg/ml at 3 and 7 wpf and collected at 1 year of age for alizarin red staining as described in the Materials and Methods section. Photographs show lateral views of the live fish and the same view after digestion and staining, revealing the bones. Arrows point to the shortened jaw structures and to the shortened operculum in the live fish and to the altered underlying bone in the stained specimen.
Quantitative measurements of the stained skeletons revealed that TCDD treatment during development had lasting consequences in both overall length and the size of the cranial structures (Table 1). The total length of the adult fish treated at 3 and 7 wpf differentiation was decreased by 9.8% compared with the control fish (p = 0.024); the length of the upper jaw was decreased by 14.7% compared with the control, indicating that cranial structures were more impacted than the other areas of the skeleton.
Table 1.
Effects of Early TCDD Exposure on F0 Adult Morphology
| Observation | Control | Embryo exposure | Sexual differentiation |
|---|---|---|---|
| Total length | 30.39±0.70mm (15) | 30.79±1.50mm (4) | 27.40±1.00 mm* (17) |
| Upper jaw length | 7.82±0.19mm (15) | 7.77±0.56mm (4) | 6.67±0.24 mm* (17) |
| Lower jaw length | 8.48±0.17mm (15) | 8.35±0.63mm (4) | 7.50±0.24 mm* (17) |
| Axial kink | 1.1±1.1% (57) | 10.0±3.7%* (37) | 53.0±11.9%* (45) |
| Cranial malformation | 0% (57) | 3.6±3.6% (37) | 49.3±11.0%* (45) |
| Jaw malformation | 0% (57) | 0% (37) | 34.1±13.6%* (45) |
Note. Zebrafish were exposed at 4 hpf or at 3 and 7 wpf and scored as described in the Materials and Methods section. Incidences are expressed as mean percentage, whereas lengths are listed as millimeters. Uncertainty is the SEM, and the numbers in parentheses indicate the n value. The asterisk indicates significant difference (p < 0.05) from the control.
The incidences of different skeletal abnormalities in the treated and control adults are shown in Table 1. The incidences of jaw malformations, cranial malformations, and spinal malformations were all increased compared with controls in which these deformities were very rarely observed. For the group of adults exposed as embryos, the impact of TCDD exposure on skeletal growth and deformity was considerably less severe.
Exposure at 3 and 7 wpf: Effect on adult sex ratio.
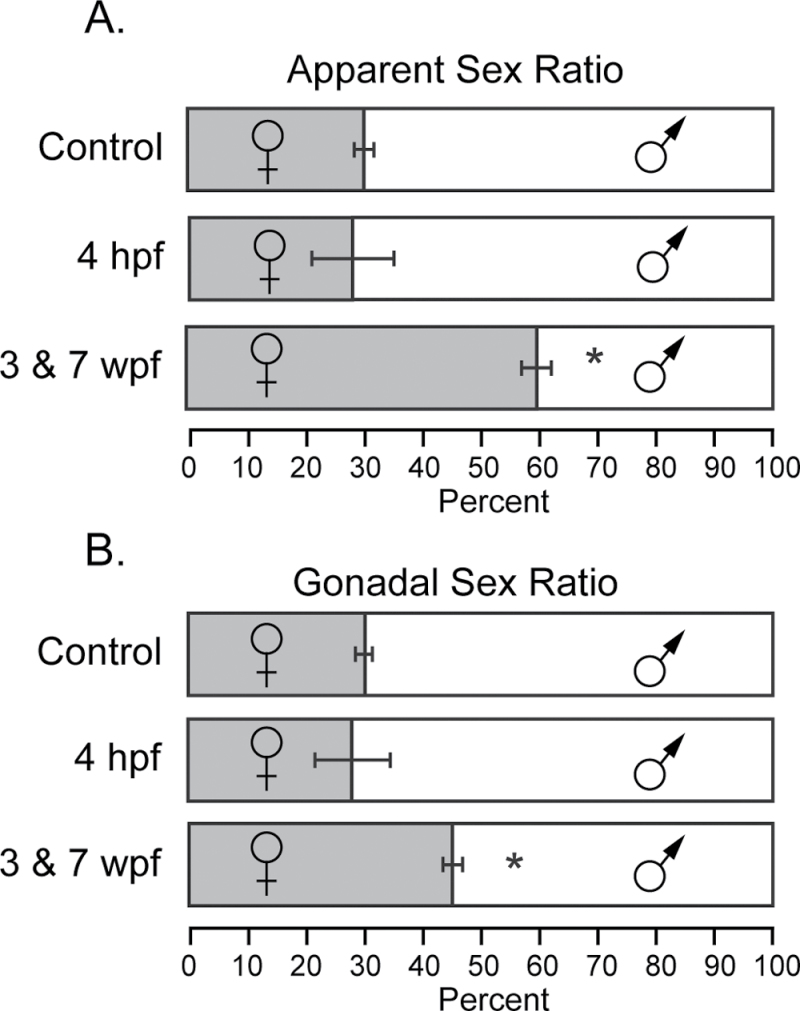
At 16 wpf, fish were scored and separated based on the most reliable secondary sex characteristics: body shape and color. We found that TCDD exposure at 3 and 7 wpf produced a pronounced shift in the sex ratio, with more than 60% of the population scored as females (Fig. 4A; p < 0.001). Although fish husbandry conditions can affect this ratio, the males are normally in the majority. Sex ratios in our control groups were statistically indistinguishable from the groups treated at 4 hpf and were consistent with the ratio we normally observe: males to females being approximately 2:1.
Fig. 4.
TCDD causes altered sex ratio in F0 adults. Zebrafish larvae were exposed to TCDD at 50 pg/ml at 3 and 7 wpf as described in the Materials and Methods section. The grey box indicates percentage of females, whereas the open box represents percentage of males. The error bars indicate SEM. (A) Sex ratios were determined by visual appearance at 12 wpf. (B) Fish were dissected at 1 year of age, and sex was scored based on gonadal sex. Control, n = 52; 4 hpf, n = 58; 3 and 7 wpf, n = 50 individuals.
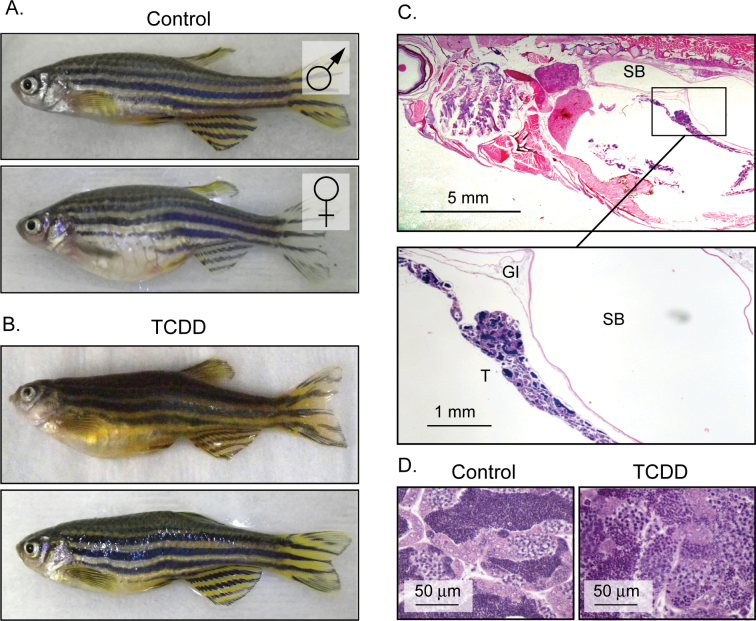
During subsequent dissection for histology, we were surprised to find frequent mismatches in which fish initially identified as females contained testes, rather than ovaries. Examples of these fish displaying the typical rounded bodies of females, yet with testes, are shown in Figure 5B. These fish are neither males nor females but have characteristics of each.
Fig. 5.
TCDD produces fish with female body form and testes in place of ovaries. Zebrafish larvae were exposed to TCDD at 50 pg/ml at 3 and 7 wpf and collected at 1 year of age to determine gonadal sex as described in the Materials and Methods section. (A) Control male and female where gonadal sex matched the scoring based on secondary sex characteristics: body shape and color. (B) Examples of fish that were scored as female based on body shape but upon histological examination contained testes. (C) Histological cross-sections (H&E stained) of an affected fish clearly showed testicular cells; the lower box is a higher magnification. (D) Higher magnification of H&E sections taken from a control male and testicular tissue taken from a fish with a female appearance.
Cross-sections of the affected fish clearly showed testicular cells in arrangements closely resembling those in a normal testis, but the overall structure was elongated and string like, not consistent with normal testicular structure (Fig. 5C). In these fish, there was no sign of ovarian tissue. Closer magnification of the sections from these fish showed what appeared to be functional testicular tissue, with spermatids moving through the normal developmental stages (Fig. 5D). These fish do not spawn; we did not determine whether they produce viable sperm.
As the fish matured and other assays were completed, we sacrificed fish from each block for dissection. This allowed us to make revised sex determinations based on the internal gonads, rather than secondary sex characteristics. By this criterion, we still observed feminization produced by TCDD exposure at 3 and 7 wpf (Fig. 4B); however, the effect was more subtle. The percentage of females rose from 29.9% in the DMSO control group to 44.5% in the TCDD-exposed group.
In the control group (n = 15), we observed no discrepancies between the secondary sex characteristics and gonadal sex. However, for those fish exposed at 3 and 7 wpf, 26.5±3.3% of those fish scored initially as females had testes (n = 30; p ≤ 0.001). Thus, TCDD treatment during sexual differentiation sometimes caused a gonad-body mismatch.
Exposure at 3 and 7 wpf: Effect on reproductive capacity.
We used spawning assays to determine the effect of early exposure to TCDD on fertility. When setting up these experiments, we were concerned that fish chosen as females might have only testes, misleading our assessment of egg production and release. We confirmed that the test fish were female by manual egg expression.
In spawning assays, TCDD exposure at 3 and 7 wpf had a significant negative impact on egg release (p < 0.001; Table 2). Egg release is the product of courtship and affected by both male and female. Therefore, alterations in the female might affect egg release, but changes in male appearance, behavior, or pheromone release might also have an effect. To better assess this, we conducted experiments in which exposed males and females were isolated for spawning with untreated control partners. We found that TCDD affected both males and females. For TCDD-exposed females, the number of eggs released per female was significantly decreased compared with the control, even when paired with untreated males (p < 0.001). The effect on the males was smaller but still significant. TCDD-exposed males elicited significantly fewer eggs from control females compared with the control pairs (p = 0.016).
Table 2.
Effect of TCDD Exposure at 3 and 7 wpf on F0 Generation Fertility
| Group | Eggs released/female | Eggs fertilized |
|---|---|---|
| Control pairs | 164.3±13.7 | 93.2±0.9% |
| TCDD pairs | 24.3±12.2* | 60.7±4.7%* |
| TCDD females | 57.6±14.3* | 86.0±2.6%* |
| TCDD males | 82.8±13.5* | 59.7±6.9* |
Note. Control and TCDD-exposed fish were spawned in groups of three males and three females as described in the Materials and Methods section. Eggs were collected, counted, and scored for fertility with an n = 30 spawns for each experimental group. The lower two rows indicate TCDD-treated fish that were crossed with control fish of the opposite sex. Numbers indicate the averages for egg release and fertility and the SEM. The asterisk indicates significant difference (p < 0.05) from the control.
Exposure to sublethal levels of TCDD at 3 and 7 wpf also significantly decreased the percentage of eggs that were fertilized (Table 2; p <0.001). As before, we crossed exposed males or females with unexposed controls to determine whether the effect had been induced in males or in females. For exposed females, we found a significant drop in percentage of fertilized eggs (Table 2; p = 0.0057). The effect on males was greater though, with TCDD-exposed males fertilizing only 60% of the eggs released by control females (p < 0.001). Thus, as with egg release, TCDD exposure during sexual development had effects on both male and female fertility.
Exposure at 3 and 7 wpf to TCDD alters ovarian development.
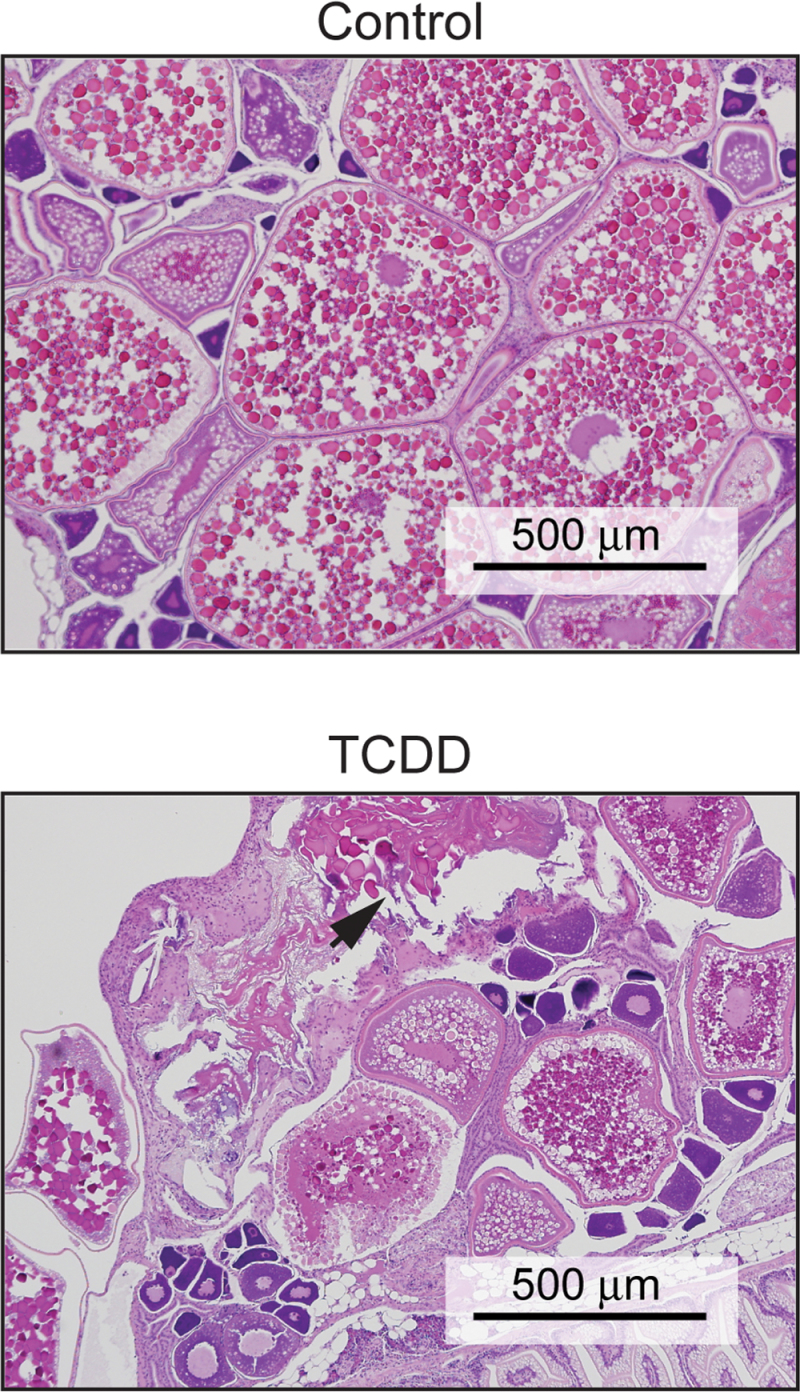
We found that TCDD exposure at 3 and 7 wpf often altered ovarian development and maturation in adult females. The top panel of Figure 6 shows an H&E-stained section of a normal adult zebrafish ovary. In this small figure, examples of all three stages of ovarian maturation are present: the small primary follicles, the intermediate secondary follicles, and the large tertiary (vitellogenic) follicles. There are no atretic follicles in this section. Fish in this age group typically have zero to one atretic follicle per 10× field.
Fig. 6.
Sublethal exposure causes abnormalities in the ovary. Zebrafish larvae were exposed to TCDD at 50 pg/ml at 3 and 7 wpf and collected at 1 year of age for hematoxylin and eosin (H&E) staining as described in the Materials and Methods section. These images are representative photomicrographs of slides from control and TCDD-exposed fish. The arrow indicates an atretic follicle.
In contrast, the section of ovary from a fish exposed at 3 and 7 wpf, as shown in Figure 6B, shows a loss of organized progression in follicle development. The section shows granulomatous inflammation, with numerous atretic follicles breaking down and few if any mature vitellogenic follicles. In some cases, the ovaries in the treated fish appeared normal (0–1 atretic follicle), but in others, the ovaries were severely disrupted, as in the figure. This disruption of ovarian organization and follicle progression was never observed in our controls but was observed in 65.6% ± 6.9 of the female fish that were exposed during sexual differentiation (n = 10; p < 0.001). We also examined the testes of exposed fish and found no histological lesions in any of the male zebrafish.
Effects of TCDD on offspring.
We also collected viable eggs from the spawning assays and scored the developing offspring for abnormalities at 7 dpf (Table 3). Rather than producing observable malformations in adulthood, the exposure at 4 hpf tended to produce abnormalities in the following generation. We found an increased incidence of uninflated swim bladders, pericardial and yolk sac edema, and curved spines in the offspring of both treated groups, but the incidence of malformations was highest in fish exposed at 4 hpf.
Table 3.
Effects of Early TCDD Exposure on F1 Generation Survival and Morphology
| Group | Mortality | Total abnormalities | Edema | Skeletal abnormalities | Uninflated swim bladder |
|---|---|---|---|---|---|
| Control | 5.2% ± 2.4 | 0.9±0.2% | 0.7±0.2% | 0.2±0.1% | 0.8±0.3% |
| 4 hpf | 19.2% ± 4.8* | 13.1±1.4%* | 8.3±0.8%* | 3.1±0.6%* | 12.0±1.3%* |
| 3 and 7 wpf | 8.6% ± 4.3* | 3.4±1.0%* | 2.2±0.6%* | 1.7±0.6%* | 3.4±1.0%* |
Note. Fish from the control and treated groups were spawned and eggs collected as described in the Materials and Methods section. Mortality was recorded daily, and fish were examined for the indicated malformations at 7 dpf. n = 30 spawns for each experimental group. Numbers indicate the average incidence and the SEM. The asterisk indicates significant difference (p < 0.05) from the control.
Survival of the offspring through development was also significantly decreased (Table 3). The malformations undoubtedly contributed to mortality, but some of the fish died with no observable cause during the 7-day period of observation. As above, offspring mortality was highest in the group treated at 4 hpf, but the offspring of fish exposed at 3 and 7 wpf were also affected.
DISCUSSION
Efforts to reduce the production and release of chlorinated and brominated aromatic hydrocarbon AHR agonists like TCDD have reduced, but not eliminated, exposure to these chemicals. An area of remaining uncertainty is the extent to which exposure during development affects health in adulthood, long after the encounter with the chemical. The zebrafish model can be useful in quickly linking exposure to latent toxicity. We found that TCDD exposures during development, producing no immediate effects at the time of exposure, predisposed the fish to problems as adults.
It is interesting that our finding of effects on skeletal formation and reproduction parallel human health outcomes thought to be caused by TCDD and related chemicals. For example, an industrial accident in Seveso, Italy, in 1976 that exposed thousands of people to TCDD was followed by a feminization of offspring from exposed individuals, prompting an article titled: Where the boys aren’t (Clapp and Ozonoff, 2000). In addition, early exposure to TCDD-like compounds has been associated with numerous types of skeletal deformities including changes in the head and axial skeleton of fish, mink, mice, rats, and hamsters (Kransler et al., 2007; Peterson et al., 1993; Takagi et al., 2000).
Gonads and Body Shape
One of our most striking results was that TCDD exposure during sexual development at 3 and 7 wpf produced fish with female body shape and male gonads. These fish cannot be characterized as males with female characteristics nor are they males with female characteristics. Instead they are intermediate, with some characteristics of each sex. They are, thus, distinct from previously discovered male fish with the phenomenon known as an ovotestis, in which oocytes develop within testes (Liney et al., 2005; Wester and Canton, 1986). We saw no signs of ovotestes or intermediate gonads in any fish examined.
We propose two different hypotheses to explain our result: It may be that TCDD changes the developmental program of some primitive gonads, originally destined to be ovaries, leading to testes formation, leaving the body shape of the fish female. An alternative is that TCDD superimposes a female body shape on a fish that was fated from the outset to develop testes.
In the first model, TCDD acts at the gonad but leaves the body form intact. In this case, there should be an increase in the total number of fish with testes and no change in the apparent sex ratio based on body shape. The second model predicts no change in the gonads, but instead a change in body shape. The results in Figure 4 agree with neither prediction perfectly but are in better agreement with the second model: There was no increase in percentage of fish with testes (rather the opposite); there was a significant increase in the percentage of fish with the female body shape.
Scoring body morphology is subjective, so finding testes in fish we scored as females naturally weakened confidence in our ability to score sex in zebrafish. In early spawning assays, several of these affected fish were placed in spawning assays with males. In these experiments, the males actively courted the fish with testes in a manner clearly indicating that to a male zebrafish, these fish appeared female. The fact that male zebrafish consistently agreed with our scoring strengthened our confidence.
Effect on Skeleton
The axial skeletal malformations that we observed would be analogous to scoliosis in humans. The effects on the head may be related to the effects on the bony palate and tooth formation reported in mammals exposed to TCDD (Bursian et al., 2013; Hornung et al., 1999; Peterson et al., 1993). Although the deformities we observed were not directly lethal under the ideal conditions of the aquarium, they would not allow survival in the wild: Craniofacial malformations made eating difficult, whereas the scoliosis reduced mobility.
One surprise was that fish could have the rounded female shape without eggs to distend the abdomen. This means that the body shape characterizing female zebrafish is not dependent on developing egg mass to make the rounded shape. This implies that TCDD had a direct effect on the formation of the body. Because the ribs play an important role in forming the abdomen, it is intriguing to think that there may be a connection between the effects we observe in the axial skeleton and this gonad to body mismatch. In many cases, we observed individual rib malformations.
Exposure Timing
We found that the timing of exposure was critical. Embryos treated at 4 hpf had fewer observable malformations as adults than their counterparts exposed at 3 and 7 wpf. The differences in effect suggest specific windows of sensitivity for the specific responses.
TCDD is known to persist in exposed individuals. Nonetheless, a fish exposed as an embryo at 4 hpf would steadily reduce its body burden of TCDD through simple dilution during growth, coupled with elimination in feces. These fish would not experience the spike of TCDD during sexual differentiation and maturation that the fish exposed at 3 and 7 wpf did. By the same token, the ratio of body mass to TCDD in the exposure solution was different for the embryonic exposure than for the later exposures. This, and the fact that in one instance the fish were exposed once and in another twice, leads to the obvious conclusion that in addition to changes in timing, the body burdens in the two groups were not comparable. Although the embryos are more sensitive to exposure than the juveniles, the chorion produces a slight barrier to uptake, and the older fish tend to more readily absorb TCDD than the embryos (Lanham et al., 2012).
Because both exposures produced birth defects in the F1 offspring, we must assume that TCDD exposure affected both the primitive and maturing germ cells.
Epidemiological studies have linked environmental exposure to TCDD and other AHR agonists with diseases including reproductive defects, heart disease, and skeletal deformations (Eskenazi et al., 2002a, b; Pelclová et al., 2006; Warner et al., 2011). However, because of the large number of confounding genetic and exposure variables for each individual, we still lack a clear connection between specific chemical exposures and specific health outcomes. Often only the adult disease consequence is noted, not the initial stressor.
A solution to this is to expose a genetically uniform population to a known amount of toxicant in early life and then follow the individuals as they mature and reproduce. The zebrafish allowed us to do just this type of experiment.
FUNDING
National Institutes of Health (NIH) (R01 ES012716) from the National Institute of Environmental Health Sciences (NIEHS) (to W.H. and R.E.P.); the University of Wisconsin Sea Grant Institute, National Sea Grant College Program, National Oceanic and Atmospheric Administration, U.S. Department of Commerce (NA 16RG2257), Sea Grant Project R/BT-22 and 25 (to W.H. and R.E.P.) and NIEHS support from T32 ES007015 and K01 OD010462 (to T.B.).
ACKNOWLEDGMENTS
We would like to thank Dorothy Nesbit, Carrie Zellmer, Kelly Mularkey, and McKinley Morganfield for technical assistance, advice, zebrafish maintenance, and husbandry. We also thank Dr. Marie Pinkerton for expertise and advice. The contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institute of Environmental Health Sciences, National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- Antkiewicz D. S., Burns C. G., Carney S. A., Peterson R. E., Heideman W. (2005). Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 84, 368–377 [DOI] [PubMed] [Google Scholar]
- Belair C. D., Peterson R. E., Heideman W. (2001). Disruption of erythropoiesis by dioxin in the zebrafish. Dev. Dyn. 222, 581–594 [DOI] [PubMed] [Google Scholar]
- Bursian S. J., Kern J., Remington R. E., Link J. E., Fitzgerald S. D. (2013). Dietary exposure of mink (Mustela vison) to fish from the upper Hudson River, New York, USA: Effects on reproduction and offspring growth and mortality. Environ. Toxicol. Chem. 32, 780–793 [DOI] [PubMed] [Google Scholar]
- Carney S. A., Chen J., Burns C. G., Xiong K. M., Peterson R. E., Heideman W. (2006a). Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol. Pharmacol. 70, 549–561 [DOI] [PubMed] [Google Scholar]
- Carney S. A., Prasch A. L., Heideman W., Peterson R. E. (2006b). Understanding dioxin developmental toxicity using the zebrafish model. Birth Defects Res. A. Clin. Mol. Teratol. 76, 7–18 [DOI] [PubMed] [Google Scholar]
- Clapp R., Ozonoff D. (2000). Where the boys aren’t: Dioxin and the sex ratio. Lancet. 355, 1838–1839 [DOI] [PubMed] [Google Scholar]
- Eskenazi B., Mocarelli P., Warner M., Samuels S., Vercellini P., Olive D., Needham L. L., Patterson D. G., Jr, Brambilla P., Gavoni N., et al. (2002a). Serum dioxin concentrations and endometriosis: A cohort study in Seveso, Italy. Environ. Health Perspect. 110, 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B., Warner M., Mocarelli P., Samuels S., Needham L. L., Patterson D. G., Jr, Lippman S., Vercellini P., Gerthoux P. M., Brambilla P., et al. (2002b). Serum dioxin concentrations and menstrual cycle characteristics. Am. J. Epidemiol. 156, 383–392 [DOI] [PubMed] [Google Scholar]
- Heindel J. J. (2007). Role of exposure to environmental chemicals in the developmental basis of disease and dysfunction. Reprod. Toxicol. 23, 257–259 [DOI] [PubMed] [Google Scholar]
- Hornung M. W., Spitsbergen J. M., Peterson R. E. (1999). 2,3,7,8- Tetrachlorodibenzo-p-dioxin alters cardiovascular and craniofacial development and function in sac fry of rainbow trout (Oncorhynchus mykiss). Toxicol. Sci. 47, 40–51 [DOI] [PubMed] [Google Scholar]
- Jørgensen A., Morthorst J. E., Andersen O., Rasmussen L. J., Bjerregaard P. (2008). Expression profiles for six zebrafish genes during gonadal sex differentiation. Reprod. Biol. Endocrinol. 6, 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kransler K. M., McGarrigle B. P., Olson J. R. (2007). Comparative developmental toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the hamster, rat and guinea pig. Toxicology. 229, 214–225 [DOI] [PubMed] [Google Scholar]
- Lanham K. A., Peterson R. E., Heideman W. (2012). Sensitivity to dioxin decreases as zebrafish mature. Toxicol. Sci. 127, 360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liney K. E., Jobling S., Shears J. A., Simpson P., Tyler C. R. (2005). Assessing the sensitivity of different life stages for sexual disruption in roach (Rutilus rutilus) exposed to effluents from wastewater treatment works. Environ. Health Perspect. 113, 1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarelli P., Gerthoux P. M., Patterson D. G., Jr, Milani S., Limonta G., Bertona M., Signorini S., Tramacere P., Colombo L., Crespi C., et al. (2008). Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ. Health Perspect. 116, 70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban L., Sreenivasan R., Olsson P. E. (2009). Long and winding roads: Testis differentiation in zebrafish. Mol. Cell. Endocrinol. 312, 35–41 [DOI] [PubMed] [Google Scholar]
- Pelclová D., Urban P., Preiss J., Lukás E., Fenclová Z., Navrátil T., Dubská Z., Senholdová Z. (2006). Adverse health effects in humans exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Rev. Environ. Health. 21, 119–138 [DOI] [PubMed] [Google Scholar]
- Peterson R. E., Theobald H. M., Kimmel G. L. (1993). Developmental and reproductive toxicity of dioxins and related compounds: Cross-species comparisons. Crit. Rev. Toxicol. 23, 283–335 [DOI] [PubMed] [Google Scholar]
- Rowlands J. C., Gustafsson J. A. (1997). Aryl hydrocarbon receptor-mediated signal transduction. Crit. Rev. Toxicol. 27, 109–134 [DOI] [PubMed] [Google Scholar]
- Safe S., Wang F., Porter W., Duan R., McDougal A. (1998). Ah receptor agonists as endocrine disruptors: Antiestrogenic activity and mechanisms. Toxicol. Lett. 102-103, 343–347 [DOI] [PubMed] [Google Scholar]
- Siegfried K. R. (2010). In search of determinants: Gene expression during gonadal sex differentiation. J. Fish Biol. 76, 1879–1902 [DOI] [PubMed] [Google Scholar]
- Skogen J. C., Overland S. (2012). The fetal origins of adult disease: A narrative review of the epidemiological literature. JRSM Short Rep. 3, 59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T. N., Matsui K. A., Yamashita K., Ohmori H., Yasuda M. (2000). Pathogenesis of cleft palate in mouse embryos exposed to 2,3,7, 8-tetrachlorodibenzo-p-dioxin (TCDD). Teratog. Carcinog. Mutagen. 20, 73–86 [DOI] [PubMed] [Google Scholar]
- Verheyden J. M., Sun X. (2008). An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature. 454, 638–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhofstad N., van Oostrom C. T., Zwart E., Maas L. M., van Benthem J., van Schooten F. J., van Steeg H., Godschalk R. W. (2011). Evaluation of benzo(a)pyrene-induced gene mutations in male germ cells. Toxicol. Sci. 119, 218–223 [DOI] [PubMed] [Google Scholar]
- Walker M. B., Kimmel C. B. (2007). A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 82, 23–28 [DOI] [PubMed] [Google Scholar]
- Warner M., Mocarelli P., Samuels S., Needham L., Brambilla P., Eskenazi B. (2011). Dioxin exposure and cancer risk in the Seveso Women’s Health Study. Environ. Health Perspect. 119, 1700–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester P. W., Canton J. H. (1986). Histopathological study of Oryzias-Latipes (Medaka) after long-term beta-hexachlorocyclohexane exposure. Aquat. Toxicol. 9, 21–45 [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). 4th ed University of Oregon Press, Eugene, OR: [Google Scholar]
- Xiong K. M., Peterson R. E., Heideman W. (2008). Aryl hydrocarbon receptor-mediated down-regulation of sox9b causes jaw malformation in zebrafish embryos. Mol. Pharmacol. 74, 1544–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]